Abstract
Background and Aims
An intense pollen–pistil interaction precedes fertilization. This interaction is of particular relevance in agronomically important species where seeds or fruits are the edible part. Over time some agronomically species have been selected for the ability to produce fruit without seeds. While this phenomenon is critical for commercial production in some species, very little is known about the events behind the production of seedless fruit. In this work, the relationship between pollen–pistil interaction and the onset of fruiting was investigated in citrus mandarin.
Methods
Pistils were sequentially examined in hand-pollinated flowers paying attention to pollen-tube behaviour, and to cytochemical changes along the pollen-tube pathway. To evaluate which of these changes were induced by pollination/fertilization and which were developmentally regulated, pollinated and unpollinated pistils were compared. Also the onset of fruiting was timed and changes in the ovary examined.
Key Results
Conspicuous changes occurred in the pistil along the pollen-tube pathway, which took place in a basipetal way encompassing the timing of pollen-tube growth. However, these changes appear to be developmentally regulated as they happened in the same way and at the same time in unpollinated flowers. Moreover, the onset of fruiting occurred prior to fertilization and the very same changes could be observed in unpollinated flowers.
Conclusions
Pollen–pistil interaction in citrus showed similarities with unrelated species and families belonging to other taxa. The uncoupling of the reproductive and fruiting processes accounts for the parthenocarpic ability of unpollinated flowers to produce fruit in citrus. However, the maintenance of a functional reproductive process reflects the potential to produce seeded fruits, providing a basis for the understanding of the production of seeded or unseeded fruits and further understanding of the process of parthenocarpy in other species.
Keywords: Citrus, flower development, mandarin, obturator, ovary, papillae, parthenocarpy, pistil, pollen-tube competition, seedlessness, stigma, stylar canal
INTRODUCTION
Pollen–pistil interaction precedes fertilization in the flower. Important changes occur in the pistil, which play a role supporting, but also controlling pollen-tube growth (Herrero and Hormaza, 1996; Hiscock and Allen, 2008; Kumar and McClure, 2010). One of the best known control systems of pollen–pistil interaction is pollen–pistil incompatibility (Heslop-Harrison, 2000; McClure and Franklin-Tong, 2010), but pollen–pistil interactions also play an important role in pollen competition and selection during compatible mating (Hormaza and Herrero, 1992; Kumar and McClure, 2010). Other changes do play a preventative role in what could be a vulnerable entrance point for pathogens (Heslop-Harrison, 2000). Changes in the pistil also regulate pollen-tube directionality (Herrero, 2000, 2003; Stewman et al., 2010), and the localization of mutants in model species is showing which genes are responsible for these interactions (Chapman and Goring, 2010; Lausser et al., 2010). Failure in these genes results in failure in seed production.
Pollen–pistil interaction is of a particular relevance in agronomically important species in which the seeds of fruit production are the edible part. However, agricultural practice over the years appears to have selected in some species the production of fruits without seeds (Varoquaux et al., 2000), as it occurs in bananas (Heslop-Harrrison and Schwarzacher, 2007) and citrus (Talon et al., 1992). This trait may be desirable in species in which seeds are a nuisance at eating. It has also been induced recently in other species such as watermelon and grapes (Bouquet and Danglot, 1996; Sugiyama et al., 2002) and has been found in a natural mutant of Annona (Lora et al., 2011). Agricultural selection has thus been towards failure in the reproductive process. However, very little is known about the mechanisms behind this lack of seeds in traditionally seedless crops. Apart from the fact that parthenocarpic fruit development is triggered by a deregulation of the hormonal balance (Vivian-Smith et al., 2001; Gorguet et al., 2005). In particular, altered levels of phytohormones have been observed during fruit growth in naturally occurring parthenocarpy in citrus plants (Talon et al., 1990, 1992). Studies in mutants of tomato (Solanum lycopersicum) and Arabidopsis thaliana have revealed that the hormonal signalling pathway is implicated in repressing fruit initiation in the absence of fertilization resulting in parthenocarpic fruit (Vivian-Smith et al., 2001; Goetz et al., 2007). A different mechanism has recently been reported in Annona, where deletion of the INO locus, responsible for ovule outer integument development, results in seedless fruits in a natural mutant (Lora et al., 2011). Citrus appear as especially interesting, because they are often seedless, but also, at least some of them as mandarins, maintain their capacity to produce seeds.
Citrus species are grown in tropical and sub-tropical climates, being the base of a prosperous industry in >100 countries, with a total annual production of over 120 million tones (FAOstat, 2009). Citrus is an old crop, having been cultivated for over 4000 years, with great diversity and apparently distant centres of origin (Khan, 2007). This variability contrasts with the permissive intercrossability between species. Indeed, the vast majority of cultivated citrus is derived from interspecific crosses between ancestral species (Nicolosi et al., 2000). In spite of this variability, a vast majority of these species are parthenocarpic and able to produce seedless fruits without fertilization. This character could have been selected early and be related to their early domestication and growing.
In mandarins, not all cultivars are equally parthenocarpic. However, the recent introduction of new mandarin cultivars to widen the production calendar has resulted in the production of seeded fruits. This occurrence is a problem that causes serious economic losses in different parts of the world, and a number of alternatives have been explored (Vardi et al., 2008), which range from the regulation of pollen flow (Chao et al., 2005) to the use of treatments such as insect repellents (Pons et al., 1996), or to reduce pollen viability (Mesejo et al., 2006). In addition, a number of breeding strategies have been explored such as the introduction of cytoplasmic male sterility (Guo et al., 2004), ploidy manipulation for increasing sterility (Grosser et al., 2000; Navarro et al., 2004; Grosser and Gmitter, 2005; Reforgiato et al., 2005), induced mutation (Spiegel-Roy and Vardi, 1981) and transgenic approaches (Li et al., 2002). However, a definitive answer is still elusive. The search for alternatives contrasts with the paucity of data on the underlying cause of the problems, namely the reproductive process.
Indeed, information on the processes underpinning fruit production in citrus, which constitutes the basis of this industry, is surprisingly thin. However, embryologists were interested in citrus early. Braun (1860) and Strasburger (1878) recorded the occurrence of polyembryony, and this research was followed by the more detailed work of Osawa (1912). Flower-bud differentiation was described long ago (Abbot, 1935) as well as the flower anatomy of the Aurantiodeae (Tillson and Bamford, 1938) and lemon (Ford, 1942). Later, the ultrastructure of stigma and style of C. limon was characterized (Ciampolini et al., 1981; Cresti et al., 1982) as well as the general structure of the gynoecium (Soost and Roose, 1996). Additionally, pollen-tube behaviour was recorded in compatible and incompatible pollinations (Ton and Krezdorn, 1966; Kahn and DeMason 1986, 1988). These studies reveal a well-conserved anatomy of the gynoecium in different species of Citrus. However, information is lacking on the reproductive process and the early changes accompanying the onset of fruiting.
