Abstract
A major goal in vision research over the past few decades has been to understand the molecular details of retinoid processing within the retinoid (visual) cycle. This includes the consequences of side reactions that result from delayed all-trans-retinal clearance and condensation with phospholipids that characterize a variety of serious retinal diseases. Knowledge of the basic retinoid biochemistry involved in these diseases is essential for development of effective therapeutics. Photoisomerization of the 11-cis-retinal chromophore of rhodopsin triggers a complex set of metabolic transformations collectively termed phototransduction that ultimately lead to light perception. Continuity of vision depends on continuous conversion of all-trans-retinal back to the 11-cis-retinal isomer. This process takes place in a series of reactions known as the retinoid cycle, which occur in photoreceptor and RPE cells. All-trans-retinal, the initial substrate of this cycle, is a chemically reactive aldehyde that can form toxic conjugates with proteins and lipids. Therefore, much experimental effort has been devoted to elucidate molecular mechanisms of the retinoid cycle and all-trans-retinal-mediated retinal degeneration, resulting in delineation of many key steps involved in regenerating 11-cis-retinal. Three particularly important reactions are catalyzed by enzymes broadly classified as acyltransferases, short-chain dehydrogenases/reductases and carotenoid/retinoid isomerases/oxygenases.
Keywords: RPE65, retinol dehydrogenase, RDH, visual cycle, retinoid cycle, isomerization, retinol, retinal, retina, retinoid isomerase, retinal degeneration, photoreceptors, lecithin:retinol acyltransferase, chromophore, LRAT
1. Introduction: regeneration of the chromophore: retinoid cycle
The pioneering studies of Boll and Kühne ca. 1877 demonstrated that exposure of frog retinas to light resulted in a series of color changes from purplish-red to yellow and then from yellow to white [1]. This process is known as photochemical bleaching and results from the sequential photoisomerization and hydrolysis of the rhodopsin chromophore [2]. A critical discovery made by Kühne was that the bleached retina could regain its purplish-red hue when repositioned in the back of the eye on top of the monolayer of cells known as the RPE and incubated in the dark [1]. This finding provided the first evidence that the RPE contained enzymatic activities necessary for regeneration of visual chromophore. The principle reactions comprising the retinoid (visual) cycle were delineated by George Wald in the 1940’s [3] but several more decades of research were required before the actual enzymes that catalyze these reactions began to be identified.
Today we know that to sustain vision, all-trans-retinal released from light-activated rhodopsin must be enzymatically isomerized back to the 11-cis isomer. This process occurs by a sequence of reactions catalyzed by membrane-bound enzymes of the retinoid cycle located in rod and cone photoreceptor cell outer segments (OS) and the retinal pigment epithelium (RPE) (Fig 1)[4–7]. The first step in the retinoid cycle involves RDH-catalyzed reduction of all-trans-retinal released from light-activated visual pigments to all-trans-retinol (see also [8]). A portion of the freed all-trans-retinal is released into the disk lumen and must be transferred to the cytosol by the ATP-binding cassette transporter 4 (ABCA4) in order to be reduced (reviewed in [9]).
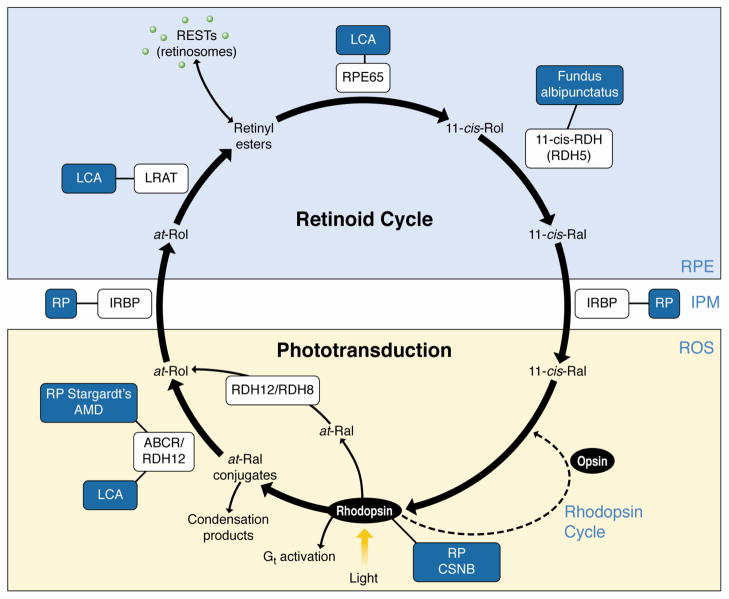
Fig. 1. The retinoid cycle regenerates 11-cis-retinal.
In rod outer segments (ROS), 11-cis-retinal (11-cis-Ral) couples to a protein opsin, forming rhodopsin [2]. Absorption of a photon of light by rhodopsin causes photoisomerization of 11-cis-Ral to all-trans-retinal (at-Ral) leading to its release from the chromophore-binding pocket of opsin. The movement of at-Ral and certain at-Ral conjugates from the intradiscal face to the cytosolic face of disk membranes is accomplished by the ABC transporter ABCR (also known as ABCA4). At-Ral then is reduced to all-trans-retinol (at-Rol) in a reversible reaction catalyzed by an NADPH-dependent all-trans-retinol dehydrogenase (RDH). At-Rol diffuses across the interphotoreceptor matrix (IPM) facilitated by the interphotoreceptor retinoid-binding protein (IRBP) into the retinal pigment epithelium (RPE) where it is esterified in a reaction catalyzed by lecithin:retinol acyltransferase (LRAT). There, all-trans-retinyl esters may be stored in retinyl ester storage particles (RESTs), also known as retinosomes, or may serve as the substrate for RPE65 that converts them to 11-cis-retinol (11-cis-Rol), which is further oxidized back to 11-cis-Ral by RDH5, RDH11 and other RDHs. 11-cis-Ral formed in the RPE diffuses back into the rod and cone outer segments, where it completes the cycle by recombining with opsins to form rhodopsin and cone pigments. Diseases that result from mutations in proteins involved in the retinoid cycle are indicated in blue boxes. AMD – age-related macular degeneration, CSNB – congenital stationary night blindness, LCA – Leber congenital amaurosis, RP – retinitis pigmentosa. Reproduced with permission from Trends in Biochemical Sciences from reference [4]. See reviews [4–7] for more details.
Several critically important reactions take place during this series of metabolic transformations. The first is the lecithin:retinol acyltransferase (LRAT) reaction that catalyzes esterification of retinols by fatty acid moieties in the RPE [10]. This reaction is important both for retaining ocular retinol generated from photoactivated visual pigments in photoreceptors and capturing retinol present in the circulation [11]. This process is facilitated by two retinoid-binding proteins: interphotoreceptor retinoid-binding protein (IRBP), which binds retinoids in the extracellular space, and cellular retinol-binding protein-1 (CRBP1) located within RPE cells [12, 13]. Resulting retinyl esters are substrate for isomerization [14]. Because of the propensity of fatty acid retinyl esters to cluster, these compounds are retained in lipid droplets of the RPE called retinosomes [15–20].
Two important redox reactions take place in photoreceptors and the RPE. Retinal released from visual pigments must be reduced to retinol in the photoreceptor OS, which then diffuses into RPE where it is esterified by LRAT. This reduction is extremely important for the health of the retina, because retinal, which can reach millimolar concentrations following a strong photobleach, is extremely toxic to photoreceptor cells and the RPE [21–27]. A high flux of retinoids through the retinoid cycle, as occurs during intense light exposure, can lead to both elevated levels of toxic retinoid intermediates, especially all-trans-retinal, and photoreceptor degeneration. However, the mechanisms by which all-trans-retinal and/or its condensation products lead to photoreceptor cell death have not been completely elucidated. The second redox reaction, in this case an oxidation, generates 11-cis-retinal from the 11-cis-retinol isomerization product. This reaction occurs in the RPE.
RPE65 catalyzes the formation of 11-cis-retinol from all-trans-retinyl esters [28–30]. This unique biochemical reaction consists of an atypical ester cleavage coupled to a trans-to-cis double bond isomerization. The reaction occurs in the RPE and is thought to be the rate-limiting step of the retinoid cycle. Although, regeneration of rhodopsin requires 11-cis-retinal supplied from the RPE, but cones are not exclusively dependent on RPE65-mediated isomerization [7, 31–36]. Biochemical studies in cone-dominant ground-squirrels and chickens [33], as well as genetic studies in zebrafish (Danio rerio), support the existence of a separate “cone visual cycle” [34, 35]. Confirmation of this alternative visual cycle awaits identification of genes that encode proteins responsible for its key enzymatic steps.
Here we focus on the three key retinoid cycle reactions catalyzed by LRAT, RDHs and RPE65 with an updated analysis of published results.
2. Lecithin:retinol acyltransferase (LRAT) - structure, catalysis, and physiological significance
Retinyl esters are bioactive storage metabolites of vitamin A. Because of their chemical stability and hydrophobicity, these compounds serve as a transport and storage form of vitamin A in vertebrates and therefore play an essential role in maintenance of retinoid homeostasis. Vitamin A esters can be formed in vivo by enzymatic transfer of activated fatty acyl moieties from acyl-CoAs or directly from a phospholipid donor. However, phospholipid-dependent synthesis is quantitatively the most dominant pathway of retinyl ester production [11, 37, 38]. An acyl-CoA-independent retinol esterification enzymatic activity was described for the first time in a microsomal fraction isolated from rat small intestine [39]. Shortly thereafter, LRAT activity was reported to be the main contributor to retinyl ester formation in liver and the RPE, and the fatty acid moiety in the sn-1 position of phosphatidylcholine (PC) was identified as an endogenous acyl source for retinyl ester formation [10, 40]. These initial studies opened the avenue for further detailed biochemical characterization of lipid-dependent acyltransferase enzymatic activity. Particularly the discovery of suicidal inhibitors such as N-Boc-l-biocytinyl-11-aminoundecane chloromethyl ketone permitted specific labeling of the protein and consequently molecular identification of this enzyme and its corresponding gene in 1999 [41].
2.1. Molecular characterization of LRAT
The LRAT gene is located in human chromosome 4 at locus 4q32.1 and encodes a membrane bound protein with a molecular weight of 25.7 kDa. LRAT expression is found in various tissues with largest level in liver, RPE, and small intestine, followed by pancreas, colon, and brain of vertebrates [41]. Based on its amino acid sequence and predicted secondary structure, LRAT is classified as a member of the NlpC/P60 thiol peptidase protein superfamily [42]. The common features shared by members of this class of proteins are three preserved residues involved in the catalytic activity: Cys, His, and a polar amino acid arranged in a similar way with respect to the conserved secondary structural elements. Interestingly, besides LRAT there are two distinct subfamilies that are part of the LRAT family present in vertebrates. These are represented by uncharacterized human neuronal-sensitive proteins (NSE1 – 2) and H-Ras-like tumor suppressor proteins (HRASLS1 – 5) which share a common 5 amino acid stretch that contains a catalytic Cys residue (NCEHFV) [42]. Interestingly, despite a highly conserved catalytic domain, HRASLS proteins display phospholipase A1/2 rather than acyltransferase enzymatic activity [43–45]. The only exception is HRASLS5 which transfers an acyl moiety from PC onto phosphatidylethanolamine (PE) to form N-acylphosphatidylethanolamines that serve as precursors of bioactive N-acylethanolamines, including the endocannabinoid anandamide [46]. Although the structure of LRAT has yet to be determined, recently deposited NMR coordinates for the LRAT-like protein, human HRASLS3, provide important insights into the molecular organization of this group of proteins [47]. The HRASLS3 structure contains a six-stranded anti-parallel β-sheet and three α helices with a β1–β2–β3–β4–α1–β5–α2–β6–α3 sequential connectivity. Consequently, whereas its overall fold is similar to that of other NlpC/P60 peptidases, there are significant topological differences derived from a circular permutation within the catalytic domain of classical NlpC/P60 proteins [42]. Conserved catalytic Cys113 and His23 residues corresponding to Cys161 and His60 in the sequence of human LRAT define the active site of HRASLS3 and are located at the N-terminus of helix α3 and β-sheet β2, respectively (Fig. 2A). Interestingly, the NMR structure of HRASLS3 permitted identification of the third residue (His35) predicted to be involved in the catalysis. Based on this finding, His72 was proposed to complete the catalytic triad in LRAT rather than the previously reported Tyr154 (Tyr105 in HRASLS3). Indeed, Tyr154 is located in a loop between β-sheet 6 and the C-terminal helix about 7.8 Å away from the sulfur atom of catalytic Cys and 9.8 Å from nearest nitrogen atom of the His residue in both the HRASLS3 structure and LRAT homology model [48]. By analogy with thiol proteases, His35 (His72 in LRAT) might play a role in properly orienting His23 (His60 in LRAT) to hydrogen bond with Cys113. This hypothesis is supported by experiments in which the His72Gln mutant of LRAT was more active than wild-type protein [49]. Moreover, replacement of Tyr154 with Ala did not affect retinyl ester formation in a COS-7 cell line overexpressing mouse LRAT (unpublished observation, Moise A. and Golczak M.).
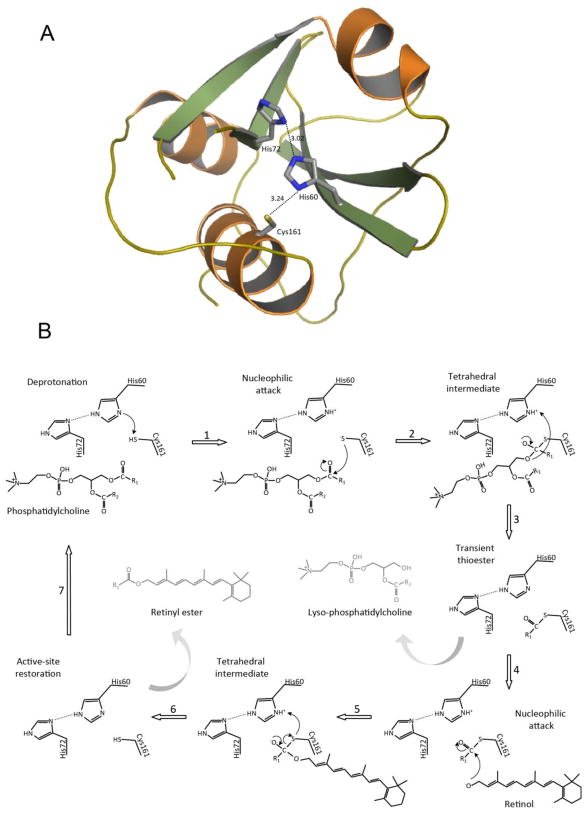
Fig. 2. Structure and catalytic strategy of LRAT.
A. Putative architecture of the LRAT catalytic domain showing positions of key residues in the active site. The overall structure, including a catalytic Cys residue at the amino terminus of a helix packed against a core of β-sheets containing the conserved His residue and its orienting polar partner, is characteristic of NlpC/P60 and structurally related proteases. A homology model of the human LRAT catalytic domain (Tyr42 – Pro173) was generated with the SWISS-MODEL server [181] based on the NMR structure of HRASLS3 (PDB accession code 2KYT). Initial model coordinates were examined with COOT [182] to optimize the stereochemistry and inter-residue contacts. The model was then energy-minimalized by using CHIMERA [183]. B. Schematic representation for the proposed mechanism of LRAT enzymatic activity showing involvement of Cys161, His60, and His72. The sequence of catalytic steps includes deprotonation of a Cys residue, a nucleophilic attack of sulfur on the carboxyl carbon of the ester bond, tetrahedral intermediate formation, and transient protein acylation accompanied by release of Lyso-PC. In the presence of retinol, the thioester is broken by nucleophilic attack of retinol’s activated hydroxyl group causing formation of a tetrahedral intermediate and subsequent acyl transfer onto retinol to form the final retinyl ester product (see text for details).
2.2. Enzymatic steps of LRAT
Because LRAT is presumably structurally similar to thiol proteases, it adapts similar catalytic strategy (Fig. 2B). Thus, in the first step of catalysis, deprotonated Cys161 serving as a nucleophile attacks the carbonyl carbon of an ester bond in a substrate forcing the carbonyl oxygen to accept a pair of electrons and transforming the sp2-hybridized carbon into an sp3-hybridized tetrahedral intermediate. Collapse of this intermediate leads to covalent acylation of the protein in the form of a transient thioester and liberation of lyso-PC. In the case of a hydrolysis reaction, an activated water molecule attacks the carbonyl carbon of the thioester causing formation of another tetrahedral intermediate. Because the hydroxyl is a poorer leaving group than the C-terminal fragment, the tetrahedral intermediate breaks down releasing a free fatty acid. However, the acyltransferase activity of LRAT requires exclusion of water and binding of retinol in LRAT’s active site. Deprotonation of the hydroxyl group of retinol permits decomposition of the thioester intermediate and formation of retinyl ester. Several lines of evidence support the above model of LRAT’s enzymatic catalysis. Initially, evidence that indicated a role for Cys161 and His60 in catalysis was derived from site-directed mutagenesis studies where replacement of these two amino acids abolished retinyl ester formation [49, 50]. Moreover, kinetic studies suggest an ordered sequence of catalytic events whereby phospholipid binding is followed by release of lyso-PC prior to retinol acceptance and final product formation (ping-pong bi-bi mechanism) [51]. However, definitive proof of the proposed mechanism was obtained by trapping the catalytic intermediate in the absence of acyl acceptor and direct detection of covalent thioester protein modification by mass spectrometry [52].
2.3. Membrane topology and access to substrates
Localized in the endoplasmic reticulum, LRAT is an integral membrane protein that assumes a single membrane-spanning topology with a cytoplasmic N-terminus and a luminal C-terminal segment [53]. Interestingly, a C-terminal truncated, soluble form of LRAT retains enzymatic activity but preferentially uses short acyl phospholipids such as 6:0 to 9:0 PCs as substrates with monomeric concentrations in the micromolar range. However, lipid substrates with acyl lengths exceeding 10 carbons that form aggregates in aqueous solution cannot be accessed by the same protein [52]. Thus, the C-terminal membrane-anchoring domain is not strictly required for the enzymatic activity but is indispensible for post-translational targeting of the protein to the membrane in transmembrane domain recognition complex dependent manner and therefore determines lipid substrate specificity of LRAT [54, 55]. Consequently, the most dominant retinyl ester found in vivo is retinyl palmitate (16:0) followed by much smaller amounts of retinyl stearate (18:0) and myristate (14:0) which reflects the cellular fatty acyl composition at the sn-1 position of PC [16]. Under physiological conditions, access to the second substrate, retinol, is facilitated by three cellular-retinol-binding proteins (CRBP-I, II, and III) which are differentially expressed in various tissues. In fact, retinol bound to CRBPs is preferred among the pool of retinoids used for retinyl ester production [39]. The importance of CRBPs is manifested by studies of knockout animal models. Depletion of CRBP-I, found in liver, eye, and kidney, causes lower levels of hepatic retinyl esters and consequently makes knockout mice more vulnerable to vitamin A deficiency if they are maintained on a vitamin A-restricted diet [56]. Moreover, accumulation of all-trans-retinol in neural retina accompanied by a reduced amount of retinyl esters in the RPE suggests that CRBP-I participates in a process that drives diffusion of all-trans-retinol to the RPE and LRAT for esterification [57]. Lack of CRBP-II, which is primarily expressed in intestine, causes a reduction of dietary retinol absorbance and increases neonatal mortality upon vitamin A restriction [58]. Finally, disruption of the Crbp-III gene leads to a decrease in the amount of retinol secreted by mammary tissue into milk [59]. Although the phenotypes described above are not severe, they clearly indicate cooperativity between CRBPs and LRAT in maintaining vitamin A homeostasis.
2.4. The physiological role of LRAT
The physiological role of LRAT has been best studied in knockout animal models. Lrat−/− mice primarily manifest an impaired uptake and accumulation of vitamin A as evidenced by complete absence of retinyl esters in nearly all examined tissues. The only exception is adipocytes that do not require LRAT for retinyl ester formation but instead employ an acyl-CoA-dependent reaction as the dominant mode for retinol esterification [60, 61]. Absence of LRAT impairs three crucial stages of retinoid metabolism, namely absorption of vitamin A from the lumen of the small intestine, its storage in liver stellate cells, and tissue specific vitamin A uptake. In addition to impaired retinoid accumulation, lack of sequestration of retinol in the form of retinyl esters makes Lrat−/− mice more susceptible to retinoic acid toxicity when they are maintained on a vitamin A sufficient diet. This is evidenced by upregulation of the Cyp26 gene in Lrat−/− mice and in model studies on the development of zebra fish embryos [11, 38, 62]. However, the most dramatic phenotype of Lrat−/− mice is manifested in the eye where, in addition to ensuring efficient vitamin A uptake in a STRA6-dependent manner [63], LRAT plays a distinctive role in directly providing substrate for visual chromophore regeneration in a reaction catalyzed by RPE65 [11, 64, 65]. Therefore, complete absence of retinyl esters in eyecups of Lrat−/− mice is accompanied by lack of 11-cis-retinoid production that results in severe attenuation of visual function at a very early age. The ensuing deficiency in rhodopsin regeneration leads to a progressive retinal degeneration manifested by a shortening of rod OS accompanied by disorganization of the synaptic terminals of photoreceptor cells. In conclusion, LRAT is an indispensable component of the vitamin A metabolic pathway that includes the retinoid cycle in the eye. It is also one of only two enzymes (besides RPE65) absolutely required for 11-cis-retinoid production in the RPE.
2.5. LRAT activity is needed to store retinylamine in the eye
Retinylamine, an inhibitor of retinoid isomerase (RPE65) was used to prevent light-induced retinal degeneration [66, 67]. Interestingly, LRAT catalyzes acyl transfer not only to retinoids with a hydroxyl group but also to those with an amino functional group [16]. Consequently, retinylamine is a good substrate for LRAT, which facilitates formation of retinylamides (predominantly retinyl palmitamide). This crucial property of LRAT permits the use of the vitamin A metabolic machinery to absorb, deliver, store, and preserve retinylamine in selected tissues. Similar to retinol, retinylamine is acylated in enterocytes and transported in chylomicrons to the liver where it is stored in the form of amides in hepatic stellate cells. Subsequently after hydrolysis, free retinylamine is secreted into the systemic circulation, either bound to RBP or albumin, where it can be picked up by peripheral tissues including the RPE. This process is driven by re-amidation of retinylamine by LRAT [68]. Newly formed retinylamides are stored in the same lipid droplets (retinosomes) as retinyl esters [16]. Retinylamine is then slowly released over a period of many days to act as a potent inhibitor of RPE65.
3. Retinol dehydrogenases
Two reactions of the retinoid cycle are catalyzed by retinol dehydrogenases (RDHs). Based on biochemical approaches, several RDHs involved in the retinoid cycle have been identified. Reduction of all-trans-retinal to all-trans-retinol in photoreceptors is catalyzed by all-trans-RDHs, whereas oxidation of 11-cis-retinol to 11-cis-retinal in the RPE is catalyzed by 11-cis-RDHs. RDHs belong to the short-chain dehydrogenase/reductase (SDR) family, which catalyzes NAD(H)-/NADP(H)-dependent oxidation/reduction reactions (Fig. 3). The SDR family consists of functionally heterogeneous proteins involved in the metabolism of retinoids, steroids, prostaglandins, aliphatic alcohols and xenobiotics [69]. To date, about 3,000 proteins from various species have been annotated in sequence databases as members of the SDR superfamily based on SDR signature features of the nucleotide-binding motif, TGXXXGXG, and the catalytic tetrad, NSYK, which constitutes the active site [69]. RDH5, RDH8, RDH10, RDH11, RDH12, RDH13, RDH14 and retSDR1 have all been identified by their expression in the retina and RPE (Fig. 4).
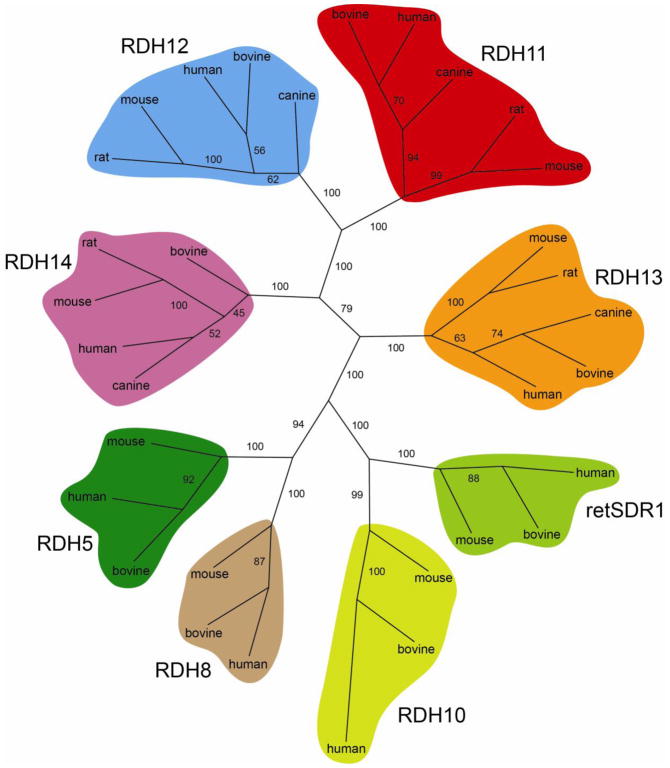
Fig. 3. Molecular phylogenetic tree of vertebrate RDHs.
Protein sequences of vertebrate RDHs from human, bovine and mouse were aligned with Tcoffee [184] and an unrooted phylogenetic tree was created using the Protdist and Neighbor programs from PHYLIP [185]. Bootstrap values from 1000 replicates are displayed on the tree branches as percentages.
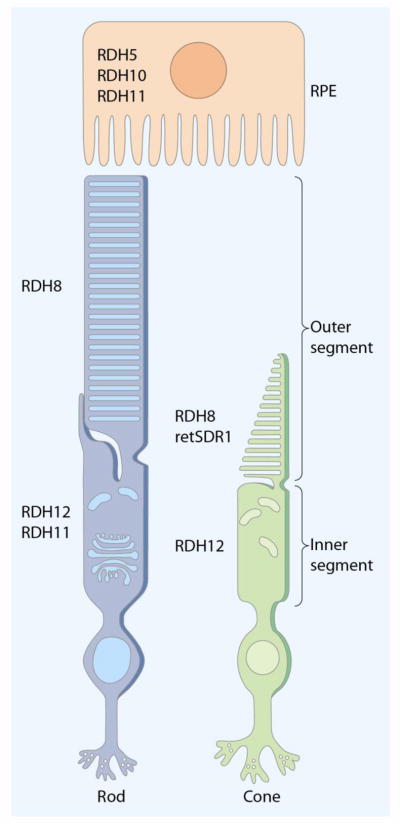
Fig. 4. Distribution of retinoid cycle RDHs in the retina and RPE.
Multiple RDHs contribute to retinoid metabolism in the eye. RDH5, RDH10 and RDH11 express in the RPE are responsible for the reaction from 11-cis-retinol to 11-cis-retinal. RDH8 expression is found in rod and cone photoreceptors, and cone OS express RDH8 and retSDR1. Expression of RDH12 is detected in rod and cone inner segments, and RDH11 is found in rod inner segments. RDHs in the photoreceptors function as all-trans-RDH that catalyzes all-trans-retinal to all-trans-retinol in the retinoid cycle.
3.1. All-trans-retinol dehydrogenases
Reduction and oxidation of retinoids are key reactions of the retinoid cycle. NADPH-dependent reduction of all-trans-retinal in photoreceptor OS is the first step in the regeneration of bleached visual pigments. RDH8 and RDH12 are the major all-trans-RDHs in rod and cone cells, and retSDR1 is a cone-specific all-trans-RDH that catalyzes this reaction [8].
3.1.1. Retinol dehydrogenases: RDH8
RDH8 (also known as photoreceptor RDH, prRDH) was identified in 2000 by Rattner and colleagues [70]. RDH8 showed significant homology and 48% identity with 17-hydroxysteroid dehydrogenase type 1. The human RDH8 gene is located in chromosome 19 at 19p13.2, and RDH8 protein expression is limited to the retina. Immunohistochemistry with anti-RDH8 antibody reveals subcellular localization of RDH8 in the OS of rod and cone photoreceptors. RDH8 demonstrates a substrate and a cofactor preference for all-trans-retinal [70] and NADPH [71]. The main phenotype of Rdh8−/− mice is delayed clearance of all-trans-retinal after bright light illumination [72], which is not accompanied by abnormal Meta-II decay of rhodopsin [72] confirming the role of RDH8 in the retinoid metabolism in the eye.
Although Rdh8−/− mice displayed: (1) accumulation of all-trans-retinal after intense illumination, (2) delayed dark adaptation, and (3) slightly increased accumulation of A2E, a product of all-trans-retinal conjugation with phosphatidylethanolamine, no significant retinal degeneration was observed under room lighting conditions [72]. Abnormal retinal morphology and retinal function recorded by scotopic and photopic electroretinograms (ERG) were not evident in Rdh8−/− mice [72]. These findings implicate the existence of other RDHs with all-trans-RDH activity in the retina.
Notably, Rdh8−/−Abca4−/− mice with a double deletion of RDH8 and ABCA4, a photoreceptor-specific ATP-binding cassette transporter which shuttles all-trans-retinal or its metabolites from the inside to the outside of disc-membranes [9], displayed a cone-rod dystrophy with characteristic features that mimic human age-related macular degeneration (AMD). These included lipofuscin accumulation, photoreceptor/RPE dystrophy, complement deposition at Bruch’s membrane and choroidal neovascularization (CNV) [27]. Even though RDH8 mutations in human retinal diseases have not been reported, a pathogenic role of all-trans-retinal in human retinal diseases must be considered because both RDH8 and ABCA4 proteins are responsible for all-trans-retinal removal from the photoreceptors.
3.1.2. Retinol dehydrogenases: RDH12
Studies of Rdh8−/− mice support an auxiliary role for RDH8 in the retinoid cycle and provide further evidence for an alternative RDH(s) that produces all-trans-retinol in photoreceptors and can compensate for the lack of RDH8. Haeseleer et al. identified RDH12 in photoreceptor cells in 2002 [73]. The RDH12 gene is located in human chromosome 14 at 14q24.1, and its highest expression occurs specifically in the retina. RDH12 expression is also detectable in kidney, pancreas, liver, prostate, testes and brain [74]. This enzyme is a 316 amino acid protein with a calculated molecular mass of 35 kDa, that is localized to the inner segments of rod and cone photoreceptors [75, 76].
RDH12 is an NADPH-dependent retinal reductase with a maximal activity toward 9-cis- and all-trans-retinoids; however, this enzyme can also utilize medium-chain aldehydes as substrates [73]. RDH12 appears to be a key enzyme involved in the visual processes, because disabling mutants of this gene lead to an early-onset rod/cone dystrophy termed Leber congenital amaurosis (LCA) [77, 78]. However, Rdh12−/− mice kept under regular laboratory lighting conditions (~50 lux) failed to display the severe retinal degeneration seen in humans with disabled RDH12 [75, 76]. Even though Rdh12−/− mice manifested a delayed all-trans-retinal clearance and a slow dark adaptation recorded by ERGs, both changes were much smaller than those found in Rdh8−/− mice. A study with Rdh8−/−, Rdh12−/− and Rdh8−/−Rdh12−/− mice revealed that RDH8 was responsible for ~70% of all-trans-RDH activity in the retinoid cycle, whereas RDH12 accounted for ~30% of this activity [79]. Although retinas from Rdh8−/−Rdh12−/− mice had lost ~98% of their all-trans-RDH activity, these mice surprisingly still converted all-trans-retinal to all-trans-retinol in vivo. Indeed Rdh8−/−Rdh12−/− mice showed only mild retinal changes at 6 months of age when kept in a regular laboratory light/dark cyclic environment. Thus, less than 2% of total all-trans-RDH activity in photoreceptors is needed to maintain sufficient retinoid homeostasis in mice under room under such conditions. The discrepancy in retinal phenotypes between humans and mice probably originate from normal differences in retinal regeneration rate. Mice are nocturnal and thus are exposed to lower levels of retinal illumination compared to humans. Consistently, the mouse retina is characterized by a higher rod to cone ratio compared to the human retina [80, 81]. Visual pigment regeneration rate in rods is slower than that in cones [7, 33]. Therefore, retinal phenotype in mice might be much milder than in humans as a result of less rapid retinoid flux through the retinoid cycle.
The primary role of RDH12 is still unclear, because RDH12 contributes less than RDH8 to the reduction of all-trans-retinal, and because RDH12 mutations cause early-onset severe retinal degeneration whereas RDH8 mutations have yet to be found in human retinal diseases. Notably in vitro experiments with COS-7 cells that exhibit human disease mutations showed that decreased RDH activity was associated with greater disease severity [77, 82], and that RDH12 loss-of-function mutations resulted in a characteristic form of early and progressive rod-cone degeneration that differs from that caused by mutations in other LCA genes [83]. Rdh12−/− mice are more susceptible to light-induced retinal degeneration than WT mice, and detoxification of aldehydes produced by light is considered one of the important functions of RDH12 [84].
3.1.3. Retinol dehydrogenases: retSDR1
Haeseleer et al. identified retSDR1 as belonging to the SDR superfamily and localizing predominantly to cone photoreceptors [85] where its expression implies an important role in reducing all-trans-retinal. The human retSDR1 gene is located on chromosome 1 at 1p36.1, and the protein is also expressed in pancreas, liver, kidney and placenta, suggesting that it is involved in retinol metabolism outside of photoreceptors. retSDR1 displayed a substrate specificity for all-trans-retinal but not for 11-cis-retinal. Homology modeling of retSDR1 with the carbonyl reductase structure used as a scaffold predicted a classical Rossmann-fold for nucleotide binding, and an N-terminal extension that could facilitate binding of the enzyme to cell membranes. Neither in vivo cellular/animal studies nor patient-oriented studies of this enzyme have yet been reported.
3.2. 11-cis-Retinol dehydrogenases
11-cis-RDH catalyzes the final oxidation step in the retinoid cycle [4, 6], namely conversion in the RPE of 11-cis-retinol to 11-cis-retinal, the chromophore of mammalian visual pigments. RDH5, RDH10 and RDH11 also have been identified to catalyze this enzymatic reaction in the RPE.
3.2.1. 11-cis-Retinol dehydrogenases: RDH5
In 1995, Simon et al. isolated RDH5 as a 32-kDa membrane-associated protein (p32) consisting of 318 amino acids, that forms a complex with RPE65 [86]. Our recent study demonstrates that RPE65 operates in a multiprotein complex with RDH5 and retinal G protein-coupled receptor (RGR) in RPE microsomes [87]. Based on the experimental data, Cys175 within the RDH5 peptide 167LAANGGGYCVSK178 and Cys231 within the RPE65 sequence 223SEIVVQFPCSDR234 became cross–linked and therefore are likely to exist in close proximity to each other in native RPE membranes. The human RDH5 gene is located on chromosome 12 at 12q13-q14, and is expressed predominantly in the RPE. COS cell-expressed RDH5 has a NADH cofactor specificity [86]. Disabling mutations of its gene cause autosomal recessive fundus albipunctatus, a rare form of human night blindness [88]. Initially, it was thought that fundus albipunctatus was a congenital stationary night blindness characterized by the appearance of numerous small white dots in the RPE together with delayed dark adaptation [89]. However, more recently it was noted that some patients with fundus albipunctatus developed progressive cone dystrophy [90, 91]. Surprisingly, Rdh5−/− mice failed to mimic the retinal phenotype observed in humans with RDH5 mutations. These mice manifested only a delay in dark adaptation along with accumulation of 11-cis- and 13-cis-retinyl esters [92]. Interestingly, a study of RPE from Rdh5−/− mice demonstrated that lack of RDH5 also leads to an accumulation of cis-retinoids, especially 13-cis-retinyl esters. Analysis of Rdh5−/− mice showed that RDH(s) responsible for the production of 11-cis-retinal display NADP-dependent specificity toward 9-cis- and 11-cis-retinal but not 13-cis-retinal. Lack of 13-cis-RDH activity could be a reason why 13-cis-isomers accumulate in the RPE of Rdh5−/− mice [93]. Indeed, Stecher et al and later Redmond et al. demonstrated that RPE65 is not inherently 11-cis-specific and can produce both 11-cis and 13-cis-retinoid isomers [94, 95]. Moreover, the 13-cis-retinoid isomer accumulation can be related to the pathology of fundus albipunctatus [15].
3.2.2. 11-cis-Retinol dehydrogenases: RDH11
RDH5 is responsible for most of the 11-cis-RDH activity in the RPE, but formation of 11-cis-retinal in Rdh5−/− mice suggests that another enzyme(s) is present. Additional members of the RDH family expressed in the eye that catalyze reduction/oxidation reactions were identified by Haseleer et al. together with RDH12 [73]. One of these enzymes, RDH11, encoded by the human RDH11 gene located on chromosome 14 at 14q24.1, was initially designated as prostate SDR 1 (PSDR1) based on its hallmark SDR protein motifs, the cofactor-binding site (TGXXXGXG), catalytic residues (YXXXK), and its high transcript expression level in the human prostate [96]. Subsequently RDH11 was identified as a gene regulated by sterol regulatory element-binding protein (SREBP), a transcription factor that coordinately regulates the expression of enzymes involved in cholesterol and fatty acid synthesis [97]. Expression of RDH11 was detected in the RPE by immunoblot [73] and its expression in photoreceptors was also demonstrated by in situ hybridization [98]. This enzyme lacks reactivity with steroid substrates but reduces other short-chain aldehydes such as 4-hydroxy-2-nonenal [97], whereas other SDR enzymes, e.g. RDH5 and RDH12 also use steroid substrates. RDH11 has NADPH specificity and catalyzes the reduction of retinals ~50-fold more efficiently than the oxidation of retinol in vitro [99]. However, the in vivo activity of RDHs probably depends upon the relative concentration of their substrates in the immediate environment, and several lines of evidence indicate that RDH11 can have 11-cis-RDH activity in the RPE. First, RDH11 is expressed in RPE cells. Second, the remaining enzymatic activity in Rdh5−/− RPE exhibited NADPH cofactor specificity and reduced levels of all-trans-, 9-cis-, and 11-cis-retinal in vitro enzyme assays using RPE protein fractions [93]. Third, this residual 11-cis-RDH activity in the Rdh5−/− RPE was membrane-associated [93], a characteristic consistent with the known subcellular location of RDH11 [100].
To investigate the role of RDH11 in the eye, Rdh11−/− mice were examined. Although these mice failed to show a significant dysfunctional retinal phenotype, 73% more cis-retinyl esters were stored in Rdh5−/−Rdh11−/− mice than in Rdh5−/− mice after light illumination. Single-flash ERGs of Rdh11−/− mice showed normal responses under dark- and light-adapted conditions, but exhibited delayed dark adaptation following high bleaching levels of light. Rdh5−/−Rdh11−/− mice also had normal ERG responses under dark- and light-adapted conditions, but featured an enhanced delay in dark adaptation relative to either Rdh11−/− or Rdh5−/− mice [101]. Taken together, these results suggest that RDH11 has a measurable backup role in regenerating visual pigments by complementing RDH5 as an 11-cis-RDH in RPE cells. They also indicate that an additional unidentified enzyme(s) oxidizes 11-cis-retinol or that an alternative pathway contributes to the retinoid cycle.
3.2.3. 11-cis-Retinol dehydrogenases: RDH10
Full-length RDH10 was cloned from human, cow, and mouse by Wu et al. in 2002 [102]. The human RDH10 gene is located in chromosome 8 at 8q21.11 and encodes a protein of 341 amino acids. Human RDH10 shares 100% and 98.6% amino acid sequence identity with the bovine and mouse proteins, respectively. RDH10 protein is predominantly expressed in the microsomal fraction of the RPE. Human RDH10 expressed in COS cells oxidized all-trans- retinol to all-trans-retinal. RDH10 displayed substrate specificity for all-trans-retinol and preferred NADP as a cofactor. Its expression was also detected in Müller cells [103], kidney, liver, small intestine, placenta, lung, heart, and skeletal muscle, with placenta, kidney and liver exhibiting the strongest expression [104]. RDH10 is essential for synthesis of embryonic retinoic acid and required for limb, craniofacial, and organ development [105]. Its catalytic role in the retinoid cycle was initially considered to involve converting all-trans-retinol to all-trans-retinal in Müller cells, thereby providing a substrate for RGR to produce 11-cis-retinal. In 2009, co-localization of RDH10 with RPE65 and CRALBP in the RPE was reported as was its more robust activity with NAD than NADP [106]. RDH10 may function in the RPE as an 11-cis-RDH, thereby partially compensating for the loss of RDH5 function in patients with fundus albipunctatus.
3.3. Additional retinol dehydrogenases in the eye
Expression of RDH13 and 14 was found in the eye, but a specific role in the retinoid cycle has not been identified.
3.3.1. Retinol dehydrogenases: RDH13
Along with other SDRs, RDH13 was identified in the eye in 2002 and its expression was examined by immunohistochemistry [73, 107]. The human RDH13 gene is found on chromosome 19 at 19q13.42 in humans. Human RDH13 shares 83% protein sequence identity with mouse RDH13 and 72% identity with frog RDH13. Moreover, the corresponding genes have a similar genomic organization, indicating that RDH13 is conserved across species. Antibodies recognizing RDH13 labeled inner segments of both rod and cone photoreceptors in human and monkey retina. RDH13 also exhibits a wide tissue distribution including heart, lung and kidney, but is a mitochondrial rather than a microsomal protein. Kinetic analysis of the purified protein shows that RDH13 is catalytically active with retinoids as substrates. This enzyme exhibits a much lower KM value for NADPH than for NADH [107]. The high degree of conservation, ubiquitous expression pattern and localization of RDH13 at the entrance to the mitochondrial matrix, suggests that this protein may function to protect mitochondria against oxidative stress associated with highly reactive retinals produced from dietary β-carotene and retinoids in the retinoid cycle. However, until recently, no enzymatic activity for RDH13 has been demonstrated in vivo.
3.3.2. Retinol dehydrogenases: RDH14
RDH14 also was discovered in the eye in 2002 along with other SDRs by Haeseleer et al. [73]. The human RDH14 gene is located in chromosome 2 at 2p24.2. mRNA for this dehydrogenase localizes in the photoreceptor nuclear layer, and appears to be expressed at low levels in the eye. Immunolabeling was clearly observed in bovine cone and rod OS with a weaker signal in Müller cells. RDH14 as well as similar SDRs such as RDH11 and RDH12, catalyzes the reaction, NADPH/retinals ↔ NADP/retinols, and employs 11-cis-retinal and all-trans-retinal equally well as substrates when they are present in a reaction mixture at identical concentrations. No steroid dehydrogenase activity has been detected for RDH14 and its role in the retinoid cycle has not been characterized.
4. RPE65: retinoid isomerase
4.1. Brief history of RPE65
RPE65, also known as p63, was identified in the early 1990’s as a major protein of bovine RPE microsomal membranes [108–112]. The function of this protein remained obscure until 1997 when it was shown to bear sequence homology to a newly identified carotenoid cleavage oxygenase involved in abscisic acid biosynthesis known as viviparous 14 (VP14) [113, 114]. In this same year RPE65 mutations were found in patients with LCA, the childhood blinding disease, establishing a critical role for this protein in maintenance of retinal health [115, 116]. The following year, the phenotype of Rpe65−/− mice was described [117]. These mice are highly visually impaired with no detectable ocular 11-cis-retinoids and an over accumulation of all-trans-retinyl esters. Despite these compelling findings, investigators were initially unable to demonstrate retinoid isomerization activity in a defined in vitro system using purified RPE65, which lead to the conclusion that RPE65 is not a retinoid isomerase itself but is a retinyl ester-binding protein that provides substrate to the true isomerase [118–122]. In 2005, three groups showed that expression of RPE65 in cell lines expressing other known components of the retinoid cycle bestowed retinoid isomerase activity upon the cells [28–30]. These findings led to the general acceptance of RPE65 as the physiological isomerase of the retinoid cycle. Recently, assay conditions have been developed under which low-level isomerase activity can be demonstrated from purified RPE65 [123].
4.2. Evolution and genetics
4.2.1. Relationship of RPE65 to the carotenoid oxygenases
On the basis of sequence homology, RPE65 belongs to a family of proteins known as carotenoid cleavage oxygenases (CCOs) [124]. Members of this family share a common core protein fold and possess four absolutely conserved His residues that coordinate an essential iron cofactor. CCOs are present in all kingdoms of life indicating that the ancestral protein appeared early during evolution; however, RPE65 is only found in vertebrates. In most species examined, the RPE65 gene encodes a protein 533 amino acids in length. RPE65 is well conserved at the protein level with sequence identity values of at least ~75% even amongst distantly related vertebrates (e.g. human vs. zebrafish). Besides RPE65, the human genome encodes two other CCO members known as ββ′-carotene monoxygenase I (BCMO I) and ββ′-carotene dioxygenase II (BCDO II). RPE65 is approximately 40% and 43% identical to BCMO I and BCDO II, respectively. The BCMO I and BCDO II protein chains are slightly longer than RPE65 with BCMO I containing an extended C-terminus and BCDO II featuring a mitochondrial-targeting N-terminal signal sequence.
Despite the structural features shared by this family of proteins, the substrate specificity and even the enzymatic activity possessed by individual members is variable [125]. The first member of this family to be cloned, a CCO from Pseudomonas, was found to oxidatively cleave non-carotenoid stilbene compounds at the α,β double bond [126–128]. CCOs found in higher organisms are thought to catalyze the cleavage of various carotenoids under physiological conditions [125]. RPE65 is the only examined member of this family for which oxidative cleavage capacity has never been demonstrated [129]. Interestingly, certain insect CCOs possess both oxidoreductase and isomerase activities [130].
4.2.2. Control of RPE65 expression
In humans, RPE65 is encoded on the short arm of chromosome 1 [131]. The gene consists of 14 exons separated by variable length introns [132]. The RPE65 protein is expressed almost exclusively in the RPE as its name indicates although there have been reports of RPE65 expression in the pineal gland of zebrafish [34] and cone photoreceptor cells of some species [133, 134]. RPE65 protein expression is detectable a few days after birth just before the photoreceptor OS develops [109]. Thereafter, the protein is thought to be expressed at high levels throughout the lifetime of the organism. Environmental cues are important for maintenance of RPE65 expression as it is quite rapidly lost in cultured RPE cells [109]. It is thought that control of RPE65 expression is achieved at both the transcriptional and post-transcriptional levels [109, 135, 136]. The human and mouse RPE65 promoter regions have been characterized in detail and contain conserved octamer, nuclear factor-1, AP-4, and E-box transcription factor consensus sites as well as non-canonical TATA-like box sequences [135, 137]. The octamer and E-box sites appear to confer RPE-specific expression of RPE65 [137]. Post-transcriptional control of RPE65 expression has been attributed to the presence of AU-rich regions in the 3′ UTR of the mRNA that target it for degradation [109, 136].
4.3. RPE65 structure
4.3.1. Chain fold and surface topography
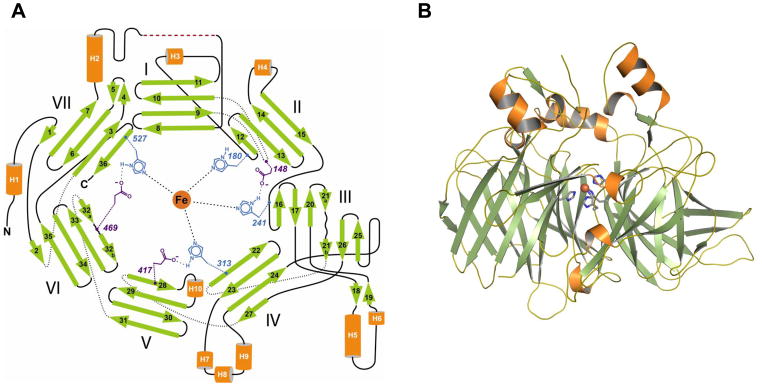
The structure of bovine RPE65 isolated from native microsomal membranes has been determined by x-ray crystallography [138]. The protein adopts a seven-bladed β-propeller structure that is sealed in a “Velcro” fashion within blade VII via interaction between the first and last strands of the core propeller fold (Fig. 5A). In addition to the core propeller, there is an N-terminal extension that provides a β-strand each to blades VI and VII as well as an internal extension that forms a two strand addition to blade III. The top face of the propeller is capped by several extended, α-helical segments that pack together and help form the active site of the enzyme (Fig. 5B). The segments connecting the strands on the bottom face are shorter and only one short α-helix is present.
Fig. 5. RPE65 structure.
A. Topology diagram of the bovine RPE65 structure. B. Cartoon representation of the structure (PDB accession code 3FSN). The structure is oriented with the top face of the propeller facing up. The catalytic iron atom is shown as a sphere bound by the conserved His residues. The iron atom is covered by a helical cap where the active site of the protein is found. The figure in panel A was reproduced and modified with permission from Proceedings of the National Academy of Sciences from ref [138].
There are two major pockets that extend into the protein interior. The first of these pockets enters the protein on the bottom face of the propeller, extends roughly along the propeller axis and terminates just prior to reaching the bound iron atom found near the center of the protein on the propeller axis. This hydrophilic pocket has an approximate volume of 1400 Å2 [139] and is formed as a consequence of the doughnut-like shape of the core propeller structure. The second pocket enters the protein at the junction between the top face of the propeller and the helical cap. This pocket houses the active site of the enzyme and has an approximate volume of 2000 Å2. Analysis of the solvent accessibility of this pocket indicates that it contains only a single mouth suggesting that substrate and product must pass into and out of the protein via the same route.
4.3.2. Active site
The hydrophobic pocket allows direct access to the catalytic iron atom and thus forms the retinoid passageway as well as the enzymatic active site. The lining residues are mainly hydrophobic with a few exceptions such as residues involved in iron coordination. A number of Phe, Tyr and Trp side chains contribute to formation of this pocket and their presence may have implication for the catalytic mechanism of RPE65 as discussed below. Notably, no Cys side chains face the interior of this pocket.
The catalytic iron is coordinated by the epsilon nitrogen atoms of the four conserved His residues 180, 241, 313 and 527 and the iron-His bond lengths are all approximately 2.1 Å. The geometry of coordination is roughly octahedral with two open coordination sides that are presumably used to promote catalysis. Delta nitrogen atoms of His residues 241, 313 and 527 form hydrogen bonding interactions with the carboxylate moieties of Glu residues 148, 417 and 469, respectively, whereas the delta nitrogen atom of His 180 hydrogen bonds with a water molecule found in the large hydrophilic cavity mentioned above. These His and Glu residues in the coordination system are absolutely and highly conserved amongst CCO members, respectively [125].
4.3.3. Mouth of the retinoid passageway
A notable feature of the protein is a cluster of amphipathic structural elements formed by residues 196–202, 234–236 and 261–271 that are situated in the area immediately surrounding the entrance to the active site-containing pocket [138]. This region is enriched in Ser, Trp, Lys and Arg residues that can interact with phospholipid headgroups as well as Phe, Leu and Ile residues that could penetrate the bilayer and interact with phospholipid acyl chains [140]. Residues 109–126 are predicted to form an amphiphilic α-helix and be located nearby the pocket entrance but these residues were unresolved in the crystal structure [87, 110, 138]. We believe that it is this surface of RPE65 that interacts with the lipid bilayer and allows uptake of retinyl esters. The crystal structures of Synechocystis apocarotenoid oxygenase (ACO) and VP14 from Zea mays feature similar amphipathic/hydrophobic patches near the entrances to their active sites suggesting a conserved mechanism for interaction with lipid membranes [141, 142].
4.3.4. “Palmitoylation switch” Cys residues
RPE65 was previously thought to cycle between a soluble and a membrane bound state via reversible palmitoylation of Cys residues 231, 329 and 330 [120]. These residues were all well-resolved in the crystal structure allowing us to assess the structural feasibility of this mechanism [138]. Although the protein used in this crystallographic study was purified from native RPE microsomal membranes, the electron density maps do not indicate the presence of S-palmitoylation at any of the abovementioned Cys residues. Furthermore, the side chains of these Cys residues are not surface exposed indicating that significant conformational changes would be required in order for them to undergo palmitoylation. These results agree with previous biochemical studies that evaluated the palmitoylation status and functional importance of these Cys residues [29, 143, 144].
4.4. RPE65 enzymology
4.4.1. Role of membranes
Rando and colleagues first isolated a membrane fraction from frog RPE that contained isomerase activity in the late 1980’s [145]. However, the exquisite inhibitory effects of detergents on the isomerase prevented molecular identification of the responsible protein(s) [146]. These studies established that the isomerase was a membrane-bound protein. The mechanism of RPE65-membrane association remains controversial with data supporting roles for both hydrophobic and electrostatic forces in mediating this interaction (reviewed in [147]). However, it is now clear that the RPE65-membrane interaction is critical for the enzymatic activity of this protein [87, 123, 148].
Besides the importance of the physical interaction between RPE65 and membranes for isomerization activity, the membrane phospholipids have been hypothesized to indirectly provide energy necessary for endergonic trans-to-cis retinoid isomerization through their use in formation of retinyl esters by LRAT as discussed below [149].
4.4.2. Substrate of the isomerase
Through the use of isotope-labeled retinoids, it was demonstrated that all-trans-retinol or a derivative in the same redox state was the compound subject to enzymatic isomerization rather than all-trans-retinal [150]. The actual substrate of the isomerase remained unclear for many years. Initial observations suggested that RPE membranes could produce 11-cis-retinol from all-trans-retinol but not all-trans-retinyl palmitate, which suggested that retinyl esters were not substrates for the isomerase [145, 146]. Later work employing improved methods for delivering retinyl esters provided compelling evidence that retinyl esters are indeed the natural substrates for the isomerase [14]. However, pulse-chase experiments have established that a large fraction of the retinyl esters in RPE cells are not immediately available to be isomerized [94]. The discovery of retinyl ester storage particles called retinosomes has provided an ultrastructural explanation for this finding [17, 18]. In the studies that established RPE65 as a retinoid isomerase, it was observed that co-expression of LRAT with RPE65 was required in order to observe robust isomerase activity, indicating that in situ generation of retinyl esters is the most effective way of providing substrate to RPE65 [28–30].
4.4.3. Mechanism of retinoid isomerization
Although RPE65 is classified as an isomerase, this protein actually catalyzes a coupled reaction consisting of a double bond geometrical isomerization and an atypical ester hydrolysis (discussed below). Thus, RPE65 is also referred to as an isomerohydrolase. The mechanism of this unusual reaction has been studied quite extensively but is still not fully elucidated. A fundamental requirement for retinoid isomerization to occur is the temporary lowering of the C11-C12 bond order so that low energy rotation to the cis configuration can occur. Additionally, energy input is required because trans-to-cis isomerization is an endergonic process. In principle, the energy released during ester hydrolysis (ΔG ≈ −5 kcal/mol) could be used to drive the uphill isomerization, which has a ΔG value of ~4 kcal/mol [149]. However, the fact that CRALBP, an 11-cis-retinol and 11-cis-retinal-binding protein, is needed for efficient production of 11-cis-retinol in in vitro assays suggests that product release is actually the rate limiting step of the isomerization process [94, 151]. The reaction could thus be driven by a downstream process that provides energy.
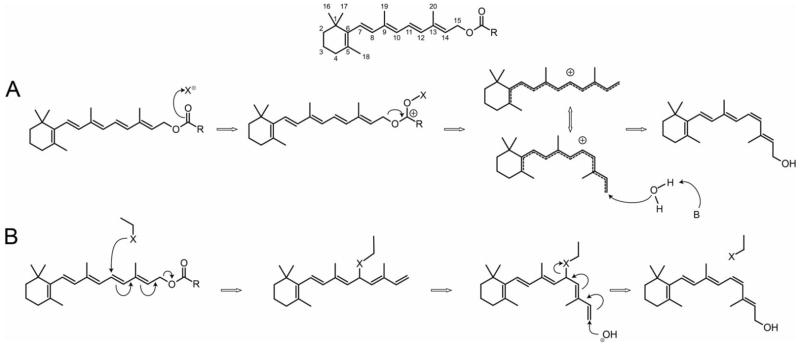
An interesting feature of the coupled reaction is that the oxygen atom bound to carbon C15 of the retinyl moiety of the retinyl ester substrate is lost and replaced with an oxygen atom derived from water in the product [138, 151, 152] (see Fig. 6 for standard retinoid carbon numbering). There are two mechanistically distinct, proposed reaction schemes that account for these observations, both of which feature an intermediate with the required reduced polyene bond order (Fig. 6).
Fig. 6.
Proposed mechanisms of trans-to-cis retinoid isomerization. Standard numbering of the retinoid carbon atoms is shown on top. A. SN1 mechanism with isomerization facilitated by the formation of a carbocation intermediate. The initial O-alkyl cleavage is promoted by interaction of the ester with a Lewis acid (X). B. SN2′ mechanism with isomerization occurring after attack of a nucleophile (X) on C11. In both mechanisms, the conjugated double bond system is restored by nucleophilic attack of water or hydroxide ion on C15. See text for details.
The first of these proposed mechanisms is an SN1 nucleophilic substitution reaction where the ester leaves via O-alkyl cleavage and is replaced by either water or a hydroxide anion [151]. Ester dissociation is thought to be promoted by interaction with a Lewis acid such as a proton or metal ion [66, 151]. The intermediate of the reaction is a resonance-stabilized retinyl carbocation. Theoretical calculations indicate that the energy barrier for C11-C12 bond rotation is reduced from ~36 kcal/mol in the ground state to ~18 kcal/mol in the carbocation intermediate consistent with the experimentally determined activation energy of ~17 kcal/mol for the isomerization reaction [151]. Because the carbocation is delocalized over several carbon atoms, multiple isomers might be expected to be generated during the reaction. Indeed, RPE65 has been shown to enzymatically produce 13-cis-retinol in addition to 11-cis-retinol and various active site mutations can alter the 13-cis/11-cis-retinol ratio [95]. Although one might predict that 9-cis-retinol could also be generated, production of this isomer is not observed. Preferential stabilization of the carbocation on the C11 and C13 atoms may account for these observations. The active site of RPE65 contains a number of aromatic residues that would be well suited to stabilize the carbocation [138]. Saturation of any of the polyene double bonds of retinol prevents the resulting compound from being isomerized indicating the entire conjugated double bond system is involved in the reaction [153]. Such conjugation may be required for the generation of a semi-stable and long-lived carbocation intermediate.
The second mechanism is best characterized as a dual SN2′ nucleophilic substitution reaction [152]. In the first step, an active site nucleophile attacks C11 of the retinoid leading to the expulsion of the ester moiety, again via an O-alkyl cleavage event. This stable, covalent intermediate has a single bond connecting C11 to C12 allowing free rotation to a cis-like state. Following this rotation, a nucleophilic hydroxide ion attacks C15 leading to dissociation of the C11-bound active site moiety and restoration of the conjugated double bond system (Fig. 6).
The former mechanism is favored by our group; however, there are several unexplained features of the isomerization reaction and the RPE65 protein that require further clarification before the enzymatic mechanism can be fully understood.
4.4.4. Role of the RPE65 iron cofactor in retinoid isomerization
RPE65, like its carotenoid cleaving relatives, requires a ferrous iron cofactor for enzymatic activity [154]. This requirement has been shown by a variety of experiments including site-directed mutagenesis of the conserved His and Glu residues involved in iron coordination [29] and exposure of RPE65 to chelating agents followed by reconstitution with various metals [138, 154]. The latter experiment demonstrated that Fe2+ but not Fe3+, Cu2+ or Mg2+ could partially restore the isomerization activity of RPE65. In the true carotenoid cleaving oxygenases the iron cofactor serves to activate molecular oxygen for addition across the double bond that will be oxidatively cleaved [155]. RPE65 is not known to perform such an oxidative cleavage reaction and the retinoid isomerization reaction does not involve a change in redox state of any carbon atom. Therefore, the specific requirement of ferrous iron for the isomerization reaction is rather puzzling. One possibility for the role of iron in retinoid isomerization is that it could serve as a Lewis acid that facilitates ester dissociation in the isomerization reaction either by binding water to facilitate proton transfer to the ester moiety or by directly interacting with the ester oxygen atoms. In the “as isolated” RPE65 crystal structure, the active site contained electron density consistent with the presence of a fatty acid interacting with the iron cofactor [138]. This observation provides support for a Lewis acid role for iron in the reaction, but further enzymatic studies and structures containing bound intact retinyl esters will be required to clarify the role of this metal in retinoid isomerization.
4.4.5. Interaction with other retinoid cycle enzymes
In a number of studies, RPE65 or its fragments have been observed to associate with other proteins that are involved in the retinoid cycle including RDH5, CRALBP and retinal G protein-coupled receptor (RGR) [86, 87, 156, 157]. The RPE65-RDH5 interaction has been observed most often and this complex can be trapped by treating RPE65 microsomal membranes with Cys crosslinking agents [87]. Although RPE65 has now been shown to function independently as a retinoid isomerase, the formation of complexes between retinoid cycle components could promote efficient transfer of retinoids in vivo.
4.5. RPE65 in disease and therapeutics
4.5.1. Diseases caused by RPE65 mutations
A major interest in RPE65 derives from its involvement in severe childhood retinopathies. In 1997, the first reports associating RPE65 mutations with the autosomal recessive, severe childhood retinopathy, LCA, were published [115, 116]. A number of RPE65 mutations have now been shown to cause LCA or a somewhat milder disease known as autosomal recessive retinitis pigmentosa (arRP). LCA and arRP are heterogeneous diseases that can be caused by defects in a variety of proteins [158, 159]. RPE65 mutations account for around 6% to 12% of LCA cases [159]. LCA resulting from loss of RPE65 function is referred to as LCA2.
4.5.2. RPE65 gene therapy
Treatment of human LCA2 via RPE65 gene therapy has been a major success story in the gene therapy field [160–164]. Initial gene therapy studies employing subretinal administration of RPE65-encoding adeno-associated virus (AAV) vectors were conducted in the Briard dog model of LCA2 and resulted in substantial visual function improvement in these animals [165]. Further successful studies in dogs, mice and primates [166–169] paved the way for the first human clinical trials using AAV2-RPE65 for the treatment of LCA in 2007 [170, 171]. These trials have demonstrated modest, but sustained improvements in visual function without evidence of toxicity. Outcomes are expected to improve as vector delivery methods are optimized and as patients with LCA2 are treated early in the disease before extensive photoreceptor degeneration has occurred [163].
4.5.3. Partial inhibition of RPE65 for the treatment of diseases associated with A2E accumulation
Rpe65−/− mice have been observed not to accumulate lipofuscin in the RPE with age like normal mice [172]. Lipofuscin is a heterogeneous, fluorescent material that consists of many types of retinal adducts [173]. A major component of RPE lipofuscin is A2E, which has toxic effects on RPE cells [174, 175]. The decreased production of A2E in Rpe65−/− mice suggested that partial inhibition of RPE65 function might protect against retinal damage in diseases associated with A2E accumulation such as Stargardt disease and age-related macular degeneration (AMD). In pursuit of this goal, an amine derivative of retinol, called all-trans-retinylamine was synthesized on the basis of its structural similarity to the putative carbocation intermediate of the retinoid isomerization reaction and was found to potently inhibit retinoid isomerization in vitro [66]. Furthermore, this compound strongly protected against light-induced retinal degeneration in mice [67]. In addition to retinylamine other compounds that potentially inhibit the visual cycle via RPE65 inhibition have been identified. Long-term treatment of individuals with the acne medication 13-cis-retinoic acid has been observed to cause night blindness [176]. This effect was reported to result from RPE65 inhibition [177] although this mechanism has been questioned [68]. Additionally, non-retinoid isoprenoid derivatives have also been studied as potential partial inhibitors of RPE65 function [178]. Although these compounds were shown to bind to RPE65, their ability to slow the visual cycle in vivo appears to be limited [68, 179]. Thus, retinylamine remains the most potent and efficacious agent for slowing the visual cycle via partial inhibition of RPE65 activity [68].
5. Conclusions
Because the retinoid cycle lies at the heart of vertebrate vision, remarkable progress has been achieved by using genetic animal models and traditional biochemistry with conjunction with structural biology. Two reactions discussed in this review are common to other tissues including acyltranfer and redox reactions of retinol. Even eye specific isomerization reaction found relevance in the context of CCO enzymes. Combined with novel, cutting-edge biophysical techniques developed in vision research to gain knowledge about the structure and function of these enzymes paved the way to understand similar reactions in other biological system. Success in this endeavor is critical to understanding the pathogenesis of retinal diseases that are related to these enzymes and the toxicity of elevated retinoid cycle intermediates in devising therapies for their successful treatment for human blinding diseases (see also [180]).
Acknowledgments
This research was supported in part by grant EY008061, and a core grant P30 EY11373 from the National Institutes of Health, and Foundation Fighting Blindness. We thank Drs. Leslie T. Webster Jr., Thomas Sundermeier and Brian Kevany for valuable comments and Michal Palczewski for advice on construction of phylogenetic trees.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ripps H. The color purple: milestones in photochemistry. FASEB J. 2008;22:4038–4043. doi: 10.1096/fj.08-1202ufm. [DOI] [PubMed] [Google Scholar]
- 2.Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wald G. Molecular basis of visual excitation. Science. 1968;162:230–239. doi: 10.1126/science.162.3850.230. [DOI] [PubMed] [Google Scholar]
- 4.von Lintig J, Kiser PD, Golczak M, Palczewski K. The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem Sci. 2010;35:400–410. doi: 10.1016/j.tibs.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamb TD, Pugh EN., Jr Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang JS, Kefalov VJ. The Cone-specific visual cycle. Prog Retin Eye Res. 2011;30:115–128. doi: 10.1016/j.preteyeres.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker RO, Crouch RK. Retinol dehydrogenases (RDHs) in the visual cycle. Exp Eye Res. 2010;91:788–792. doi: 10.1016/j.exer.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsybovsky Y, Molday RS, Palczewski K. The ATP-binding cassette transporter ABCA4: structural and functional properties and role in retinal disease. Adv Exp Med Biol. 2010;703:105–125. doi: 10.1007/978-1-4419-5635-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saari JC, Bredberg DL. Lecithin:retinol acyltransferase in retinal pigment epithelial microsomes. J Biol Chem. 1989;264:8636–8640. [PubMed] [Google Scholar]
- 11.Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redmond TM, Wiggert B, Robey FA, Nguyen NY, Lewis MS, Lee L, Chader GJ. Isolation and characterization of monkey interphotoreceptor retinoid-binding protein, a unique extracellular matrix component of the retina. Biochemistry. 1985;24:787–793. doi: 10.1021/bi00324a038. [DOI] [PubMed] [Google Scholar]
- 13.Edwards RB, Adler AJ. Exchange of retinol between IRBP and CRBP. Exp Eye Res. 1994;59:161–170. doi: 10.1006/exer.1994.1094. [DOI] [PubMed] [Google Scholar]
- 14.Moiseyev G, Crouch RK, Goletz P, Oatis J, Jr, Redmond TM, Ma JX. Retinyl esters are the substrate for isomerohydrolase. Biochemistry. 2003;42:2229–2238. doi: 10.1021/bi026911y. [DOI] [PubMed] [Google Scholar]
- 15.Maeda A, Maeda T, Imanishi Y, Golczak M, Moise AR, Palczewski K. Aberrant metabolites in mouse models of congenital blinding diseases: formation and storage of retinyl esters. Biochemistry. 2006;45:4210–4219. doi: 10.1021/bi052382x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golczak M, