Abstract
The short circulating half-life and side effects of IFNα affect its dosing schedule and efficacy. Fusion of IFNα to a tumor-targeting mAb (mAb-IFNα) can enhance potency because of increased tumor localization and improved pharmacokinetics. We used the Dock-and-Lock method to generate C2-2b-2b, a mAb-IFNα comprising tetrameric IFNα2b site-specifically linked to hL243 (humanized anti–HLA-DR). In vitro, C2-2b-2b inhibited various B-cell lymphoma leukemia and myeloma cell lines. In most cases, this immunocytokine was more effective than CD20-targeted mAb-IFNα or a mixture comprising the parental mAb and IFNα. Our findings indicate that responsiveness depends on HLA-DR expression/density and sensitivity to IFNα and hL243. C2-2b-2b induced more potent and longer-lasting IFNα signaling compared with nontargeted IFNα. Phosphorylation of STAT1 was more robust and persistent than that of STAT3, which may promote apoptosis. C2-2b-2b efficiently depleted lymphoma and myeloma cells from whole human blood but also exhibited some toxicity to B cells, monocytes, and dendritic cells. C2-2b-2b showed superior efficacy compared with nontargeting mAb-IFNα, peginterferonalfa-2a, or a combination of hL243 and IFNα, using human lymphoma and myeloma xenografts. These results suggest that C2-2b-2b should be useful in the treatment of various hematopoietic malignancies.
Introduction
In the United States, there were > 137 000 new cases of hematopoietic neoplasias (65 540 non-Hodgkin lymphoma [NHL], 20 580 multiple myeloma [MM], and 43 050 leukemia) and 54 020 deaths from these diverse diseases in 2009.1 IFNα exhibits clinical activity in NHL therapy,2,3 and its addition to rituximab immunotherapy has shown some clinical advantage.4,5 IFNα has been used for therapy of hairy cell leukemia and chronic myelogenous leukemia.6,7 For > 30 years, IFNα has been used in various ways for the management of MM. However, despite considerable efforts, numerous clinical trials, and 2 large meta-analyses, its exact role in the treatment of MM still remains unclear.8 IFNα can have direct cytotoxic activity on tumors, inhibit angiogenesis, and stimulate both innate and adaptive immunity; however, its use in cancer therapy has been limited because of its short circulating half-life and systemic toxicity. Fusion of IFNα to a tumor-targeting mAb could enhance direct and indirect effects of IFNα and increase the therapeutic index by improved pharmacokinetics (Pk), increased local concentration, prolonged tumor retention, and limited systemic exposure of IFNα. HLA-DR is an attractive target because it is expressed on the cell surface of many hematopoietic malignancies.9 IMMU-114 (or hL243γ4p) is a humanized IgG4 version of L243, a mouse anti–HLA-DR mAb, which was engineered to prevent the formation of half-IgG molecules associated with the IgG4 isotype.10 IMMU-114 has direct cytotoxicity on various types of hematopoietic cell lines in vitro and in vivo; as an IgG4 variant, its effector functions, particularly complement-dependent cytotoxicity (CDC), are minimized.11
We previously used the modular Dock-and-Lock (DNL) method12,13 to generate a novel immunocytokine, named 20-2b-2b (formerly 20-2b), which comprises 4 IFNα2b groups tethered to veltuzumab (humanized anti-CD20 mAb), and showed potent in vitro and in vivo activity in human NHL xenograft models.14 With the use of the DNL method, we subsequently engineered a bispecific mAb-IFNα (20-C2-2b) comprising veltuzumab fused to a stabilized hL243 F(ab)2 and dimeric IFNα2b, which exhibited potent cytotoxicity against NHL and MM cell lines.15 Because HLA-DR is highly expressed on many types of hematopoietic cancers in which CD20 expression is largely limited to B-cell lymphoma,9 we generated an HLA-DR–targeting mAb-IFNα (C2-2b-2b) comprising tetrameric IFNα fused to hL243 IgG1. Comparative studies with 20-2b-2b presented herein indicate that C2-2b-2b is more potent against NHL and has a much broader potential usage. C2-2b-2b may be useful for therapy of many hematopoietic neoplasias, including a variety of types of lymphoma, leukemia, and myeloma.
Methods
Abs and reagents
The following mAbs were provided by Immunomedics Inc: veltuzumab (anti-CD20 IgG1); hL243γ4p (IMMU-114, anti-HLA-DR IgG4); hL243 IgG1; a murine anti-IFNα mAb; hMN-14 (labetuzumab); a rat anti-idiotype mAb hL243 (WT). Peginterferon alfa-2a (Hoffmann-La Roche) and recombinant IFNα (Schering Corp) were used as control reagents.
Cell culture
Heat-inactivated FBS was obtained from Hyclone. All other cell culture media and supplements were purchased from Invitrogen Life Technologies. Sp/ESF cells, a cell line derived from Sp2/0-Ag14 with superior growth properties, were maintained in Hybridoma Serum-Free Media. MEC-1 was grown in IMDM with 10% FBS, 1mM sodium pyruvate, 10mM l-glutamine, and 25mM HEPES. RS4;11 and Granta-519 were grown in MEM with 10% FBS. All the other lines were grown in RPMI 1640 medium with 10% FBS (20% FBS for Jeko-1, Kasumi-3, and KMS12-BM), 1mM sodium pyruvate, 10mM l-glutamine, and 25mM HEPES. Daudi, Ramos, Raji, RL Jeko-1, NCI-H929, U266, GDM-1, and Kasumi-3 cells were purchased from American Type Culture Collection. MEC-1, REH, 697, HC-1, RS4;11, WSU-FSCCL, Granta-519, and NCI-H929 cells were from the German Collection of Microorganisms and Cell Cultures. The sources of MM cell lines are as follows: KMS11, KMS12-PE, and KMS12-BM from Dr Takemi Otsuki (Kawasaki Medical School); CAG, OPM-6, and MM.1R from Dr Joshua Epstein (University of Arkansas), Dr Kenji Oritani (Osaka University), and Dr Steven Rosen (Northwestern University), respectively.
DNL constructs
mAb-IFNα (C2-2b-2b, 20-2b-2b, 734-2b-2b, and 14-2b-2b) were generated by the combination of a CH3-AD2-IgG module (Figure 1A) with the IFNα2b-DDD2 (Figure 1B) module with the use of the DNL method, as described previously.14,16 C2-2b-2b comprises a CH3-AD2-IgG module derived from hL243 IgG1. The 734-2b-2b and 14-2b-2b, which comprise tetrameric IFNα2b fused with mAb h734 (humanized anti-indium diethylene triamine pentaacetic acid IgG1) and hMN-14 (humanized anti-CEACAM5 IgG1), respectively, were used as a nontargeting control mAb-IFNα. Detailed methods for the production and purification of the DNL modules used to generate C2-2b-2b, as well as the generation of C2-2b-2b by DNL, are provided in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
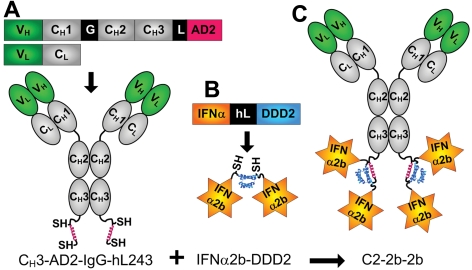
Figure 1.