In this work, the pollen–pistil interaction was examined in a widely grown mandarin ‘Nova’ [tangelo Orlando (C. paradisi × C. reticulata) × C. clementina] and the sequence of events related with those occurring at the onset of fruiting. The sequential comparison, of pollinated and unpollinated flowers, sheds light on which of these processes are induced by pollination/fertilization and which are developmentally regulated, as well as providing a reference line for understanding the fruiting process in this genus.
MATERIALS AND METHODS
Plant material
Adult trees of the ‘Nova’ and ‘Fortune’ (Citrus clementina Hort. ex Tan. × C. reticulata Blanco) cultivars grown at the ‘Primosole’ experimental farm of the University of Catania in Sicily were used for this study. These were chosen for their widespread use in major citrus-producing Mediterranean countries.
Pollination procedures
Pollen was obtained from ‘Fortune’. For this purpose, anthers from flowers were collected 1 d prior to anthesis. The anthers were left to dehisce on paper at room temperature (about 25 °C) for 1 d, and fresh pollen was immediately used for pollination. Additionally, ‘Nova’ flowers were emasculated 1 d before anthesis, pollinated with pollen from ‘Fortune,’ and bagged with cotton tissue. A batch of pistils, similarly emasculated and bagged, was left unpollinated.
Microscopy
Pollinated and unpollinated pistils of ‘Nova’ were sampled sequentially every 2 d, seven times, until 14 d after pollination. The collected pistils were cut into three sections (stigma, style and ovary). Ten pistils per treatment were fixed in 2·5 % glutaraldehyde in 50 mm phosphate buffer (pH 7·2), dehydrated in a graded ethanol series up to 70 % ethanol, and stored at 4 °C. Subsequently, they were dehydrated in 100 % ethanol, embedded in JB-4 methacrylate (Polysciences Co. Ltd, Eppelheim, Germany), and sectioned at 3 µm on a rotary microtome (Leica, Wetzlar, Germany). Ten additional pistils were sampled and treated each day. These samples were fixed in FAA solution (5 mL formalin/5 mL glacial acetic acid/90 mL 70 % ethanol, v/v/v; Johansen, 1940), washed in 50 % ethanol for 1 h, and stored at 4 °C. Subsequently, they were dehydrated and transferred to paraffin using tertiary butyl alcohol as the intermediate solvent. The samples were left in liquid paraffin at 60 °C for 3 weeks and embedded.
Paraffin sections, 10 µm thick, were stained with 0·1 % aniline blue in 0·1 n K3PO4 (Linskens and Esser, 1957) to observe pollen tubes. Additionally, starch was localized with 2 % IKI (Johansen, 1940). JB-4 sections were stained with periodic acid–Schiff's reagent (PAS) (Jensen, 1962) for carbohydrates, 0·07 % calcofluor (Hughes and McCully, 1975) for cellulose, 0·01 % auramine (Heslop-Harrison, 1977) for cutin, 0·01 % acridine orange (Fleming et al., 1993) for RNA, and 0·02 % toluidine blue (O'Brien et al., 1964) for general staining.
Sections stained with PAS, toluidine blue and IKI were observed with bright field microscopy. Sections stained with aniline blue, calcoflour, auramine, and acridine orange were observed using a fluorescence microscope (Leica DM 2500; Leica, Wetzlar, Germany), using the I3 filter (excitation 450–490 nm) for aniline blue and calcoflour, the N2·1 filter (515–560 nm) for auramine and the A filter (340–380 nm) for acridine orange.
Evaluation of ovary growth
To evaluate the onset of fruiting, ten flowers/fruitlets, were individually weighed every 5 d from anthesis to 20 d later. This procedure was performed in pollinated and unpollinated flowers.
RESULTS
Pistil anatomy
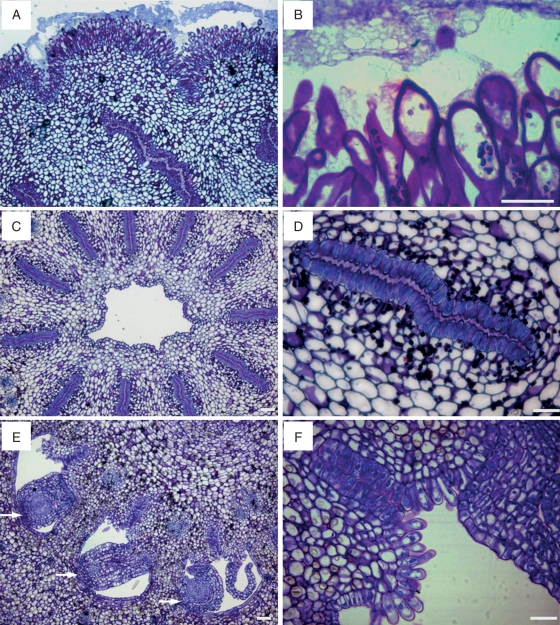
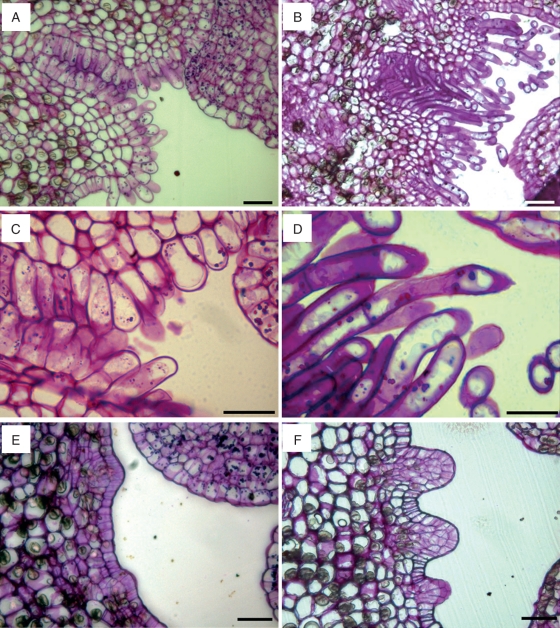
The gynoecium was formed from 10 or 11 carpels fused together, which gathered around an inner channel. While they all shared a continuous common stigma (Fig. 1), each carpel has an independent stylar canal leading to each locule, which hosted three or four ovules.
Fig. 1.
Pistil anatomy of the ‘Nova’ mandarin. (A) A common stigmatic surface gives way to different stylar canals. (B) The stigma surface is covered by unicellular and multicellular papillae varying in size. (C) A cross-section of a style with the stylar canals located radially and an inner channel (D) Each stylar canal is bordered by papillar cells. (E) Stylar canals come out in the ovary locule facing the ovules (arrows). (F) Papillar cells bordering on the stylar canals are continuous with the papilla in the placenta. JB-4-embedded longitudinal (A, B) and transverse (C–F) sections of the pistil stained with PAS and toluidine blue. Scale bars = 50 µm.
