Abstract
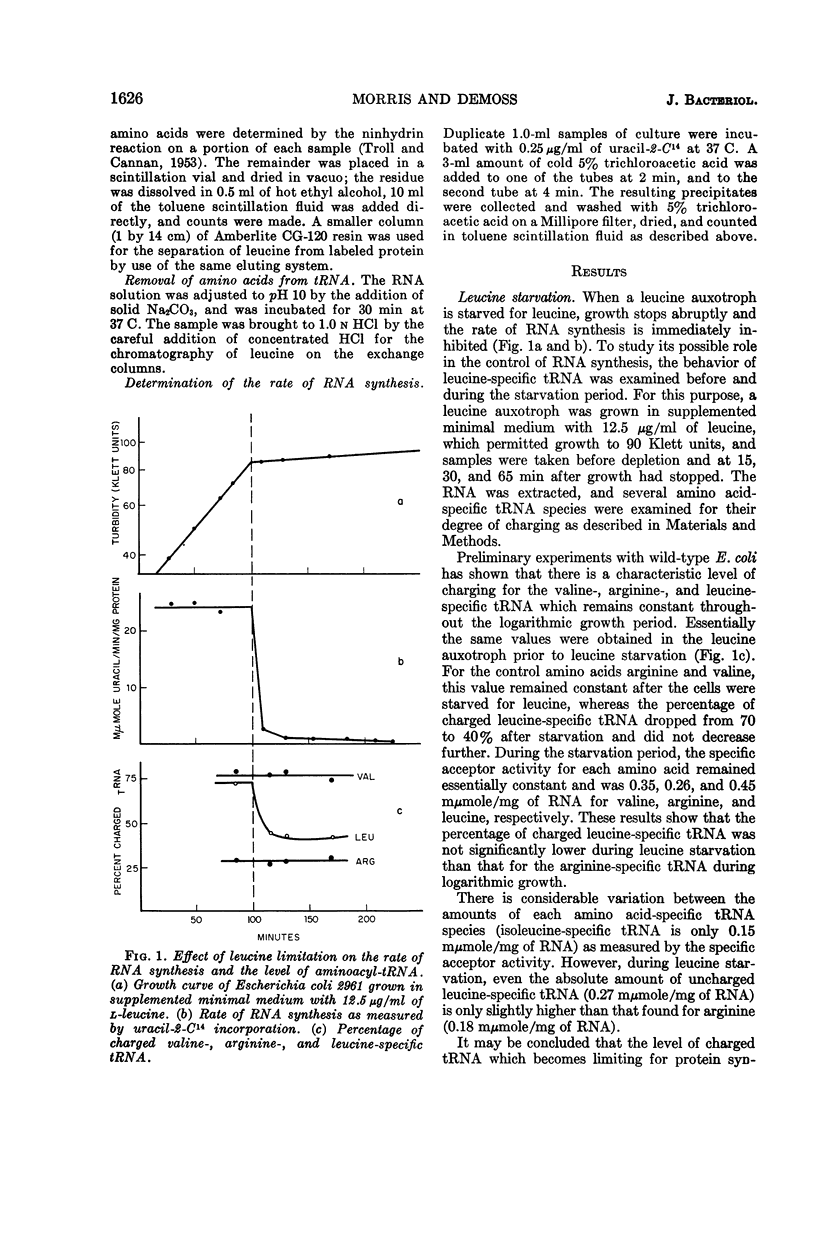
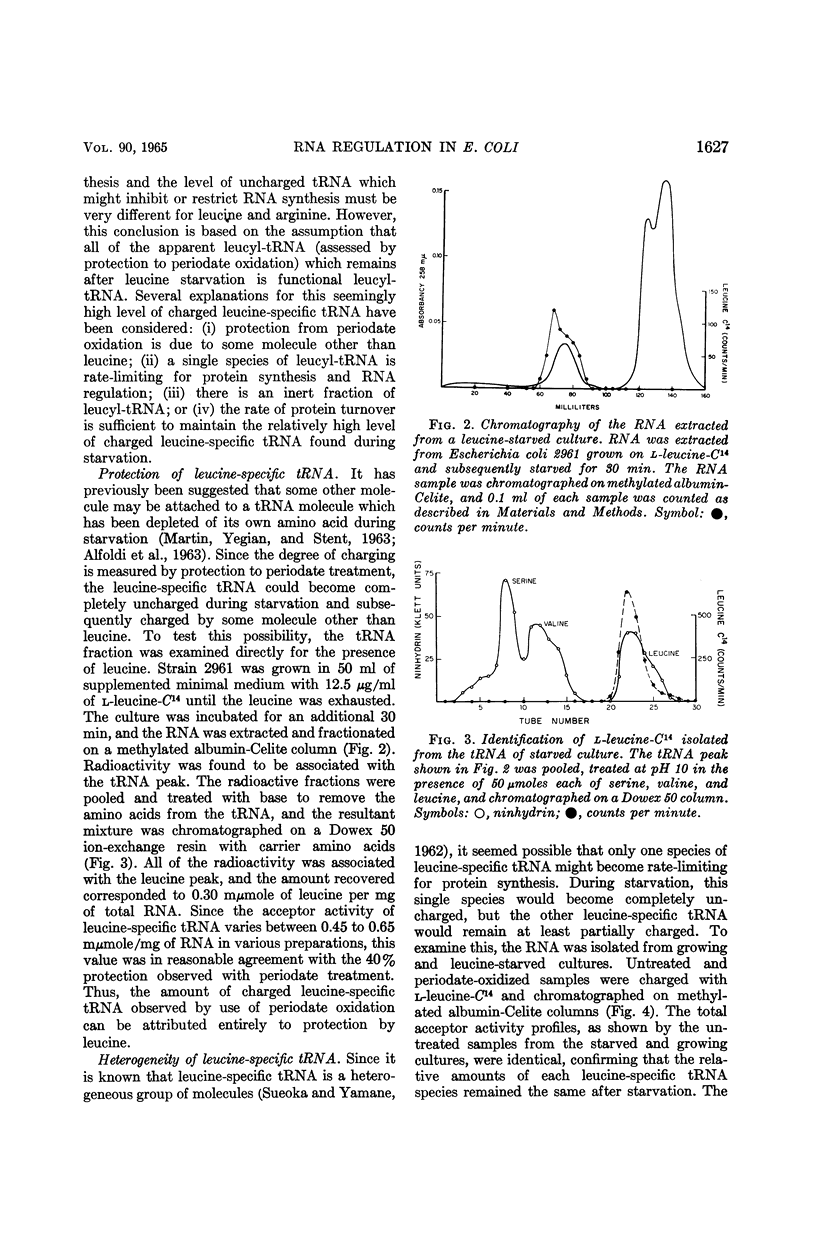
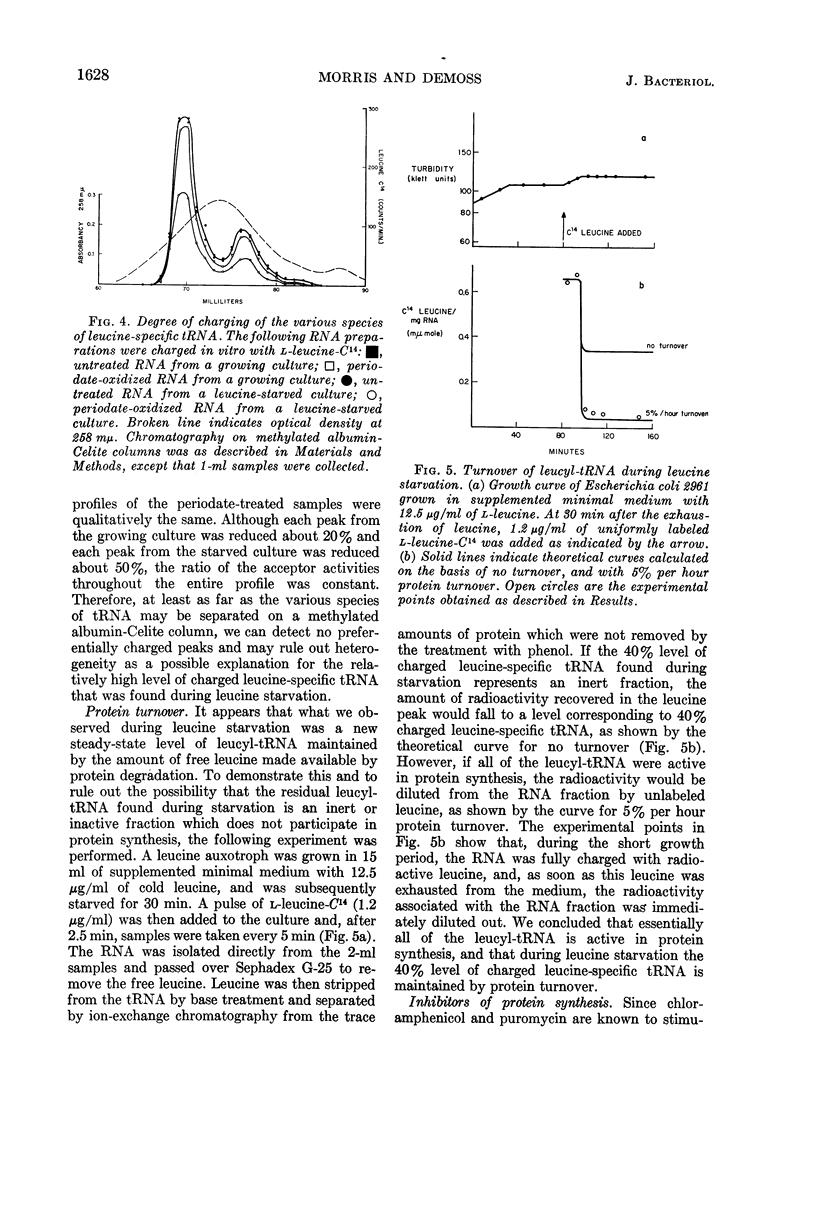
Morris, D. W. (University of California, San Diego), and J. A. DeMoss. Role of aminoacyl-transfer ribonucleic acid in the regulation of ribonucleic acid synthesis in Escherichia coli. J. Bacteriol. 90:1624–1631. 1965.—A leucine auxotroph of Escherichia coli was examined for its rate of ribonucleic acid (RNA) synthesis and the level of charged leucine-, arginine-, and valine-specific transfer RNA (tRNA) during the exponential growth period and when growth was limited by leucine starvation. During the logarithmic growth period, the leucine-specific tRNA was 70% charged, arginine-specific tRNA was 30% charged, and the valine-specific tRNA was 80% charged. When leucine became limiting, RNA synthesis was inhibited and the levels of charged arginine- and valine-specific tRNA remained constant, whereas the level of charged leucine-specific tRNA dropped to 40%. Examination of the leucyl-tRNA during the leucine starvation period showed that this 40% level is maintained by protein turnover. Addition of chloramphenicol or puromycin