Abstract
The Bcl-2 nineteen kilodalton interacting protein 3 (BNIP3) is a hypoxia-inducible pro-apoptotic member of the Bcl-2 family that induces cell death by associating with the mitochondria. Under normal conditions, BNIP3 is expressed in skeletal muscle and in the brain at low levels. In many human solid tumors, BNIP3 is upregulated in hypoxic regions but paradoxically, this BNIP3 expression fails to induce cell death. Herein, we have determined that BNIP3 is primarily localized to the nucleus of glial cells of the normal human brain, as well as in the malignant glioma cell line U251. Upon exposure of U251 cells to hypoxia, BNIP3 expression in the cytoplasm increases and localizes with the mitochondria, contributing to induction of cell death. In contrast, when BNIP3 is forcibly over expressed in the nucleus, it fails to induce cell death. Expression of N-terminal BNIP3 (lacking the transmembrane and conserved domains) in U251 cells blocks hypoxia-induced cell death acting as a dominant negative protein by binding to wild-type BNIP3 and blocking its association with the mitochondria. In glioblastoma multiforme (GBM) tumors, BNIP3 expression is increased in hypoxic regions of the tumor and is primarily localized to the nucleus in approximately 80% of tumors. Hence, BNIP3 is sequestered in the nucleus within the brain but under hypoxic conditions, BNIP3 becomes primarily cytoplasmic promoting cell death. In GBMs, BNIP3 expression is increased but it remains sequestered in the nucleus in hypoxic regions, thereby blocking BNIP3’s ability to associate with the mitochondria providing tumor cells with a possible survival advantage.
Keywords: BNIP3, astrocytes, glial cells, glioblastoma multiforme, hypoxia, mitochondria, cell death, nuclear localization
Introduction
The Bcl-2 nineteen kilodalton interacting protein 3 (BNIP3) is a pro-cell death Bcl-2 family member that localizes to the mitochondria upon over-expression causing opening of the permeability transition (PT) pore, loss of mitochondrial membrane potential (Δψm) and reactive oxygen species (ROS) production resulting in cell death 1. BNIP3 also induces an autophagic response in cells contributing to cell death 2, 3. BNIP3-induced cell death is independent of mitochondrial cytochrome c release and caspase activation 1, 4. The BNIP3 protein has four major domains: a PEST domain that targets BNIP3 for degradation, a putative BH3 (Bcl-2 homology 3) domain that is homologous to other Bcl-2 family members, a CD (conserved) domain that is conserved from C. elegans to humans, and a TM (transmembrane) domain, which targets BNIP3 to the mitochondria and is essential for BNIP3-induced cell death 5–7. Deletion of the CD or BH3 domains fails to affect BNIP3’s ability to induce cell death while deletion of the TM domain blocks BNIP3-induced cell death due to its inability to associate with the mitochondrial membrane 8.
BNIP3 is expressed in normal tissues such as skeletal muscle and brain and its expression is increased under hypoxic conditions correlating with hypoxia-induced cell death 9. Many solid tumors have regions of hypoxia and express BNIP3. The hypoxic response in tumors is triggered to a large extent by the transcription factor hypoxia inducible factor 1 (HIF-1), which is a heterodimer made up of HIF-1α and HIF-1β subunits 10. HIF-1α levels increase during hypoxia correlating with BNIP3 expression. In breast and lung tumors, both HIF-1α and BNIP3 expression are found in viable cells surrounding necrotic regions of these tumors 11. This correlates with poor prognosis in these patients. The mechanism allowing BNIP3 expression in viable tumor cells without induction of cell death is unknown.
Glioblastoma mutiforme (GBM) is the most malignant form of brain cancer with a high mortality rate 12. The aggressive biological behavior of GBMs may be, in part, due to tumor hypoxia 12, 13. One hypothesis that has been proposed to explain why hypoxic areas are common in glioblastoma is that GBM are swiftly developing tumors that outgrow their vasculature, which leads to resistance to chemotherapy and radiation therapy 14, 15. The functional significance of BNIP3 expression in GBM tumors is unknown.
Herein, we demonstrate that for the first time BNIP3 is localized to the nucleus in glial cells. Under hypoxia BNIP3 is localized to the cytoplasm and mediates hypoxia-induced cell death. However, in GBM tumors BNIP3 expression in viable tumor cells remains predominantly nuclear under hypoxic conditions, contributing to cell survival. This could explain why hypoxic regions in solid tumors are poor prognostic indicators.
MATERIALS AND METHODS
Cell Culture and Transfections
Human glioblastoma cell lines U251 and U87 (obtained from Dr. V.W. Yong, University of Calgary and Dr. C. Hao, University of Alberta, respectively) were cultured in Dulbecco’s modified essential medium (DMEM), supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 1 mM MEM sodium pyruvate, 0.3% glucose and 100 units/ml penicillin/streptomycin. Normal human astrocytes (Cambrex) were cultured in ABM media (Cambrex) as per the manufacturer’s instructions. The cell lines were grown in a humidified incubator in the presence of 5% CO2 at 37°C. Cells were maintained under hypoxic conditions (less than 1% oxygen) at 37°C within a hypoxic chamber (Forma Scientific) filled with 5% CO2 balanced with N2. Transfection experiments were carried out with the U87 and U251 cell lines. The cells were plated 48 hours prior to transfection to achieve approximately 40% confluence. The U87 cell line was transfected using Geneporter (GTS) and the U251 cell line using Effectene (Qiagen) as per the manufacturer’s instructions.
Western blotting
U251 cells were lysed for total protein or nuclear proteins as previously described (9). The lysates (60μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked in 5% skim milk and western blotted with monoclonal antibodies against BNIP3 (1:1000, University of Manitoba), pro-caspase 8 (1:1000, Upstate Biotech.), HDAC1 (1:1000, Upstate Biotech), or β–actin (1:50, Sigma). The immuno-blots were visualized with chemiluminescence (NEN-Dupont).
Immunostaining and Confocal Microscopy
Paraffin-embedded primary glioblastoma multiforme tumor section slides were obtained from the Brain Tumor Tissue Bank (London, Canada) and baked in an oven (70°C) for 20 minutes. The slides were deparaffinized, rehydrated and washed with H2O for 5 minutes. Antigen presentation was completed by incubating the slides in a pressure cooker for 20 minutes filled with citrate buffer (10mM citric acid monohydrate, pH to 6.0). The slides were removed, cooled to room temperature (RT), and then washed three times for 5 minutes in PBS (0.5% Triton X100). Blocking solution (1X PBS, 0.2% Triton X100, 0.02% sodium azide, 5% goat serum and 0.1% bovine serum albumin) was added to each slide for 2 hours at RT. Primary antibodies (polyclonal anti-BNIP3 1:700 dilution 5, anti-GFAP 1:50 dilution (Dako), anti-HIF-1α 1:100 dilution (Novus), cytochorme c oxidase 1:100 dilution (Santa Cruz), and anti-cytochrome c 1:100 dilution (gift from Dr. Andrews, McMaster University) were diluted in blocking solution and added to slides. The slides were incubated at 4°C overnight and subsequently washed. The appropriate biotinylated secondary antibody (1:200 dilution, Vector Inc.) was prepared in blocking solution and added to the slides for 2 hours at RT, then washed three times. Strepavidin conjugated to the appropriate fluorochrome (Texas Red or Oregon Green, Vector Inc.) in blocking solution (15μg/ml) was added to slides and incubated for 2 hours at RT in the dark. Vectashield with DAPI stain (Vector) was added to each slide and a coverslip was placed and sealed. For double immunofluorescence, a second primary antibody was added and incubated overnight following the same procedure as the first primary antibody.