The stigma surface had unicellular and multicellular papillae (Fig. 1A), which varied in size and averaged 64·6 ± 18·9 µm. The papillae showed an increase in length toward the upper part of the stigma, and they were smaller in the stigmatic stylar canal openings. Small amyloplasts were observed in the papillae at the flower opening. Later in development, the papillae became highly vacuolated cells and produced an exudate, which covered the stigmatic surface (Fig. 1B) and were stained by auramine, acridine orange and PAS.
Stylar canals were layered radially in a common cylindrical style that had a central hollow channel in the middle (Fig. 1C). Each stylar canal looked like a buttonhole bordered by papillar cells. The stylar canal cells had a rich cytoplasm, and the cell walls facing the stylar canal were thick and secretory, with a secretion filling this canal (Fig. 1D).
Stylar canals descend towards the ovary and lead into the ovary locule, at the inner angle of each locule (Fig. 1E), in an area continuous with the stylar canal papillae that is covered by long starch-rich papilla (Fig. 1F). The placenta bears three or four anatropous ovules per locule. The ovule exostome, formed by the opening of the outer integument of the ovule, was also rich in amyloplasts and faced the papillae. However, at anthesis, these papillae did not reach the ovule exostome, and left a gap between them.
Pollen-tube growth
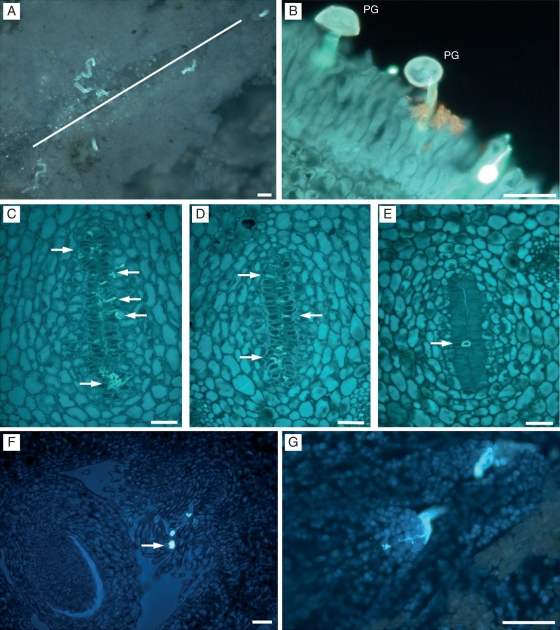
Pollen grains germinated on the stigmatic surface (Fig. 2A) and the pollen tubes reached the upper part of the style 48 h after pollination. Once germination had occurred, pollen grains appeared empty of their cytoplasmic content (Fig. 2B). The stylar canals appeared on the surface of the stigma and pollen tubes grew towards them and along the stylar canal close to the papillae bordering cells. The first pollen tubes reached the base of the style some 6 d after pollination.
Fig. 2.
Pollen tube growth, in ‘Nova’ mandarin. (A) Germinating pollen grains in the stigma with pollen tubes oriented toward the stylar canals opening (white line) on the surface of the stigma. (B) Germinated pollen grains (PG) with pollen tubes growing between the stigmatic papillae. (C–E) Pollen tube growth in the style in the upper (C), middle (D) and lower (E) part, showing a reduction in pollen-tube numbers (arrows) along the style, which is accompanied by a reduction in surface area and in the number of cells bordering the stylar canal. (F) Pollen tubes (arrow) reaching the ovary growing on the long papillae, and (G) penetrating the ovule through the micropyle. Paraffin embedded longitudinal (A, B) and transverse (C–G) sections of the pistil stained with aniline blue. Scale bars = 50 µm.
About 75 % of pollen grains germinated on the stigmatic surface and over 100 pollen tubes reached the upper part of the style. This number decreased as the pollen tubes grew along the stylar canals, and only some 8–12 pollen tubes reached the base of the style. This reduction was accompanied by a reduction in the size of the stylar canals (Figs. 2C–E), which were reduced in surface area from approx. 277 µm to 142 µm, as they descended towards the ovary. This reduction was due to a reduction in the number of cells bordering the stylar canal (Table 1). Pollen tubes reached the ovary locule through the long papillar hairs, which were continuous with the stylar canals (Fig. 2F). The pollen tube penetrated into the micropyle (Fig. 2G) and traversed the nucellus. This event occurred 12 d after pollination. Sometimes it was possible to see pollen tubes growing out of the stylar canals within the central channel of the style.
Table 1.
Radial length and number of cells on one side of the stylar canals at different levels of the style
| Upper style | Middle style | Lower style | |
|---|---|---|---|
| Radial length (μm) | 277 ± 35 | 193 ± 14 | 143 ± 15 |
| Number of cells | 57 ± 5 | 37 ± 4 | 28 ± 3 |
Changes in the stigma–style
Conspicuous changes occurred in the stigma–style after flower opening. These changes occurred along the internal space of the style and also along the pollen-tube pathway in the stigma and the style.
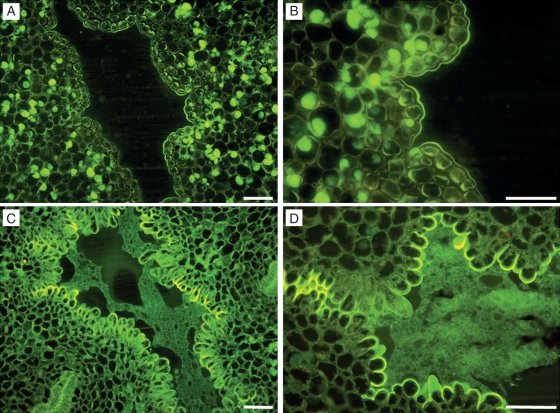
At anthesis, the internal channel, around which the different carpels gathered, appeared as an empty space (Fig. 3A) bordered by cells with thick walls (Fig. 3B). These cells appeared the same along the length of the channel. However, later, these cells changed their appearance and entered a secretory phase, which involved the production of a secretion that filled this area (Fig. 3C, D). The secretion was basipetally produced, which started at the upper part of the style, continued through the style, and all the way down to the ovary. Secretory activity began 4 d after anthesis in the upper part and filled the entire hollow inner space 6 d later. Both the walls of these cells and the secretion reacted positively to staining with auramine, acridine orange and PAS. These changes occurred in the same way and at the same times in pollinated and unpollinated flowers.
Fig. 3.
Central channel modification during flower maturation in ‘Nova’ mandarin. (A) At anthesis, in the central channel, an empty space can be observed, which is bordered by cells with thick walls (B). Some of the cells in (A) and (B) show stained lipidic contents. (C) Four days after flower-opening, cells bordering the central channel changed their appearance and entered a secretory phase (D) as the cells became papillated. JB-4-embedded transverse sections of the central canal stained with auramine. Scale bars = 50 µm.
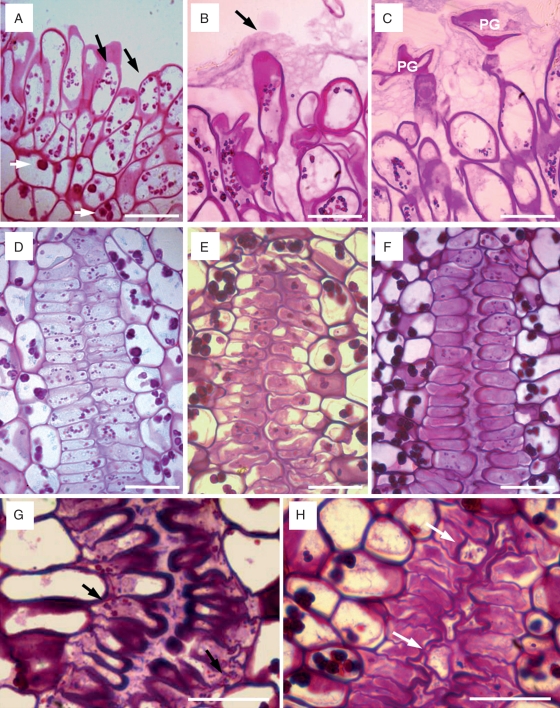
Clear changes also occurred along the pollen-tube pathway. The stigmatic papillae had starch at anthesis, although variability in the amount of starch between the different flowers of a same tree could be observed. Smaller amyloplasts of a different colour (blue, red, orange, brown) than in parenchymatic cells were observed in the papillae (Fig. 4A). However, shortly after flower opening, starch disappeared as the stigmatic secretion increased. The papillae vacuolated and secretion could be observed mainly in the papillar tips (Fig. 4B). This event occurred both in pollinated and unpollinated flowers. However, in pollinated stigmas, the stigmatic secretion had a more open appearance (Fig. 4C) than in unpollinated stigmas, which appeared denser.
Fig. 4.
Changes in the stigma and style in ‘Nova’ mandarin. (A) Stigmatic papillae at anthesis with small starch grains (black arrows) with a different appearance than starch in parenchymatic cells (white arrows). (B) Stigmatic papillae 2 d after anthesis covered with a stigmatic compact secretion (black arrow). (C) Germinated pollen grain (PG) in degraded stigmatic secretion in a flower 2 d after pollination. (D) Cells bordering the stylar canal with starch at anthesis. (E, F) Progressive degradation of starch reserves in the cells and secretion in the stylar canal 2 and 4 d after anthesis. (G) Stylar canal of an unpollinated flower 6 d after anthesis with a conspicuous secretion and small starch grains (black arrows) still present. (H) Stylar canal of pollinated flower 6 d after pollination, without starch grains, and the pollen tubes (white arrows) growing in the stylar canal. JB-4-embedded longitudinal (A–C) and transverse (D–H) sections of the stigma and style stained with PAS. Scale bars = 50 µm.
Cells bordering the stylar canal also had small starch grains at anthesis (Fig. 4D). During pollen-tube growth, starch reserves in the stylar canal cells were degraded, as a secretion appeared (Fig. 4E) to fill the internal canal (Fig. 4F). In unpollinated flowers, the stylar canal cells also entered a secretory phase, but kept some of their starch reserves (Fig. 4G). This occurred at the same time as in pollinated flowers, but the appearance of the style was completely different in pollinated flowers following pollen-tube passage. In these styles, the cells were depleted of starch and the secretion had also vanished, while profiles of pollen tubes occupied the secretion area (Fig. 4H).
Changes in the ovary
In the ovary, the papillar cells in the placenta, which are continuous with the stylar canal cells, also exhibited conspicuous changes. These papillar cells were short at anthesis (Fig. 5A), but grew and extended as the flower aged (Fig. 5B), approaching the ovule exostome. Ten to twelve days after anthesis, epidermal hairs became two-celled by a transverse division and appeared >2-fold longer than at anthesis. At anthesis, papillar cells had some starch and a well-defined cell wall (Fig. 5C). However, later on, the elongated papilla entered a secretory phase (Fig. 5D) and starch vanished in the most external part close to the pollen tubes. Secretions were stained with auramine, acridine orange and PAS. Following the production of the secretion, these cells became highly vacuolated. Secretion in this area started 6 d after anthesis and was most conspicuous 10 d after anthesis. Pollen tubes were observed traversing this area 12 d after pollination. A sequential comparison of pollinated and unpollinated flowers showed that papillar cell elongation and the production of secretion occurred in the same way and at the same time in both pollinated and unpollinated flowers.
Fig. 5.
Changes in the ovary in ‘Nova’ mandarin. Comparison of the ovary at anthesis (A, C, E), and 10 d later (B, D, F). (A) Stylar canal lined with papillar cells come out into the ovary locule. (B) Papillae have elongated 10 d after anthesis and are approaching the ovule. (C) Short papillae at anthesis with starch grains. (D) Elongated papillar cells with secretion. (E) Ovary locule at anthesis with small protuberances. (F) Development of juice vesicles 10 d after anthesis. JB-4-embedded transverse sections of the ovary stained with PAS. Scale bars = 50 µm.
Conspicuous changes were also observed in the ovary locules. At anthesis, some protuberances appeared in the locule wall (Fig. 5E), which continued to develop into the juice vesicles. These changes were most conspicuous 10 d after pollination (Fig. 5F) and prior to fertilization and could also be observed at this time in unpollinated flowers.
Early fruit growth
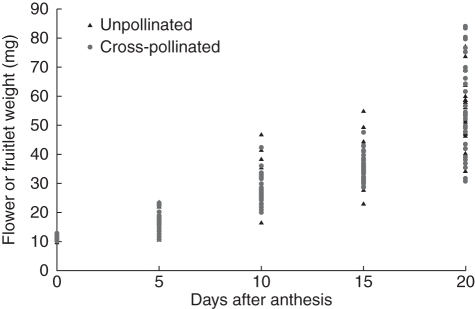
To evaluate the onset of fruiting, ten flowers/fruitlets were weighed every 5 d in both pollinated and unpollinated flowers. Clear differences existed between flowers/fruitlets within one sampling day, and these differences were most apparent on the most advanced dates. Ovary growth could be observed some 5 d after anthesis and was clearly apparent 10 d after pollination (Fig. 6). This growth occurred prior to fertilization. Surprisingly, unpollinated flowers had a similar continuous growth that was similar in weight to that of pollinated flowers.
Fig. 6.
Onset of fruiting. Weight of flowers/fruitlets, from anthesis to 20 d later, in pollinated and in unpollinated flowers. Unpollinated flowers had a similar continuous growth that paralleled in weight to that of pollinated flowers.
DISCUSSION
The anatomical features of the mandarin gynoecium are conserved with those described in other Citrus species. However, the developmental approach of this work reveals that the pistil exhibited conspicuous changes during the flower lifespan. These changes played a clear part supporting, but also constraining, pollen tube passage in both the style and also in the ovary. Most of them appeared to be developmentally regulated because they occurred the same way in unpollinated species. This observation also applied to the onset of fruiting, which occurred independently of fertilization.