Schematics of C2-2b-2b and its constituent DNL modules. Structures and expression cassettes of CH3-AD2-IgG-hL243 (A) and IFNα2b-DDD2 (B), and C2-2b-2b (C). Blue and red helices represent DDD2 and AD2, respectively; SH indicates sulfhydryl groups of engineered cyseines; hL243 V, variable (green); C, constant (gray); G, hinge; L, linker; and hL, 6-His-linker.
Analytical methods
Size-exclusion high-performance liquid chromatography (SE-HPLC) was performed with an Alliance HPLC System with a BioSuite 250, 4-μm UHR SEC column (Waters Corp). Immunoreactivity was assessed by mixing excess WT or anti-IFNα with C2-2b-2b before analysis of the resulting immune complex by SE-HPLC.
SDS-PAGE was performed with 4%-20% gradient Tris-glycine gels (Invitrogen). Immunoblot analysis was performed as described previously,9 with all primary and HRP-conjugated second Abs from Cell Signaling Technology.
Electrospray ionization time of flight (TOF) liquid chromatography/mass spectrometry was performed with a 1200-series HPLC coupled with a 6210 TOF MS (Agilent Technologies). C2-2b-2b was reduced with 50mM tris(2-carboxyethyl)phosphine and resolved by reversed-phase HPLC at 60°C, using a 20-minute gradient of 40%-80% acetonitrile in 0.1% aqueous formic acid with a Jupiter 300 column (Phenomenex). For the TOF MS, the capillary and fragmentor voltages were set to 5500 and 200 V, respectively.
IFNα2b-specific activities were determined with the iLite Human Interferon Alpha Cell-Based Assay Kit (PBL Interferon Source), as described previously.14 Cell binding, apoptosis, and in vitro cytotoxicity assays were performed as described previously.15
Ex vivo and in vivo methods
Blood specimens were collected under a protocol approved by the New England Institutional Review Board. All animal studies were approved by the Center for Molecular Medicine and Immunology Institutional Animal Care and Use Committee and were performed in accordance with regulations from the Association for Assessment and Accreditation of Laboratory Animal Care, US Department of Agriculture, and Department of Health and Human Services. In vitro Ab-dependent cellular cytotoxicity (ADCC) and CDC activity were assayed as described previously.17 For ex vivo experiments, Daudi or CAG cells (5 × 104) were mixed with heparinized whole blood (150 μL) from healthy volunteers and incubated with mAbs or mAb-IFNα at 1nM for 2 days at 37°C and 5% CO2. The CAG plus whole blood mixtures were stained in triplicate with allophycocyanin (APC)–BDCA-1, PE-CD14, and FITC-CD19 for analysis of myeloid dendritic cell 1 (mDC-1), monocytes, and B cells; APC–BDCA-3, FITC-CD3, and PE-CD19 for analysis of mDC-2 and T cells; and FITC–BDCA-2 and APC-CD138 for analysis of plasmacytoid DC (pDC) and CAG cells. Labeled mAbs were purchased from BD Biosciences (FITC-CD19, FITC-CD3, and CD138) and Miltenyi Biotec Inc (APC–BDCA-1, APC–BDCA-3, FITC–BDCA-2, PE-CD14, and PE-CD19). DCs were not analyzed in the Daudi plus whole blood studies. Daudi cells were detected as CD19+ cells in the monocyte gate; the normal B and T cells are CD19+ and CD3+ cells, respectively, in the lymphocyte gate in the side scatter versus forward scatter dot plot. Analyses were performed by flow cytometry with the use of a FACSCalibur (BD Biosciences).
In vivo efficacy in mice
Female 8- to 12-week-old C.B.17 homozygous SCID mice (Taconic) were inoculated intravenously with 1.5 × 107 Daudi or 1.0 × 107 CAG cells on day 0. Treatments were administered as a single subcutaneous injection on day 7. Saline was used as a control treatment. Animals, monitored daily, were humanely killed when hind-limb paralysis developed or if they became otherwise moribund. In addition, mice were killed if they lost > 20% of initial body weight. Survival curves were analyzed with Kaplan-Meier plots, using the Prism (Version 4.03) software package (GraphPad Software).
Statistical analyses
Statistical significance (P < .05) was determined with Student t tests for all results except for the in vivo survival curves, which were evaluated by log-rank analysis.
Results
Generation and characterization of C2-2b-2b
The HLA-DR–targeting IgG-AD2 module, CH3-AD2-IgG-hL243 (Figure 1A), was combined with 2 molar equivalents of IFNα2b-DDD2 (Figure 1B) and, following a mild redox reaction, C2-2b-2b (Figure 1C) was purified by Protein A affinity chromatography. SDS-PAGE (supplemental Figure 1) and SE-HPLC (supplemental Figure 2) analyses of the purity, molecular size, and immunoreactivity of C2-2b-2b are detailed as supplemental Results. Liquid chromatography/mass spectrometry analysis confirmed the mass of each of the 3 polypeptides comprising C2-2b-2b, with the experimental masses being consistent with the calculated masses from their deduced amino acid sequences and predicted posttranslational modifications, including O-linked glycosylation on a portion of the IFNα2b-DDD2,18 as well as N-linked glycosylation and amino-terminal pyroglutamate on the HC-AD2 polypeptide (supplemental Table 1; supplemental Figure 3).
Biologic activity
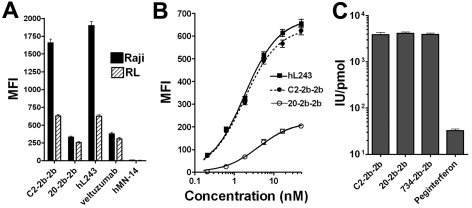
The mAb-IFNα bound similarly to live HLA-DR+/CD20+ cells as their parental mAbs (Figure 2A). The Ag density of HLA-DR is ∼ 6-fold greater than CD20 in these cells,9 allowing more binding of C2-2b-2b compared with 20-2b-2b. Binding curves with the use of RL cells, which were analyzed with a one-site binding nonlinear regression model, showed that C2-2b-2b can achieve a similar Bmax and Kd (1.8nM) as hL243γ4p. C2-2b-2b achieved a 3-fold greater Bmax and stronger binding affinity than with 20-2b-2b (Kd = 4.2nM) for these cells (Figure 2B).
Figure 2.
Biologic activity of C2-2b-2b. (A-B) Binding of mAbs and mAb-IFNα to live NHL cells. After binding of the indicated constructs, cells were probed with PE-conjugated goat anti–human Fc and analyzed by flow cytometry. MFI indicates mean fluorescence intensity; n = 5000 cells; error bars, 95% confidence interval. (A) Raji (black bars) or RL (hatched bars) cells were incubated at 4°C for 1 hour in the presence of 5nM of the indicated construct. (B) RL cells were incubated at 4°C for 1 hour in the presence of 0.2-50nM of hL243 IgG1, C2-2b-2b, or 20-2b-2b. (C) IFNα2-specific activities were determined with a cell-based reporter gene assay.
The specific activities for the mAb-IFNα used in this study were measured with a cell-based reporter gene assay and compared with peginterferonalfa-2a (Figure 2C). The measured specific activities were similar among the 3 mAb-IFNα (3880-4146 IU/pmol, not significantly different), which were > 100-fold (P < .001) greater than that of peginterferonalfa-2a (32 IU/pmol).