After transfection of U87 and U251 cell lines with 2 μg of pCDNA3, pCDNA3-BNIP3, pCDNA3-BNIP3ΔTM, or pCDNA3-N-terminal BNIP3, the cells were incubated for 24, 48 and 72 hrs, under normoxic and hypoxic conditions, then trypsinized, centrifuged and resuspended in 2 ml of media. A 200 μl aliquot was cytospun (ThermoShandon) at 1000 RPM for 5 minutes with medium-low acceleration. The slides obtained from the cytospin were immediately fixed with 3.7% formaldelyde in 1x PBS at RT for one hour. Following three washes with 1x PBS (0.1% NP40), slides were incubated with primary BNIP3 antibody (1:700 dilution in 10x FBS, 1x PBS, 0.1% NP40) for 1 hour at RT. The slides were then washed three times in PBS (0.1% NP40) and incubated with goat anti-rabbit fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Sigma) for 1 hour. After three more washes, slides were mounted with coverslips containing Vectashield mounting media with Hoescht dye (to counter-stain for nuclei). 200 or more cells were counted per sample to assess DNA condensation. Fluorescence was visualized and captured using an Olympus BX51 fluorescent microscope with a Photometrics Cool Snap CF camera and an Olympus IX70 inverted confocal laser microscope using Fluoview 2.0 software.
The U251 cell line and normal human astrocytes (glial cells) were grown on coverslips in 6 well dishes and then incubated for 0 and 24 or 48 hours in hypoxia, stained with mitotracker (Molecular Probes) for 45 minutes and then fixed with 3.7% formaldelyde in 1x PBS at RT for one hour. Following three washes with 1x PBS (0.1% NP40), slides were incubated with primary BNIP3 antibody (1:700 dilution in 10x FBS, 1x PBS, 0.1% NP40) for 1 hour at RT. The slides were then washed three times in PBS (0.1% NP40) and incubated with goat anti-rabbit Texas red-conjugated secondary antibody (Sigma) for 1 hour. After three more washes, slides were mounted with coverslips containing Vectashield mounting media with Hoescht dye (to counter-stain for nuclei). Images were obtained with a Olympus IX70 inverted confocal laser microscope using Fluoview 2.0 software.
Subcellular Fractionation
U87 cells were resuspended in CFS buffer (10 mM Hepes, pH 7.4, 220 mM mannitol, 68 mM sucrose, 2 mM NaCl, 2.5 mM KH2PO4, 0.5 mM EGTA, 2mM MgCl2, 0.1 mM phenylmethylsulfonylfluoride, and 1 mM dithiothreitol) and Dounce-homogenized (45 strokes) with a tight pistil. The cells were spun down at 3000 rpm for 5 min at 4° C. The supernatant was then centrifuged for 15 minutes at 12,800 rpm at 4° C. The pellet was resuspended in 30 μl of H buffer (300 mM sucrose, 5mM 2-[2-hydroxy-1,1- bis(hydroxymethyl)ethyl]amino-ethanesulfonic acid, and 200 μM EGTA; represents the heavy membrane fraction. The supernatant was spun down at 41,000 rpm for 1 h, and represents the cytosolic fraction. The fractions were loaded onto SDS-polyacrylamide electrophoresis and western blotted.
Determination of Mitochondrial Membrane Potential
U251 cells were grown in chamber slides and were co-transfected with pEGFP N1 (Clontech) and pCDNA3, pCDNA3-BNIP3, pCDNA3-BNIP3ΔTM, and pCDNA3-N-terminal BNIP3 using Geneporter (GTS) as per the manufacturer’s instructions. After 16 hours of incubation, the slides were washed twice with 1X Hepes-buffered saline solution (HBSS) and tetramethylrhodamine (TMRM; Molecular Probes) was added to the slides at 150 μm final concentration and incubated in the dark at RT for 10 min. The slides were then viewed on an Olympus IX70 inverted confocal laser microscope using Fluoview 2.0 software.
Immunohistochemical staining of GBM tumors
Upon deparaffinization and dehydration, GBM tumor slides were then placed in a humidity chamber and 200μl of blocking solution was placed on each slide and incubated for 2 hours at RT. Primary antibody was added to the slides (anti-BNIP3 1:200) and incubated at 4°C overnight. The slides were then washed three times with 1X PBS (0.05% Triton X100). The secondary antibody (biotinylated goat anti-rabbit 1:200, Vector Inc.) was prepared in blocking solution and added to each slide. The slides were then incubated for 2 hours at RT, then washed and incubated in 0.3% H2O2 for 30 minutes at RT. The slides were then washed again, Elite ABC solutions (Vector, Inc) were placed on the slides and incubated for 30 minutes at RT. After washing, DAB substrate was added to the slides and incubated from 2–5 minutes until staining was detected. The reaction was stopped by the addition of water for 5 minutes. Nuclei were stained with Meyer’s hematoxylin by incubating the slides in a hematoxylin solution in a Coplin’s jar for 3 minutes and then in basic water for 1 minute (1 drop of NH3OH in 10 ml H2O). The slides were dehydrated through consecutive washes in graded ethanols (50–100%) and xylene and then mounted with Permount (Fisher). As a negative control, tissue samples were incubated with secondary antibodies alone. As a positive control, mouse skeletal muscle was immuno-stained for BNIP3, which is endogenously expressed at high levels in this tissue.
Cell Death Assay
U251 cells (1×105 cells) were seeded in 6 well plates. The next day, using lipofectamine reagent (Invitrogen) cells were transfected with 0.2 μg of pcDNA3-β-galactosidase (pcDNA3-β-gal) plasmid plus 1 μg of pcDNA3-BNIP3ΔTM, N-terminal BNIP3, or vector alone. After 4 hours transfection, cells were incubated either in DMEM under normoxic or hypoxic conditions for 48 hours. Following incubation, cells were fixed in 0.2 % glutaraldehyde and washed three times with 0.1M phosphate buffer saline (PBS) and stained in X-gal buffer (0.5 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside, 3mM K3Fe(CN)6, 3 mM K4Fe(CN)6•3H2O, 1 mM MgCl2 in 0.1 M PBS) to detect for β-galactosidase expression as described previously (Miura et al. 1993). The percentage of dead cells was calculated by assessing the number of round, condensed, blue cells in the total population of flat blue cells. A total number of at least 200 cells were counted for each experiment. Another cell death assay used was DNA staining with DAPI as described above. The slide was viewed on a fluorescence microscope using a fluorescein filter. The percentage of dead cells was calculated by counting the changes in nuclear morphology such as breakage of DNA into large irregular bodies. A total number of at least 250 cells were counted for each assay.
