Abstract
Purpose
Several studies have suggested that bisphosphonates have an antitumor effect. We sought to evaluate whether the use of bisphosphonates increased the rates of pathological complete response (pCR) in breast cancer patients.
Methods
We identified 1449 breast cancer patients receiving taxane and anthracycline-based neoadjuvant chemotherapy between 1995 and 2007 at M.D. Anderson Cancer Center. We also identified those patients that while receiving chemotherapy received bisphosphonates for osteopenia or osteoporosis. Primary outcome was the proportion of patients achieving a pCR. Groups were compared using the chi-squared test. A multivariable logistic regression model was fit to examine the relationship between the use of bisphosphonates and pCR. An exploratory survival analysis using the Kaplan-Meier method was performed; groups were compared using the log-rank test.
Results
From the 1449 patients included, 39 (2.7%) received bisphosphonates. Those receiving bisphosphonates were older (p<0.001) and less likely to be obese (p=0.04). The pCR rate was 25.4% in the bisphosphonate group and 16% in the non-bisphosphonate group (p=.11). In the multivariable model, patients treated with bisphosphonates tended to have higher rates of pCR (OR 2.18; 95%CI 0.90–5.24); however the difference was not statistically significant. With a median follow up of 55 months (3–145), no differences in recurrence or in survival were observed.
Conclusions
The use of bisphosphonates at the time of neoadjuvant chemotherapy was not associated with a statistically significant increase in the rates of pCR. The observed estimates suggest a positive effect; however, the small proportion of patients receiving bisphosphonates likely affected the power to detect a statistically significant difference.
Keywords: bisphosphonates, neoadjuvant chemotherapy, breast cancer, pathological complete response
Background
Breast cancer is the second most common cause of cancer death among women in the United States. It is estimated that during 2009 over 194,280 new cases were diagnosed and 40,610 deaths occurred1. Despite significant advances in the treatment of patients with breast cancer, approximately 10 to 60% of patients with initial localized breast cancer will suffer a systemic relapse. Neoadjuvant systemic therapy (NST), is the standard approach to treat women with locally advanced and inflammatory breast cancer, and is now being used in patients with earlier stage disease. By down-staging tumors; NST probably improves available surgical options while concurrently allowing for in vivo assessment of chemo-sensitivity. Furthermore, attaining a pathological complete response (pCR) following NST has been shown by a number of investigators to be a surrogate marker for improved long-term outcome, possibly due to the eradication of distant micrometastatic residual disease 2–8. Unfortunately, NST using conventional anthracyline and/or taxane-based regimens, results in pCR rates of only 8–31% 4, 6, 9, 10.
Bisphosphonates are analogs of pyrophosphates that bind to hydroxyapatite crystals and inhibit bone resorption by osteoclasts. They are widely used for the treatment of osteoporosis and to prevent both, skeletal complications in patients with bone metastases, and the bone loss associated with cancer treatment 11, 12. There is clinical and pre-clinical data suggesting that bisphosphonates have osteoclast-independent effects that can be associated with an anti-tumor effect 13; proposed mechanisms include the induction of apoptosis, synergistic effect with chemotherapy or the inhibition of tumor cells by affecting adhesion, migration, invasion and cell proliferation 14–16. Recent epidemiological studies have shown that the use of bisphosphonates decreases the incidence of invasive breast cancer in postmenopasal women17, 18.
The purpose of this study was to determine whether the use of bisphosphonates increased the rates of pathological complete response (pCR) in a cohort of breast cancer patients treated with taxane and anthracycline-based neoadjuvant chemotherapy.
