Abstract
Ischemia/reperfusion (I/R) injury in the kidney is a major cause of acute kidney injury (AKI) in humans and is associated with significantly high mortality. To identify genes that modulate kidney injury and repair, we conducted genome-wide expression analysis in the rat kidneys after I/R and found that the mRNA levels of fibrinogen (Fg)α, Fgβ, and Fgγ chains significantly increase in the kidney and remain elevated throughout the regeneration process. Cellular characterization of Fgα and Fgγ chain immunoreactive proteins shows a predominant expression in renal tubular cells and the localization of immunoreactive Fgβ chain protein is primarily in the renal interstitium in healthy and regenerating kidney. We also show that urinary excretion of Fg is massively increased after kidney damage and is capable of distinguishing human patients with acute or chronic kidney injury (n = 25) from healthy volunteers (n = 25) with high sensitivity and specificity (area under the receiver operating characteristic of 0.98). Furthermore, we demonstrate that Fgβ-derived Bβ15-42 peptide administration protects mice from I/R-induced kidney injury by aiding in epithelial cell proliferation and tissue repair. Given that kidney regeneration is a major determinant of outcome for patients with kidney damage, these results provide new opportunities for the use of Fg in diagnosis, prevention, and therapeutic interventions in kidney disease.
Introduction
Kidney disease is a major public health concern receiving increased global attention owing to the significantly increased prevalence and high mortality rates.1,2 Renal ischemia/reperfusion (I/R) accounts for a significant number of acute kidney injury (AKI) cases in humans. Studies suggest that damage to the renal microvascular architecture and deterioration of the angiogenic response constitutes the early steps in the complex multiple pathways involved in both early and chronic ischemic renal injury.3 Restoration of blood flow to the site of injured tissue is crucial for developing a successful repair response that involves the surviving dedifferentiated cells spreading over the denuded basement membrane, undergoing mitogenesis and ultimately redifferentiating to re-establish and restore functional integrity of the nephron.4,5 Although these processes are well described at the pathologic level, very little is known about the cellular and molecular mechanisms of action of blood proteins within the kidney and their contribution to pathogenesis of renal disease.
Fibrinogen (Fg), a 340-kDa dimeric blood protein, is made up of 2 sets of 3 different polypeptide chains, Fgα, Fgβ, and Fgγ, that span a length of 50 kb on chromosome 4 in region q28.6 Although the primary site for Fg synthesis is shown to be liver, extrahepatic synthesis of Fg by epithelial cells of intestine,7 cervix,8 and lung9,10 has been reported, suggesting that it may function in other structural and functional capacities. Furthermore, endogenous expression of Fgα and Fgβ chain mRNA has been shown in the normal rat kidneys, levels of which significantly increase at 2 hours after the onset of brain death injury,11,12 potentially restoring hemostasis by supporting extracellular matrix and wound repair processes after injury.12 In addition to its major role in blood clotting and circulation via interaction with platelets,13 Fg has been recognized as an important regulator of inflammation,14 wound healing,15 angiogenesis,16 and neoplasia17 via its action on a wide range of cellular receptors such as integrins, intracellular adhesion molecule-1, and vascular endothelial cadherin.18
In this study, we identify Fgα, Fgβ, and Fgγ chain mRNA to be significantly up-regulated in the kidney after bilateral renal I/R injury. We provide evidence suggesting the ability of urinary Fg to serve as a translational, noninvasive, and sensitive biomarker for early detection of AKI. We also demonstrate a therapeutic potential of Fg-derived Bβ15-42 peptide that elicits ∼ 50% protection from bilateral I/R injury by increasing renal tissue repair and decreasing apoptosis. These results provide novel insight into the role of Fg as a diagnostic biomarker and therapeutic candidate in kidney disease and suggest that the presence of Fg in the kidney could serve as a protective mechanism against ischemic injury to facilitate tubular epithelial cell proliferation and tissue repair.
Methods
Peptides and proteins
Endotoxin-free Bβ15-42 (GHRPLDKKREEAPSLRPAPPPISGGGYR) and random peptide (DRGAPAHRPPRGPISGRSTPEKEKLLPG) were custom synthesized (Invitrogen) with 95% modification and N-terminal amine group addition and free acid modification.
Animals
Male Wistar rats (280-320 g) and male C57Bl/6 mice (22-25 g) were purchased from Harlan and Charles River Laboratories, respectively. The animals were maintained in central animal facility over wood chips free of any known chemical contaminants under conditions of 21 ± 1°C and 50%-80% relative humidity at all times in an alternating 12-hour light-dark cycle. Animals were fed with commercial rodent chow (Teklad rodent diet 7012), given water ad libitum, and were acclimated for 1 week before use. All animal maintenance and treatment protocols were in compliance with the “Guide for Care and Use of Laboratory Animals” as adopted and promulgated by the National Institutes of Health and were approved by the Harvard Medical School Animal Care and Use Committees.
Selection of participants
Critically ill patients in the intensive care unit with elevated serum creatinine (SCr; > 1.5 mg/dL) were recruited. Causes of AKI or chronic kidney disease (CKD) were obtained by detailed chart review including the treating nephrologist's consultation note and evaluation of laboratory data by a coauthor not involved in the patients' care (S.S.W.). Healthy volunteers were recruited from the staff at Brigham and Women's Hospital. Healthy volunteers were excluded if they reported a recent hospitalization, diagnosis of CKD, or treatment with nephrotoxic medications (nonsteroidal anti-inflammatory drugs were allowed). All participants were patients or employees (healthy volunteers) of Brigham and Women's Hospital, a tertiary care teaching hospital. The Institutional Review Board approved the protocols for recruitment and sample collection.
Human kidney biopsy sections
Human kidney biopsy sections were obtained through Brigham and Women's Hospital's pathology service core, classified as patient without evidence of acute tubular injury (ATI): 58-year-old female diagnosed with renal oncocytoma and patient with ATI: 78-year-old male diagnosed with active glomerulonephritis with diffuse proliferative and crescentic pattern of injury, active interstitial nephritis, active tubular injury involving focal tubular atrophy, and interstitial fibrosis (30%).
Experimental design
Rat studies.
For whole genome expression profiling studies, 9 male Wistar rats underwent I/R surgery, and 3 rats underwent sham surgery simulating I/R. To perform I/R surgery, the rats were anesthetized using pentobarbital sodium (30 mg/kg ip), and renal ischemia was induced by nontraumatic vascular clamps over the pedicles for 20 minutes as described previously.19,20 On release of the clamps, the incision was closed in 2 layers with 2-0 sutures. The sham rats underwent anesthesia and a laparotomy only and were killed after 24 hours. The rats in I/R group were further divided in subgroups of 3 rats each and killed after 6, 24, and 120 hours of reperfusion. To confirm the results of gene expression analysis, 20 male Wistar rats underwent 20 minutes bilateral I/R surgery and 5 rats underwent sham surgery as described above and were killed at 6, 24, 72 and 120 hours after reperfusion (n = 5/time point).