Results
BNIP3 expression is localized to the nucleus in human glial cells
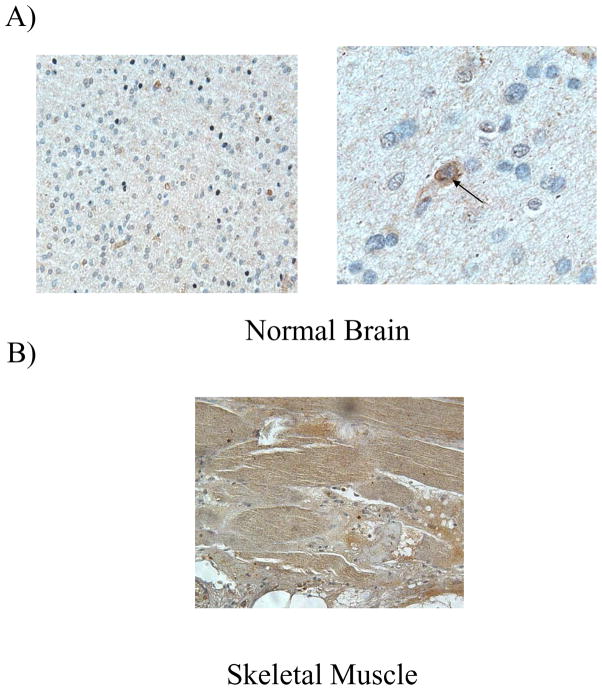
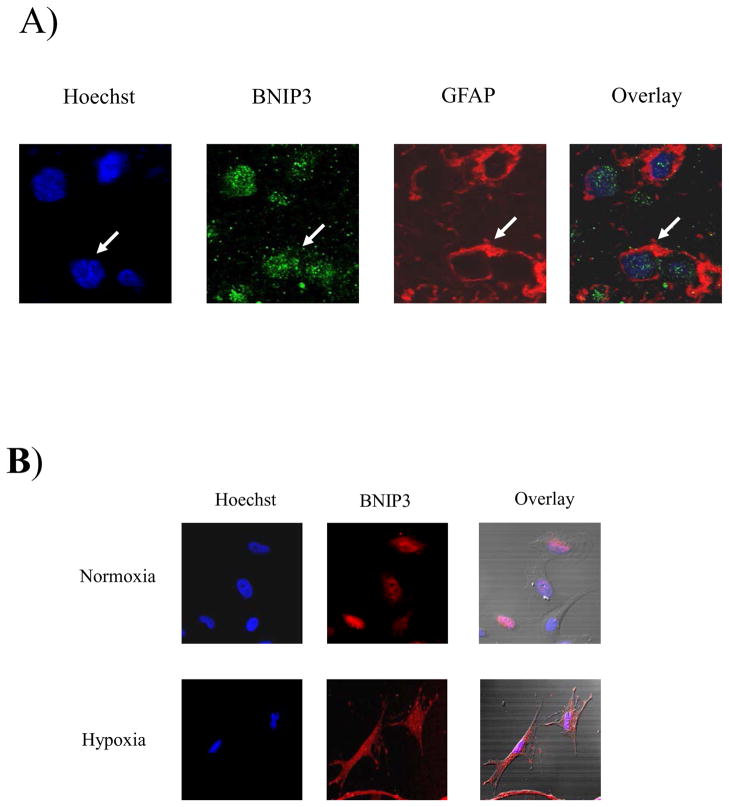
It has been previously demonstrated that BNIP3 is expressed in skeletal muscle and brain 1, 16, 17. It is not known however, what type of cells in the human brain express BNIP3 or where BNIP3 is localized. We have determined that BNIP3 is expressed at low levels in normal human brain and its expression is localized primarily to the nucleus with some perinuclear immunostaining (Figure 1A). This is in contrast to skeletal muscle where BNIP3 expression is more robust and localized throughout the cell (Figure 1B). In order to distinguish which specific cells in the brain express BNIP3, we immuno-stained normal human brain tissue sections with an antibody to glial fibrillary acidic protein (GFAP) an intermediate-filament (IF) protein that is highly specific for cells of astroglial lineage. The cells were also immuno-stained for antibodies against BNIP3. This showed that BNIP3 was predominantly expressed in the nucleus of glial cells with some staining surrounding the nucleus (Figure 2A). To further confirm our results, we cultured primary human glial cells in both normoxic and hypoxic conditions and immuno-stained with antibodies against BNIP3. We found that BNIP3 was primarily localized to the nucleus with some cytoplasmic staining under normoxia. Under hypoxia, cytoplasmic staining for BNIP3 was significantly increased (Figure 2B).
Figure 1. BNIP3 is expressed in normal brain and skeletal muscle.
A) Paraffin-embedded normal brain tissue was immunostained with antibodies against BNIP3 (brown). DNA was counter-stained with hematoxylin (blue). Low magnification is on the left hand side with high magnification on the right hand side. The arrow shows a cell expressing BNIP3. B) Mouse skeletal muscle tissue was immunostained for BNIP3 (brown) and counter-stained with hematoxyin (blue). The images were captured on a microscope (Olympus) with digital imaging software (ImagePro Plus 4.5). These results are representative of three independent experiments.
Figure 2. Wild-type BNIP3 is expressed in nucleus of human glial cells.
A) Normal brain tissue was immunostained for BNIP3 and GFAP (marker for glial cells). DNA was stained with Hoescht dye and the slides were analyzed on a confocal microscope (Olympus). B) Normal human glial cells were stained with BNIP3 and counterstained with Hoescht dye for DNA.
Under hypoxic conditions, BNIP3 localization changes from nuclear to cytoplasmic in U251 glioma cells
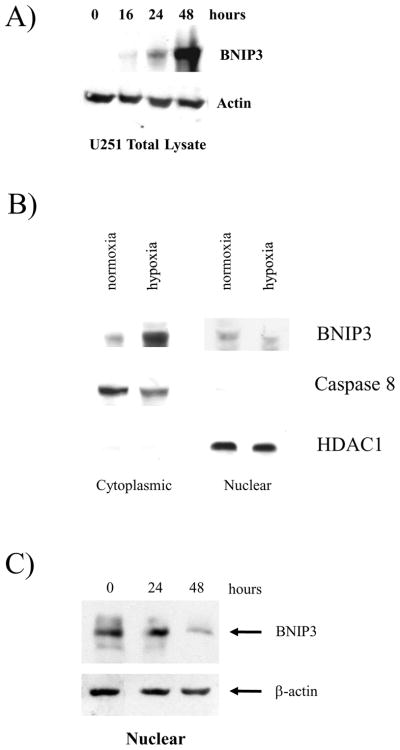
To examine the localization of BNIP3 in malignant glioma cells, we immuno-blotted for BNIP3 expression in the human glioma cell line U251 under hypoxia over a 48 hour time course. BNIP3 protein levels increased under hypoxia with the highest level of expression detected after 48 hours of hypoxia (Figure 3A). The subcellular localization of BNIP3 in U251 cells was determined under normoxic and hypoxic conditions through isolation of nuclear and cytoplasmic protein fractions. These protein fractions were then immuno-blotted for BNIP3 expression. BNIP3 protein was detected in nuclear fractions isolated from U251 cells under normoxic conditions but its expression in the nucleus decreased under hypoxic conditions. In contrast, BNIP3 expression was dramatically increased in the cytoplasmic fraction under hypoxic conditions but remained almost undetectable in the cytoplasm under normoxic conditions. As a control for subcellular localization, we used caspase 8 as a cytoplasmic marker since it is not localized to the nucleus and is involved in cell death and HDAC1 as a nuclear marker (Figure 3B). These controls confirmed that the nuclear and cytoplasmic lysates had low levels of cross-contamination. The level of nuclear BNIP3 expression was determined over a 48 hour time course of hypoxia. At 48 hours of hypoxia, the levels of BNIP3 nuclear expression were significantly decreased in contrast to total BNIP3 expression (Figure 3C). Our results indicate that BNIP3 is expressed in the nucleus of glioma cells in GBM tissue samples and under hypoxic conditions in vitro, the amount of cytoplasmic BNIP3 increases corresponding with the increase in total BNIP3 protein levels.
Figure 3. Wild-type BNIP3 localizes to the nucleus in U251 glioma cells under normal conditions, but under hypoxia BNIP3 expression increases in the cytoplasm.