A support-constrain strategy
The stigma of the ‘Nova’ mandarin is very similar to the stigma of lemon (Cresti et al., 1982), with unicellular and multi-cellular papillae that are variable in size, and covered with a conspicuous secretion that plays a part in pollen capture, adhesion and germination as it occurs in other species (Dumas et al., 1984; Swanson et al., 2004, Hiscock and Allen, 2008). While stigmatic secretion was produced equally in pollinated and unpollinated stigmas, this secretion changed in appearance following pollen-grain germination.
The stylar canals were continuous with the papillar surface of the stigma and were also filled with a secretion. However, this secretion was not present at the beginning of anthesis and was produced in a basipetal way, starting underneath the stigma and continuing down to the base of the style. Interestingly, the timing of secretion production was concomitant with pollen-tube growth along these stylar parts. While no evident changes were observed in the parenchymatic cells of the style, small amyloplasts in the cells bordering the stylar canal cells completely disappeared during pollen-tube growth – this has also been observed in species with a transmitting tissue (Herrero and Dickinson, 1979; Ciampolini et al., 1981). There is mounting evidence that pollen-tube growth, in solid or hollow styles, is heterotrophic at the expanse of the stylar reserves (Herrero and Dickinson, 1981). In hollow styles, this extracellular secretion has been shown to be incorporated into the growing pollen tubes (Kroh et al., 1971; Labarca and Loewus, 1973), and more recent evidence supports this observation in other species (McClure, 2009).
The pistil of mandarin appears to be a structure especially well adapted to support pollen-tube growth as it occurs in other species (Herrero and Hormaza, 1996). However, this support apparently is not unrestricted, and a reduction in the number of pollen tubes growing along the style was observed. A reduction in the number of pollen tubes has been reported previously in other Citrus species (Kahn and DeMason, 1986). Similarly, a reduction in the number of pollen tubes travelling along the style has been recorded in compatible pollinations in a number of unrelated species (Cruzan and Barrett, 1996; Herrero and Hormaza, 1996), and it is especially noticeable in species where the number of germinating pollen grains is higher than the number of ovules available for fertilization (Hormaza and Herrero, 1994). This pollen-tube attrition has been proposed to constitute a substrate for pollen-tube competition and prezygotic selection (Mulcahy, 1979; Hormaza and Herrero, 1994; Erbar, 2003).
The anatomy of the stylar canals in mandarin could support this strategy because they become smaller as they descend towards the ovary. This reduction in size has not been reported before, and it is due to a decrease in the number of cells that border the stylar canal. A similar fact has been described in other species with a solid style. In Prunus persica, a reduction in stylar reserves appears to be caused by a progressive reduction in the physical space occupied by the stylar transmitting tissue and also by a reduction in the amount of carbohydrates stored in this region (Herrero, 1992). It has been proposed that these anatomical changes could play a role favouring pollen-tube competition and thus facilitating pollen selection (Tilton et al., 1984; Hormaza and Herrero, 1994), with possible evolutionary and adaptive implications (Hedhly et al., 2007, 2009).
Pollen-tube growth in the ovary
In the ovary, conspicuous changes occurred during development. The papillae, which are continuous with the cells bordering the stylar canals, elongated, reaching proportions 2-fold longer and thinner than at anthesis. This growth allowed them to approach the ovule exostome as they entered a secretory phase. Pollen tubes reached the ovules through these elongated papillar cells concomitantly with this secretory phase. Thus, these papillar hairs appeared to play a role similar to an obturator, a placental proturberance that becames secretory at a particular time (Arbeloa and Herrero, 1987), which then acts as a drawbridge connecting the ovule with the base of the style.
Obturators have been observed in a number of unrelated species and families, such as Euphorbiaceae, Rosaceae and Liliaceae, as well as in many other taxa. They also have different morphological structures, such as a pad or swelling, hairs, filaments or tufts (Tilton and Horner, 1980) and appear to establish contact between the ovule micropyle and the placenta (Endress and Matthews, 2006). A chemotropic and guidance function was proposed by Tilton et al. (1984), and in some species, like Prunus, it has been shown that the obturator regulates pollen-tube access to the ovule through the production of a secretion at a particular time (Arbeloa and Herrero, 1987). Likewise, the physical approaching of the area of pollen-tube growth towards the ovule also occurs in other species, like in Pistacio, with the development of a ponticulus (Martínez-Pallé and Herrero, 1998).
Although a function for the papillar hairs in citrus described here has not been reported previously, the presence of papillar hairs in this area is ubiquitous in a wide range of Citrus species. These structures were first described in C. limon (Ford, 1942) and later in C. grandis (Banerji, 1954) and Poncirus trifoliata (Boeswinkel, 1978), and they can be multicelled as in C. australasica or unicelled as in C. australis (Clarke and Prakash, 2001). It might be worth determining whether such a similar function also occurs in other Citrus species.
A similar mechanism relating to the control of pollen-tube entrance into the ovary has been described in Zea mays, in which papillar hairs also cover the ovary entrance. As the pollen tubes pass by, these hairs loose turgidity once fertilization has occurred, preventing other pollen tubes from entering this region (Heslop-Harrison et al., 1985). Intraovarian trichomes are taxonomically widespread in monocotyledons, but they are rare in dicotyledons. In contrast to dicotyledons, monocot intraovarian trichomes are always associated with mucilage secretion (Rudall et al., 1998). It might be worth determining whether or not they could also be involved in pollen-tube passage and regulating pollen-tube access to the ovule. Pollen-tube guidance in the ovary is an active field where sporophytic (Chapman and Goring, 2010; Stewman et al., 2010) and gametophytic (Okuda et al., 2009; Yang et al., 2010) signals are supplied for pollen-tube orientation. The present results provide a frame to elucidate the function of these signals. Likewise, multicellular stigmatic hairs with independent functions from stigma and style were studied for comparative studies of pollen-tube growth in early angiosperms (Prychid et al., 2011).
Developmental changes in unpollinated flowers
The pistil in mandarin appears well adapted to support pollen-tube growth. A basipetal maturation starting at the stigma and continuing down to the ovary is carried out by a number of changes that occur at particular times, which support pollen germination and pollen-tube growth along the style, but also pollen-tube access to the ovule in the ovary. The fact that these changes are not dependent on the action of pollen tubes, but occur in the same places and at the same time in unpollinated flowers, supports the idea that these changes are developmentally regulated. Pistil development appears to be a continuous process throughout the flower lifespan and this has been described in other species (Herrero and Dickinson, 1980; Herrero and Gascón, 1987; Herrero and Arbeloa, 1989). Maturation occurred in a basipetal way, starting at the stigma and proceeding down to the ovary as is also the case in other species (Herrero and Arbeloa, 1989; Lora et al., 2010). Changes in the pistil appeared to occur during the growth of the pollen tube or, rather, pollen-tube growth encompassed pistil development (Herrero and Arbeloa, 1989).