to a leucine-starved culture derepressed RNA synthesis. In the presence of chloramphenicol, the leucine-specific tRNA was fully charged; however, in the presence of puromycin the amount of charged leucine-specific tRNA remained at the starved level. Therefore, during leucine starvation the level of uncharged leucine-specific tRNA is not invariably correlated with the rate of RNA synthesis. We propose that it is the availability of charged tRNA and not the amount of uncharged tRNA which is the important factor in the amino acid control of RNA synthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALFOELDI L., STENT G. S., HOOGS M., HILL R. PHYSIOLOGICAL EFFECTS OF THE RNA CONTROL (RC) GENE IN E. COLI. Z Vererbungsl. 1963 Nov 21;94:285–302. doi: 10.1007/BF00894773. [DOI] [PubMed] [Google Scholar]
- BROCK T. D. ACTION OF STREPTOMYCIN AND RELATED ANTIBIOTICS. Fed Proc. 1964 Sep-Oct;23:965–975. [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. PROTEIN AND NUCLEIC ACID SYNTHESIS IN TWO MUTANTS OF ESCHERICHIA COLI WITH TEMPERATURE-SENSITIVE AMINOACYL RIBONUCLEIC ACID SYNTHETASES. J Bacteriol. 1965 Mar;89:706–711. doi: 10.1128/jb.89.3.706-711.