In vitro cytotoxicity
A total of 22 cell lines comprising NHL (3 Burkitt leukemias, 2 mantle cell leukemias, and 1 follicular leukemia), leukemias (2 acute myeloid [AMLs], 2 chronic lymphocytic [CLLs], 3 acute lymphoblastic [ALLs], and 1 hairy cell), and MM (8 lines) were evaluated for direct cytotoxicity of C2-2b-2b in vitro. The results, including the relative Ag densities of HLA-DR and CD20 and response to C2-2b-2b as well as control treatments with 20-2b-2b, hL243γ4p, nontargeting mAb-IFNα (734-2b-2b), and a combination of hL243γ4p and 734-2b-2b (hL243 + 734-2b), are summarized in Table 1, and the individual dose-response curves are shown in supplemental Figures 4-6. The 734-2b-2b, which uses an IgG-AD2 module that does not bind specifically to any cells or tissues, is the preferred control for nontargeting IFNα, because it shares similar properties with C2-2b-2b, including structure, IFNα specific activity, and Pk.
Table 1.
In vitro cytotoxicity of nAb-IFNα on hematopoietic tumor cell lines
| Cell line | Type | HLA-DR expression, MFI | CD20 expression, MFI | C2-2b-2b |
20-2b-2b |
734-2b-2b |
hL243γ4p |
hL243γ4p + 734-2b-2b |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50, nM | TI, fold | Imax, % | IC50, nM | TI, fold | Imax, % | IC50, nM | Imax, % | IC50, nM | Imax, % | IC50, nM | Imax, % | ||||
| Daudi | BL | 2092* | 500* | 3 × 10−5 | 117 | 95 | 1 × 10−4 | 25 | 95 | 3 × 10−3 | 95 | 5.13 | 67 | 4 × 10−3 | 95 |
| Raji | BL | 2317* | 391* | 0.35 | 23 | 70 | 3.89 | 2 | 60 | 8.13 | 62 | > 20† | 45 | 0.56 | 70 |
| Ramos | BL | 373* | 516* | 0.90 | > 20 | 82 | 7.76 | > 3 | 70 | > 20† | 35 | > 20† | 25 | > 20† | 43 |
| WSU-FSCCL | FL | 2030* | 37* | 0.19 | > 100 | 80 | > 20† | 22 | > 20† | 24 | 0.37 | 85 | 0.46 | 73 | |
| Jeko-1 | MCL | 539* | 250* | 0.10 | > 200 | 100 | 1.1 | > 20 | 59 | > 20† | 21 | 0.4 | 98 | 0.18 | 90 |
| Granta-519 | MCL | 1797* | 805* | 4.0 | > 5 | 66 | > 20† | 31 | > 20† | 14 | 6.4 | 59 | 3.5 | 66 | |
| CAG | MM | 2804* | 4* | 3 × 10−3 | 55 | 98 | 0.13 | 1 | 85 | 0.16 | 85 | 20 | 52 | 0.08 | 98 |
| NCI-H929 | MM | 4‡ | 1‡ | 0.13 | 1 | 98 | 0.14 | 1 | 98 | 0.11 | 98 | > 20† | 0 | 0.12 | 98 |
| KMS12-PE | MM | 940* | 3* | 0.63 | 6 | 80 | 3.28 | 1 | 72 | 3.9 | 68 | > 20† | 27 | 0.97 | 76 |
| KMS12-BM | MM | 1502* | 318* | 0.51 | > 50 | 73 | 7.82 | > 3 | 55 | > 20† | 22 | 3.47 | 59 | 0.61 | 73 |
| MM1R | MM | 79‡ | 1‡ | 2 × 10−3 | 10 | 100 | 0.05 | 0.5 | 95 | 0.02 | 97 | > 20† | 10 | 0.03 | 98 |
| KMS11 | MM | 615* | 3* | 1.85 | > 10 | 66 | > 20† | 21 | > 20† | 24 | 2.19 | 62 | 1.72 | 66 | |
| OPM6 | MM | 41‡ | 2‡ | 4 × 10−3 | 2 | 99 | 8 × 10−3 | 1 | 99 | 8 × 10−3 | 99 | > 20† | 6 | 8 × 10−3 | 99 |
| U266 | MM | 223‡ | 2‡ | 9 × 10−4 | 22 | 96 | 0.02 | 1 | 93 | 0.02 | 92 | > 20† | 10 | 8 × 10−3 | 91 |
| HC-1 | HCL | 5501* | 1079* | 0.08 | 134 | 91 | 0.47 | 23 | 69 | 10.72 | 59 | 0.59 | 89 | 0.49 | 89 |
| Kasumi-3 | AML | 445* | 5* | 6 × 10−3 | 18 | 87 | 0.10 | 1 | 78 | 0.11 | 76 | > 20† | 0 | 0.09 | 81 |
| GDM-1 | AML | 553* | 4* | 8 | > 3 | 68 | > 20† | 11 | > 20† | 35 | > 20† | 0 | > 20† | 35 | |
| MEC-1 | CLL | 2421* | 271* | 1.2 | 17 | 78 | 20 | > 1 | 50 | > 20† | 34 | > 20† | 30 | 20 | 50 |
| WAC | CLL | 799* | 211* | 2.19 | > 10 | 79 | > 20† | 26 | > 20† | 20 | > 20† | 37 | 5.89 | 60 | |
| REH | ALL | 2583* | 15* | 2.14 | 12 | 67 | 13.80 | 2 | 57 | 26.30 | 52 | 7.46 | 64 | 4.95 | 64 |
| 697 | ALL | 1108* | 13* | > 20† | 40 | > 20† | 40 | > 20† | 23 | > 20† | 18 | > 20† | 34 | ||
| RS4/11 | ALL | 2522* | 3* | > 20† | 41 | > 20† | 39 | > 20† | 36 | > 20† | 19 | > 20† | 41 | ||
Cultures were grown in 48-well plates until the density of untreated cells increased a minimum of 10-fold (3-7 days).
MFI indicates mean fluorescent intensity; IC50, concentration (nM) resulting in 50% growth inhibition compared with untreated cells; TI, targeting index = fold reduction in EC50 compared with nontargeted IFNα (734-2b-2b); Imax, maximal percentage decrease in viable cells compared with untreated cells; BL, Burkitt lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; HCL, hairy cell leukemia; AML, acute myeloid leukemia; CLL, chronic lymphoid leukemia; and ALL, acute lymphoblastic leukemia.
Previously reported in Rossi et al.15
Treatment failed to reach 50% inhibition.
Previously reported in Rossi et al.14
The responsiveness of the cells to IFNα (determined with 734-2b-2b) varied widely, whereby 12 were insensitive (IC50 > 20nM), 3 were moderately sensitive (20nM > IC50 > 0.2nM), and 7 were highly sensitive (IC50 < 0.2nM). With respect to hL243, 13 cell lines were categorized as insensitive (IC50 > 20nM) and 9 were sensitive (IC50 < 20nM). Six and 3 of the lines were either insensitive or sensitive to both single agents, respectively.
Treatment with C2-2b-2b resulted in a > 50% maximal inhibition for all of the lines but 2 (of 3) ALLs, which were nonresponsive to both hL243γ4p and IFNα. The IC50 for C2-2b-2b varied widely (3 × 10−14M to 8 × 10−9M) among cell lines. The principal feature correlating with high potency of C2-2b-2b is the sensitivity of the cell line to IFNα. Among the tested lines, the ALL and CLL were less responsive to C2-2b-2b; however, the sample size is too small to make any generalization about these leukemias. Similarly, the responsiveness of AML cannot be generalized, because one line was highly sensitive (IC50 = 6pM) to C2-2b-2b, whereas the other was only moderately responsive (IC50 = 8nM).