Methods
Patient Population
Patients treated with NST were identified in a prospectively maintained data base in the Breast Medical Oncology Department at The University of Texas, M. D. Anderson Cancer Center. One thousand four hundred and forty nine patients diagnosed with invasive primary breast cancer between 1995 and 2007 and treated with anthracycline-and taxane-based NST were included. We excluded patients with metastatic disease at diagnosis, bilateral breast cancer, or males. The variables recorded included patient demographics (race, age, menopausal status), tumor characteristics (histology, grade, lymphovascular invasion, ER, PR and Her2 status), clinical stage at diagnosis (based on the criteria proposed by the American Joint Committee on Cancer Criteria version VI19), body mass index (BMI), pathological stage, and recurrence and survival information. Patients that received bisphosphonates for other indication (osteopenia and osteoporosis) while receiving NST were identified by chart review (physician’s notes, medication records and pharmacy records).
All pathology specimens were reviewed by dedicated breast pathologists at our institution. Histologic type and grade were defined according to the WHO classification system 20 and modified Black’s nuclear grade system 21, respectively. All surgical breast and axillary lymph node specimens were reviewed to identify the presence or absence of residual disease. pCR was defined as no residual invasive cancer in both the breast and the axillary lymph nodes.
Treatment
All patients were treated with a multidisciplinary approach and they received NST with an anthracycline and taxane-based regimen. Taxanes administered included paclitaxel 175–250mg/m2 intravenously (IV) on day 1 every 21 days for four cycles; paclitaxel 80 mg/m2 weekly for 12 doses; or docetaxel 100mg/m2 IV on day 1 every 3 weeks for four cycles. Anthracycline regimens included 3 to 6 cycles of one of the following: fluorouracil 500mg/m2, epirubicin 100mg/m2 and cyclophosphamide 500mg/m2 IV on day 1, every 3 weeks; fluorouracil 500mg/m2 on days 1 and 4, epirubicin 75mg/m2 and cyclophosphamide 500mg/m2 IV on day 1, every 3 weeks (FEC 75); fluorouracil 500 mg/m2 IV on days 1 and 4, doxorubicin 50mg/m2 IV by continuous infusion over 72 hours and cyclophosphamide 500mg/m2 on day 1 every 3 weeks; or doxorubicin 60mg/m2 and cyclophosphamide 600mg/m2 IV on day 1, every 3 weeks. There were a total of 258 patients with HER2-neu overexpressed tumors; none of them received trastuzumab in the neoadjuvant setting. At the completion of NST all patients underwent definitive surgery, eligibility for breast conservation was determined based on recommendations made by the multidisciplinary team and patient preferences. All patients had axillary staging with axillary lymph node dissection or sentinel lymph node biopsy. Radiation therapy was delivered if patients underwent breast conservation surgery, or had locally advanced disease, primary tumor larger than 5 cm or four or more involved lymph nodes. Adjuvant hormonal therapy was administered according to standard practice. The Institution Review Board of The University of Texas, M. D. Anderson Cancer Center approved the study.
Statistical Analysis and Outcome Measures
We computed descriptive statistics. Patient characteristics were compared based on whether patients received or did not receive bisphosphonates, groups were compared using chi-square test. Rates of pCR were compared among groups. An exploratory analysis was done to compare the pCR rates by bisphosphonate status according to tumor subtype. A multivariable logistic regression model was fit to examine the relationship between the use of bisphosphonates and pCR. Variables in the model include age, stage, tumor grade, tumor subtype, lymphovascular invasion (LVI) and BMI. Exploratory survival analyses were carried out to examine the recurrence-free survival (RFS) and overall survival (OS) between groups. RFS was calculated from the date of diagnosis to the date of first documented local or distant recurrence or last follow-up. Patients who died before experiencing a disease recurrence were considered censored at their date of death; OS was calculated from the date of diagnosis to the date of death or last follow-up. Kaplan-Meier product limit method was used to estimate the survival outcomes and groups were compared with the log-rank statistics. P-values less than 0.05 were considered statistically significant; all tests were two-sided. Statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC) and S-Plus 7.0 (Insightful Corporation, Seattle, WA).
Results
A total of 1449 patients were included, among whom only 39 (2.7%) received bisphosphonates concurrently with NST. Among the patients that received bisphosphonates, 66.67% (n=26); 28.20% (n=11) and 5.13% (n=2) received alendronate, risedronate and ibandronate respectively. Patients that received bisphosphonates were more likely to be older and postmenopausal (both, p<0.001), and less likely to be overweight (p=0.04). Patient and tumor characteristics stratified by bisphosphonate use are summarized in Table-1.
Table 1.
Patient and tumor characteristics according to bisphosphonate use.
| Non-Bisphosphonate (N=1410) | Bisphosphonate (N=39) | ||||
|---|---|---|---|---|---|
| N | (%) | N | (%) | P | |
|
| |||||
| Age (median) | 49 | 58 | |||
|
| |||||
| Age | |||||
| < 50 | 726 | (51.5) | 2 | (5.1) | |
| ≥ 50 | 684 | (48.5) | 37 | (94.9) | < 0.001 |
|
| |||||
| Menopausal Status | |||||
| Pre | 674 | (47.9) | 6 | (15.4) | |
| Post | 733 | (52.1) | 33 | (84.6) | < 0.001 |
|
| |||||
| Body Mass Index | |||||
| Normal/underweight | 454 | (33.3) | 18 | (47.4) | |
| Overweight | 439 | (32.2) | 14 | (36.8) | |
| Obese | 472 | (34.6) | 6 | (15.8) | 0.04 |
|
| |||||
| Race | |||||
| White/other | 1202 | (85.2) | 37 | (94.9) | |
| Black | 208 | (14.8) | 2 | (5.1) | 0.09 |
|
| |||||
| Clinical Stage | |||||
| I | 56 | (4.0) | 2 | (5.1) | |
| II | 759 | (54.0) | 27 | (69.2) | |
| III | 590 | (42.0) | 10 | (25.6) | 0.12 |
|
| |||||
| Nuclear Grade | |||||
| I | 50 | (3.7) | 1 | (2.6) | |
| II | 443 | (32.4) | 18 | (47.4) | |
| III | 876 | (64.0) | 19 | (50.0) | 0.15 |
|
| |||||
| LVI | |||||
| Negative | 932 | (68.5) | 30 | (78.9) | |
| Positive | 428 | (31.5) | 8 | (21.1) | 0.17 |
|
| |||||
| Tumor subtype | |||||
| Hormone receptor + | 767 | (55.1) | 24 | (61.5) | |
| Her2neu + | 250 | (17.9) | 8 | (20.5) | |
| Triple negative | 376 | (27.0) | 7 | (17.9) | 0.45 |
Among the patients not treated with bisphosphonates, 16.0% achieved a pCR. A higher rate of pCR (25.6%) was observed among the patients treated with bisphosphonates, however this difference did not achieve statistical significance (p= 0.11). When the rates of pCR in the bisphosphonate and non-bisphosphonate groups were compared according to tumor subtype, the same trend was observed, but in all cases the results were not statistically significant. In the hormone positive tumors 7.6% vs 16.7% (p=0.10); in Her2/neu overexpressed tumors 26.8% vs 37.5% (p=0.50) and among triple receptor negative tumors 26.9% vs 42.9% (p=0.35) for patients in the non-bisphosphonate and bisphosphonate group, respectively. The results of the multivariable model for pCR are shown in Table-2. After adjusting for age, stage, tumor subtype, grade, LVI and BMI, bisphosphonate use was associated with higher pCR rates (OR 2.16; 95%CI 0.90–5.24, p=0.08), however statistical significance was not reached.
Table 2.
Multivariable logistic regression model for pCR
| Odds Ratio | 95% CI | P | |
|---|---|---|---|
|
| |||
| Bisphosphonates use: yes v. no | 2.18 | 0.90 to 5.24 | 0.08 |
| Age: ≥ 50 versus < 50 | 0.66 | 0.48 to 0.92 | 0.015 |
| Stage: III vs I/II | 0.69 | 0.5 to 0.96 | 0.026 |
| Grade: III vs I/II | 3.80 | 2.37 to 6.07 | <.001 |
| Subtype: HER2 positive vs Hormone positive | 3.02 | 1.97 to 4.64 | <.001 |
| Subtype: Triple negative vs Hormone positive | 2.66 | 1.8 to 3.93 | 0.011 |
| LVI: positive vs negative | 0.38 | 0.26 to 0.57 | <.001 |
| BMI: overweight vs normal | 0.69 | 0.46 to 1.04 | 0.022 |
| BMI: obese vs normal | 1.09 | 0.75 to 1.59 | 0.10 |
pCR = pathologic complete response; HER2 = human epidermal growth factor receptor 2; LVI = lymphovascular invasion; BMI = body mass index; CI = confidence interval.
Median follow-up was 55 months (range 3–155 months). Among all patients, there were 413 recurrences, RFS at 5 years was 71.0% (95%CI 68.0%–73.0%) among patients that did not receive bisphosphonates and 81% (95%CI 64%–90%) among those who received bisphosphonates (p=0.28). There were a total of 359 deaths, OS at 5 years was 77.0% (95%CI 74.0%–79.0%) among those not receiving bisphosphonates, compared to 83% (95%CI 65.0%–92.0%) among those that received bisphosphonates (p=0.42). The RFS and OS estimates by bisphosphonate group according to tumor subtype are shown in Table-3. Kaplan Meier curves for RFS and OS by bisphosphonate group are presented in Figure-1.
Table 3.
5 year Relapse-Free Survival and 5 year Overall Survival among patients that received and did not receive bisphosphonates. Estimates are shown for all patients and according to tumor subtype.
| N Patients | Relapse-Free Survival
|
Overall Survival
|
|||||
|---|---|---|---|---|---|---|---|
| N Events | 5-Year Estimates (95% CI) | Log-rank P | N Events | 5-Year Estimates (95% CI) | Log-rank P | ||
| All patients | |||||||
| No BIS | 1410 | 405 | 0.71(0.68, 0.73) | 352 | 0.77(0.74, 0.79) | ||
| Yes BIS | 39 | 8 | 0.81(0.64, 0.9) | .28 | 7 | 0.83(0.65, 0.92) | .42 |
|
| |||||||
| Hormone Positive | |||||||
| No BIS | 767 | 171 | 0.77(0.73, 0.8) | 144 | 0.84(0.81, 0.87) | ||
| Yes BIS | 24 | 6 | 0.77(0.54, 0.9) | .72 | 5 | 0.8(0.53, 0.92) | .59 |
|
| |||||||
| HER2 Positive | |||||||
| No BIS | 250 | 91 | 0.65(0.59, 0.71) | 69 | 0.78(0.72, 0.83) | ||
| Yes BIS | 8 | 1 | 0.87(0.36, 0.98) | .24 | 1 | 0.88(0.39, 0.98) | .43 |
|
| |||||||
| Triple Negative | |||||||
| No BIS | 376 | 138 | 0.62(0.57, 0.67) | 135 | 0.61(0.55, 0.66) | ||
| Yes BIS | 7 | 1 | 0.86(0.33, 0.98) | .25 | 1 | 0.86(0.33, 0.98) | .30 |
Figure 1.