Mouse studies.
Forty male C57Bl/6 wild-type mice were anesthetized using pentobarbital sodium (30 mg/kg ip) and subjected to 27 minutes of bilateral renal I/R surgery by the retroperitoneal approach. Sham surgery was performed with exposure of both kidneys but without induction of ischemia. Immediately on the start of reperfusion, 3.6 mg/kg Bβ15-42 or random peptide were administered intravenously to the mice via tail vein. One milliliter of warm saline (37°C) was injected intraperitoneally 3 hours after surgery for volume supplementation. Mice (n = 5-10/group) in the respective groups (sham or I/R-administered Bβ15-42 or random peptide) were killed at 24 and 48 hours after reperfusion using overdose of pentobarbital (180 mg/kg ip).
Blood and urine analysis
At sacrifice, blood was collected from dorsal aorta in heparinized tubes. SCr concentrations were measured using a Creatinine Analyzer II (Beckman Coulter). Blood urea nitrogen (BUN) was measured spectrophotometrically at 340 nm using a commercially available kit (Thermo Fisher Scientific) as described previously.21
Urines were collected by placing animals in individual metabolic cages, and 1 mL of RNAlater (for rats and 40 μl for mice; Ambion) was added to the tubes to preserve RNA. Urinary kidney injury molecule-1 (Kim-1 in rats and KIM-1 in humans) was measured using previously established Luminex-based assays.20,22 Urinary Kim-1 in mice was measured using a Luminex-based assay in Dr J. V. Bonventre's laboratory (Brigham and Woman's Hospital, Harvard Medical School). Urinary N-acetyl-β-d-glucosaminidase (NAG; Roche Diagnostics) was measured spectrophotometrically according to the manufacturer's protocols. Urinary creatinine concentration was used to normalize biomarker measurements to account for the influence of urinary dilution on biomarker concentrations. Fg in mouse, rat, and human urines was measured using commercially available species-specific Luminex-based assay kit from Millipore.
Whole genome expression profiling and hierarchical clustering
For genome-wide expression analysis, RatRef-12 bead array (Illumina) was used that contains ∼ 22 523 50-mer oligonucleotide probes primarily based on National Center for Biotechnology Information RefSeq Release 16 database. Gene expression and hybridization array data set has been submitted to the National Center for Biotechnology Information Gene expression omnibus (accession:GSE27274; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=drkjxksyckeqcrq&acc=GSE27274). Total RNA was extracted from 30 mg of frozen tissue samples using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Integrity of the isolated total RNA was determined by 1% agarose gel electrophoresis, and the RNA concentration was measured by ultraviolet light absorbance at 260 nm using a Nanodrop 2000C spectrophotometer (Thermo Fisher Scientific). Aliquots of RNA were converted into double-stranded cRNA and biotinylated using the Illumina TotalPrep RNA amplification kit (Ambion). The cRNA samples were then labeled with streptavidin-cyanin 3 (Cy3) and hybridized onto RatRef-12 Expression BeadChip (Illumina). The image was scanned using a BeadArray reader (Illumina), and the data were analyzed by BeadStudio software (Version 3.3.7; Illumina). For nonredundant 22 523 symbols, the intensity profiles were quantile normalized. We used median absolute deviation (MAD) to select highly variable genes (MAD > 0.4; n = 1571) for subsequent analysis. Hierarchical clustering was performed using 1 − Pearson correlation coefficient as distance with average linkage option.
Real-time RT-PCR
The isolated RNA was treated with QuantiTect Reverse Transcription kit (QIAGEN Sciences). Real-time PCR of the tissue samples was performed with QuantiFast SYBR Green (QIAGEN Sciences) using CFX96 RT-PCR instrument (Bio-Rad Laboratories).21 Primers were designed to amplify a 120- to 150-base pair fragment with the following cycle conditions: 95°C for 3 minutes and then the following steps were repeated 40 times: 95°C for 30 seconds, 55°C for 30 seconds. Forward and reverse primer sequences for rat- and mouse-specific genes were designed using MacVector software (MacVector) and are listed in Table 1.
Table 1.
Real-time PCR primers used for the quantification of mRNA expression levels in the study
| Gene | F/R | Sequence |
|---|---|---|
| CD68 | F | TCTTTCTCCAGCTGTTCACC |
| R | ATGATGAGAGGCAGCAAGAG | |
| Gapdh | F | TCCGCCCCTTCTGCCGATG |
| R | CACGGAAGGCCATGCCAGTGA | |
| ICAM-1 | F | TGTTTTGCTCCCTGGAAGGC |
| R | AGTCACTGCTGTTTGTGCTCTCC | |
| IL-1β | F | ACCTGTCCTGTGTAATGAAAGACG |
| R | TGGGTATTGCTTGGGATCC | |
| IL-6 | F | CAAGAGACTTCCATCCAGTTGCC |
| R | CATTTCCACGATTTCCCAGAGAAC | |
| IL-10 | F | CAGCCTTGCAGAAAAGAGAG |
| R | GGAAGTGGGTGCAGTTATTG | |
| Mouse Fgα chain | F | TGTGGAGAGACATCAGAGTCAATG |
| R | CGTCAATCAACCCTTTCATCC | |
| Mouse Fgβ chain | F | CTATGGCTGCTGCTGCTATTG |
| R | GGCTCTTCCTTTCTCCTGTCAAC | |
| Mouse Fgγ chain | F | TGTGGCTACCAGAGATAACTGTTG |
| R | ATGTCTTCCAGCGTTCGGAG | |
| Mouse Kim-1 | F | GGAGATACCTGGAGTAATCACACTG |
| R | TAGCCACGGTGCTCACAAGG | |
| Rat Fgα chain | F | AGCAGCCCAGCGACAAGAAAAG |
| R | GCAGTCAGAACCATCATCCGAAG | |
| Rat Fgβ chain | F | TAGCCTAAGACCTGCCCCTC |
| R | ACCGTGAACACAGCCTCCTG | |
| Rat Fgγ chain | F | GGACAATGACAACGACAAGTTCG |
| R | ATACCAGCGGGTTTTCCAGG | |
| Rat Kim-1 | F | TATTTGGGGGAACAGGTTGC |
| R | CAAGTCACTCTGGTTAGCCGTG | |
| TNFα | F | AGAAGAGGCACTCCCCCAAAAG |
| R | TTCAGTAGACAGAAGAGCGTGGTG |
F indicates forward; and R, reverse.