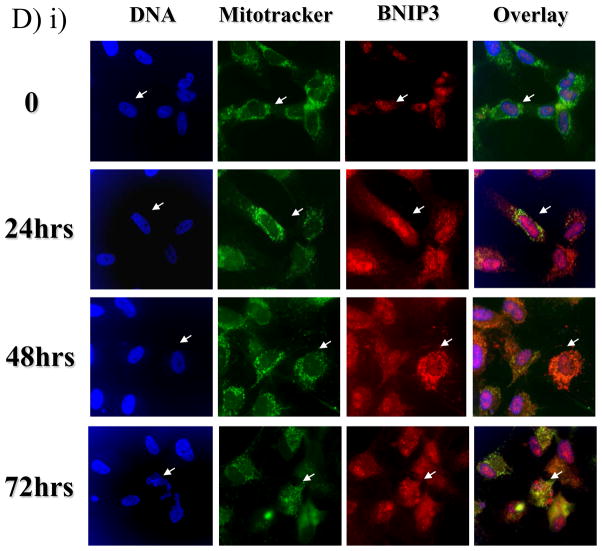
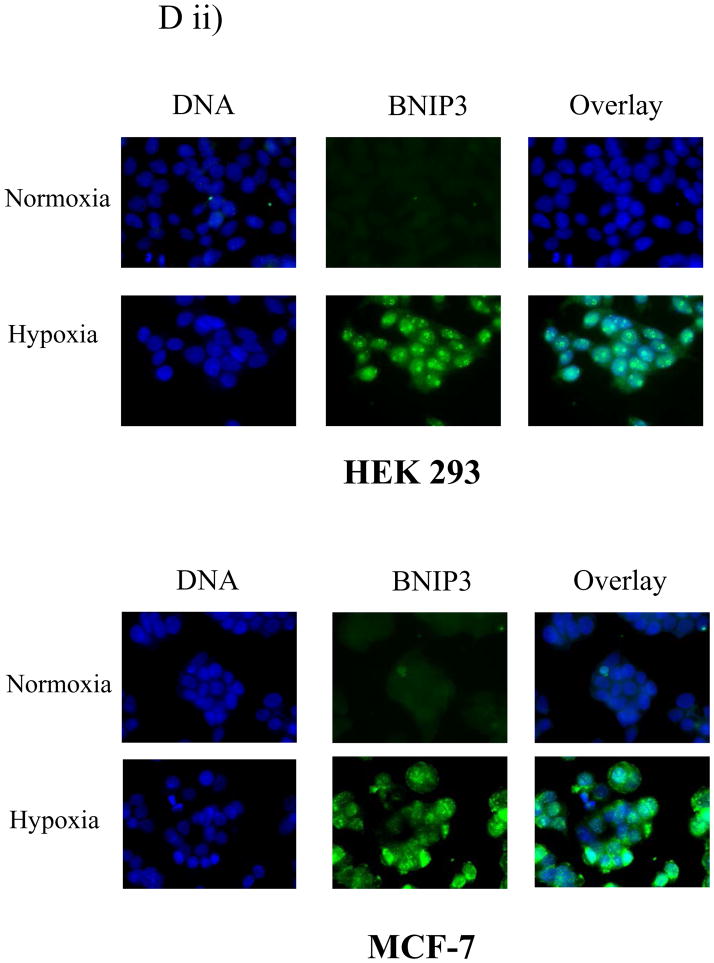
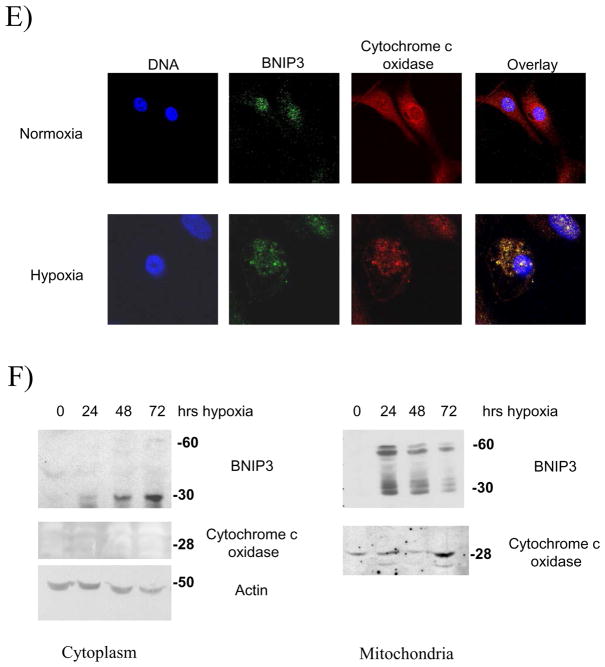
A) Total lysates from U251 cells were western blotted for BNIP3 under a time course of hypoxia and the blot was reprobed with actin as a loading control. B) Cytoplasmic and nuclear fractions of U251 cells were isolated and lysed under normoxic and hypoxic conditions (48 hours). The lysate was western blotted for BNIP3 expression and the blots were stripped and reprobed with antibodies against caspase 8 (cytoplasmic protein) and HDAC1 (nuclear protein) as controls. C) U251 cells were placed under hypoxia conditions for 48 hours and nuclear lysate was obtained as described in Materials and Methods section. The lysate was western blotted for BNIP3. The blot was stripped and reprobed for actin as a loading control. D) i) U251 cells were placed under normoxic or hypoxic conditions and immunostained with antibodies against BNIP3 (red) and with Mitotracker (green). Images were captured on a confocal laser microscope. Yellow staining represents co-localization of BNIP3 with Mitotracker staining. Arrows represent the same cell in each panel. ii) HEK 293 and MCF-7 cells were placed under normoxic or hypoxia conditions and then stained with antibodies against BNIP3. DNA was visualized with DAPI staining. E) U251 cells were stained with antibodies against BNIP3 and cytochrome c oxidase under normoxic and hypoxic conditions. DNA was stained as previously mentioned. F) Cytoplasmic and mitochondrial fractions were isolated from U87 cells over a time course of hypoxia as described in Materials and Methods section. The lysate was western blotted for BNIP3, and cytochrome c oxidase. As a loading control, cytoplasmic fraction was western blotted with actin antibodies.
BNIP3-induced cell death is mediated through the mitochondria 4, 18. The glioma cell line U251 was exposed to hypoxia over a 72 hour time course. At each time point, U251 cells were immunostained with BNIP3 and stained for Mitotracker (a mitochondria-specific dye). In U251 cells under normoxic conditions, endogenous BNIP3 was expressed at low levels in the nucleus (Figure 3D). BNIP3 expression increased in the cytoplasm after 24 and 48 hours of hypoxia, with 17% of cells undergoing cell death as determined by nuclear morphology (data not shown). By 72 hours, BNIP3 levels were still elevated and localized within the cytoplasm. However, BNIP3 was primarily localized to the mitochondria (visualized by Mitotracker) with 36% of cells undergoing cell death (Figure 3Di). Other cell types were also immuno-stained for BNIP3 to compare BNIP3 expression under normoxic or hypoxic conditions. HEK 293 (human embryonic kidney cell line) and MCF-7 (human breast cancer cell line) cells were placed under normoxic and hypoxic conditions. BNIP3 was not detected under normoxic conditions (unlike U87 cells) but under hypoxia BNIP3 was expressed throughout the cell (Figure 3 Dii). To further confirm that BNIP3 expression under hypoxia localizes with the mitochondria, U87 cells were immuno-stained for cytochrome c oxidase and BNIP3. Under hypoxia, BNIP3 staining co-localized with cytochrome c oxidase similar to using Mitotracker (Figure 3E). In addition, subcellular fractionation of cytoplasmic and mitochondria was performed. Under normoxic conditions, BNIP3 was not detected in cytoplamic or mitochondria fractions. Upon hypoxia exposure, BNIP3 expression increased in both cytoplasmic and mitochondrial fractions where only the 60kDa homodimer was found in the mitochondrial fraction (Figure 3F).