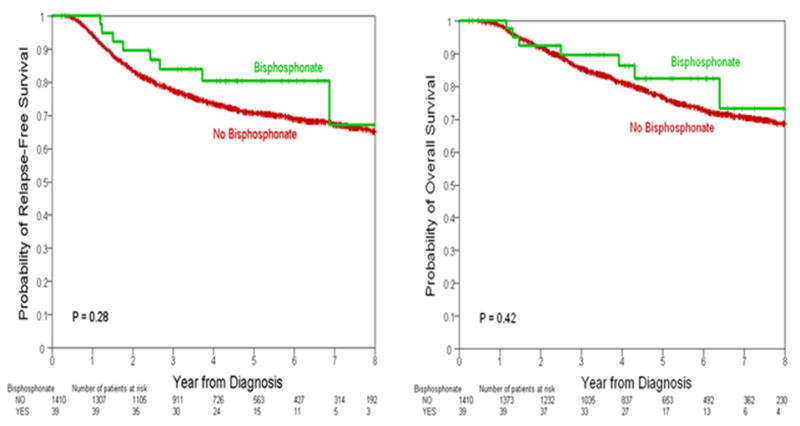
Relapse-Free Survival and Overall Survial by bisphosphonate intake.
Discussion
The aim of this study was to describe the effect of bisphosphonate use in the pCR rates among breast cancer patients treated with anthracycline and taxane-based neoadjuvant chemotherapy. In this retrospective, single-institution study, we show that pCR rates were higher among patients that received bisphosphonates, however the difference was not statistically significant. Similarly, in a multivariable model, patients that received oral bisphosphonates during their treatment with NST tended to have higher rates of pCR, however this association only approached statistical significance. We did not find an association between bisphosphonate use and RFS or OS.
Our results, despite being non-conclusive, could suggest that bisphosphonates may have tumor effects outside the bone, maybe even enhancing the effect of chemotherapy. We observed that 16% of the patients that did not receive bisphosphonates achieved a pCR compared with 25.6% on the patients that received bisphosphonates. These results are in concordance to previously reported data. The AZURE trial evaluated whether zoledronic acid (ZA) added to systemic chemotherapy improved disease-related outcomes. In a subset analysis evaluating 195 patients that received NST22, the mean residual tumor size was smaller in the ZA group compared with the non-ZA group (p=0.024). The rates of pCR favored the group treated with ZA (6.9% vs. 11.7%), and in the multivariable analysis, the use of ZA increased the odds of achieving a pCR (OR 2.1; 95%CI 0.8–6.3)22. The estimate did not achieve statistical significance, and the authors attributed it to the small number of patients achieving a pCR. Aft et al, in a phase II clinical trial (n=120) evaluated the addition of ZA to anthracycline and taxane-based NST23. In an exploratory analysis evaluating tumor responses at the time of surgery, a pCR rate of 15.5% was observed in the patients that did not receive ZA, compared with 21.6% in the patients that received ZA (p=0.63). Also, in a tumor subtype-analysis, they observed an effect among patients with triple negative tumors, with a pCR rate of 10.5% in the non-ZA group compared with a pCR rate of 28.6% in the ZA acid group. More recently, the ANZAC trial, randomized (n=40) patients to receive chemotherapy with or without ZA. The biomarker analysis showed a greater reduction in VGEF and in cell turnover at day 5 in the ZA arm; however this markers recovered by day 21; suggesting that ZA may have relevant biologic effects that need further exploration24.
Despite being non significant, our results and some of the previously discussed studies suggest that bisphosphonates may have an antitumor effect when used in combination with NST, however the small numbers mandate a cautious interpretation of the data. Bisphosphonates, and in particular ZA, has show to have synergistic anti-tumor effect in preclinical models when used in combination with doxorubicin14, 15, and all the patients included in the previously discussed studies were treated with anthracyclines. The possible anti-tumor effect of bisphosphonates is also supported by epidemiological studies, suggesting that the use of bisphosphonates is associated with reduced risk of developing breast cancer. Data from the Women’s Health Initiative evaluated the association between the use of bisphosphonates and the development of invasive breast cancer. In a large cohort of 154,768 postmenopausal women, a statistically significant association between the use of oral bisphosphonates and a lower incidence of invasive breast cancer (HR 0.68; 95% CI 0.52–0.88) was observed17. Similarly, Rennert et al18 reported that the use of bisphosphonates for more than one year was associated with a 28% reduction in the risk of postmenopausal breast cancer.