Immunofluorescence staining
Kidney tissues were perfused with cold PBS before harvesting and then fixed in formalin for 16 hours and embedded in paraffin. The sections incubated overnight at 4°C in rabbit monoclonal anti-Fgα (Epitomics), rabbit polyclonal anti-Fgβ (ProteinTech Group), rabbit polyclonal anti-Fgγ (ProteinTech Group), anti–rat Fg (Nordic Immunologic Laboratories), anti–human fibrinogen (Sigma-Aldrich), and rabbit monoclonal anti-Ki67 (Vector Laboratories). The primary antibody was detected using goat anti–rabbit Cy3–labeled (red) and donkey anti–goat Cy3–labeled secondary antibodies (Jackson ImmunoResearch Laboratories). 4,6-Diamidino-2-phenylindole (Sigma-Aldrich) was used for nuclear staining (blue). The tissue sections were mounted using ProLong Gold antifade reagent (Invitrogen). The images were captured by Nikon DS-QiMc camera attached to Nikon eclipse 90i flourescence microscope using oil immersion objectives (100× magnification for rats and 60× magnification for mice and humans) by Nikon NIS elements AR Version 3.2 software.
TUNEL assay
Apoptosis was measured in kidney tissues by TUNEL assay using the In Situ Cell Death detection kit (Roche Applied Science) according to the manufacturer's instructions.21
In vitro experimental design
The renal tubular epithelial cell line LLC-PK1 established from pig renal cortex was obtained from ATCC and maintained in DMEM containing 10% FBS. Two thousand and five hundred LLC-PK1 cells were plated in a 96-well plate for 24 hours in DMEM and 10% FBS at which time they formed a 50% confluent monolayer in the well. They were pretreated with 6μM Bβ15-42 or random peptide for 6 hours and were immersed in 100 μL of mineral oil on top of DMEM without any serum for 6 hours. This oil immersion simulates in vivo ischemic conditions by restricting cellular exposure to oxygen and nutrients as well as limiting metabolite washout.23 After 6 hours, the mineral oil was removed, and cells were incubated with 6μM Bβ15-42 or random peptide for 48 hours in serum-free conditions. 5-Bromo-2′-deoxyuridine (BrdU) was measured as an index of cell proliferation by incubating cells with BrdU for 2 hours before harvesting, and the absorbance was quantified using a spectrophotometer (Millipore) at 450 nm as per the manufacturer's instructions. Absorbance obtained from untreated cells was taken as 100% (n = 6 wells/group), and the experiment was repeated twice.
Statistics
Data are expressed as average + standard error. Statistical difference (P < .05) as calculated by 1-way ANOVA or Student t test. P < .05 is considered significant and is represented by a single asterisk (*) where applicable. All graphs were generated by Prism software (GraphPad Software). Diagnostic performance (ie, the ability of a urinary biomarker to identify kidney injury) was assessed by evaluating sensitivity and specificity using the receiver operating characteristic (ROC) curve. The area under the ROC curve and 95% confidence interval were calculated using the nonparametric method.22 The area under the ROC curve for a diagnostic test ranges from 0.5 (no better than chance alone) to 1.0 (perfect test, equivalent to the standard). Statistical analyses were performed with MedCalc for Windows (Version 11.5; Mariakerke).
Results
mRNA expression of α, β, and γ chains of Fg increases in the kidney after I/R injury
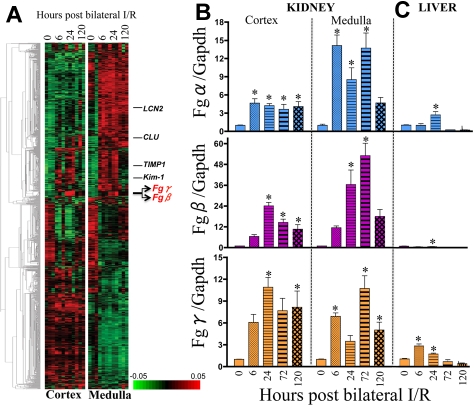
In the quest to identify early genes modulating kidney injury and repair process, we conducted gene expression profiling in the cortex and medulla of rat kidney after 20 minutes of bilateral renal I/R. This reversible model of kidney injury results in elevated kidney dysfunction (measured by SCr and BUN; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and proximal tubular injury (measured by Kim-1 mRNA levels and histopathologic findings characterized by proximal tubular necrosis and apoptosis; supplemental Figure 1B-C) at 24 hours of reperfusion followed by recovery at 120 hours. We selected highly variable genes (MAD > 0.4; n = 1571) and performed hierarchical clustering to investigate their coexpression pattern during kidney regeneration after ischemic injury (Figure 1A). The selected genes include the previously identified candidate genes lipocalin-2 (LCN2),24 clusterin (CLU),25 tissue inhibitor of metalloproteinase-1 (TIMP1),26 and kidney injury molecule-1 (Kim-1).20 Although the up-regulation of LCN2 and CLU were more dominant in renal medulla compared with cortex, TIMP1 and Kim-1 were up-regulated both in cortex and medulla at 24 hours after ischemic injury. We observed a local cluster of genes that includes Fgβ and Fgγ chains (Figure 1A arrow) whose expression pattern is similar to Kim-1, ie, clear up-regulation after 24 hours of ischemic injury both in cortex and medulla. The probe for Fgα chain is absent in the RatRef-12 chip from Illumina. Therefore, we further evaluated the expression profile of Fgα, Fgβ, and Fgγ chains by real-time PCR in kidney (cortex and medulla; Figure 1B); liver (Figure 1C); and lung, spleen, and heart (supplemental Figure 2) over time, and we found that there was a modest elevation (3-fold) of Fgα and Fgγ chains in the liver at early time points (Figure 1C), whereas there was no significant change in the mRNA levels of any of the chains in lung, spleen, and heart (supplemental Figure 2). The medulla showed significantly higher expression of all 3 chains compared with cortex with the highest up-regulation after 72 hours of reperfusion (Fgα chain, 14-fold; Fgβ chain, 50-fold; and Fgγ chain, 10-fold; Figure 1B).
Figure 1.
Fg (Fgα, Fgβ, and Fgγ chains) is significantly up-regulated in kidney cortex and medulla of male Wistar rats after 20 minutes of bilateral renal I/R injury compared with sham surgery. (A) A heatmap shows the expression levels of the most variable 1571 genes that were selected from 22 523 transcripts (see “Whole genome expression profiling and hierarchical clustering”). Coexpressed genes are grouped by hierarchical clustering. The samples are presented in order of time after ischemic injury (0, 6, 24, and 120 hours; triplicate for each). (B) Real-time PCR analysis in kidney. (C) Liver tissues for Fgα, Fgβ, and Fgγ chains, normalized using a housekeeping gene (Gapdh) and fold change determined over sham group (n = 5/group). *P < .05 as determined by 1-way ANOVA in comparison with sham rats.