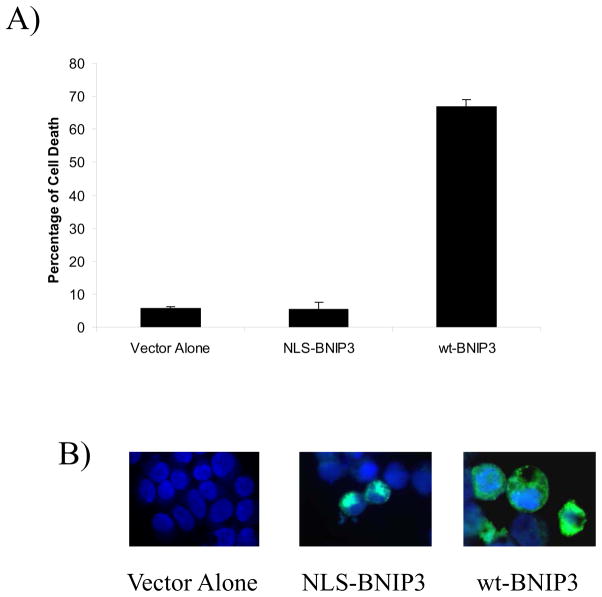
To further confirm that nuclear BNIP3 is not involved in inducing cell death, human embryonic kidney (HEK) 293 cells that lack BNIP3 staining under normoxic conditions were transfected with BNIP3 containing a nuclear localization signal (NLS). These cells remained viable after 24 hours similar to cells transfected with vector alone but cells transfected with wild type BNIP3 underwent cell death. The cells were immunostained for BNIP3 indicating nuclear localization of NLS-BNIP3 and cytoplasmic staining for wt-BNIP3 (Figure 4). These results suggest a mechanism whereby glioma cells may sequester BNIP3 under normoxic conditions in the nucleus, but under hypoxic conditions BNIP3 associates with the mitochondria and induces cell death.
Figure 4. Nuclear localized BNIP3 fails to induce cell death.
HEK293 cells were transfected with vector alone, NLS-BNIP3 and wt-BNIP3. The cells were immunostained with antibodies against the T7-tagged BNIP3 and DAPI to visualize DNA. The amount of cell death was determined by changes in nuclear morphology. Standard error was calculated from three independent experiments.
Expression of dominant negative BNIP3 blocks hypoxia-induced cell death in glioma cells through association with wild type BNIP3
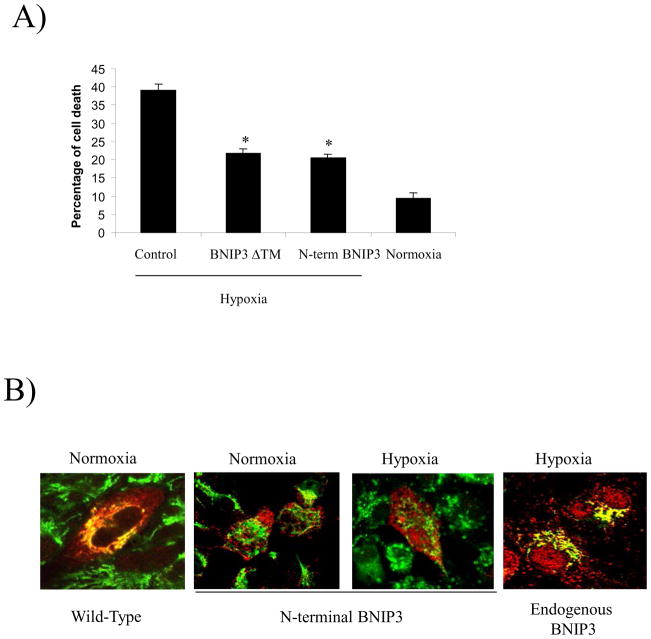
BNIP3’s contribution to hypoxia-induced cell death is negated by expression of truncated BNIP3 constructs in HEK293 cells 9. To determine whether this occurs in glioma cells, BNIP3ΔTM, N-terminal BNIP3 and vector alone cDNA were transiently transfected into U251 cells, then the cells were subjected to hypoxic conditions for 72 hours. We found that cells transfected with vector alone showed 40% cell death primarily due to induction of endogenous BNIP3 expression under hypoxia. However, cells transfected with BNIP3ΔTM or N-terminal BNIP3 under hypoxia showed 20% cell death (a 50% reduction), compared to cells placed under normoxic conditions, which had only 10% cell death (Figure 5A). These results support BNIP3’s role in hypoxia-induced cell death in glioma cells and that both N-terminal and BNIP3ΔTM may act in a dominant negative manner against wild-type BNIP3 function.
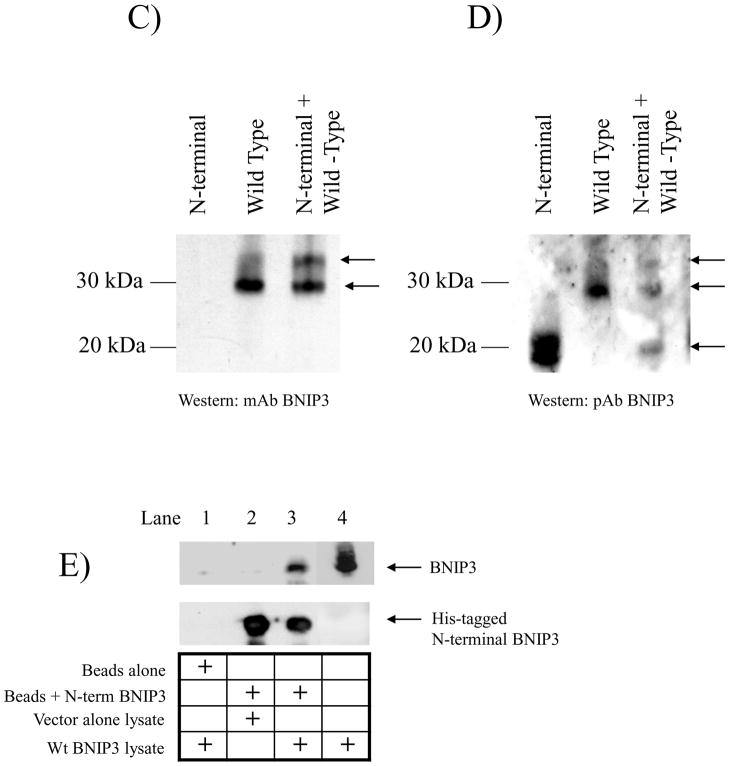
Figure 5. Expression of BNIP3 ΔTM and N-terminal BNIP3 exhibit a dominant negative effect through inhibition of hypoxia-induced cell death by blocking endogenous BNIP3 from associating with the mitochondria.
A) U251 cells were transfected with cDNA for BNIP3 ΔTM, N-terminal BNIP3 or empty vector (control) in combination with a β-gal expression vector. The cells were placed under hypoxic conditions for 72 hours and the amount of cell death was determined in cells expressing β-galatosidase as described in the Material and Methods section. Normoxia represents the amount of cell death in U251 cells transfected with empty vector under normal oxidative conditions. Error bars represent the standard error determined from three independent experiments. * denotes a p value <0.001 representing statistical significance between empty vector (control) transfected cells under hypoxia and cells transfected with BNIP3 ΔTM or N-terminal BNIP3 under hypoxia. B) U251 cells were transfected with cDNA for wt BNIP3 or N-terminal BNIP3 in an expression vector and placed under normoxic or hypoxic conditions. Forty-eight hours after transfection, the cells were stained with Mitotracker (mitochondrial stain, green) and with antibodies against BNIP3 (red). The cells were analyzed using a confocal microscope. These results represent three independent experiments. Yellow staining represents co-localization of BNIP3 and mitochondria. C) U87 cells were transfected with wild-type BNIP3 and N-terminal BNIP3 either alone or in combination. The cells were then lysed and western blotted with monoclonal antibodies against BNIP3 (recognizes only wild-type BNIP3) or D) polyclonal antibodies (recognize both wild-type and N-terminal BNIP3) E) His-tagged N-terminal BNIP3 was bound to nickel-agarose beads. i) HEK293 cells transfected with vector alone or wt BNIP3 were lysed and added to these beads. The resulting elution was western blotted for wild-type (wt) BNIP3. As a negative control, beads alone were incubated with lysate expressing wt BNIP3. Lysate expressing wt BNIP3 was used as a positive control. The blots were stripped and reprobed with anti-His tag antibodies. The lower arrow points to the predicted size of the truncated form of BNIP3, the middle arrow points to the 30kDa form of wt BNIP3 and the upper arrow points to the predicted heterodimeric complex of truncated and wild-type BNIP3. ii) HEK 293 cells under hypoxic conditions were lysed and added the his-tagged N-terminal BNIP3 bound beads. The elution was western blotted for BNIP3. F) U87 cells were lysed under normoxic or hypoxic conditions and added to beads bound to His-tagged N-terminal BNIP3. As a positive control, wild type BNIP3 was transiently transfected into U87 cells and lysed. The lysate was also added to beads bound to His-tagged N-terminal BNIP3. The resultant elution was western blotted for anti BNIP3 or his-tagged antibodies.