The potency of C2-2b-2b, which was directly associated with the cells' response to 734-2b-2b, was enhanced by targeting of HLA-DR in all Ag-positive cells. The targeting index (TI) represents the fold-increase in potency of a targeted mAb-IFNα compared with 734-2b-2b. Generally, the TI of C2-2b-2b was greater for cells with higher HLA-DR Ag density, whereas cells with very low Ag density, such as OPM6 (MM), exhibited a low TI. However, the TI is not directly proportional to Ag density. Notably, for each HLA-DR–positive line, the TI of C2-2b-2b is ≥ 2 and greater than that of 20-2b-2b, even for Ramos, which has higher CD20 Ag density compared with HLA-DR. The results suggest that hL243-induced signaling can contribute to the TI of C2-2b-2b.
There were 8 lines that were sensitive to IFNα but not hL243. Half of these were more effectively inhibited by hL243 + 734-2b compared with 734-2b-2b alone. The combination enhanced cytotoxicity on moderately IFNα-sensitive cells but showed little or no improvement on highly IFNα-sensitive lines, which are killed by IFNα at lower than the effective concentrations for hL243. These results suggest that the actions of hL243 and IFNα can have an additive effect on cell killing. Besides NCI-H929 (HLA-DR− MM), 7 of the 8 lines were more responsive to C2-2b-2b than to 734-2b-2b, and in each case C2-2b-2b was superior to hL243 + 734-2b, indicating that, in addition to the additive actions of hL243 and IFNα, targeting contributes to enhanced toxicity.
Six lines were sensitive to hL243 but not IFNα. Compared with hL243γ4p alone, only one of these was more effectively inhibited with hL243 + 734-2b, whereby 4 were more responsive to C2-2b-2b. The increase in cytotoxicity was relatively small for C2-2b-2b over hL243γ4p with the IFNα-insensitive cells. Surprisingly, 4 of the 6 lines that were insensitive to both IFNα and hL243 were effectively inhibited by C2-2b-2b but not hL243 + 734-2b. C2-2b-2b was substantially more potent than 734-2b-2b, hL243γ4p, or hL243 + 734-2b against each of the 3 lines that were sensitive to both IFNα and hL243.
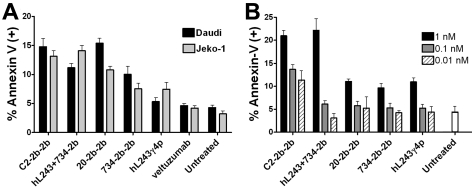
Apoptosis
Apoptosis was induced in Daudi (Burkitt leukemia) with only 1pM of any mAb-IFNα but not with 10pM of hL243γ4p (Figure 3A). Treatment with C2-2b-2b or 20-2b-2b resulted in significantly more apoptotic cells than 734-2b-2b or hL243 + 734-2b (P < .001). There was no significant difference observed between 734-2b-2b and the mixture.
Figure 3.
Apoptosis in NHL and MM cells. Cells were treated for 48 hours before quantification of the percentage of annexin-V–positive cells by flow cytometry. (A) For Daudi cells, hL243γ4p was 10pM; C2-2b-2b, 20-2b-2b, and hL243 + 734-2b were 1pM. For Jeko-1 cells, all treatments were at 0.5nM. (B), CAG was treated at 1nM, 0.1nM, and 0.01 nM. Error bars are the SD from triplicate samples.
Treatment of Jeko-1 (mantle cell leukemia), which was sensitive to hL243γ4p but not IFNα in the cytotoxicity assay, with 0.5nM of hL243γ4p or 734-2b-2b induced similar levels of apoptosis, and their effects are apparently additive, because treatment with hL243 + 734-2b resulted in approximately twice the number of annexin-V–positive cells compared with either agent alone (Figure 3A). Both C2-2b-2b and hL243 + 734-2b were superior to 20-2b-2b (P < .002), because of the action of hL243.
Apoptosis of CAG (MM) was evident after treatment with hL243γ4p, 20-2b-2b, 734-2b-2b, or hL243 + 734-2b at 1nM, but not at 0.1 or 0.01nM. C2-2b-2b induced apoptosis even at 0.01nM and is ≥ 100-fold more potent than either single agent alone and ≥ 10-fold more potent than the hL243 + 734-2b combination (Figure 3B).
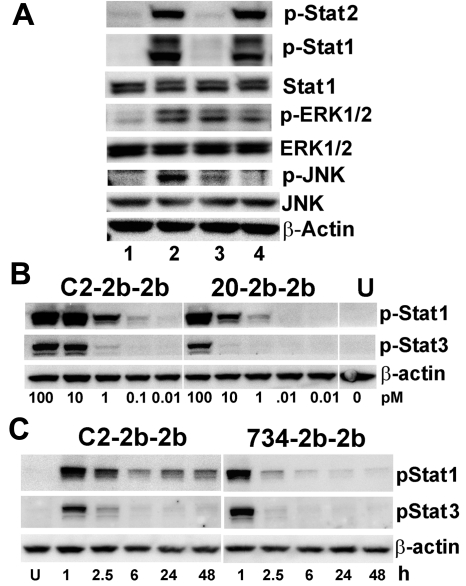
Signaling
We have recently reported that binding of hL243γ4p to HLA-DR activates ERK and JNK MAPK signaling pathways.9 In Daudi, C2-2b-2b (20nM) induced more tyrosine phosphorylation of ERK1/2 than hL243γ4p or 734-2b-2b, which each increased p-ERK1/2 levels compared with untreated cells (Figure 3A). C2-2b-2b treatment increased p-JNK1/2 levels appreciably more than hL243γ4p, and no p-JNK1/2 was evident in cells treated with 734-2b-2b (Figure 4A).
Figure 4.
Immunoblot analysis of cell signaling induced by C2-2b-2b. (A) Daudi cells were untreated (lane 1) or treated for 2 hours at 20nM with C2-2b-2b (lane 2), hL243γ4p (lane 3), or 734-2b-2b (lane 4) and evaluated with loading 20 μg of total protein/lane. (B) CAG cells were treated for 1 hour with C2-2b-2b (left) or 20-2b-2b (middle). The picomolar concentration of mAb-IFNα is indicated at the bottom of each lane. Phospho-Stat-1 and phospho-Stat-3 were measured with loading 15 μg of total protein/lane. (C) Daudi cells were treated for 1 hour with 0.1nM of C2-2b-2b (left) or 734-2b-2b (right), washed, and then incubated an additional 48 hours. Phospho-Stat-1 and phospho-Stat-3 were measured with loading 10 μg of total protein/lane. Time points indicate hours after washing; and U, untreated. β-actin was used to verify equal loading. Specific Ab probes are indicated to the right of each panel.
