Abstract
The efficacy of retreatment with immunomodulatory drugs (IMiDs) among patients with multiple myeloma who received this class of drugs for initial therapy is unknown. We studied 140 patients who received either thalidomide-dexamethasone (81; 58%) or lenalidomide-dexamethasone (59; 42%) as first-line therapy of multiple myeloma followed by repeat IMiD (thalidomide [34; 24%] or lenalidomide [106; 76%]) as one of the salvage regimens. A median of 2 treatments (range, 1-6), including a stem cell transplant in 105 patients (75%), were administered before IMiD-based salvage therapy. The median time from diagnosis to repeat exposure to IMiD was 28 months. Among the 113 evaluable patients, 50 (44%) achieved at least a partial response, and 63 (56%) achieved less than a partial response to repeat IMiD. Response rates with lenalidomide retreatment were higher than with repeat administration of thalidomide.
Introduction
The unique antiangiogenic and immunomodulatory properties of thalidomide provided a rationale for its use in multiple myeloma (MM), resulting in a response in almost one third of patients with relapsed or refractory disease.1 Over the last decade, derivatives of thalidomide such as lenalidomide and pomalidomide (immunomodulatory drugs; IMiDs) have been developed that enhanced the potency and reduced the toxicity of the parent compound. Indeed, the striking improvement seen in the overall survival of MM patients in the last decade, both at diagnosis and in the post–stem cell transplant (SCT) relapse setting, is in large part attributable to the advent of novel agents (thalidomide, lenalidomide, and bortezomib).2 Various studies have demonstrated remarkable activity of IMiDs, either used alone or in combination with dexamethasone, in patients with previously untreated or relapsed MM.1,3–7 Thalidomide and lenalidomide are commonly used as initial therapy in MM. Despite recent improvements in the overall survival with novel agents, curative therapy does not exist, and MM patients eventually relapse, which requires a treatment change.8 Therefore, a considerable proportion of MM patients previously treated with first-line IMiDs undergo salvage therapy with IMiDs for relapsed or refractory disease; however, data on their retreatment efficacy is unknown. We designed the present study to ascertain the depth and durability of response that can be achieved with IMiD retreatment in patients with newly diagnosed MM who received first-line therapy with either thalidomide-dexamethasone or lenalidomide-dexamethasone.
Methods
After obtaining approval from the Mayo Foundation Institutional Review Board, we studied 410 sequential patients from a prospectively maintained database who received either thalidomide-dexamethasone or lenalidomide-dexamethasone as initial therapy for newly diagnosed MM between February 2000 and November 2008. Among these, 140 patients who received thalidomide or lenalidomide (with or without dexamethasone) as one of the salvage regimens for relapsed or refractory disease at some time during their MM disease course formed the study group. The diagnosis of MM was made by conventional criteria.9 The choice of treatment was dictated by the routine clinical practice and drug availability. The disease response after initial therapy and salvage therapy was assessed according to the International Myeloma Working Group uniform response criteria.10 The best response on a particular regimen is reported here. The study was performed in accordance with the principles of the Helsinki Declaration, with approval from the Institutional Review Board.
Results and discussion
The median (range) age of the study population was 60 (29-78) years at the time of salvage therapy; 87 (62%) were male. Thalidomide and lenalidomide were used as initial therapy in 81 (58%) and 59 (42%) patients, respectively. The study group received a median of 2 treatments (range, 1-6; including an SCT where applicable) before repeat therapy with an IMiD. The median (range) time to repeat IMiD was 28 (3-102) months from diagnosis. An autologous SCT was performed in 105 patients (75%) before repeat use of an IMiD. Bortezomib was used as one of the treatments (before the repeat IMiD) in 34 patients (24%). The proportion of patients with high-risk cytogenetics was similar across the groups studied (Table 1).
Table 1.