While a number of developmental changes in the pistil have also been found in other species, in most species, fruiting is a response to fertilization (Lee, 1988). Flowering plants usually require fertilization to form fruit and seed and to initiate floral organ abscission in structures that do not contribute to the fruit (Vivian-Smith et al., 2001). Fertilization of the ovule generally triggers the development of the ovary into a fruit (Gillaspy et al., 1993). Ovule abortion or failures along the reproductive process have been observed in mutants of arabidopsis and tomato revealing that, while fruit initiation is normally inhibited in the absence of fertilization, alterations in the hormonal signalling results in parthenocarpic fruit (Vivian-Smith et al., 2001; Goetz et al., 2007).
However, the onset of fruiting in mandarin appeared to be independent of fertilization time because fruit growth started prior to fertilization. Additionally, the same observation was seen in the development of the juice vesicles. Surprisingly, these changes also occurred in the same way and time in unpollinated flowers, which reflects an uncoupling of the fertilization and fruiting time processes. This uncoupling supports the parthenocarpic tendency long reported in mandarins and in most Citrus species (Talon et al., 1992) and these results help elucidate the parthenocarpic tendency in this genus. Finally, the approach of this work may help to elucidate the events behind parthenocarpy in agriculturally important species.
ACKNOWLEDGEMENTS
This work was supported by: the Italian Ministry of the University – Project PRIN “The productive process in fruit tree species: molecular, physiological and agronomical aspects of floral incompatibility and strategies for its control”; the Minister of Science and Innovation – EU Feder [Project grant CICYT AGL-2006-13529-CO2-01/AGR and AGL-2009-12621-CO2-01/AGR]; the Aragon Government A-43 support to the group.
LITERATURE CITED
- Abbott CE. Blossom-bud differentiation in citrus trees. American Journal of Botany. 1935;22:476–485. [Google Scholar]
- Arbeloa A, Herrero M. The significance of the obturator in the control of the pollen tube entry into the ovary in peach (Prunus persica) Annals of Botany. 1987;60:681–685. [Google Scholar]
- Banerji I. Morphological and cytological studies on Citrus grandis Osbeck. Phytomorphology. 1954;4:390–396. [Google Scholar]
- Boeswinkel FD. Development of ovule and testa in Rutaceae. III. Some representatives of Aurantioideae. Acta Botanica Neerlandica. 1978;27:341–354. [Google Scholar]
- Bouquet A, Danglot Y. Inheritance of seedlessness in grapevine (Vitis vinifera L.) Vitis. 1996;35:35–42. [Google Scholar]
- Braun A. Über Polyembryony und Keimung von Caelebogyne. 1860:109–263. Ein Nachtrag zu der Abhandlung über Parthenogenesis bei Pflanzen. Abhandlungen der königlichen Akademie der Wissenschaften Berlin 1859. [Google Scholar]
- Chao CCT, Fang J, Devanand PS. Long distance pollen flow in mandarin orchards determined by AFLP markers: implications for seedless mandarin production. Journal of the American Society for Horticultural Science. 2005;130:374–380. [Google Scholar]
- Chapman LA, Goring DR. Pollen–pistil interactions regulating successful fertilization in the Brassicaceae. Journal of Experimental Botany. 2010;7:1987–1999. doi: 10.1093/jxb/erq021. [DOI] [PubMed] [Google Scholar]
- Ciampolini F, Cresti M, Sarfatti G, Tiezzi A. Ultrastructure of the stylar canal cells of Citrus limon (Rutaceae) Plant Systematics and Evolution. 1981;138:263–274. [Google Scholar]
- Clarke K, Prakash N. Floral morphology and embryology of two Australian species of Citrus (Rutaceae) Australian Journal of Botany. 2001;49:199–207. [Google Scholar]
- Cresti M, Ciampolini F, Van Went JL, Wilms HJ. Ultrastructure and histochemistry of Citrus limon (L.) stigma. Planta. 1982;156:1–9. doi: 10.1007/BF00393436. [DOI] [PubMed] [Google Scholar]
- Cruzan MB, Barrett SCH. Postpollination mechanisms influencing mating patterns and fecundity: an example from Eichornia paniculata. American Naturalist. 1996;147:576–598. [Google Scholar]
- Dumas C, Knox RB, Gaude T. Pollen − pistil recognition: new concepts from electron microscopy and cytochemistry. International Review of Cytology. 1984;90:239–272. [Google Scholar]
- Endress PK, Matthews ML. First steps towards a floral structural characterization of the major rosid subclades. Plant Systematics and Evolution. 2006;260:223–251. [Google Scholar]
- Erbar C. Pollen tube transmitting tissue: place of competition of male gametophytes. International Journal of Plant Sciences. 2003;164:265–277. [Google Scholar]
- FAOstat. Faostat statistical database. 2009 Food and Agriculture Organisation of the United Nations. (http://faostat.fao.org/faostat. ) [Google Scholar]
- Fleming AJ, Mandel T, Roth I, Kuhlemeier C. The pattern of gene expression in the tomato shoot apical meristem. The Plant Cell. 1993;5:297–309. doi: 10.1105/tpc.5.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES. Anatomy and histology of the eureka lemon. Botanical Gazette. 1942;104:288–305. [Google Scholar]
- Gillaspy G, Ben-David H, Gruissem W. Fruits: a developmental perspective. The Plant Cell. 1993;5:1439–1451. doi: 10.1105/tpc.5.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz M, Hooper LC, Johnson SD, Rodrigues JCM, Vivian-Smith A, Koltunow AM. Expression of aberrant forms of AUXIN RESPONSE FACTOR8 stimulates parthenocarpy in Arabidopsis and tomato. Plant Physiology. 2007;145:351–366. doi: 10.1104/pp.107.104174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorguet B, van Heusden AW, Lindhout P. Parthenocarpic fruit development in tomato. Plant Biology. 2005;7:131–139. doi: 10.1055/s-2005-837494. [DOI] [PubMed] [Google Scholar]
- Grosser JW, Gmitter FG., Jr 2004 SIVB Congress Symposium Proceedings ‘Thinking outside the cell’: applications of somatic hybridization and cybridization in crop improvement, with citrus as a model. In vitro Cellular & Developmental Biology-Plant. 2005;41:220–225. [Google Scholar]
- Grosser JW, Ollitrault P, Olivares-Fuster O. Somatic hybridization in citrus: an effective tool to facilitate variety improvement. In vitro Cellular & Developmental Biology – Plant. 2000;36:434–449. [Google Scholar]