BNIP3 induces cell death by associating with the outer membrane of the mitochondria causing a loss of mitochondrial membrane potential 1, 5, 7. To determine whether N-terminal BNIP3 localizes to the mitochondria and if its expression affects the localization of endogenous BNIP3, U251 cells were transfected with either wild-type BNIP3 or N-terminal BNIP3. The cells were immunostained for BNIP3 and Mitotracker and visualized on a confocal microscope. Wild-type (wt) BNIP3 was localized with the mitochondria as indicated by dual fluorescence with Mitotracker (yellow staining), whereas N-terminal BNIP3 failed to associate with the mitochondria (Figure 5B). In U251 cells under hypoxia, endogenous BNIP3 localized to the mitochondria (yellow staining) as determined by immunostaining for BNIP3 and using Mitotracker (Figure 5B).
Previous studies have shown that BNIP3 dimerizes and these homodimers associate with the mitochondria 8. BNIP3 also binds to Bcl-2, blocking BNIP3’s ability to localize to the mitochondria and induce cell death. To determine whether N-terminal BNIP3 acts in a dominant negative manner by interacting with wild-type BNIP3, we transfected wild-type BNIP3 and N-terminal BNIP3 into U87 glioma cells and performed western blots with a monoclonal BNIP3 antibody that only recognizes wild-type BNIP3. When wild-type BNIP3 is expressed, the monomeric form of BNIP3 (a 30 kDa protein) was observed. But when both wild-type and N-terminal BNIP3 were co-expressed, an additional higher molecular weight protein band was detected (Figure 5C). This band was confirmed by using a polyclonal antibody that recognizes both wild-type and N-terminal BNIP3 (Figure 5D). Cells were also transfected with wild-type BNIP3 or vector alone and the lysates were immunoprecipitated through a nickel-agarose column with bound recombinant His-tagged N-terminal BNIP3 protein (Figure 5E). We found that wild-type BNIP3 bound to the His-tagged N-terminal BNIP3 (lane 3) but not the nickel-agarose beads alone, indicating that wild-type and N-terminal BNIP3 form a heterodimeric complex. Since N-terminal BNIP3 lacks the CD and TM domains, this interaction is likely mediated by the PEST domain or surrounding regions of the BNIP3 protein. In addition, we found that endogenous BNIP3 expressed under hypoxic condition binds to the N-terminal BNIP3 (Figure 5F). Taken together, this supports a mechanism for truncated BNIP3 contributes to the protection of cells against hypoxia-induced cell death by forming a heterodimeric complex with endogenous wild-type BNIP3.
BNIP3 is expressed in the nucleus of human glioblastoma tumors
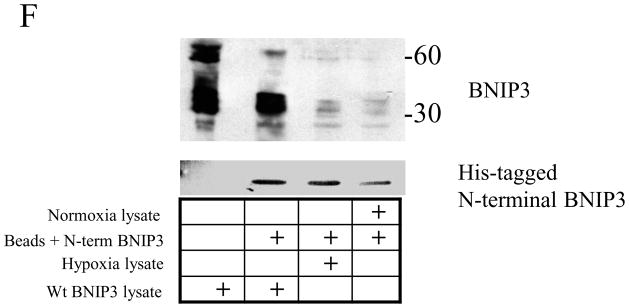
BNIP3 protein levels have been found to be elevated in breast, ovarian, head and neck, colorectal, and kidney tumors 11, 19, 20. To determine BNIP3 expression in primary GBM tumors derived from glial cells, we immunohistochemically stained paraffin embedded sections of primary GBM tumors with antibodies against BNIP3 and counterstained for DNA. We found that in GBM tumors the level of BNIP3 expression is significantly increased compared to normal brain (refer to Figure 1A). We also observed that BNIP3 immunostaining in GBM tumors surrounded necrotic regions, similar to BNIP3 expression patterns observed in other tumors 11 (Figure 6A). To confirm that BNIP3 expression was in malignant glial cells, GBM tumors were immunostained with an antibody to GFAP. BNIP3 expression was co-expressed with GFAP (Figure 6B) in malignant glial cells within these primary GBM tumors.
Figure 6. Malignant glial cells in GBM tumors express high levels of BNIP3 in necrotic regions that is primarily localized in the nucleus.
A) Primary glioblastoma multiforme (GBM) paraffin-embedded tumor tissues were immunostained by antibodies against BNIP3 (brown). DNA was counter-stained with hematoxylin (blue). The left panel shows a necrotic region (N) within a GBM immuno-stained for BNIP3 (brown) and counter-stained for DNA (blue). The arrow shows a cell expressing BNIP3. B) GBM tumor tissue was immunostained for BNIP3 (green) and GFAP (marker for glial cells, red). The slides were analyzed on a confocal microscope (Olympus). Arrows represent the same cell in each panel.
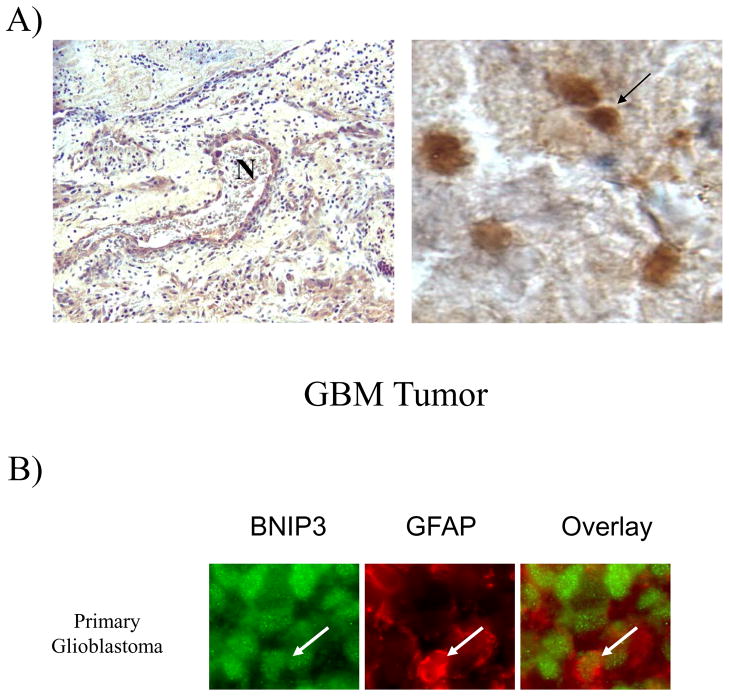
BNIP3 expression has also been demonstrated in hypoxic regions of lung and breast tumors 11, 21. HIF-1α transcriptionally regulates BNIP3 expression by binding to a hypoxia response element located within the promoter region of BNIP3 9. To further investigate whether BNIP3 is expressed in the nucleus of cells in hypoxic regions of GBM tumors, BNIP3 and HIF-1α co-expression was examined by immuno-staining. In GBM tumors, HIF-1α is co-expressed with BNIP3, while both proteins were undetectable in normal brain under the same experimental conditions (Figure 7A). Furthermore, HIF-1α and BNIP3 expression were co-localized to the nucleus in GBM tumors. These results agree with previous reports that BNIP3 expression is found in hypoxic regions in other solid tumors.