Baseline characteristics and response to retreatment grouped by initial response to IMiD
| Len→Len* n = 48 | Len→Thal* n = 11 | Thal→Len* n = 58 | Thal→Thal* n = 23 | |
|---|---|---|---|---|
| Median age, y (range) | 63 (29-78) | 58 (33-72) | 60 (38-77) | 57 (38-71) |
| Males, % | 58 | 55 | 62 | 74 |
| High-risk MM, n (%) | 6 (13) | 2 (18) | 12 (21) | 3 (13) |
| Median no. of prior treatments | 2 | 1 | 2 | 2 |
| SCT before repeat IMiD, % | 79 | 55 | 71 | 87 |
| Dex plus repeat IMiD, % | 92 | 100 | 86 | 87 |
| Median duration of first IMiD, mo (IQR) | 4 (4-6) | 5 (4-8) | 4 (3-6) | 4 (3-5) |
| Median time from diagnosis to repeat IMiD, mo (IQR) | 26 (18-38) | 13 (4-23) | 31 (23-49) | 23 (18-36) |
| Median duration of second IMiD, mo (IQR) | 7 (3-18) | 3 (2-4) | 7 (3-14) | 6 (2-18) |
| Response to first-line IMiD† | ||||
| ≥ VGPR(%)† | 5 ≥ VGPR (45) | 1 PR (33) | 2 ≥ VGPR (33) | 1 PR (25) |
| 3 PR (27) | 2 < PR (67) | 1 PR (17) | 3 < PR (75) | |
| 3 < PR (27) | 3 < PR (50) | |||
| PR (%)† | 4 ≥ VGPR (18) | 1 ≥ VGPR (25) | 2 ≥ VGPR (8) | 5 PR (45) |
| 7 PR (32) | 3 < PR (75) | 7 PR (29) | 6 < PR (55) | |
| 11 < PR (50) | 15 < PR (63) | |||
| < PR (%)‡ | 2 ≥ VGPR (33) | 3 < PR (100) | 1 ≥ VGPR (7) | 5 < PR (100) |
| 4 < PR (67) | 8 PR (57) | |||
| 5 < PR (36) | ||||
| ORR (> PR)‡ (n = 140), % | 54 | 20 | 48 | 30 |
| N§ | 44 (92%) | 7 (64%) | 50 (86%) | 22 (96%) |
| RR§ (n = 123; 88%)‡, % | 57 | 17 | 47 | 32 |
| N‖ | 4 (8%) | 4 (36%) | 8 (14%) | 1 (4%) |
| RR‖ (n = 17; 12%)‡, % | 25 | 25 | 50 | 0 |
≥ VGPR includes patients with complete response; VGPR and < PR includes patients with stable and progressive disease and nonevaluable (n = 9) patients after first-line IMiD. High-risk multiple myeloma was determined by fluorescent in situ hybridization and conventional cytogenetics risk stratification of myeloma.
Thal indicates thalidomide; Len, lenalidomide; Dex, dexamethasone; IQR, interquartile range; ≥ VGPR, very good partial response; PR, partial response; ORR, overall response rate; and RR, response to retreatment.
Primary-salvage IMiD combination.
Response to retreatment with an IMiD is grouped by initial response to IMiD.
Patients with nonevaluable disease response (n = 27) were not used to calculate the percentage.
Number of patients and response rate among patients who discontinued first-line IMiD for reasons other than disease progression (transplant, toxicity, choice, and alternative treatment).
Number of patients and response rate among patients who discontinued first-line IMiD because of disease progression.
More than one half (86; 61%) of the patients completed first-line therapy to undergo a planned SCT, whereas drug toxicity (19; 14%), disease progression (17; 12%), patient's choice (10; 7%), and alternative treatment options (8; 6%) were the other reasons that led to a treatment change. The IMiD used for salvage therapy was thalidomide or lenalidomide in 34 patients (24%) and 106 patients (76%), respectively. The majority (89%) of patients received the repeat IMiD along with dexamethasone therapy. The response rates to salvage IMiD therapy and the initial responses from IMiD with or without dexamethasone are summarized in Table 1. Overall, 50 patients (44%) achieved a partial response (PR) or better and 63 (56%) achieved less than a PR (stable and progressive disease) to repeat IMiD therapy, whereas the remaining 27 patients were nonevaluable for response. Retreatment with lenalidomide and thalidomide produced a PR or better in 48%-54% and 20%-30% of patients, respectively (Table 1). Similarly, among patients who discontinued initial IMiD because of progression (n = 17), retreatment with lenalidomide produced a PR or better in 25%-50%, whereas only a few (0%-25%) patients responded after repeat thalidomide therapy (Table 1). Nineteen (58%) of the 33 patients who had a PR or better to initial lenalidomide therapy obtained a PR or better with repeat use of lenalidomide. Similarly, 6 (40%) of the 15 patients with a PR or better with initial thalidomide treatment had a similar response with repeat use of thalidomide. Patients who progress on first-line IMiD can still achieve a response with repeat IMiD therapy, especially with lenalidomide used as second-line therapy.
The higher response rate observed with retreatment with lenalidomide than with thalidomide in the present study likely reflects an underlying difference in their mode of action that limits cross-resistance in previously treated MM patients. Thalidomide appears to have more antiangiogenic potential than lenalidomide,11 whereas lenalidomide has greater immunomodulation and tumor-inhibiting properties than thalidomide.12,13 In fact, compared with thalidomide, lenalidomide has been found to be significantly (100-1000 times) more potent than thalidomide in activating T-cell and cytokine (IFN-γ and IL-2) production while inhibiting proinflammatory cytokines such as TNF-α, IL-1, and IL-6.14 A previous in vitro study in the MM.1S cell line observed a 50% inhibition of DNA synthesis with a smaller dose of lenalidomide (0.1-1.0μM) compared with 15% inhibition with a higher dose of thalidomide (100μM).13
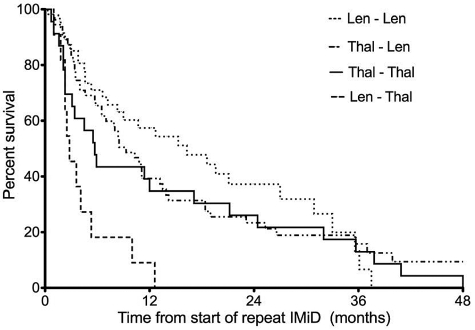
Previous investigations have revealed the benefit of lenalidomide therapy in MM patients previously treated with thalidomide.15–17 Lenalidomide-dexamethasone has been shown to be more effective than dexamethasone alone in relapsed or refractory MM patients independent of prior thalidomide use in a post hoc analysis of patients from the MM-009 and MM-010 trials.15 The response rate (RR) of 42% and 50% reported in that study with lenalidomide-dexamethasone in patients who had relapsed or were refractory to thalidomide,15 respectively, is similar to the RR (48%) observed in the present study with lenalidomide treatment after initial thalidomide use. In addition, the retreatment RR (48%-54%) with lenalidomide in the present study is comparable to the RR (55%-60%) seen with lenalidomide-dexamethasone in relapsed MM.4,18 The higher RR observed with repeat lenalidomide use translated into a median time to progression of 9 and 16 months after initial thalidomide and lenalidomide use, respectively. On the other hand, the median time to progression with thalidomide after initial therapy with thalidomide and lenalidomide was 6 and 3 months, respectively (Figure 1). Overall, the median time to progression for the entire study population was 9.0 months (95% confidence interval 6-12 months) from the start of repeat IMiD therapy. We did not specifically examine overall survival, because it would be significantly affected by the available treatments for salvage therapy, which have changed significantly over the time period of the study. In conclusion, the results of the present study support the choice of repeat therapy with IMiDs when initial IMiD therapy is discontinued because of the common causes that lead to a treatment change.