- Guo WW, Prasad D, Cheng YJ, Serrano P, Deng XX, Grosser JW. Targeted cybridization in citrus: transfer of Satsuma cytoplasm to seedy cultivars for potential seedlessness. Plant Cell Report. 2004;22:752–758. doi: 10.1007/s00299-003-0747-x. [DOI] [PubMed] [Google Scholar]
- Hedhly A, Hormaza JI, Herrero M. Warm temperatures at bloom reduce fruit set in sweet cherry. Journal of Applied Botany and Food Quality. 2007;81:158–164. [Google Scholar]
- Hedhly A, Hormaza JI, Herrero M. Global warming and sexual plant reproduction. Trends in Plant Science. 2009;14:30–36. doi: 10.1016/j.tplants.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Herrero M. Mechanisms in the pistil that regulate gametophyte population in peach (Prunus persica) In: Ottaviano E, Mulcahy DL, Sari-Gorla M, Mulcahy GB, editors. Angiosperm pollen and ovules. Berlin: Springer; 1992. pp. 377–381. [Google Scholar]
- Herrero M. Changes in the ovary related to pollen tube guidance. Annals of Botany. 2000;85:79–85. [Google Scholar]
- Herrero M. Male and female synchrony and the regulation of mating in flowering plants. Philosophical Transactions of the Royal Society of London Series B – Biological Sciences. 2003;358:1019–1024. doi: 10.1098/rstb.2003.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero M, Arbeloa A. Influence of the pistil on pollen-tube kinetics in peach (Prunus persica) American Journal of Botany. 1989;76:1441–1447. [Google Scholar]
- Herrero M, Dickinson HG. Pollen–pistil incompatibility in Petunia hybrida: changes in the pistil following compatible and incompatible intraspecific crosses. Journal of Cell Science. 1979;36:1–18. doi: 10.1242/jcs.36.1.1. [DOI] [PubMed] [Google Scholar]
- Herrero M, Dickinson HG. Ultrastructural and physiological differences between buds and mature flowers of Petunia hybrida prior to and following pollination. Planta. 1980;148:138–145. doi: 10.1007/BF00386414. [DOI] [PubMed] [Google Scholar]
- Herrero M, Dickinson HG. Pollen tube development in Petunia hybrida following compatible and incompatible intraspecific matings. Journal of Cell Science. 1981;47:365–383. doi: 10.1242/jcs.47.1.365. [DOI] [PubMed] [Google Scholar]
- Herrero M, Gascón M. Prolongation of embryo sac viability in pear (Pyrus communis) following pollination or treatment with gibberellic acid. Annals of Botany. 1987;60:287–293. [Google Scholar]
- Herrero M, Hormaza JI. Pistil strategies controlling pollen tube growth. Sexual Plant Reproduction. 1996;9:343–347. [Google Scholar]
- Heslop-Harrison JS, Schwarzacher T. Domestication, genomics and the future for banana. Annals of Botany. 2007;100:1073–1084. doi: 10.1093/aob/mcm191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop-Harrison V. The pollen stigma interaction: pollen tube penetration in crocus. Annals of Botany. 1977;41:913–922. [Google Scholar]
- Heslop-Harrison Y. Control gates and micro-ecology: the pollen–stigma interaction in perspective. Annals of Botany. 2000;85:5–13. [Google Scholar]
- Heslop-Harrison Y, Heslop-Harrison J, Roger BJ. The pollen stigma interaction in the grasses: pollen-tube guidance and the regulation of tube number in Zea mays. Acta Botanica Neerlandica. 1985;34:193–211. [Google Scholar]
- Hiscock SJ, Allen AM. Diverse cell signalling pathways regulate pollen–stigma interactions: the search for consensus. New Phytologist. 2008;179:286–317. doi: 10.1111/j.1469-8137.2008.02457.x. [DOI] [PubMed] [Google Scholar]
- Hormaza JI, Herrero M. Pollen selection. Theoretical and Applied Genetics. 1992;83:663–672. doi: 10.1007/BF00226682. [DOI] [PubMed] [Google Scholar]
- Hormaza JI, Herrero M. Gametophytic competition and selection. In: Williams EG, Clarke AE, Knox RB, editors. Genetic control of self-incompatibility and reproductive development in flowering plants. Dordrecht: Kluwer; 1994. pp. 372–400. [Google Scholar]
- Hughes J, McCully ME. The use of an optical brightener in the study of plant structure. Stain Technology. 1975;50:319. doi: 10.3109/10520297509117082. [DOI] [PubMed] [Google Scholar]
- Jensen WA. Botanical histochemistry. San Francisco, CA: Freeman and Co; 1962. [Google Scholar]
- Johansen DA. Plant microtechnique. New York, NY: McGraw-Hill; 1940. [Google Scholar]
- Kahn TL, DeMason DA. A quantitative and structural comparison of Citrus pollen tube development in cross-compatible and self-incompatible gynoecia. Canadian Journal of Botany. 1986;64:2548–2555. [Google Scholar]
- Kahn TL, DeMason DA. Citrus pollen tube development in cross-compatible gynoecia, self-incompatible gynoecia, and in vitro. Canadian Journal of Botany. 1988;66:2527–2532. [Google Scholar]
- Khan I, editor. Citrus genetics, breeding and biotechnology. Wallingford: CABI Publishing; 2007. [Google Scholar]
- Kroh M, Labarca L, Loewus F. Use of pistil exudate for pollen tube wall byosynthesis in Lilium longiflorum. In: Heslop-Harrison J, editor. Pollen development and physiology. London: Butterworths; 1971. pp. 273–278. [Google Scholar]
- Kumar A, McClure B. Pollen–pistil interactions and the endomembrane system. Journal of Experimental Botany. 2010;61:2001–2013. doi: 10.1093/jxb/erq065. [DOI] [PubMed] [Google Scholar]
- Labarca C, Loewus F. Nutritional role of pistil exudate in pollen tube wall formation in Lilium longiflorum. II. Production and utilization of exudate from stigma and stylar canal. Plant Physiology. 1973;52:87–92. doi: 10.1104/pp.52.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lausser A, Kliwer I, Srilunchang KO, Dresselhaus T. Sporophytic control of pollen tube growth and guidance in maize. Journal of Experimental Botany. 2010;61:673–682. doi: 10.1093/jxb/erp330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TD. Patterns of fruit and seed production. In: Lovett-Doust J, Lovett-Doust L, editors. Plant reproductive ecology: patterns and strategies. New York, NY: Oxford University Press; 1988. pp. 179–201. [Google Scholar]
- Li DD, Shi W, Deng XX. Agrobacterium-mediated transformation of embryogenic calli of Ponkan mandarin and the regeneration of plants containing the chimeric ribonuclease gene. Plant Cell Report. 2002;21:153–156. [Google Scholar]
- Linskens HF, Esser K. Über eine spezifische Anfärbung der Pollenschläuche in Griffel und die Zahl der Kallossepfropten nach elbstung und Fremdung. Naturwissenschaften. 1957;44:16. [Google Scholar]