Immunoreactivity of Fgα, Fgβ, and Fgγ chains in the kidney
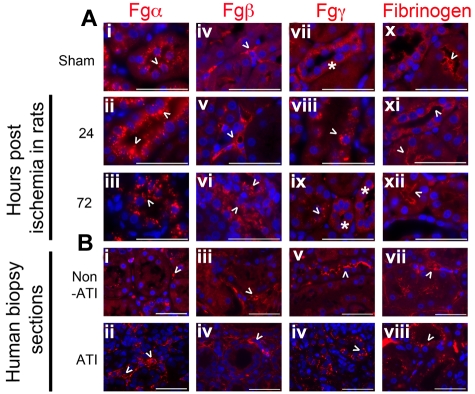
Immunostaining, to evaluate the cellular expression profile of Fg whole protein and Fgα, Fgβ, and Fgγ chains, revealed immunoreactive protein for all 3 chains and Fg whole molecule in renal tubular cells. Positive staining was observed using the anti-Fgβ chain and anti-Fg whole molecule antibodies in the interstitial spaces, indicative of extracellular Fg. In sham kidneys (Figure 2Ai), the Fgα chain is expressed with fine granular cytoplasmic reactivity and more pronounced expression at the peak of injury by 24 hours (Figure 2Aii). Fgα chain immunoreactive protein, as detected by the monoclonal antibody, continued to be expressed in the proximal tubular epithelial cells and in the glomeruli throughout the time course of injury (Figure 2Aiii). Fgβ chain immunoreactive molecules in the uninjured kidneys, assessed by a polyclonal antibody against Fgβ chain, showed focal reactivity in the renal interstitium (Figure 2Aiv). At the peak of injury by 24 hours, Fgβ immunoreactivity distinctly outlined the peritubular capillaries (Figure 2Av), and a small proportion of tubular epithelial cells expressed the Fgβ chain immunoreactive component in their cytoplasm. By 72 hours, intense, irregular and coarse distal tubular staining (Figures 2Avi) and a distinct luminal outline along proximal tubules featured. The Fgγ chain staining, assessed by a polyclonal antibody against Fgγ chain, was primarily located in the distal tubules and collecting ducts with a diffuse cytoplasmic distribution that gravitated along the basolateral side in uninjured kidneys (Figure 2Avii). At the peak of injury by 24 hours, the Fgγ chain immunoreactive protein stained in a coarse granular pattern, distributed centrally in the cytoplasm in the distal tubules and collecting ducts (Figure 2Aviii). By 24 hours, the Fgγ chain in the cortex was confined toward the apical side of the proximal tubules, whereas in the medulla, the cellular debris of injured S3 segments nonspecifically stained for the Fgγ chain as well. By 72 hours, Fgγ chain immunoreactive proteins showed a mixed pattern that resembled sham and 24-hour injured kidneys in the staining and distribution patterns (Figure 2Aix). The expression of Fg whole molecule was identified in a linear pattern along the apical surface of epithelial cells (Figures 2Ax-xii) as well as along the glomerular basement.
Figure 2.
Fg (immunoreactive Fgα, Fgβ, and Fgγ chains) protein is expressed in the kidney of rats and humans. (A) Representative formalin-fixed paraffin-embedded kidney tissue sections of sham male Wistar rats and those from rats that had undergone 20 minutes of bilateral ischemia reperfusion stained for immunoreactive proteins to Fgα (i-iii), Fgβ (iv-vi), Fgγ (vii-ix), and Fg (x-xii) after 24 and 72 hours of reperfusion and compared with healthy (sham) kidneys. Bar represents 50μm. (B) Representative formalin-fixed human-biopsied kidney sections of AKI and non-AKI patients, stained for immunoreactive molecules recognizing Fgα (i-ii), Fgβ (iii-iv), Fgγ (v-vi), and Fg (vii-viii). Bar represents 50 μm. Arrowheads indicate respective immunoreactive molecules. Asterisks in panels Bvii and Bix mark tubules with similar expression pattern along the basolateral side of respective tubules.
A consistent pattern of tubulo-interstitial expression of Fg and its chains was observed in human kidney biopsy sections obtained from patients pathologically diagnosed with ATI compared with patient without evidence of ATI (Figure 2Bi-viii). All 3 chains along with Fg were present in the interstitium in both ATI and non-ATI patients in addition to which Fgγ chain (Figure 2Bvi) and Fg (Figure 2Bviii) was predominantly expressed on the apical side of the tubules in the ATI patient.
Increased urinary levels of Fg in rats and humans serve as a potential biomarker for AKI
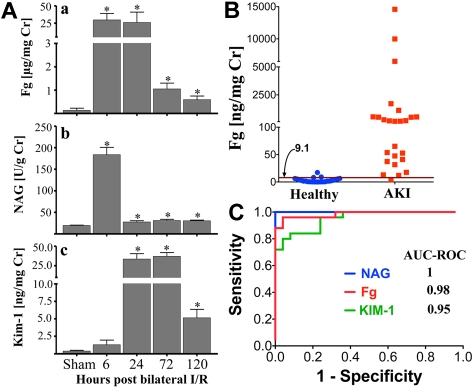
We hypothesized that if Fg was secreted into the urine on injury, then urinary Fg may serve as a biomarker for kidney injury. After 20 minutes of bilateral renal I/R injury in rats, we observed ∼ 100-fold increase in urinary Fg concentration as early as 6 hours (Figure 3Aa) that remained higher than baseline until day 5 (∼ 4-fold) after reperfusion, correlating with proximal tubular necrosis as assessed by histopathologic injury (supplemental Figure 1C) and elevated urinary NAG (Figure 3Aii) as well as urinary Kim-1 (Figure 3Aiii). There was no increase in plasma Fg (supplemental Figure 3) levels after sham or kidney I/R injury compared with rats that did not undergo sham or I/R surgery. To evaluate the performance of urinary Fg in distinguishing healthy volunteers against patients with CKD and/or AKI, urinary Fg was measured in 25 patients admitted to the intensive care unit with abnormal serum creatinine (> 1.5 mg/dL) with established kidney damage from a variety of causes and 25 healthy volunteers. We also compared urinary Fg against 2 other well-studied AKI or CKD biomarkers, NAG and KIM-1. Demographic and clinical data are shown in Table 2. Median urinary concentration of Fg was significantly higher in patients with AKI and CKD than in healthy volunteers (P < .001; Figure 3B) and corresponded with the increased levels of urinary NAG and KIM-1 (supplemental Figure 4). The diagnostic ability of urinary Fg to distinguish between patients with AKI or CKD versus patients without kidney injury was 0.98 as calculated by area under the ROC curve (Figure 3C).
Figure 3.
A significant increase in urinary Fg after kidney injury in rats and humans. (A) Urinary Fg was compared with tubular injury biomarkers NAG and Kim-1 in rats subjected to 20 minutes of bilateral renal I/R injury (n = 5/group). *P < .05 as determined by Student t test in comparison with sham rats. (B) Urinary Fg was measured in a human cross-sectional study with clinically established multifactorial AKI and/or CKD (n = 25) versus healthy volunteers (n = 25). Magenta line and corresponding number marked by arrow indicate a threshold cutoff value at 95% specificity. (C) ROC comparing the sensitivity and specificity of urinary Fg, NAG, and KIM-1 to distinguish patients with AKI and/or CKD from healthy volunteers.
Table 2.