Figure 1.
Median time to progression from start of repeat IMiD therapy. Lenalidomide followed by lenalidomide = 16 months, thalidomide followed by lenalidomide = 9 months, thalidomide followed by thalidomide = 6 months, and lenalidomide followed by thalidomide = 3 months. Overall, the median time to progression for the entire study population was 9 months.
Acknowledgments
The authors sincerely appreciate the support of their colleagues (Division of Hematology, Mayo Clinic, Rochester, MN) in the preparation of this manuscript.
This work was supported in part by research grant CA 62242 from the National Cancer Institute (S.K.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.M. and S.K. designed the study, collected the data, wrote the manuscript, and performed statistical analysis; and S.M., M.Q.L., A.D., M.A.G., F.B., S.R.H., K.D-S., D.D., S.Z., J.L., P.R.G., S.V.R., and S.K. interpreted the data and edited the manuscript.
Conflict-of-interest disclosure: M.Q.L. has received clinical trial funding from Celgene. A.D. has received research funding from Celgene and Millenium. M.G. has received honoraria from Celgene & Millenium. S.K. has served as the principal investigator on Celgene-supported clinical trials. The remaining authors declare no competing financial interests.
Correspondence: Shaji Kumar, MD, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: kumar.shaji@mayo.edu.
References
- 1.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341(21):1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 2.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar S, Gertz MA, Dispenzieri A, et al. Response rate, durability of response, and survival after thalidomide therapy for relapsed multiple myeloma. Mayo Clin Proc. 2003;78(1):34–39. doi: 10.4065/78.1.34. [DOI] [PubMed] [Google Scholar]
- 4.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357(21):2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 5.Lacy MQ, Gertz MA, Dispenzieri A, et al. Long-term results of response to therapy, time to progression, and survival with lenalidomide plus dexamethasone in newly diagnosed myeloma. Mayo Clin Proc. 2007;82(10):1179–1184. doi: 10.4065/82.10.1179. [DOI] [PubMed] [Google Scholar]
- 6.Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106(13):4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Lilienfeld-Toal M, Hahn-Ast C, Furkert K, et al. A systematic review of phase II trials of thalidomide/dexamethasone combination therapy in patients with relapsed or refractory multiple myeloma. Eur J Haematol. 2008;81(4):247–252. doi: 10.1111/j.1600-0609.2008.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attal M, Harousseau JL, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108(10):3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 9.Kyle RA. Diagnostic criteria of multiple myeloma. Hematol Oncol Clin North Am. 1992;6(2):347–358. [PubMed] [Google Scholar]
- 10.International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749–757. [PubMed] [Google Scholar]
- 11.Lu L, Payvandi F, Wu L, et al. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc Res. 2009;77(2):78–86. doi: 10.1016/j.mvr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163(1):380–386. [PubMed] [Google Scholar]
- 13.Hideshima T, Chauhan D, Shima Y, et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96(9):2943–2950. [PubMed] [Google Scholar]
- 14.Kotla V, Goel S, Nischal S, et al. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009;2:36. doi: 10.1186/1756-8722-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M, Dimopoulos MA, Chen C, et al. Lenalidomide plus dexamethasone is more effective than dexamethasone alone in patients with relapsed or refractory multiple myeloma regardless of prior thalidomide exposure. Blood. 2008;112(12):4445–4451. doi: 10.1182/blood-2008-02-141614. [DOI] [PubMed] [Google Scholar]
- 16.Guglielmelli T, Bringhen S, Berruti A, et al. Sequential therapy with IMID's in relapsed-refractory multiple myeloma patients. Blood. 2009;114 Abstract 2888. [Google Scholar]
- 17.Richardson PG, Schlossman RL, Weller E, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100(9):3063–3067. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- 18.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]