Abstract
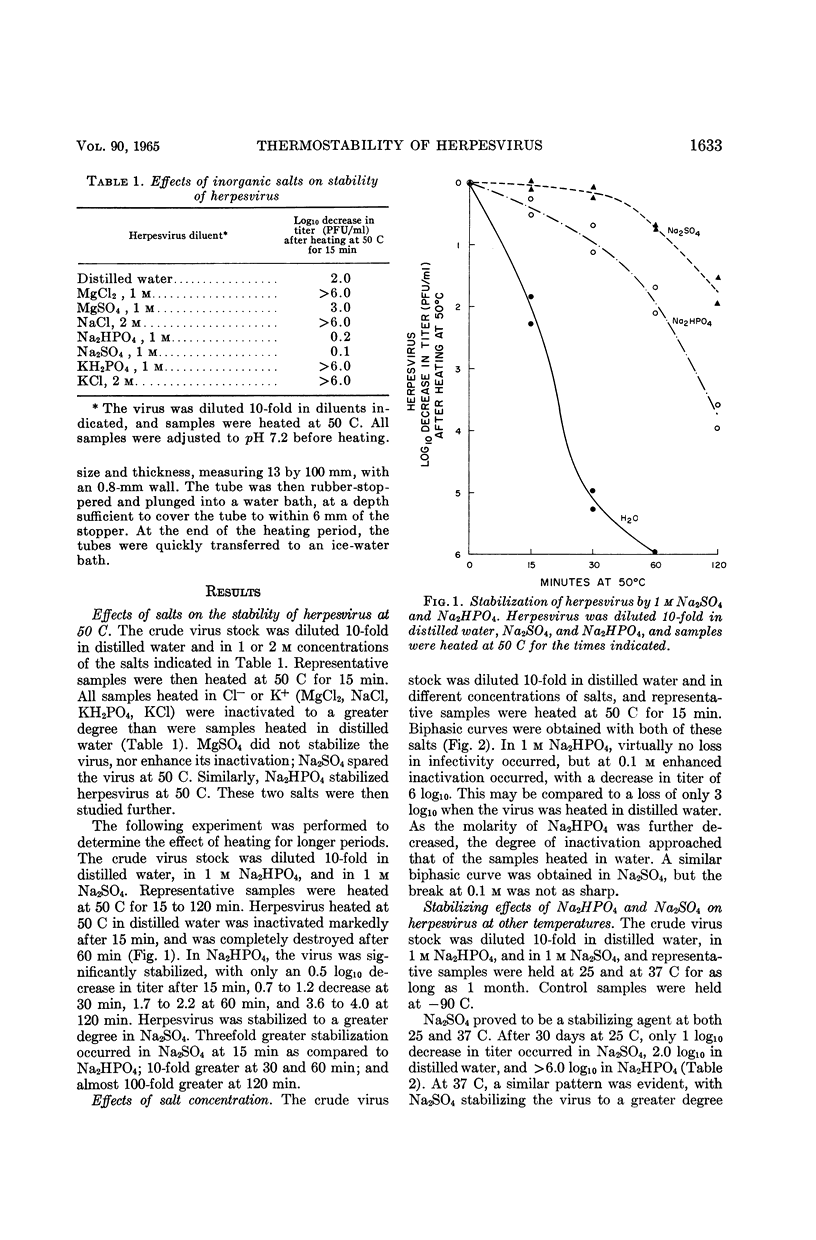
Wallis, Craig (Baylor University College of Medicine, Houston, Tex.), and Joseph L. Melnick. Thermostabilization and thermosensitization of herpesvirus. J. Bacteriol. 90:1632–1637. 1965.—Herpesvirus, long considered as one of the most thermolabile of viruses, was stabilized by 1 m Na2SO4 or Na2HPO4 so that it withstood heating at 50 C, but the virus was not protected by 1 m MgCl2, MgSO4, or KH2PO4, or 2 m KCl or NaCl; 1 m Na2SO4 also stabilized herpesvirus at 25 and 37 C. In contrast, herpesvirus was made extremely thermosensitive in the presence of isotonic salt concentrations or of isotonic tris(hydroxymethyl)aminomethane buffer, especially at pH 7.2 or above. Partially purified virus was relatively thermostable when suspended in distilled water at pH 7.2, but in Earle's salt solution the virus immediately became thermosensitive. As found in tissue culture harvests, herpesvirus was thermolabile, but the virus was rendered stable at 50 C by simple dilution in distilled water. Protection by proteins or amino acids, generally accepted as virus-stabilizing agents, did not seem to be the result of a direct effect upon herpesvirus. The present data suggest that the added proteins counteract in part thermosensitizing effects of the salts contained in the virus harvest.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The effect of the temperature of incubation on the formation and release of herpes simplex virus in infected FL cells. Virology. 1959 Aug;8:508–524. doi: 10.1016/0042-6822(59)90052-2. [DOI] [PubMed] [Google Scholar]
- KAPLAN A. S. A study of the herpes simplex virus-rabbit kidney cell system by the plaque technique. Virology. 1957 Dec;4(3):435–457. doi: 10.1016/0042-6822(57)90078-8. [DOI] [PubMed] [Google Scholar]
- MUNK K., ACKERMANN W. W. Some properties of herpes simplex virus. J Immunol. 1953 Dec;71(6):426–430. [PubMed] [Google Scholar]
- NAKAMURA M., UENO Y. INFECTIOUS RIBONUCLEIC ACID (RNA) OF JAPANESE B ENCEPHALITIS (JBE) VIRUS: HIGH YIELDS OF RNA AND ITS STABILIZATION. Proc Soc Exp Biol Med. 1964 Dec;117:700–704. doi: 10.3181/00379727-117-29672. [DOI] [PubMed] [Google Scholar]
- PLUMMER G., LEWIS B. THERMOINACTIVATION OF HERPES SIMPLEX VIRUS AND CYTOMEGALOVIRUS. J Bacteriol. 1965 Mar;89:671–674. doi: 10.1128/jb.89.3.671-674.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Butel J. S., Wallis C. Protection of Measles Virus by Sulfate Ions Against Thermal Inactivation. J Bacteriol. 1965 Jul;90(1):132–135. doi: 10.1128/jb.90.1.132-135.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT T. F., McLEOD D. L., TOKUMARU T. A biologic comparison of two strains of Herpesvirus hominis. J Immunol. 1961 Jan;86:1–12. [PubMed] [Google Scholar]
- WALLIS C., MENICK J. L. Cationic stabilization--a new property of enteroviruses. Virology. 1962 Apr;16:504–506. doi: 10.1016/0042-6822(62)90234-9. [DOI] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L., Rapp F. Different effects of MgC1-2 and MgSO-4 on the thermostability of viruses. Virology. 1965 Aug;26(4):694–699. doi: 10.1016/0042-6822(65)90332-6. [DOI] [PubMed] [Google Scholar]