IFNα activity is mediated through phosphorylation of STATs after binding of type I IFN receptors. C2-2b-2b and 734-2b-2b both induce robust phosphorylation of STAT1 and STAT2 in Daudi (Figure 4A). Whereby STAT1 activation is required for IFNα-mediated cell death,19 STAT3 is a main survival factor in MM,20 and possibly other tumor types. In CAG (HLA-DR+/CD20− MM), C2-2b-2b induced more p-STAT1 and p-STAT3 than 20-2b-2b (nontargeting for these cells) because of targeting. Notably, phosphorylation of STAT1 is more robust than STAT3, particularly at lower concentrations (Figure 3B). Induction of STAT3 phosphorylation is not a unique property of mAb-IFNα, because it was up-regulated similarly with rIFNα2b and peginterferonalfa-2a (supplemental Figure 7). In addition to increasing potency (lower IC50), targeting of IFNα to HLA-DR (or another Ag) can increase the tumor residence time and prolong IFNα signaling. This effect was studied with Daudi cells, which were treated for 1 hour at 0.1nM with C2-2b-2b or 734-2b-2b and then washed before further incubation for 48 hours (Figure 4C). Unlike cells treated with the nontargeting 734-2b-2b, whereby p-STAT1was negligible as early as 6 hours after washing, p-STAT1 persisted for 48 hours after washing in cells treated with C2-2b-2b. Phospho-STAT1 persisted longer than p-STAT3, which was minimal by 6 hours.
Effector functions
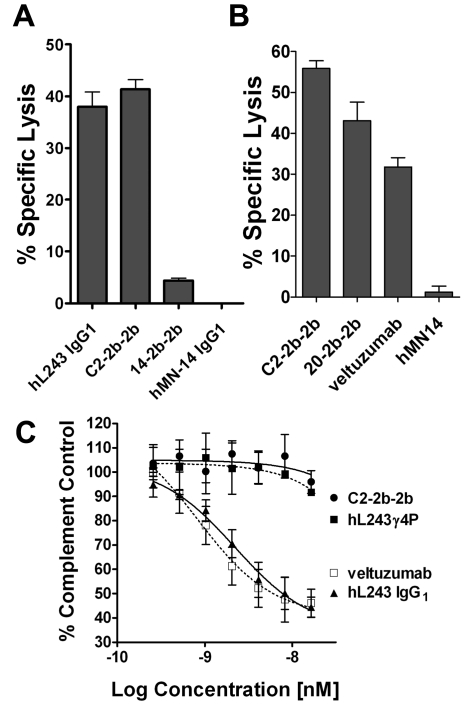
By design, hL243γ4p has diminished ADCC,11 yet hL243 IgG1 exhibits potent ADCC (Figure 5A). ADCC was greater for C2-2b-2b (41.4% lysis) than for hL243 IgG1 (38.0%). Although significant (P = .009), the enhancement was modest compared with that achieved with 20-2b-2b over its parent, veltuzumab (Figure 5B), as was also reported previously.14 The relatively small enhancement is probably because of the potent ADCC of hL243 IgG1. C2-2b-2b induced significantly (P = .002) greater ADCC than 20-2b-2b (supplemental Figure 9B). Only hL243 IgG1, but not hL243γ4p or C2-2b-2b, induced CDC in vitro (Figure 5C).
Figure 5.
Effector functions. ADCC was evaluated with Raji (A) or Daudi (B) cells. Cells were incubated with the indicated mAb or mAb-IFNα (10 replicates/treatment) at 33nM in the presence of freshly isolated PBMCs) for 4 hours before quantification of cell lysis with CytoTox-One (Promega). Effector/target ratio = 50:1. Error bars indicate SD. The hMN-14 (anti-CEACAM5 mAb) and 14-2b-2b (mAb-IFNα of hMN-14) were used as a nontargeting mAb and mAb-IFNα, respectively. (C) CDC. Daudi cells were incubated with serial dilutions of C2-2b-2b, hL243γ4p, hL243 IgG1, or veltuzumab in the presence of human complement (1/20 final dilution; Quidel Corp) for 2 hours at 37°C and 5% CO2. Viable cells were then quantified with the Vybrant Cell Metabolic Assay Resazurin kit (Invitrogen). Controls included cells treated with 0.25% Triton X-100 (100% lysis) and cells treated with complement alone (background). The percentage of complement control (number of viable cells in the test sample compared with cells treated with complement only) was plotted against the log of the molar concentration. Error bars indicate SDs.
Ex vivo depletion of lymphoma and myeloma from whole human blood
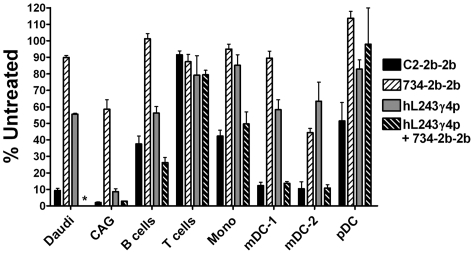
Daudi (NHL) and CAG (MM) cells were depleted from whole blood (ex vivo) more effectively by C2-2b-2b than with hL243γ4p, 734-2b-2b, or hL243 + 734-2b. C2-2b-2b was less toxic to normal B cells than with Daudi or CAG (Figure 6). Under these conditions, B cells were depleted by C2-2b-2b (62%) and hL243γ4p (44%), hL243 + 734-2b (74%), but not by 734-2b-2b. None of the treatments significantly depleted T cells. Monocytes were depleted more by C2-2b-2b (58%) and hL243 + 734-2b (50%) than with hL243γ4p (15%) or 734-2b-2b (5%). C2-2b-2b and hL243 + 734-2b depleted DCs, with mDC-1 and mDC-2 being more sensitive than pDCs. The mDC-1s were depleted by hL243γ4p (41%), and the mDC-2s were depleted by both hL243γ4p (38%) and 734-2b-2b (55%).
Figure 6.
Enhanced depletion of lymphoma and myeloma cells from whole blood. Fresh heparinized human blood was mixed with Daudi or CAG cells and incubated with 1nM of C2-2b-2b, 734-2b-2b, hL243γ4p, or hL243γ4p+734-2b for 48 hours. The number of CAG, Daudi, B, T, monocyte, and dendritic (mDC-1, mDC-2, and pDC) cells in treated blood samples was plotted as a percentage of the number of the specific cell type counted in untreated blood by flow cytometry. Error bars indicate SDs. *Daudi cells were not tested with hL243γ4p+734-2b.
In vivo therapy of lymphoma and myeloma xenografts
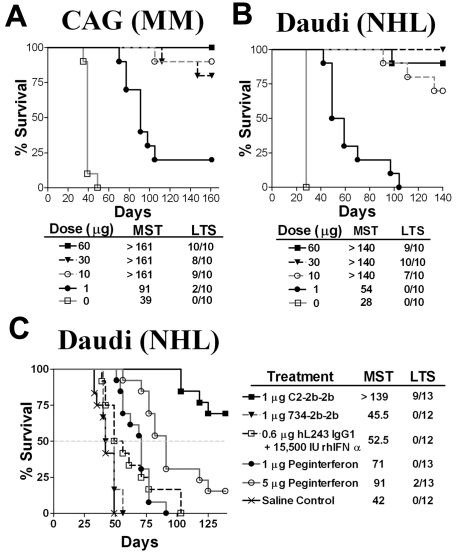
The efficacy of C2-2b-2b was evaluated in xenografts with the use of established Daudi or CAG disease models, whereby a single-dose treatment was given 7 days after tumor inoculation (Figure 7). In preliminary dose-escalation experiments, a 1-μg (15 500 IU) dose of C2-2b-2b significantly (P < .001) improved survival in both models (Figure 7A-B). A 10-μg dose of C2-2b-2b resulted in 70% and 80% long-term survivors (LTSs) for Daudi and CAG, respectively. On termination of the study, necropsies performed on the LTSs showed no visible evidence of disease, indicating that the animals were cured. Even at the highest dose (60 μg), there was no evidence of acute or chronic toxicity in any animal. In a comparative study (Figure 7C), a single 1-μg (15 500 IU) dose of C2-2b-2b significantly (P < .001) increased the median survival time (MST) (> 139 days) of Daudi-bearing mice than for groups treated with saline control (MST = 42 days); an equivalent 1-μg (15 500 IU) dose of 734-2b-2b (MST = 45.5 days); a mixture of 0.6 μg of hL243 IgG1 and 15 500 IU of rhIFNα2b (MST = 52.5 days); 1 μg of peginterferonalfa-2a (MST = 71 days); 5 μg of peginterferonalfa-2a (MST = 91 days).