Demographic and clinical characteristics of human subjects
| AKI or CKD (n = 25) | Healthy volunteers* (n = 25) | |
|---|---|---|
| Mean age,† years + SD | 64.8 + 19.5 | 35.6 + 10.7 |
| Female,‡ % | 64 | 68 |
| Black,§ % | 20 | 16 |
| Cause of elevated sCr | AKI from shock or sepsis (72%), obstruction (4%), multifactorial (12%), prerenal (4%), CKD (8%) | |
| Mean (SD) peak sCr | 4.5 (4.7) mg/dL |
Healthy volunteers were excluded if they reported a diagnosis of CKD; sCR was not measured.
P < .001.
P = .73.
P = .68.
Fibrinogen Bβ15-42 protects the kidney against I/R injury
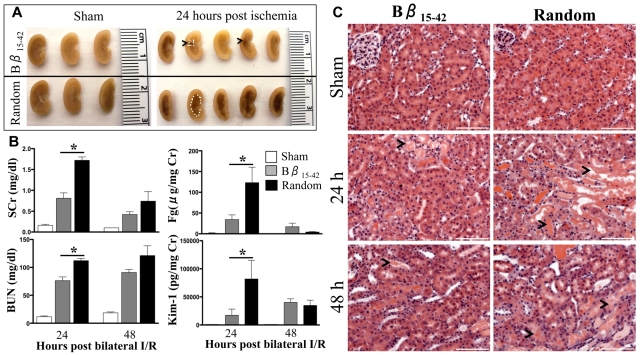
Given that in our model Fgβ chain was the highest up regulated gene after kidney injury among the 3 chains (Figure 1B) and the fact that exogenous Bβ15-42 peptide administration has been shown to protect against myocardial I/R injury27–29 and lung injury,30 we next evaluated the therapeutic potential of Bβ15-42 peptide in renal I/R injury. Bβ15-42 or random peptide (3.6 mg/kg) was administered intravenously 1 minute after reperfusion after 27 minutes of bilateral renal I/R injury in C57Bl/6 mice (n = 5-10/group). A significant reduction in the vascular congestion (outlined by white dots in Figure 4A) was observed. Approximately 50% reduction in kidney dysfunction (measured by SCr and BUN) and kidney proximal tubular injury (measured by urinary levels of Kim-1 and Fg; Figure 4B) and a significant decrease in proximal tubular damage in the outer stripe of outer medulla (histopathological evaluation of H&E stained kidney sections; Figure 4C) were recorded at 24 hours after I/R injury in the mice administered Bβ15-42 peptide compared with random peptide. The kidney injury and dysfunction parameters decreased by 48 hours, suggesting the onset of a complete structural and functional recovery in both groups.
Figure 4.
Bβ15-42 peptide protects mice from renal I/R injury. Male C57Bl/6 mice were subjected to 27 minutes of bilateral renal I/R injury or sham surgery and 3.6 mg/kg Bβ15-42 peptide or random peptide was administered intravenously 1 minute after reperfusion. (A) The medullary congestion after ischemia was significantly less in the Bβ15-42 peptide–administered mice compared with mice administered random peptide. Outline of vascular congestion traced by white dots. (B) SCr and BUN as indicators of renal dysfunction and urinary levels of Fg and Kim-1 as indicators of kidney injury were measured at 24 and 48 hours in all the groups (n = 5/group of sham, n = 10/group at 24 hours, and n = 5/ group at 48 hours). *P < .05 as determined by 1-way ANOVA in comparison with sham mice. (C) Representative H&E stained kidney sections comparing sham, Bβ15-42 and random peptide–administered groups of mice at 24 and 48 hours after ischemia. Bar represents 100 μm (20× magnification).
Decreased apoptosis and increased tissue repair in the kidneys of Bβ15-42–treated mice compared with mice treated with random peptide after I/R injury
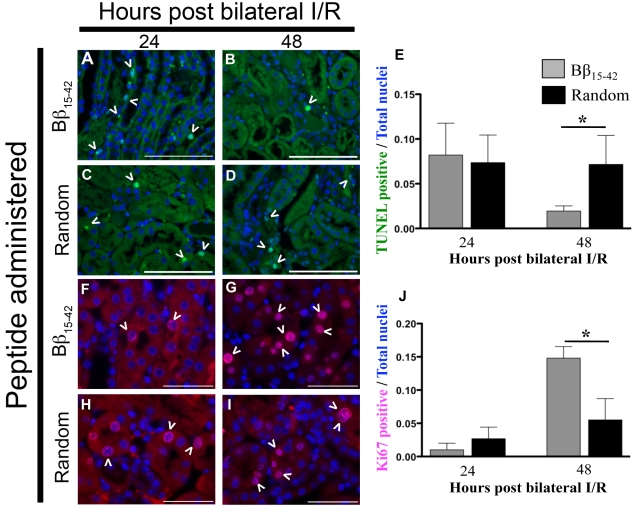
To elucidate the mechanism of Bβ15-42–induced protection in I/R mice, we measured candidate markers of inflammation, leukocyte infiltration, apoptosis, and proliferation in kidney tissues over time. There was no difference in mRNA levels of inflammatory cytokines (IL-1β, IL-6, IL-10, TNFα, and intracellular adhesion molecule-1 [ICAM-1]) or macrophage marker (CD68) between the Bβ15-42 and random peptide treatment groups (supplemental Figure 5). Similarly, leukocyte infiltration (measured by myeloperoxidase staining) also seemed to be similar between the 2 groups (supplemental Figure 6). The number of TUNEL-positive apoptotic cells in the renal medulla was similar at 24 hours (Figure 5A,C). However, at 48 hours there was a significant decrease in apoptosis (P < .05) in the mice administered Bβ15-42 compared with random peptide (Figure 5B,D). Interestingly, a significant number of cells seemed to be in a proliferative state (Ki67 positive) in the renal medulla at 48 hours after administration of the Bβ15-42 peptide compared with random peptide administration (Figure 5G). In vitro experiments using proximal tubular epithelial cells (LLC-PK1) mimicked the in vivo findings in demonstrating a protective effect of Bβ15-42 from hypoxic injury by stimulating renal epithelial cell proliferation (supplemental Figure 7), suggesting that the Bβ15-42 peptide promotes an efficient resolution of ischemic injury by inducing rapid tissue regeneration response, thereby decreasing the necrosis and apoptosis in the kidney.
Figure 5.
Bβ15-42 peptide aids in the resolution of injury by decreasing necrosis/apoptosis and inducing rapid tissue regeneration. Paraffin-embedded kidneys of mice subjected to 27 minutes of bilateral I/R that were administered either Bβ15-42 or random peptides were compared at 24 and 48 hours for number of apoptotic cells (green; A) by TUNEL assay (bar represents 100 μm, 40× magnification). Arrowheads indicate TUNEL-positive nuclei. (B) Number of proliferative cells determined by Ki67-positive staining cells (red; bar represents 50 μm, 60× magnification). The numbers of positive staining TUNEL and Ki67 nuclei are represented graphically on the right of respective photomicrographs. Arrowheads indicate Ki67 positively stained nuclei. *P < .05 as determined by Student t test between the 2 groups within the same time point.
