Abstract
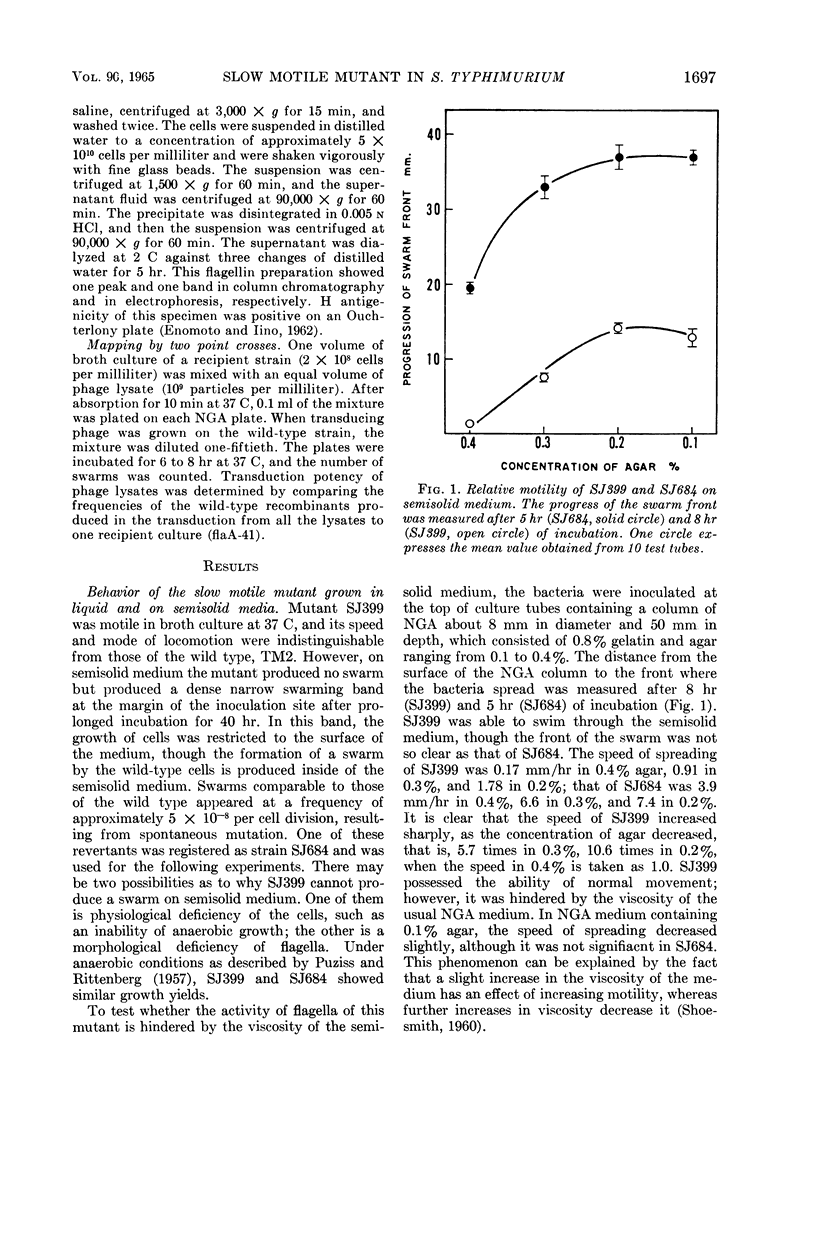
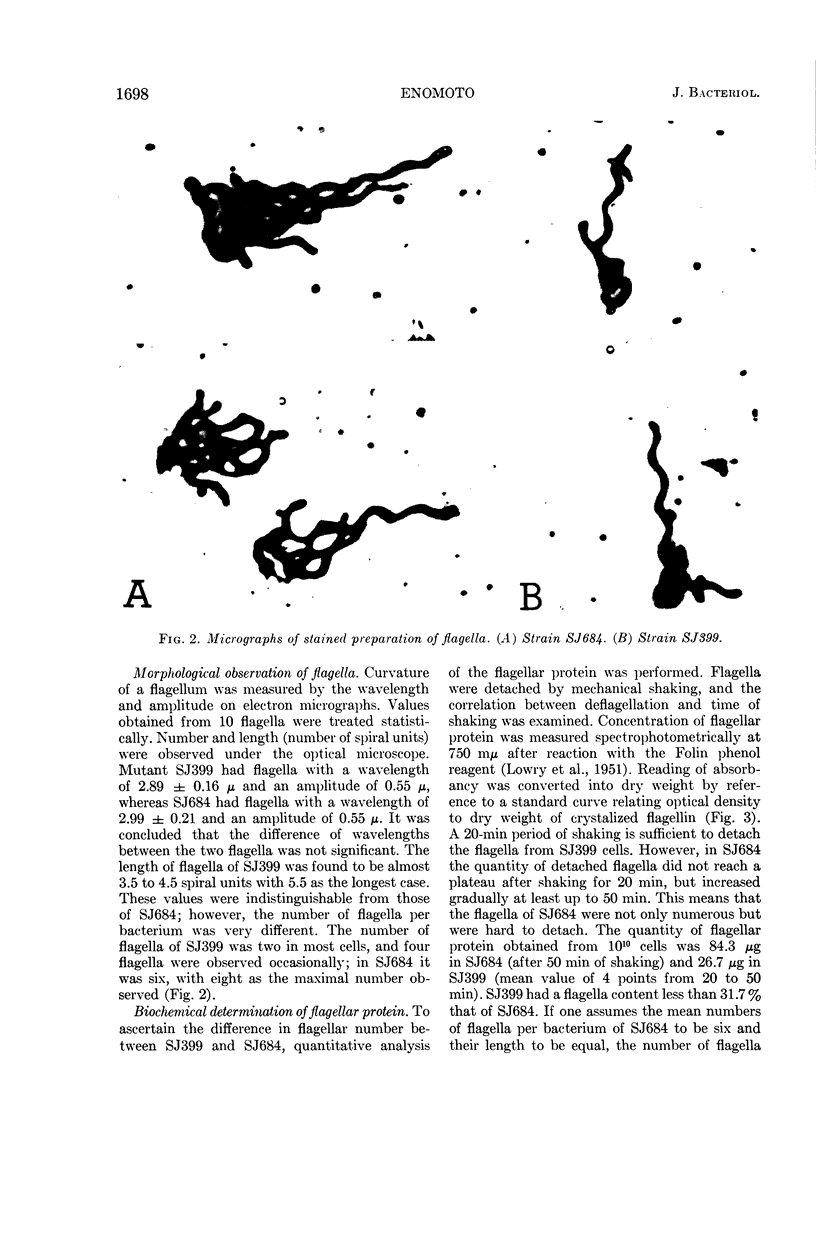
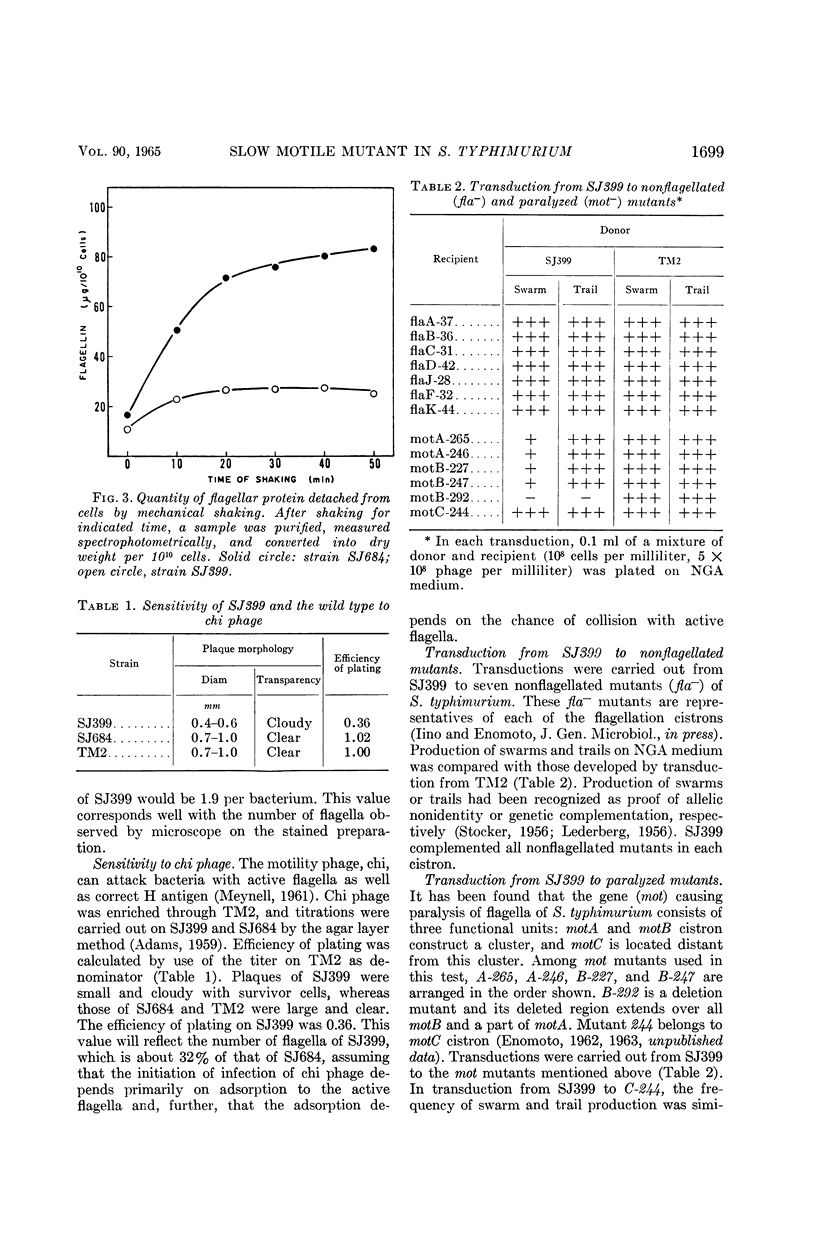
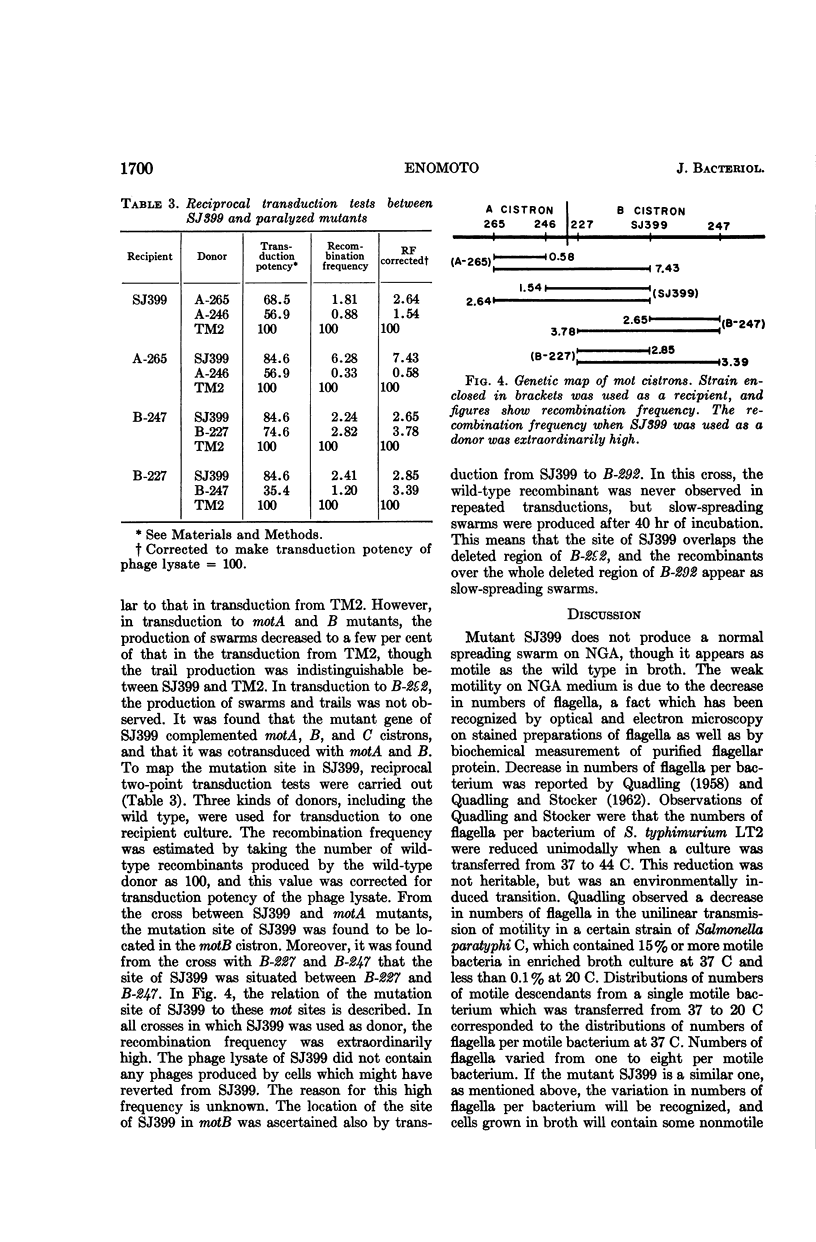
Enomoto, Masatoshi (National Institute of Genetics, Misima, Japan). Slow motile mutant in Salmonella typhimurium. J. Bacteriol. 90:1696–1702. 1965.—A slow motile mutant, SJ399, was isolated from a wild-type strain of Salmonella typhimurium TM2. The mutant was as motile as the wild type in broth culture at 37 C. However, on semisolid medium it produced a much narrower swarming band than TM2. The motility of this mutant was hindered by the viscosity of semisolid medium. H antigenicity and morphological characters of flagella of the mutant were the same as those of the wild type. The motility phage, chi, responded differently to SJ399 and the wild type. Plaques of SJ399 were small and cloudy, whereas on the wild type they were large and clear. The efficiency of plating on SJ399 was 0.36 as compared with 1 with the wild type. Stained preparations revealed that the mutant had about one-third the number of flagella of the wild type. The reduction of the number of flagella also was ascertained by biochemical measurement of flagellar protein which was purified after deflagellation from