The potential antitumor effect of bisphosphonates has been reported in the adjuvant setting, but the data is still inconclusive. Diel and colleagues25 randomized (n=302) patients to receive clodronate for two years versus standard of care. After 36 months of follow up, a reduction in the incidence of bone and visceral metastases (p=0.003) and an increase in OS (p=0.001) in the clodronate arm was observed. However, after an extended follow up of 53 months the effect of clodronate on visceral metastases was no longer significant. A similar large multicenter study 26, compared the use of clodronate against placebo in 1069 breast cancer patients. After 2 years of treatment, patients in the clodronate group had a reduction in the incidence of bone metastases; however the effect did not reach statistical significance. In contrast Saarto et al27 did not observe any reduction in the incidence of bone metastases in the patients that were randomized to receive clodronate. Actually, the patients treated with clodronate had higher risk of non-skeletal recurrences (43 vs 25%, P=.0007), leading to lower disease-free survival in the treatment arm.
The ABCSG-12 study (n=1803), a phase-III 2×2, 4-arm trial, randomized patients to receive groserelin or tamoxifen with or without ZA28. No difference was observed among the tamoxifen and groserelin arms, however; patients that received ZA experienced improved RFS (HR=0.64; 95%CI 0.46–0.91) but no difference in OS was seen. A recently published meta-analysis29 including data from 13 clinical trials involving evaluated the use of bisphosphonates in the adjuvant setting in 6886 breast cancer patients. Treatment with ZA was not associated with any statistically significant difference in death (OR 0.642; 95%CI, 0.388–1.063) or bone metastasis rates (OR, 0.661 95%CI, 0.379–1.151). The AZURE trial22 randomized 3360 breast cancer patients to receive 5 years of ZA or not. With a median follow up of 58.6 months, no differences in rates of local or distant recurrence or in DFS (HR 0.98; 95%CI 0.85–1.10) or OS (HR 0.88; 95%CI 0.72–1.01) were seen. However, in an analysis according to menopausal status, and improvement in DFS and OS was observed among patients with established menopause, suggesting that ZA may have a benefit when use in a low estrogen environment.
To appreciate the findings of our study, some strengths and limitations need to be addressed. Our study is retrospective in nature, therefore is associated to the limitations inherent to this study design. All the patients were treated at M.D. Anderson Cancer Center. Despite the different chemotherapy regimens used, patients were treated in similar way whether they were treated or not with bisphosphonates, therefore, if differences in treatment occur, they were non-differential. Information regarding bisphosphonate intake during NST was obtained by chart review. However, despite a thorough review of physician’s notes, medication records and pharmacy records, it is possible that not all the patients receiving bisphosphonates were correctly identified, likely diluting any possible association. The observed estimates suggest that bisphosphonates may have a positive effect increasing the rates of pCR among breast cancer patients; however, the small proportion of patients receiving bisphosphonates likely affected the power to detect a statistically significant difference. Also, it is important to mention that all the patients identified as bisphosphonate users, received oral bisphosphonates. We cannot exclude that the use or intravenous, more potent bisphosphonates, could have a significant effect in the rates of pCR. Additionally, we did not observe any differences in RFS or OS, it is important to mention that the impact of adjuvant radiation, endocrine and trastuzumab therapy is not taken into account in our analysis. It is possible that any potential differences in survival were diluted by differences in treatment in the adjuvant setting.
In conclusion, this study makes observation that in this cohort of patients, bisphosphonate use was not associated with pCR rates. All our estimates suggest a positive effect; however, the small proportion of patients receiving bisphosphonates likely affected the power to detect a statistically significant difference. Given the preclinical and clinical data suggesting bisphosphonate-induced antitumor effects, prospective studies evaluating the effect of potent bisphosphonates are needed. Several questions regarding the use of bisphosphonates remain unanswered, it is unclear what dose and frequency will be appropriate and further studies should address this issue. Also further epidemiological studies and molecular characterization of the tumors is needed in order to explore whether the antitumor effect of bisphosphonates predominates in specific breast cancer patient subsets.