Discussion
Although it has been recognized that progressive kidney disease is characterized by gradual deterioration of the renal endothelium, which correlates with the development of tubulo-interstitial injury, fibrosis, and glomerulosclerosis,3 there has been little effort to characterize the regulatory role of blood proteins in pathophysiology of AKI. Here, we show that (1) in whole genome expression profiling studies Fgβ and Fgγ chains are among the highly up-regulated genes after 24 hours of ischemic injury both in kidney cortex and medulla; (2) Fg could serve as an effective safety and efficacy biomarker for kidney injury not only because of the marked increase in urinary Fg after kidney damage (Figure 3) but also because of their reduced levels on Bβ15-42 peptide mediated protection from kidney injury (Figure 5), demonstrating responsiveness to both injury and recovery; and (3) Fg-derived Bβ15-42 administration protects mice from I/R-induced kidney injury by aiding kidney tissue repair, thereby demonstrating for the first time its therapeutic potential in AKI. These findings not only highlight the important role of Fg in renal tissue injury and repair but also offer a therapeutic strategy to enhance kidney regeneration as opposed to simply preventing further injury or deleterious inflammation in the damaged tissue.
The presence of Fgα, Fgβ, and Fgγ chain transcripts in the kidney at baseline11 as well as its up-regulation after nephrotoxicity31 or brain death–induced vascular endothelial activation in kidneys12,32 has been observed previously. Here, we characterized the cellular expression patterns of Fg and its individual chains in the renal tubule after injury. Because we used polyclonal antibodies for immunostaining of the Fgβ and Fgγ chain, respectively, there is no way to distinguish between the intact fibrinogen versus intermediates such as α/γ, β/γ, or the half-molecule of one each α/β/γ polypeptide chains. However, we found distinct expression patterns of Fgα, Fgβ, and Fgγ chains in the renal tubular epithelial cells, glomeruli, and interstitium at baseline and during the regeneration in the injured kidney. The increased Fg expression after injury can potentially be a consequence of plasma leakage because of organ damage, as seen after spinal cord injury,33 but the observation of detectable transcript levels of Fgα, Fgβ, and Fgγ chains (Figure 1) and corresponding immunoreactivity of all 3 chains as well as whole Fg molecule in sham rats and in patient without any evidence of tubular injury (Figure 2) suggests that the protein could be potentially synthesized and assembled in the kidney.
Plasma Fg has been associated with vascular disease in numerous epidemiologic studies.34 Although we did not find any increase in plasma Fg levels after I/R injury (supplemental Figure 3), urinary Fg levels increased massively as early as 6 hours after I/R injury that decreased over time but remained modestly elevated during the resolution phase of injury (Figure 3). In 1974, Naish et al35 reported higher levels of urinary fibrin degradation products (FDPs) in patients with glomerulonephritis. Subsequently, urinary FDPs were shown to be able to make a diagnosis of 25 of 26 acute rejection episodes at least 24 hours before deterioration in renal function and the elevation of FDPs preceded the rise in NAG in 9 of 11 patients.36 Consistent with these published reports, we show in a cross-sectional study of individuals with and without kidney damage that urinary Fg performed very well in differentiating between patients with and without AKI/CKD, with an area under the ROC of 0.98. The urine samples were obtained well after the diagnosis of kidney injury was made; therefore, this study does not address the issue of early diagnosis before a rise in SCr. However, it does suggest that the sensitivity and specificity of urinary Fg was comparable with the other more advanced biomarkers of AKI such as NAG and KIM-1,19,20,22,37 and further warrants an investigation to evaluate the comparative efficacy and temporal pattern of urinary Fg excretion with other AKI/CKD biomarkers in longitudinal studies. Urinary fragments of Fgβ (ROC of 0.85) and Fgα chains (ROC of 0.74) were found to distinguish patients with malignant versus benign ovarian cancer38 and plasma levels of Fgγ′ chain (a splice variant of Fgγ chain) associated with several traditional cardiovascular risk factors in Framingham Offspring cohort.39 The current assay is a sandwich ELISA-based Luminex assay using 2 polyclonal antibodies against Fg protein; therefore, it will be interesting to use a more targeted approach such as liquid chromatography-multiple reaction monitoring/mass spectrometry to identify whether there is a predominant excretion of Fgβ chain polypeptides in the urine after kidney injury that would correlate with the highest Fgβ chain mRNA levels in the kidney.
We tested the therapeutic efficacy of Fgβ chain–derived peptide (Bβ15-42) in mice subjected to bilateral renal I/R injury and found that Bβ15-42 substantially reduces acute tubular injury. Others have investigated the role of Fg during kidney damage using the Malayan pit viper venom ancrod and found that it provided remarkable protection in experimental allergic glomerulonephritis in rabbits.40 This provides an indirect evidence for protective role of Bβ15-42 because ancrod reduces plasma levels of Fg and simultaneously increases FDPs such as the bioactive polypeptide (Bβ15-42).41 Bβ15-42 is a naturally occurring, 28-amino acid product cleaved from fibrin fragments, and at suprapharmacologic doses it has shown to protect from myocardial infarction28 and acute lung injury30,42 in animal models. In a multicentered phase IIa clinical trial with Bβ15-42 peptide administration, successful protective effects were seen in patients with acute myocardial infarction, whose endothelial barrier integrity had not been compromised.27,29 The peptide also has been shown to be vasculoprotective in models of vascular leak in a Fyn-dependent pathway.42 Bβ15-42 has been shown to mediate platelet spreading, proliferation, capillary tube formation, and von Willebrand factor release, and it has a binding site for heparin.42,43 Bβ15-42 also has low-affinity interactions with vascular endothelial-cadherin, efficiently disrupting the interaction between Fg with its receptors vascular endothelial-cadherin on endothelial cells, thereby stabilizing endothelial barriers that in turn elicits beneficial anti-inflammatory properties.28,42
Here, we show a unique mechanism of Bβ15-42 peptide–mediated protection in vivo and in vitro that results in increased proliferation of renal tubular epithelial cells resulting in decreased necrosis and apoptosis after damage (Figure 5). The fact that among the 3 chains of Fg, Fgβ chain transcript levels increase the highest (∼ 50-fold) at 72 hours (Figure 1B), the peak of regeneration in this model,44 further underscores the finding that Fg is up-regulated in the kidney as a protective mechanism to aid in regeneration. This result would be consistent with the studies conducted thus far, suggesting that Fg seems to be most important in tissue repair when cells must infiltrate and organize fluid-filled areas such as internal dead space created by injury.15 In a cutaneous wound healing model, Fg deficient (Fg−/−) mice revealed an abnormal pattern of tissue repair, including misguided epithelium, delayed wound closure, reduced tensile strength, and diminished ability to organize a dead space.15 Fg has been shown to have multiple binding partners, such as β3-integrin45 and ICAM-1,46 regulating the tissue repair process in neurons and lung epithelial cells.47 Given that both β3-integrin and ICAM-1 expression on renal epithelial cells increases after kidney injury,48–50 it remains to be investigated whether Fg or the Bβ15-42 peptide aids in renal epithelial proliferation and extracellular remodeling via one of the known ligands and pathways. Kidney regeneration, after an episode of AKI, is a major determinant of outcome for patients with AKI and therefore the use of Bβ15-42 peptide offers a novel therapy to improve the rate or effectiveness of the tissue repair process after ischemic kidney damage.