- Lora J, Hormaza JI, Herrero M. The progamic phase of an early-divergent angiosperm, Annona cherimola (Annonaceae) Annals of Botany. 2010;2:221–231. doi: 10.1093/aob/mcp276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lora J, Hormaza JI, Herrero M, Gasser CS. Seedless fruits and the disruption of a conserved genetic pathway in angiosperm ovule development. Proceedings of the National Academy of Science of the USA. 2011;108:5461–5465. doi: 10.1073/pnas.1014514108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure B. Darwin's foundation for investigating self-incompatibility and the progress toward a physiological model for S-RNase-based SI. Journal of Experimental Botany. 2009;60:1069–1081. doi: 10.1093/jxb/erp024. [DOI] [PubMed] [Google Scholar]
- McClure B, Franklin-Tong N. Special issue: pollen tube dynamics, tip growth and the control of pollination – preface. Journal of Experimental Botany. 2010;61:iii–iv. [Google Scholar]
- Martínez-Pallé E, Herrero M. Pollen tube pathway in chlazogamous Pistacia vera L. International Journal of Plant Sciences. 1998;159:566–574. [Google Scholar]
- Mesejo C, Martínez-Fuentes A, Reig C, Rivas F, Agustí M. The inhibitory effect of CuSO4 on pollen germination and pollen tube growth and its application for the production of seedless fruit. Plant Science. 2006;170:37–43. [Google Scholar]
- Mulcahy DL. The rise of angiosperms: a gynecological factor. Science. 1979;206:20–23. doi: 10.1126/science.206.4414.20. [DOI] [PubMed] [Google Scholar]
- Navarro L, Olivares-Fuster O, Juárez J, et al. Applications of biotechnology to citrus improvement in Spain. Acta Horticulture. 2004;632:221–234. [Google Scholar]
- Nicolosi E, Deng ZN, Gentile A, La Malfa S, Continella G, Tribulato E. Citrus phylogeny and genetic origin of important species as investigated by molecular markers. Theoretical and Applied Genetics. 2000;100:1155–1166. [Google Scholar]
- O'Brien TP, Fedre N, McCully ME. Polychromatic staining of plant cell wall by Toluidine blue ‘O. Protoplasma. 1964;59:367–373. [Google Scholar]
- Okuda S, Tsutsui H, Shiina K, et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature. 2009;458:357–362. doi: 10.1038/nature07882. [DOI] [PubMed] [Google Scholar]
- Osawa I. Cytological and experimental studies in Citrus. Journal of the College of Agriculture. 1912;4:83–116. Imperial University of Tokyo. [Google Scholar]
- Pons J, Pastor J, Polls M, Reverter AJ. Polinización cruzada en cítricos. III. Polinización entomófila. Efecto de repelentes. Levante Agrícola. 1996;337:291–295. [Google Scholar]
- Prychid CJ, Sokoloff DD, Remizowa MV, Tuckett RE, Yadav SR, Rudall PJ. Unique stigmatic hairs and pollen-tube growth within the stigmatic cell wall in the early-divergent angiosperm family Hydatellaceae. Annals of Botany. 2011 doi: 10.1093/aob/mcr021. doi:10.1093/aob/mcr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reforgiato G, Russo G, Recupero S. New promising citrus triploid hybrids selected from crosses between monoembryonic diploid female and tetraploid male parents. Hortscience. 2005;40:516–520. [Google Scholar]
- Rudall PJ, Prychid CJ, Jones C. Intra-ovarian trichomes mucilage secretion and hollow styles in monocotyledons. In: Owens SJ, Rudall PJ, editors. Reproductive biology. Richmond: Royal Botanic Gardens, Kew; 1998. pp. 219–230. [Google Scholar]
- Soost RK, Roose ML. Citrus. In: Janick J, Moore JN, editors. Fruit breeding. New York, NY: John Wiley; 1996. pp. 257–323. [Google Scholar]
- Spiegel-Roy P, Vardi A. ‘Yafit’ and ‘Norit’: two new easy peeling mandarin hybrids. Proceedings of the International Society of Citriculture. 1981;1:57–59. [Google Scholar]
- Stewman SF, Jones-Rhoades M, Bhimalapuram P, Tchernookov M, Preuss D, Dinner AR. Mechanistic insights from a quantitative analysis of pollen tube guidance. BMC Plant Biology. 2010;10 doi: 10.1186/1471-2229-10-32. [online article, http://www.biomedcentral.com/1471-2229/10/32. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasburger E. Üeber Polyembryonie. Jenaische Zeitschrift für Naturwissenschaft. 1878;12:647–670. [Google Scholar]
- Sugiyama K, Morishita M, Nishino E. Seedless watermelons produced via soft X-ray irradiated pollen. HortScience. 2002;37:292–295. [Google Scholar]
- Swanson R, Edlund AF, Preuss D. Species specificity in pollen–pistil interactions. Annual Review of Genetics. 2004;38:793–818. doi: 10.1146/annurev.genet.38.072902.092356. [DOI] [PubMed] [Google Scholar]
- Talon M, Hedden P, Primo-Millo E. Gibberellins in Citrus sinensis: a comparison between seeded and seedless varieties. Journal of Plant Growth Regulation. 1990;9:201–206. [Google Scholar]
- Talon M, Zacarias L, Primo-Millo E. Gibberellins and parthenocarpic ability in developing ovaries of seedless mandarins. Plant Physiology. 1992;99:1575–1581. doi: 10.1104/pp.99.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillson AH, Bamford R. The floral anatomy of the Aurantiodeae. American Journal of Botany. 1938;25:780–793. [Google Scholar]
- Tilton VR, Horner T. Stigma, style, and obturator of Ornithogalum caudatum (Liliaceae) and their function in the reproductive process. American Journal of Botany. 1980;67:1113–1131. [Google Scholar]
- Tilton VR, Wilcox LW, Palmer RG, Albertsen MC. Stigma, style and obturator of soybean, Glycine max (L.) Merr. (Leguminoseae) and their function in the reproductive process. American Journal of Botany. 1984;71:676–686. [Google Scholar]
- Ton LD, Krezdorn AH. Growth of pollen tubes in three incompatible varieties of citrus. Proceeding of the American Society for Horticultural Science. 1966;89:211–215. [Google Scholar]
- Vardi A, Levin I, Carmi N. Induction of seedlessness in citrus: from classical techniques to emerging biotechnological approaches. Journal of the American Society for Horticultural Science. 2008;133:117–126. [Google Scholar]
- Varoquaux F, Blanvillan R, Delseny M, Gallois P. Less is better: new approaches for seedless fruit production. Trends Biotechnology. 2000;18:233–242. doi: 10.1016/s0167-7799(00)01448-7. [DOI] [PubMed] [Google Scholar]
- Vivian-Smith A, Luo M, Chaudhury A, Koltunow A. Fruit development is actively restricted in the absence of fertilization in Arabidopsis. Development. 2001;128:2321–2331. doi: 10.1242/dev.128.12.2321. [DOI] [PubMed] [Google Scholar]
- Yang WC, Shi DQ, Chen YH. Female gametophyte development in flowering plants. Annual Review of Plant Biology. 2010;61:89–108. doi: 10.1146/annurev-arplant-042809-112203. [DOI] [PubMed] [Google Scholar]