Acknowledgments
This work was funded by NIH K23 CA121994 for Ana M. Gonzalez-Angulo.
Footnotes
Disclaimers: The authors have no potential conflicts of interest.
References
- 1.American Cancer Society. Estimated new cancer deaths by sex. USA: 2009. http://www.cancer.org. [Google Scholar]
- 2.Feldman LD, Hortobagyi GN, Buzdar AU, Ames FC, Blumenschein GR. Pathological assessment of response to induction chemotherapy in breast cancer. Cancer Res. 1986;46(5):2578–81. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3697997. [PubMed]
- 3.Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672–85. doi: 10.1200/JCO.1998.16.8.2672. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9704717. [DOI] [PubMed]
- 4.O’Regan RM, Von Roenn JH, Carlson RW, Malik U, Sparano JA, Staradub V, et al. Final results of a phase II trial of preoperative TAC (docetaxel/doxorubicin/cyclophosphamide) in stage III breast cancer. Clin Breast Cancer. 2005;6(2):163–8. doi: 10.3816/CBC.2005.n.019. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16001995. [DOI] [PubMed]
- 5.Sataloff DM, Mason BA, Prestipino AJ, Seinige UL, Lieber CP, Baloch Z. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: a determinant of outcome. J Am Coll Surg. 1995;180(3):297–306. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7874340. [PubMed]
- 6.Stearns V, Singh B, Tsangaris T, Crawford JG, Novielli A, Ellis MJ, et al. A prospective randomized pilot study to evaluate predictors of response in serial core biopsies to single agent neoadjuvant doxorubicin or paclitaxel for patients with locally advanced breast cancer. Clin Cancer Res. 2003;9(1):124–33. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12538460. [PubMed]
- 7.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–22. doi: 10.1200/JCO.2007.10.6823. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17785706. [DOI] [PubMed]
- 8.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001(30):96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11773300. [DOI] [PubMed]
- 9.Colleoni M, Viale G, Zahrieh D, Pruneri G, Gentilini O, Veronesi P, et al. Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: a study of preoperative treatment. Clin Cancer Res. 2004;10(19):6622–8. doi: 10.1158/1078-0432.CCR-04-0380. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15475452. [DOI] [PubMed]
- 10.Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17(2):460–9. doi: 10.1200/JCO.1999.17.2.460. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10080586. [DOI] [PubMed]
- 11.Coleman RE. Bisphosphonates: clinical experience. Oncologist. 2004;9(Suppl 4):14–27. doi: 10.1634/theoncologist.9-90004-14. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15459426. [DOI] [PubMed]
- 12.Pavlakis N, Schmidt R, Stockler M. Bisphosphonates for breast cancer. Cochrane Database Syst Rev. 2005;(3):CD003474. doi: 10.1002/14651858.CD003474.pub2. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16034900. [DOI] [PubMed]
- 13.Naidu A, Dechow PC, Spears R, Wright JM, Kessler HP, Opperman LA. The effects of bisphosphonates on osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(1):5–13. doi: 10.1016/j.tripleo.2008.03.036. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18504149. [DOI] [PubMed]
- 14.Ottewell PD, Lefley DV, Cross SS, Evans CA, Coleman RE, Holen I. Sustained inhibition of tumor growth and prolonged survival following sequential administration of doxorubicin and zoledronic acid in a breast cancer model. Int J Cancer. 126(2):522–32. doi: 10.1002/ijc.24756. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19621384. [DOI] [PubMed]
- 15.Ottewell PD, Monkkonen H, Jones M, Lefley DV, Coleman RE, Holen I. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J Natl Cancer Inst. 2008;100(16):1167–78. doi: 10.1093/jnci/djn240. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18695136. [DOI] [PubMed]