In summary, our study provides evidence that Fg may function as a key molecular link between tubulo-vascular damage and regeneration in the kidney and provides new opportunities for the use of Fg in diagnosis, prevention, and therapeutic interventions in kidney disease.
Supplementary Material
Acknowledgments
The authors thank Dr Vanesa Bijol, MD, a pathologist in the renal division of the Brigham and Women's Hospital, for help with unbiased inferences and interpretations of immunostained sections of kidney.
V.S.V.'s laboratory is supported by the National Institute of Environmental Health Sciences Pathway to Independence award program (ES16723).
Footnotes
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.K., A.K.A., and V.S.V. designed research; A.K., A.K.A, D.H., T.-M.K., V.R., and G.C. performed research; N.A.B. and S.S.W. contributed biospecimens from rodents and humans; T.-M.K. provided bioinformatics support and data analysis; A.K., A.K.A., D.H., S.S.W., and V.S.V. analyzed data; and A.K. and V.S.V. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vishal S. Vaidya, Laboratory of Kidney Toxicology and Regeneration, Renal Division, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Department of Environmental Health, Harvard School of Public Health, Harvard Institutes of Medicine, Rm 562, 77 Avenue Louis Pasteur, Boston, MA 02115; e-mail: vvaidya@partners.org.
References
- 1.Eckardt KU, Kasiske BL. Kidney disease: improving global outcomes. Nat Rev Nephrol. 2009;5(11):650–657. doi: 10.1038/nrneph.2009.153. [DOI] [PubMed] [Google Scholar]
- 2.Szczech LA, Harmon W, Hostetter TH, et al. World Kidney Day 2009: problems and challenges in the emerging epidemic of kidney disease. J Am Soc Nephrol. 2009;20(3):453–455. doi: 10.1681/ASN.2009010041. [DOI] [PubMed] [Google Scholar]
- 3.Lerman LO, Chade AR. Angiogenesis in the kidney: a new therapeutic target? Curr Opin Nephrol Hypertens. 2009;18(2):160–165. doi: 10.1097/MNH.0b013e32831ec1db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basile DP. Novel approaches in the investigation of acute kidney injury. J Am Soc Nephrol. 2007;18(1):7–9. doi: 10.1681/ASN.2006111228. [DOI] [PubMed] [Google Scholar]
- 5.Reinders ME, Rabelink TJ, Briscoe DM. Angiogenesis and endothelial cell repair in renal disease and allograft rejection. J Am Soc Nephrol. 2006;17(4):932–942. doi: 10.1681/ASN.2005121250. [DOI] [PubMed] [Google Scholar]
- 6.Kant JA, Fornace AJ, Jr, Saxe D, Simon MI, McBride OW, Crabtree GR. Evolution and organization of the fibrinogen locus on chromosome 4: gene duplication accompanied by transposition and inversion. Proc Natl Acad Sci U S A. 1985;82(8):2344–2348. doi: 10.1073/pnas.82.8.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molmenti EP, Ziambaras T, Perlmutter DH. Evidence for an acute phase response in human intestinal epithelial cells. J Biol Chem. 1993;268(19):14116–14124. [PubMed] [Google Scholar]
- 8.Lee SY, Lee KP, Lim JW. Identification and biosynthesis of fibrinogen in human uterine cervix carcinoma cells. Thromb Haemost. 1996;75(3):466–470. [PubMed] [Google Scholar]
- 9.Simpson-Haidaris PJ, Courtney MA, Wright TW, Goss R, Harmsen A, Gigliotti F. Induction of fibrinogen expression in the lung epithelium during Pneumocystis carinii pneumonia. Infect Immun. 1998;66(9):4431–4439. doi: 10.1128/iai.66.9.4431-4439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson-Haidaris PJ, Wright TW, Earnest BJ, Hui Z, Neroni LA, Courtney MA. Cloning and characterization of a lung-specific cDNA corresponding to the gamma chain of hepatic fibrinogen. Gene. 1995;167(1–2):273–278. doi: 10.1016/0378-1119(95)00679-6. [DOI] [PubMed] [Google Scholar]
- 11.Baumhueter S, Mendel DB, Conley PB, et al. HNF-1 shares three sequence motifs with the POU domain proteins and is identical to LF-B1 and APF. Genes Dev. 1990;4(3):372–379. doi: 10.1101/gad.4.3.372. [DOI] [PubMed] [Google Scholar]
- 12.Morariu AM, Schuurs TA, Leuvenink HG, van Oeveren W, Rakhorst G, Ploeg RJ. Early events in kidney donation: progression of endothelial activation, oxidative stress and tubular injury after brain death. Am J Transplant. 2008;8(5):933–941. doi: 10.1111/j.1600-6143.2008.02166.x. [DOI] [PubMed] [Google Scholar]
- 13.Lord ST. Fibrinogen and fibrin: scaffold proteins in hemostasis. Curr Opin Hematol. 2007;14(3):236–241. doi: 10.1097/MOH.0b013e3280dce58c. [DOI] [PubMed] [Google Scholar]
- 14.Degen JL. Hemostatic factors and inflammatory disease. Thromb Haemost. 1999;82(2):858–864. [PubMed] [Google Scholar]
- 15.Drew AF, Liu H, Davidson JM, Daugherty CC, Degen JL. Wound-healing defects in mice lacking fibrinogen. Blood. 2001;97(12):3691–3698. doi: 10.1182/blood.v97.12.3691. [DOI] [PubMed] [Google Scholar]
- 16.Palumbo JS, Degen JL. Hemostatic factors in tumor biology. J Pediatr Hematol Oncol. 2000;22(3):281–287. doi: 10.1097/00043426-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Palumbo JS, Kombrinck KW, Drew AF, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96(10):3302–3309. [PubMed] [Google Scholar]
- 18.Adams RA, Schachtrup C, Davalos D, Tsigelny I, Akassoglou K. Fibrinogen signal transduction as a mediator and therapeutic target in inflammation: lessons from multiple sclerosis. Curr Med Chem. 2007;14(27):2925–2936. doi: 10.2174/092986707782360015. [DOI] [PubMed] [Google Scholar]
- 19.Vaidya VS, Ford GM, Waikar SS, et al. A rapid urine test for early detection of kidney injury. Kidney Int. 2009;76(1):108–114. doi: 10.1038/ki.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaidya VS, Ozer JS, Dieterle F, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28(5):478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnamoorthy A, Clement ME, O'Leary E, Bonventre JV, Vaidya VS. TIM2 gene deletion results in susceptibility to cisplatin-induced kidney toxicity. Toxicol Sci. 2010;118(1):298–306. doi: 10.1093/toxsci/kfq240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaidya VS, Waikar SS, Ferguson MA, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1(3):200–208. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meldrum KK, Meldrum DR, Hile KL, Burnett AL, Harken AH. A novel model of ischemia in renal tubular cells which closely parallels in vivo injury. J Surg Res. 2001;99(2):288–293. doi: 10.1006/jsre.2001.6201. [DOI] [PubMed] [Google Scholar]