Figure 7.
Survival curves showing therapeutic efficacy of C2-2b-2b in disseminated Burkitt lymphoma (Daudi) and multiple myeloma (CAG) xenograft models. Female CB17 SCID mice were inoculated with CAG (A) or Daudi (B-C) cells intravenously on day 0. Treatment was administered as a single subcutaneous dose on day 7. Survival curves were analyzed with Prism software. (A-B) Dose-escalation study. Groups of 10 mice were treated with C2-2b-2b at 1 μg, 10 μg, 30 μg, or 60 μg. (C) Efficacy compared with hL243 and IFNα. Groups of 12 or 13 mice were given a single dose of C2-2b-2b (1 μg, 4pmol, 15 500 IU), 734-2b-2b (1 μg, 4pmol, 15 500 IU), 0.6 μg (4pmol) hL243 IgG1, + 15 500 IU rIFNα2b or peginterferonalfa-2a [1 μg, 16pmol, and 5 μg, 80pmol).
Discussion
We developed an immunocytokine comprising tetrameric IFNα2b site-specifically tethered to the humanized anti–HLA-DR mAb, hL243, and evaluated its potential use for therapy of a variety of hematopoietic neoplasms. The results show that the HLA-DR–targeted mAb-IFNα, C2-2b-2b, is more potent and effective than either IFNα or hL243 alone or in combination. In addition, C2-2b-2b is more potent and more widely applicable than CD20-targeted mAb-IFNα (20-2b-2b).
Clinically, the use of mAb-targeted IFNα may allow less frequent dosing of a single agent and may reduce or eliminate side effects associated with IFNα therapy. The literature suggests that targeted IFNα might also induce an acute tumor-directed immune response and evoke immune memory.21,22 Because murine cells are considerably less sensitive (∼ 4 logs) than human cells to human IFNα2b,23,24 the antitumor activity of mAb-IFNα in murine models is primarily because of the direct action of IFNα2b on the tumor cells and cannot be attributed to immunoactivation in the host. In the ex vivo studies, C2-2b-2b depleted normal B cells, monocytes, and DCs. This potential pharmacodynamic effect that might be associated with anti–HLA-DR as well as IFNα therapy would probably be transient, because the cell populations should be repopulated from hematopoietic stem cells. The therapeutic potential of the mAb-IFNα, including efficacy as well as side effects and limitations, must be evaluated in patients, whereby careful monitoring of toxicity will be important for this potent agent.
Even without immunoactivation, C2-2b-2b effectively eliminated NHL and MM xenografts in vivo. Fusion of IFNα to hL243 increases its in vivo potency by prolonging circulation and tumor targeting. C2-2b-2b was significantly more potent than either 734-2b-2b or peginterferonalfa-2a (given at a 20-fold higher dose), showing that tumor targeting by the anti–HLA-DR mAb is critical for its superior potency and efficacy. In the Daudi model, a remarkably low dose of 1 μg (4 pmol) of C2-2b-2b significantly improved survival and was superior to similar doses of 734-2b-2b or hL243 IgG1 combined with rIFNα2b. A single C2-2b-2b dose of ≥ 10 μg resulted in a high percentage of cures in both models. We previously reported that 20-2b-2b, which targets IFNα to CD20 on B-cell lymphoma, exhibited similarly high potency and efficacy in a comparable Daudi model, whereby a single 1.7-μg dose of 20-2b-2b significantly increased survival time, and greater doses resulted in a high percentage of LTS.14 The in vivo efficacy with Daudi correlates with the in vitro cytotoxicity results, whereby both C2-2b-2b and 20-2b-2b show subpicomolar IC50.
The effect of targeting was evident in the in vitro cytotoxicity study, which indicated that HLA-DR Ag density and sensitivity to the actions of IFNα2b and hL243 each affect the in vitro responsiveness of a particular cell line to C2-2b-2b. Most of the lines were inhibited more effectively by C2-2b-2b than by hL243 + 734-2b, which may be due solely to an increased local concentration of IFNα as a result of tumor targeting. Alternatively, crosslinking with HLA-DR may prevent the internalization or down-regulation of the type I IFN receptors, resulting in a more prolonged and effective IFNα-induced signal.
In some cases, hL243 and IFNα signaling may act additively, resulting in more potent killing. We have reported that hL243 induces apoptosis in some malignant (and some normal) cell types by signaling pathways that involve the MAPKs ERK and JNK, which are phosphorylated in response to HLA-DR binding on sensitive, but not insensitive, cell lines and CLL patient samples.9 In addition, hL243-induced apoptosis was abolished with a combination of ERK and JNK inhibitors.9 C2-2b-2b up-regulated phosphorylation of ERK1/2 and JNK1/2 more robustly than hL243γ4p, suggesting that IFNα signaling may potentiate hL243-induced apoptosis under certain conditions. In cases in which the effective concentrations of IFNα and hL243 overlap, including moderately IFNα-sensitive (20nM > IC50 > 0.2nM) cells, such as Raji or KMS12-PE, and poorly IFNα-sensitive cells, such as Jeko-1 or WAC, an additive effect was observed for hL243 + 734-2b, which was more potent than either single agent.
STAT3 is constitutively active in a number of human cancers25 and is a key mediator of the prosurvival function of IL-6 in MM and NHL.26,27 In contrast, STAT1 is a promoter of apoptosis,28–30 and its activation can counteract the prosurvival effects of STAT3.31 Thyrell et al32 showed that treatment of MM cell lines with IFNα resulted in decreased STAT3 activity, diminished STAT3/3 (homodimer) DNA binding, and a shift from STAT3/3 to STAT3/1 heterodimers, which lead to the down-regulation of the STAT3 targets, BCL-XL, Mcl-1, and survivin. MAb-IFNα (targeted or nontargeted) induced phosphorylation of both STAT1 and STAT3; however, phosphorylation of STAT1 was more robust, stimulated at lower concentrations of mAb-IFNα, and longer lasting than that of STAT3. Targeting with C2-2b-2b results in increased potency and extends the duration of IFNα signaling. In experiments designed to mimic in vivo conditions, whereby cells were washed after a short treatment with C2-2b-2b, p-STAT3 diminished to negligible levels by 6 hours after washing, yet p-STAT1 persisted for ≥ 48 hours. The levels of both p-STAT1 and p-STAT3 decreased rapidly after washing of cells treated with nontargeting mAb-IFNα. Extended activation of STAT1 over STAT3, which may sustain tumor cells in a proapoptotic state, might be an important advantage of targeted mAb-IFNα immunotherapy with constructs such as C2-2b-2b.