- 16.Tenta R, Tiblalexi D, Sotiriou E, Lembessis P, Manoussakis M, Koutsilieris M. Bone microenvironment-related growth factors modulate differentially the anticancer actions of zoledronic acid and doxorubicin on PC-3 prostate cancer cells. Prostate. 2004;59(2):120–31. doi: 10.1002/pros.10363. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15042612. [DOI] [PubMed]
- 17.Chlebowski RT, Chen Z, Cauley JA, Anderson G, Rodabough RJ, McTiernan A, et al. Oral bisphosphonate use and breast cancer incidence in postmenopausal women. J Clin Oncol. 28(22):3582–90. doi: 10.1200/JCO.2010.28.2095. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20567009. [DOI] [PMC free article] [PubMed]
- 18.Rennert G, Pinchev M, Rennert HS. Use of bisphosphonates and risk of postmenopausal breast cancer. J Clin Oncol. 3577–81;28(22) doi: 10.1200/JCO.2010.28.1113. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20567021. [DOI] [PubMed]
- 19.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, et al. Staging system for breast cancer: revisions for the 6th edition of the AJCC Cancer Staging Manual. Surg Clin North Am. 2003;83(4):803–19. doi: 10.1016/S0039-6109(03)00034-3. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12875597. [DOI] [PubMed]
- 20.The world Health Organization Histological Typing of Breast Tumors--Second Edition. The World Organization. Am J Clin Pathol. 1982;78(6):806–16. doi: 10.1093/ajcp/78.6.806. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7148748. [DOI] [PubMed]
- 21.Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet. 1957;105(1):97–102. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=13442910. [PubMed]
- 22.Coleman RE, Winter MC, Cameron D, Bell R, Dodwell D, Keane MM, et al. The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: exploratory evidence for direct anti-tumour activity in breast cancer. Br J Cancer. 102(7):1099–105. doi: 10.1038/sj.bjc.6605604. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20234364. [DOI] [PMC free article] [PubMed]
- 23.Aft R, Naughton M, Trinkaus K, Watson M, Ylagan L, Chavez-MacGregor M, et al. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol. 11(5):421–8. doi: 10.1016/S1470-2045(10)70054-1. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20362507. [DOI] [PMC free article] [PubMed]
- 24.Winter MC, Syddall SP, Cross SS, Evans CA, Ingram CE, Jolley IJ, et al. ANZAC: A Randomized Neoadjuvant Biomarker Study Investigating the Anti-Tumor Activity of the Addition of Zoledronic Acid to Chemotherapy in Breast Cancer. Cancer Res. 2010;70(24) [Google Scholar]
- 25.Diel IJ, Solomayer EF, Costa SD, Gollan C, Goerner R, Wallwiener D, et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339(6):357–63. doi: 10.1056/NEJM199808063390601. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9691101. [DOI] [PubMed]
- 26.Powles T, Paterson S, Kanis JA, McCloskey E, Ashley S, Tidy A, et al. Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J Clin Oncol. 2002;20(15):3219–24. doi: 10.1200/JCO.2002.11.080. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12149294. [DOI] [PubMed]
- 27.Saarto T, Blomqvist C, Virkkunen P, Elomaa I. Adjuvant clodronate treatment does not reduce the frequency of skeletal metastases in node-positive breast cancer patients: 5-year results of a randomized controlled trial. J Clin Oncol. 2001;19(1):10–7. doi: 10.1200/JCO.2001.19.1.10. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11134190. [DOI] [PubMed]
- 28.Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360(7):679–91. doi: 10.1056/NEJMoa0806285. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19213681. [DOI] [PubMed]
- 29.Mauri D, Valachis A, Polyzos NP, Tsali L, Mavroudis D, Georgoulias V, et al. Does adjuvant bisphosphonate in early breast cancer modify the natural course of the disease? A meta-analysis of randomized controlled trials. J Natl Compr Canc Netw. 8(3):279–86. doi: 10.6004/jnccn.2010.0020. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20202461. [DOI] [PubMed]