Figure 7. BNIP3 is expressed in hypoxic regions of GBM tumors but does not co-localize with the mitochondria.
A) Normal brain and primary GBM sections were immuno-stained with antibodies against BNIP3 and HIF-1α. DNA was stained with Hoescht dye and the slides were analyzed on a confocal microscope. These results represent three independent experiments. B) Glioblastoma tumors were immuno-stained with antibodies against BNIP3 (green) and cytochrome c (marker for mitochondria, red) as described in the Materials and Methods section. DNA was stained with Hoescht dye (blue). The images were captured on a fluorescent microscope with deconvolution software. Similar results were obtained from other GBM tumors. In each panel, arrows represent staining of the same cell expressing BNIP3.
BNIP3 induces cell death by associating with the mitochondria 1, 16. We investigated the localization of BNIP3 to the mitochondria in GBM tumors. GBM tumors were immunostained for BNIP3, cytochrome c (a mitochondrial marker), and DNA. In tumors where BNIP3 was predominantly nuclear (some cytoplasmic staining was also observed), BNIP3 failed to localize with cytochrome c expression (Figure 7B, upper panel). Similarly, in tumors where BNIP3 was predominantly cytoplasmic, BNIP3 (some nuclear staining was also observed) also failed to localize with cytochrome c (Figure 7B, lower panel). These results suggest that BNIP3 fails to associate with the mitochondria in viable tumor cells but its localization can be both nuclear and/or cytoplasmic. To determine the extent of nuclear localization in GBM tumors, we graded 22 primary GBMs according to whether BNIP3 expression was predominantly nuclear, cytoplasmic or in both subcellular compartments (Table 1). The expression of BNIP3 was observed to be predominantly in the nucleus in 17 out of 22 tumors examined (approximately 80%).
Table 1.
| Tumor number | Nuclear staining * | Cytoplasmic staining ** |
|---|---|---|
| 819 | +++ | +/− |
| 288 | +++ | + |
| 412 | +++ | + |
| 450 | +++ | + |
| 667 | +++ | + |
| 814 | +++ | + |
| 886 | +++ | + |
| 870 | +++ | + |
| 580 | ++ | +/− |
| 231 | ++ | +/− |
| 618 | ++ | +/− |
| 817 | ++ | +/− |
| 812 | ++ | +/− |
| 821 | ++ | +/− |
| 886 | ++ | + |
| 345 | ++ | + |
| 447 | ++ | + |
| 276 | + | ++ |
| 775 | + | +/− |
| 346 | +/− | +++ |
| 409 | +/− | +++ |
| 785 | +/− | − |
Nuclear staining was graded as follows: +++ for predominantly nuclear staining, ++ for significant nuclear staining, + for minimal nuclear staining, and +/− for negligible nuclear staining.
Cytoplasmic staining was graded as follows: +++ for predominantly cytoplasmic staining, ++ for significant cytoplasmic staining, + for minimal cytoplasmic staining, and +/− for negligible cytoplasmic staining.
Discussion
In the rat brain, BNIP3 is expressed in the nucleus of hippocampal neurons after transient global brain ischemia, reflecting cellular stress or damage, but appears not to mediate cell death. Under normal conditions, BNIP3 is not expressed in neurons 17. We have shown in human brain that BNIP3 is expressed in the nucleus of glial cells. Under hypoxic conditions, BNIP3 expression is primarily cytoplasmic and localizes with mitochondria contributing to hypoxia-induced cell death9, 22. This is consistent with the ability of BNIP3 to induce cell death under hypoxic conditions in other cell types. Thus, nuclear localization of BNIP3 negates its pro-cell death function. BNIP3 expression is also detected in the nucleus of cells in normal human brain but the function for this localization is unknown. BNIP3 is expressed in skeletal muscle but its expression differs from brain tissue where BNIP3 expression is throughout the tissue. BNIP3 expression has not been described in other normal tissues to date. It is important to note that some cytoplasmic staining for BNIP3 was detected in primary glial cells, and glioblastoma tumors, suggesting that there may be other regulatory mechanisms that control BNIP3’s cell death function.
Under hypoxia, BNIP3 expression is increased, corresponding to hypoxia-induced cell death 9. This increased BNIP3 expression is regulated by HIF-1α 9, 11. In GBM tumors, we have determined that BNIP3 expression correlates with HIF-1α expression and is highly expressed in hypoxic regions within the tumor. Unlike, human glial cells under hypoxic stress where BNIP3 expression increases in the cytoplasm, BNIP3 remains primarily localized to the nucleus in hypoxic regions of GBM tumors. Other proteins that are regulated by HIF-1α have been found to localize to the nucleus under cellular stress 23. As well, the Bcl-2 family member A1 has been shown to be sequestered in the nucleus affecting its function 24. The nuclear localization of BNIP3 effectively prevents BNIP3 from binding to the mitochondria inhibiting its ability to induce cell death. This could be a selective mechanism for tumor cells to escape hypoxia-induced cell death by preventing BNIP3’s association with the mitochondria.
The role BNIP3 plays in hypoxia-induced cell death in glioma cells is unknown. Using anti-sense oligonucleotides against BNIP3, the ability of HEK293 cells to induce hypoxia-induced cell death is diminished 9. Blockage of BNIP3 expression in mouse cardiomyocytes inhibits ischemia-induced cell death 25. By eliminating the transmembrane domain of BNIP3 and expressing this transmembrane-deleted form in HEK293 cells, the amount of hypoxia-induced cell death was also reduced. This suggests that these truncated forms of BNIP3 can act in a dominant negative manner blocking hypoxia. We found similar results in U251 glioma cells. Furthermore in U251 cells transfected with wild-type BNIP3, BNIP3ΔTM (lacking the transmembrane domain), N-terminal BNIP3 (lacking both the CD and TM domains), or vector alone, only wild-type BNIP3-induced cell death (data not shown). The mechanism for the dominant negative effect of N-terminal BNIP3 is unknown but our results suggest that N-terminal BNIP3 could bind to wild type BNIP3 preventing its cell killing activity.
BNIP3 induces cell death when over expressed in a wide variety of cell types. BNIP3 induced cell death is caspase independent and does not results in apoptotic morphologies. Recently, it has been suggested that BNIP3 induces programmed cell death type II (autophagy) in glioma cells 2, 3. The role for autophagy in hypoxia is unknown but it has been demonstrated that necrosis can occur under hypoxic conditions.
BNIP3 lacks a consensus nuclear localization signal and when over-expressed in epithelial cells, BNIP3 is exclusively localized to the cytoplasm (data not shown). However, when the NLS-BNIP3 is expressed, it fails to induce cell death. The mechanism underlying BNIP3’s nuclear localization in normal glial cells or GBM tumors remains undefined. In addition, the function of BNIP3 within the nucleus is also unknown. Although BNIP3 could be sequestered from the mitochondria to prevent cell death, it is also possible that BNIP3’s location in the nucleus could be influencing gene expression in cells under hypoxic stress. Defining this mechanism and the function of nuclear localization for BNIP3 will be the focus of future investigations.