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekiel D. H. Intracellular charging of soluble ribonucleic acid in Escherichia coli subjected to isoleucine starvation and chloramphenicol treatment. Biochem Biophys Res Commun. 1964;14:64–68. doi: 10.1016/0006-291x(63)90212-2. [DOI] [PubMed] [Google Scholar]
- FANGMAN W. L., NEIDHARDT F. C. PROTEIN AND RIBONUCLEIC ACID SYNTHESIS IN A MUTANT OF ESCHERICHIA COLI WITH AN ALTERED AMINOACYL RIBONUCLEIC ACID SYNTHETASE. J Biol Chem. 1964 Jun;239:1844–1847. [PubMed] [Google Scholar]
- GALE E. F., FOLKES J. P. The assimilation of amino-acids by bacteria. XV. Actions of antibiotics on nucleic acid and protein synthesis in Staphylococcus aureus. Biochem J. 1953 Feb;53(3):493–498. doi: 10.1042/bj0530493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASH J. H., WISHNICK M., MILLER P. A. ON THE MODE OF ACTION OF THE TETRACYCLINE ANTIBIOTICS IN STAPHYLOCOCCUS AUREUS. J Biol Chem. 1964 Jun;239:2070–2078. [PubMed] [Google Scholar]
- KURLAND C. G., MAALOE O. Regulation of ribosomal and transfer RNA synthesis. J Mol Biol. 1962 Mar;4:193–210. doi: 10.1016/s0022-2836(62)80051-5. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- MARTIN E. M., YEGIAN C., STENT G. S. SPECIFICITY OF THE AMINO ACID TRANSFER REACTION IN E. COLI STRAINS CARRYING DIFFERENT ALLELES OF THE RNA CONTROL (RC) GENE. Z Vererbungsl. 1963 Nov 21;94:303–315. doi: 10.1007/BF00894774. [DOI] [PubMed] [Google Scholar]
- NATHANS D. INHIBITION OF PROTEIN SYNTHESIS BY PUROMYCIN. Fed Proc. 1964 Sep-Oct;23:984–989. [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUEOKA N., YAMANE T. Fractionation of amino acyl-acceptor RNA on a methylated albumin column. Proc Natl Acad Sci U S A. 1962 Aug;48:1454–1461. doi: 10.1073/pnas.48.8.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TISSIERES A., BOURGEOIS S., GROS F. Inhibition of RNA polymerase by RNA. J Mol Biol. 1963 Jul;7:100–103. doi: 10.1016/s0022-2836(63)80024-8. [DOI] [PubMed] [Google Scholar]
- TRAUT R. R., MONRO R. E. THE PUROMYCIN REACTION AND ITS RELATION TO PROTEIN SYNTHESIS. J Mol Biol. 1964 Oct;10:63–72. doi: 10.1016/s0022-2836(64)80028-0. [DOI] [PubMed] [Google Scholar]
- TROLL W., CANNAN R. K. A modified photometric ninhydrin method for the analysis of amino and imino acids. J Biol Chem. 1953 Feb;200(2):803–811. [PubMed] [Google Scholar]