cells. The content of flagellin in SJ399 was about 32% of that of the wild type. Phage P22-mediated transductions from SJ399 to nonflagellated (fla−) and paralyzed (mot−) mutants showed that the mutant SJ399 complements seven fla− and three mot− strains which are representative mutants of flagellation and motility cistrons, respectively. The mutation site of SJ399 was cotransduced with both motA and B cistrons. The two point cross tests between SJ399 and mot mutants revealed that the mutation site of SJ399 is located in the motB cistron. The insertion of the genetic region containing the mutation site of SJ399 to the motB cistron is discussed in relation to intracistronic complementation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- CRICK F. H., ORGEL L. E. THE THEORY OF INTER-ALLELIC COMPLEMENTATION. J Mol Biol. 1964 Jan;8:161–165. doi: 10.1016/s0022-2836(64)80156-x. [DOI] [PubMed] [Google Scholar]
- ENOMOTO M., IINO T. COLONIAL DIMORPHISM IN NONMOTILE SALMONELLA. J Bacteriol. 1963 Sep;86:473–477. doi: 10.1128/jb.86.3.473-477.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINCHAM J. R. Genetically determined multiple forms of glutamic dehydrogenase in Neurospora crassa. J Mol Biol. 1962 Apr;4:257–274. doi: 10.1016/s0022-2836(62)80004-7. [DOI] [PubMed] [Google Scholar]
- Iino T. A Stabilizer of Antigenic Phases in Salmonella Abortus-Equi. Genetics. 1961 Nov;46(11):1465–1469. doi: 10.1093/genetics/46.11.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIFSON E., HUGH R. Variation in shape and arrangement of bacterial flagella. J Bacteriol. 1953 Mar;65(3):263–271. doi: 10.1128/jb.65.3.263-271.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIFSON E. Staining, shape and arrangement of bacterial flagella. J Bacteriol. 1951 Oct;62(4):377–389. doi: 10.1128/jb.62.4.377-389.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lederberg J, Iino T. Phase Variation in Salmonella. Genetics. 1956 Sep;41(5):743–757. doi: 10.1093/genetics/41.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg J. Linear Inheritance in Transductional Clones. Genetics. 1956 Nov;41(6):845–871. doi: 10.1093/genetics/41.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYNELL E. W. A phage, phi chi, which attacks motile bacteria. J Gen Microbiol. 1961 Jun;25:253–290. doi: 10.1099/00221287-25-2-253. [DOI] [PubMed] [Google Scholar]
- PUZISS M., RITTENBERG S. C. Studies on the anaerobic metabolism of Bacillus anthracis and Bacillus cereus. J Bacteriol. 1957 Jan;73(1):48–51. doi: 10.1128/jb.73.1.48-51.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUADLING C., STOCKER B. A. An environmentally-induced transition from the flagellated to the non-flagellated state in Salmonella typhimurium: the fate of parental flagella at cell division. J Gen Microbiol. 1962 Jun;28:257–270. doi: 10.1099/00221287-28-2-257. [DOI] [PubMed] [Google Scholar]
- QUADLING C. The unilinear transmission of motility and its material basis in Salmonella. J Gen Microbiol. 1958 Feb;18(1):227–237. doi: 10.1099/00221287-18-1-227. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER M. J., LEVINTHAL C. Hybrid protein formation of E. coli alkaline phosphatase leading to in vitro complementation. J Mol Biol. 1963 Jul;7:1–12. doi: 10.1016/s0022-2836(63)80014-5. [DOI] [PubMed] [Google Scholar]
- STENT G. S. THE OPERON: ON ITS THIRD ANNIVERSARY. MODULATION OF TRANSFER RNA SPECIES CAN PROVIDE A WORKABLE MODEL OF AN OPERATOR-LESS OPERON. Science. 1964 May 15;144(3620):816–820. doi: 10.1126/science.144.3620.816. [DOI] [PubMed] [Google Scholar]
- STOCKER B. A. Abortive transduction of motility in Salmonella; a nonreplicated gene transmitted through many generations to a single descendant. J Gen Microbiol. 1956 Dec;15(3):575–598. doi: 10.1099/00221287-15-3-575. [DOI] [PubMed] [Google Scholar]