- 24.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14(10):2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 25.Dieterle F, Perentes E, Cordier A, et al. Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol. 2010;28(5):463–469. doi: 10.1038/nbt.1622. [DOI] [PubMed] [Google Scholar]
- 26.Amin RP, Vickers AE, Sistare F, et al. Identification of putative gene based markers of renal toxicity. Environ Health Perspect. 2004;112(4):465–479. doi: 10.1289/ehp.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallén J, Petzelbauer P, Schwitter J, Geudelin B, Buser P, Atar D. Impact of time to therapy and presence of collaterals on the efficacy of FX06 in acute ST elevation myocardial infarction: a substudy of the F.I.R.E., the efficacy of FX06 in the prevention of myocardial reperfusion injury trial. EuroIntervention. 5(8):946–952. [PubMed] [Google Scholar]
- 28.Petzelbauer P, Zacharowski PA, Miyazaki Y, et al. The fibrin-derived peptide Bbeta15-42 protects the myocardium against ischemia-reperfusion injury. Nat Med. 2005;11(3):298–304. doi: 10.1038/nm1198. [DOI] [PubMed] [Google Scholar]
- 29.Atar D, Petzelbauer P, Schwitter J, et al. Effect of intravenous FX06 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction results of the F.I.R. E. (efficacy of FX06 in the prevention of myocardial reperfusion injury) trial. J Am Coll Cardiol. 2009;53(8):720–729. doi: 10.1016/j.jacc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Matt U, Warszawska JM, Bauer M, et al. Bbeta(15-42) protects against acid-induced acute lung injury and secondary pseudomonas pneumonia in vivo. Am J Respir Crit Care Med. 2009;180(12):1208–1217. doi: 10.1164/rccm.200904-0626OC. [DOI] [PubMed] [Google Scholar]
- 31.Thukral SK, Nordone PJ, Hu R, et al. Prediction of nephrotoxicant action and identification of candidate toxicity-related biomarkers. Toxicol Pathol. 2005;33(3):343–355. doi: 10.1080/01926230590927230. [DOI] [PubMed] [Google Scholar]
- 32.Schuurs TA, Gerbens F, van der Hoeven JA, et al. Distinct transcriptional changes in donor kidneys upon brain death induction in rats: insights in the processes of brain death. Am J Transplant. 2004;4(12):1972–1981. doi: 10.1111/j.1600-6143.2004.00607.x. [DOI] [PubMed] [Google Scholar]
- 33.Schachtrup C, Lu P, Jones LL, et al. Fibrinogen inhibits neurite outgrowth via beta3 integrin-mediated phosphorylation of the EGF receptor. Proc Natl Acad Sci U S A. 2007;104(28):11814–11819. doi: 10.1073/pnas.0704045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinhart WH. Fibrinogen–marker or mediator of vascular disease? Vasc Med. 2003;8(3):211–216. doi: 10.1191/1358863x03vm494ra. [DOI] [PubMed] [Google Scholar]
- 35.Naish P, Evans DJ, Peters DK. Urinary fibrinogen derivative excretion and intraglomerular fibrin deposition in glomerulonephritis. Br Med J. 1974;1(5907):544–546. doi: 10.1136/bmj.1.5907.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia B, Thompson AE, Tucker SM, Wing AJ. Urinary excretion of NAG and FDP in acute renal graft rejection. Proc Eur Dial Transplant Assoc. 1975;11:311–319. [PubMed] [Google Scholar]
- 37.Vaidya VS, Niewczas MA, Ficociello LH, et al. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, KIM-1 and NAG. Kidney Int. 2011;79(4):464–470. doi: 10.1038/ki.2010.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petri AL, Simonsen AH, Yip TT, et al. Three new potential ovarian cancer biomarkers detected in human urine with equalizer bead technology. Acta Obstet Gynecol Scand. 2009;88(1):18–26. doi: 10.1080/00016340802443830. [DOI] [PubMed] [Google Scholar]
- 39.Lovely RS, Kazmierczak SC, Massaro JM, D'Agostino RB, O'Donnell CJ, Farrell DH. Fibrinogen: evaluation of a new assay for study of associations with cardiovascular disease. Clin Chem. 2010;56(5):781–788. doi: 10.1373/clinchem.2009.138347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomson NM, Moran J, Simpson IJ, Peters DK. Defibrination with ancrod in nephrotoxic nephritis in rabbits. Kidney Int. 1976;10(5):343–347. doi: 10.1038/ki.1976.120. [DOI] [PubMed] [Google Scholar]
- 41.Dempfle CE, Argiriou S, Kucher K, Muller-Peltzer H, Rubsamen K, Heene DL. Analysis of fibrin formation and proteolysis during intravenous administration of ancrod. Blood. 2000;96(8):2793–2802. [PubMed] [Google Scholar]
- 42.Gröger M, Pasteiner W, Ignatyev G, et al. Peptide Bbeta(15-42) preserves endothelial barrier function in shock. PLoS ONE. 2009;4(4):e5391. doi: 10.1371/journal.pone.0005391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3(8):1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 44.Sabbahy ME, Vaidya VS. Ischemic kidney injury and mechanisms of tissue repair. Wiley Interdiscip Rev Syst Biol Med. 2011 doi: 10.1002/wsbm.133. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheresh DA, Berliner SA, Vicente V, Ruggeri ZM. Recognition of distinct adhesive sites on fibrinogen by related integrins on platelets and endothelial cells. Cell. 1989;58(5):945–953. doi: 10.1016/0092-8674(89)90946-x. [DOI] [PubMed] [Google Scholar]
- 46.Languino LR, Plescia J, Duperray A, et al. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1-dependent pathway. Cell. 1993;73(7):1423–1434. doi: 10.1016/0092-8674(93)90367-y. [DOI] [PubMed] [Google Scholar]
- 47.Ryu JK, Davalos D, Akassoglou K. Fibrinogen signal transduction in the nervous system. J Thromb Haemost. 2009;7:151–154. doi: 10.1111/j.1538-7836.2009.03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arrizabalaga P, Sole M, Abellana R, et al. Tubular and interstitial expression of ICAM-1 as a marker of renal injury in IgA nephropathy. Am J Nephrol. 2003;23(3):121–128. doi: 10.1159/000068920. [DOI] [PubMed] [Google Scholar]
- 49.Kim S, Nadel JA. Fibrinogen binding to ICAM-1 promotes EGFR-dependent mucin production in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297(1):L174–183. doi: 10.1152/ajplung.00032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simon EE. Potential role of integrins in acute renal failure. Nephrol Dial Transplant. 1994;9(suppl 4):26–33. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.