Overall, we propose for the first time that BNIP3 is localized to the nucleus of glial cells, and upon induction of hypoxic stress BNIP3 expression localizes to the cytoplasm and mitochondria leading to cell death. The pathogenesis of GBM tumors may select for nuclear BNIP3 expression as opposed to cytoplasmic expression under hypoxic stress. Understanding how hypoxia-induced cell death is regulated in the brain may lead to more effective treatments against GBMs.
Supplementary Material
Acknowledgments
We would like to thank Dr. David Andrews and Dr. Jeannick Cizeau for supplying important reagents. This work was supported by a CancerCare Manitoba Foundation grant to Drs. D. Eisenstat and S. Gibson. Dr. S. Gibson is a Canadian Institute of Health Research New Investigator and Dr. P. Baijal was supported by a post-doctoral fellowship from the Manitoba Health Research Council.
References
- 1.Vande Velde C, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S, Hakem R, Greenberg AH. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol. 2000;20:5454–68. doi: 10.1128/mcb.20.15.5454-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daido S, Kanzawa T, Yamamoto A, Takeuchi H, Kondo Y, Kondo S. Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res. 2004;64:4286–93. doi: 10.1158/0008-5472.CAN-03-3084. [DOI] [PubMed] [Google Scholar]
- 3.Kanzawa T, Zhang L, Xiao L, Germano IM, Kondo Y, Kondo S. Arsenic trioxide induces autophagic cell death in malignant glioma cells by upregulation of mitochondrial cell death protein BNIP3. Oncogene. 2005;24:980–91. doi: 10.1038/sj.onc.1208095. [DOI] [PubMed] [Google Scholar]
- 4.Kim JY, Cho JJ, Ha J, Park JH. The carboxy terminal C-tail of BNip3 is crucial in induction of mitochondrial permeability transition in isolated mitochondria. Arch Biochem Biophys. 2002;398:147–52. doi: 10.1006/abbi.2001.2673. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Cizeau J, Vande Velde C, Park JH, Bozek G, Bolton J, Shi L, Dubik D, Greenberg A. Nix and Nip3 form a subfamily of pro-apoptotic mitochondrial proteins. J Biol Chem. 1999;274:7–10. doi: 10.1074/jbc.274.1.7. [DOI] [PubMed] [Google Scholar]
- 6.Farooq M, Kim Y, Im S, Chung E, Hwang S, Sohn M, Kim M, Kim J. Cloning of BNIP3h, a member of proapoptotic BNIP3 family genes. Exp Mol Med. 2001;33:169–73. doi: 10.1038/emm.2001.29. [DOI] [PubMed] [Google Scholar]
- 7.Yasuda M, D’Sa-Eipper C, Gong XL, Chinnadurai G. Regulation of apoptosis by a Caenorhabditis elegans BNIP3 homolog. Oncogene. 1998;17:2525–30. doi: 10.1038/sj.onc.1202467. [DOI] [PubMed] [Google Scholar]
- 8.Ray R, Chen G, Vande Velde C, Cizeau J, Park JH, Reed JC, Gietz RD, Greenberg AH. BNIP3 heterodimerizes with Bcl-2/Bcl-X(L) and induces cell death independent of a Bcl-2 homology 3 (BH3) domain at both mitochondrial and nonmitochondrial sites. J Biol Chem. 2000;275:1439–48. doi: 10.1074/jbc.275.2.1439. [DOI] [PubMed] [Google Scholar]
- 9.Kothari S, Cizeau J, McMillan-Ward E, Israels SJ, Bailes M, Ens K, Kirshenbaum LA, Gibson SB. BNIP3 plays a role in hypoxic cell death in human epithelial cells that is inhibited by growth factors EGF and IGF. Oncogene. 2003;22:4734–44. doi: 10.1038/sj.onc.1206666. [DOI] [PubMed] [Google Scholar]
- 10.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–71. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 11.Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res. 2001;61:6669–73. [PubMed] [Google Scholar]
- 12.Gurney JG, Kadan-Lottick N. Brain and other central nervous system tumors: rates, trends, and epidemiology. Curr Opin Oncol. 2001;13:160–6. doi: 10.1097/00001622-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–25. doi: 10.1093/jnen/61.3.215. discussion 26–9. [DOI] [PubMed] [Google Scholar]
- 14.Kayama T, Yoshimoto T, Fujimoto S, Sakurai Y. Intratumoral oxygen pressure in malignant brain tumor. J Neurosurg. 1991;74:55–9. doi: 10.3171/jns.1991.74.1.0055. [DOI] [PubMed] [Google Scholar]
- 15.Sharp FR, Bergeron M, Bernaudin M. Hypoxia-inducible factor in brain. Adv Exp Med Biol. 2001;502:273–91. doi: 10.1007/978-1-4757-3401-0_18. [DOI] [PubMed] [Google Scholar]
- 16.Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci U S A. 2002;99:12825–30. doi: 10.1073/pnas.202474099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt-Kastner R, Aguirre-Chen C, Kietzmann T, Saul I, Busto R, Ginsberg MD. Nuclear localization of the hypoxia-regulated pro-apoptotic protein BNIP3 after global brain ischemia in the rat hippocampus. Brain Res. 2004;1001:133–42. doi: 10.1016/j.brainres.2003.11.065. [DOI] [PubMed] [Google Scholar]
- 18.Yasuda M, Han JW, Dionne CA, Boyd JM, Chinnadurai G. BNIP3alpha: a human homolog of mitochondrial proapoptotic protein BNIP3. Cancer Res. 1999;59:533–7. [PubMed] [Google Scholar]
- 19.Sowter HM, Ferguson M, Pym C, Watson P, Fox SB, Han C, Harris AL. Expression of the cell death genes BNip3 and NIX in ductal carcinoma in situ of the breast; correlation of BNip3 levels with necrosis and grade. J Pathol. 2003;201:573–80. doi: 10.1002/path.1486. [DOI] [PubMed] [Google Scholar]
- 20.Zamora R, Alarcon L, Vodovotz Y, Betten B, Kim PK, Gibson KF, Billiar TR. Nitric oxide suppresses the expression of Bcl-2 binding protein BNIP3 in hepatocytes. J Biol Chem. 2001;276:46887–95. doi: 10.1074/jbc.M101865200. [DOI] [PubMed] [Google Scholar]
- 21.Giatromanolaki A, Koukourakis MI, Sowter HM, Sivridis E, Gibson S, Gatter KC, Harris AL. BNIP3 expression is linked with hypoxia-regulated protein expression and with poor prognosis in non-small cell lung cancer. Clin Cancer Res. 2004;10:5566–71. doi: 10.1158/1078-0432.CCR-04-0076. [DOI] [PubMed] [Google Scholar]
- 22.Guo K, Searfoss G, Krolikowski D, Pagnoni M, Franks C, Clark K, Yu KT, Jaye M, Ivashchenko Y. Hypoxia induces the expression of the pro-apoptotic gene BNIP3. Cell Death Differ. 2001;8:367–76. doi: 10.1038/sj.cdd.4400810. [DOI] [PubMed] [Google Scholar]
- 23.Sawa A, Khan AA, Hester LD, Snyder SH. Glyceraldehyde-3-phosphate dehydrogenase: nuclear translocation participates in neuronal and nonneuronal cell death. Proc Natl Acad Sci U S A. 1997;94:11669–74. doi: 10.1073/pnas.94.21.11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somogyi RD, Wu Y, Orlofsky A, Prystowsky MB. Transient expression of the Bcl-2 family member, A1-a, results in nuclear localization and resistance to staurosporine-induced apoptosis. Cell Death Differ. 2001;8:785–93. doi: 10.1038/sj.cdd.4400879. [DOI] [PubMed] [Google Scholar]
- 25.Regula KM, Ens K, Kirshenbaum LA. Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circ Res. 2002;91:226–31. doi: 10.1161/01.res.0000029232.42227.16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.