The modular DNL method has been invaluable for our development and evaluation of immunocytokines. It has allowed facile construction of defined multifunctional structures that maintain the biologic properties of their individual components, as well as ideal control structures, such as 734-2b-2b, which have the same architecture and specific activity as C2-2b-2b, but do not target cells. Immunocytokines produced with the DNL method are stable in plasma and are suitable for in vivo applications.14
We and others have reported that fusion proteins comprising CD20-targeting mAbs and IFNα are more effective against NHL than are combinations of mAb and IFNα in xenograft and syngeneic mouse models, indicating that mAb-IFNα can overcome the toxicity and Pk limitations associated with IFNα.14,33 The prototype mAb-IFNα, 20-2b-2b,14 is under development for CD20-targeted immunotherapy of NHL. CD20 is a preferred target for this disease because it is expressed at high levels on the cell surface of many B-cell NHLs, and its expression on normal cells is essentially limited to B cells. The potential benefits of therapy with 20-2b-2b will probably be limited to patients with NHL and possibly with CLL. Our prior surveys of NHL, MM, AML, ALL, and CLL cells found high-level HLA-DR and CD20 expression associated with 79% and 45% of the cell lines, respectively, and most often the Ag density of HLA-DR was markedly greater than that of CD20.9,15 The broader range and higher-level of HLA-DR expression makes C2-2b-2b attractive for use in therapy of diverse malignancies. We recently described the first bispecific mAb-IFNα, 20-C2-2b, which targets dimeric IFNα to both HLA-DR and CD20.15 Although the bispecific immunocytokine may be highly effective for therapy of tumors expressing both Ags, including NHL and the proposed myeloma cancer stem cells, it may be less attractive for therapy of CD20− tumors than C2-2b-2b, which has double the IFNα activity and would be subjected to less of an Ag sink.
More than 20 years ago, Bridges et al34 demonstrated that an antimurine MHC class II mAb could cure B-cell lymphoma in a mouse model and suggested class II Ags as potential therapeutic targets. HLA-DR expression on many normal cell types may be a limitation because of the Ag sink and potential toxicity.35 However, the Ag for approved Abs such as rituximab (CD20) and cetuximab (epidermal growth factor receptor) as well as many additional Abs under clinical investigation are also expressed, sometimes widely, on normal cells, showing that these limitations can be managed. Anti–HLA-DR mAbs, including hL243 IgG1, induce apoptosis, ADCC, and CDC.9,11,36 Although ADCC may enhance therapeutic potential, CDC is largely responsible for the side effects associated with the mAb infusion37 and may have limited the clinical use of another anti–HLA-DR mAb, Hu1D10.38 The hL243γ4p variant (IMMU-114), which was engineered for improved clinical safety by using the constant region of the human IgG4 isotype, maintains the ability to induce apoptosis but has diminished ADCC and CDC.9,11 Clinical investigation of IMMU-114 will be useful for identifying potential toxicity issues that may be associated with C2-2b-2b. C2-2b-2b, which is derived from hL243 IgG1, also maintains signaling/apoptotic properties and ADCC of its parent mAb, but it lacks the toxicity associated with CDC, similar to hL243γ4p. This unique combination should be advantageous for immunotherapy. We anticipate little toxicity, because of the lack of CDC and the anticipated low-level dosing that would be used for C2-2b-2b.
We have demonstrated a high degree of variability among tumor lines with respect to their sensitivity to IFNα, hL243, and C2-2b-2b in vitro. However, ADCC and the actions of immune effector cells, which can be stimulated by the high local concentration of IFNα, may augment tumor killing in vivo. Therefore, even HLA-DR+ tumors that are not responsive to the direct actions of hL243 or IFNα might still be responsive to C2-2b-2b immunotherapy.
Supplementary Material
Acknowledgments
The authors thank John Kopinski, Susan Chen, Diana Pilas, Anju Nair, Maria Zalath, Roberto Arrojo, and Preeti Trisal for excellent technical assistance.
This work was partially supported by the National Cancer Institute, National Institutes of Health (grant P01-CA103985; D.M.G.).
The authors dedicate this article to Ralph A. Reisfeld on the occasion of his 85th birthday.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.A.R designed the study, performed laboratory work, analyzed data, and wrote the paper; D.M.G. and C.-H.C. analyzed data and wrote the paper; and D.L.R., T.M.C., and R.S. performed key laboratory experiments.
Conflict-of-interest disclosure: All authors except R.S. are employees or hold stock or both in Immunomedics Inc or IBC Pharmaceuticals Inc.
Correspondence: Edmund A. Rossi, Immunomedics Inc 300 American Rd, Morris Plains, NJ 07950; e-mail: erossi@immunomedics.com; or David M. Goldenberg, Garden State Cancer Center, Center for Molecular Medicine and Immunology, 300 American Rd, Morris Plains, NJ 07950; e-mail: dmg.gscancer@att.net.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Armitage AE, Armitage JD, Armitage JO. Alpha-interferon for relapsed non-Hodgkin's lymphoma. Bone Marrow Transplant. 2006;38(10):701–702. doi: 10.1038/sj.bmt.1705509. [DOI] [PubMed] [Google Scholar]
- 3.Armitage JO, Coiffier B. Activity of interferon-alpha in relapsed patients with diffuse large B-cell and peripheral T-cell non-Hodgkin's lymphoma. Ann Oncol. 2000;11(3):359–361. doi: 10.1023/a:1008384506227. [DOI] [PubMed] [Google Scholar]
- 4.Davis TA, Maloney DG, Grillo-Lopez AJ, et al. Combination immunotherapy of relapsed or refractory low-grade or follicular non-Hodgkin's lymphoma with rituximab and interferon-alpha-2a. Clin Cancer Res. 2000;6(7):2644–2652. [PubMed] [Google Scholar]
- 5.Kimby E, Jurlander J, Geisler C, et al. Long-term molecular remissions in patients with indolent lymphoma treated with rituximab as a single agent or in combination with interferon alpha-2a: a randomized phase II study from the Nordic Lymphoma Group. Leuk Lymphoma. 2008;49(1):102–112. doi: 10.1080/10428190701704647. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed S, Rai KR. Interferon in the treatment of hairy-cell leukemia. Best Pract Res Clin Haematol. 2003;16(1):69–81. doi: 10.1016/s1521-6926(02)00084-1. [DOI] [PubMed] [Google Scholar]
- 7.Baccarani M, Rosti G, de Vivo A, et al. A randomized study of interferon-alpha versus interferon-alpha and low-dose arabinosyl cytosine in chronic myeloid leukemia. Blood. 2002;99(5):1527–1535. doi: 10.1182/blood.v99.5.1527. [DOI] [PubMed] [Google Scholar]
- 8.Khoo TL, Vangsted AJ, Joshua D, Gibson J. Interferon-alpha in the treatment of multiple myeloma. Curr Drug Targets. 2011;12(3):437–446. doi: 10.2174/138945011794815329. [DOI] [PubMed] [Google Scholar]
- 9.Stein R, Gupta P, Chen X, et al. Therapy of B-cell malignancies by anti-HLA-DR humanized monoclonal antibody, IMMU-114, is mediated through hyper-activation of ERK and JNK MAP kinase signaling pathways. Blood. 2010;115(25):5180–5190. doi: 10.1182/blood-2009-06-228288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith LM, Petty HR, Parham P, McConnell HM. Cell surface properties of HLA antigens on Epstein-Barr virus-transformed cell lines. Proc Natl Acad Sci U S A. 1982;79(2):608–612. doi: 10.1073/pnas.79.2.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein R, Qu Z, Chen S, et al. Characterization of a humanized IgG4 anti-HLA-DR monoclonal antibody that lacks effector cell functions but retains direct antilymphoma activity and increases the potency of rituximab. Blood. 2006;108(8):2736–2744. doi: 10.1182/blood-2006-04-017921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang CH, Rossi EA, Goldenberg DM. The dock and lock method: a novel platform technology for building multivalent, multifunctional structures of defined composition with retained bioactivity. Clin Cancer Res. 2007;13(18 Pt 2):5586s–5591s. doi: 10.1158/1078-0432.CCR-07-1217. [DOI] [PubMed] [Google Scholar]
- 13.Rossi EA, Goldenberg DM, Cardillo TM, et al. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc Natl Acad Sci U S A. 2006;103(18):6841–6846. doi: 10.1073/pnas.0600982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossi EA, Goldenberg DM, Cardillo TM, Stein R, Chang CH. CD20-targeted tetrameric interferon-alpha, a novel and potent immunocytokine for the therapy of B-cell lymphomas. Blood. 2009;114(18):3864–3871. doi: 10.1182/blood-2009-06-228890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi EA, Rossi DL, Stein R, Goldenberg DM, Chang CH. A bispecific antibody-IFNalpha2b immunocytokine targeting CD20 and HLA-DR is highly toxic to human lymphoma and multiple myeloma cells. Cancer Res. 2010;70(19):7600–7609. doi: 10.1158/0008-5472.CAN-10-2126. [DOI] [PubMed] [Google Scholar]
- 16.Rossi EA, Goldenberg DM, Cardillo TM, Stein R, Chang CH. Hexavalent bispecific antibodies represent a new class of anticancer therapeutics: 1. Properties of anti-CD20/CD22 antibodies in lymphoma. Blood. 2009;113(24):6161–6171. doi: 10.1182/blood-2008-10-187138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi EA, Goldenberg DM, Cardillo TM, et al. Novel designs of multivalent anti-CD20 humanized antibodies as improved lymphoma therapeutics. Cancer Res. 2008;68(20):8384–8392. doi: 10.1158/0008-5472.CAN-08-2033. [DOI] [PubMed] [Google Scholar]
- 18.Adolf GR, Kalsner I, Ahorn H, Maurer-Fogy I, Cantell K. Natural human interferon-alpha 2 is O-glycosylated. Biochem J. 1991;276(Pt 2):511–518. doi: 10.1042/bj2760511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arulampalam V, Kolosenko I, Hjortsberg L, et al. Activation of STAT1 is required for interferon-alpha-mediated cell death. Exp Cell Res. 2011;317(1):9–19. doi: 10.1016/j.yexcr.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Oancea M, Mani A, Hussein MA, Almasan A. Apoptosis of multiple myeloma. Int J Hematol. 2004;80(3):224–231. doi: 10.1532/IJH97.04107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13(2):119–134. doi: 10.1016/s1359-6101(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 22.Huang TH, Chintalacharuvu KR, Morrison SL. Targeting IFN-alpha to B cell lymphoma by a tumor-specific antibody elicits potent antitumor activities. J Immunol. 2007;179(10):6881–6888. doi: 10.4049/jimmunol.179.10.6881. [DOI] [PubMed] [Google Scholar]
- 23.Kramer MJ, Dennin R, Kramer C, et al. Cell and virus sensitivity studies with recombinant human alpha interferons. J Interferon Res. 1983;3(4):425–435. doi: 10.1089/jir.1983.3.425. [DOI] [PubMed] [Google Scholar]
- 24.Weck PK, Apperson S, May L, Stebbing N. Comparison of the antiviral activities of various cloned human interferon-alpha subtypes in mammalian cell cultures. J Gen Virol. 1981;57(Pt 1):233–237. doi: 10.1099/0022-1317-57-1-233. [DOI] [PubMed] [Google Scholar]
- 25.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19(21):2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 26.Catlett-Falcone R, Landowski TH, Oshiro MM, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10(1):105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 27.Alas S, Bonavida B. Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin's lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin Cancer Res. 2003;9(1):316–326. [PubMed] [Google Scholar]
- 28.Chin YE, Kitagawa M, Kuida K, Flavell RA, Fu XY. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol Cell Biol. 1997;17(9):5328–5337. doi: 10.1128/mcb.17.9.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A, Commane M, Flickinger TW, Horvath CM, Stark GR. Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science. 1997;278(5343):1630–1632. doi: 10.1126/science.278.5343.1630. [DOI] [PubMed] [Google Scholar]
- 30.Lee KY, Anderson E, Madani K, Rosen GD. Loss of STAT1 expression confers resistance to IFN-gamma-induced apoptosis in ME180 cells. FEBS Lett. 1999;459(3):323–326. doi: 10.1016/s0014-5793(99)01283-1. [DOI] [PubMed] [Google Scholar]
- 31.Dimberg LY, Dimberg AI, Ivarsson K, et al. Ectopic and IFN-induced expression of Fas overcomes resistance to Fas-mediated apoptosis in multiple myeloma cells. Blood. 2005;106(4):1346–1354. doi: 10.1182/blood-2004-04-1322. [DOI] [PubMed] [Google Scholar]
- 32.Thyrell L, Arulampalam V, Hjortsberg L, et al. Interferon alpha induces cell death through interference with interleukin 6 signaling and inhibition of STAT3 activity. Exp Cell Res. 2007;313(19):4015–4024. doi: 10.1016/j.yexcr.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Xuan C, Steward KK, Timmerman JM, Morrison SL. Targeted delivery of interferon-alpha via fusion to anti-CD20 results in potent antitumor activity against B-cell lymphoma. Blood. 2010;115(14):2864–2871. doi: 10.1182/blood-2009-10-250555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bridges SH, Kruisbeek AM, Longo DL. Selective in vivo antitumor effects of monoclonal anti-I-A antibody on B cell lymphoma. J Immunol. 1987;139(12):4242–4249. [PubMed] [Google Scholar]
- 35.Stein R, Balkman C, Chen S, et al. Evaluation of anti-human leukocyte antigen-DR monoclonal antibody therapy in spontaneous canine lymphoma. Leuk Lymphoma. 2011;52(2):273–284. doi: 10.3109/10428194.2010.535182. [DOI] [PubMed] [Google Scholar]
- 36.Rech J, Repp R, Rech D, et al. A humanized HLA-DR antibody (hu1D10, apolizumab) in combination with granulocyte colony-stimulating factor (filgrastim) for the treatment of non-Hodgkin's lymphoma: a pilot study. Leuk Lymphoma. 2006;47(10):2147–2154. doi: 10.1080/10428190600757944. [DOI] [PubMed] [Google Scholar]
- 37.van der Kolk LE, Grillo-Lopez AJ, Baars JW, Hack CE, van Oers MH. Complement activation plays a key role in the side-effects of rituximab treatment. Br J Haematol. 2001;115(4):807–811. doi: 10.1046/j.1365-2141.2001.03166.x. [DOI] [PubMed] [Google Scholar]
- 38.Shi JD, Bullock C, Hall WC, et al. In vivo pharmacodynamic effects of Hu1D10 (remitogen), a humanized antibody reactive against a polymorphic determinant of HLA-DR expressed on B cells. Leuk Lymphoma. 2002;43(6):1303–1312. doi: 10.1080/10428190290026376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.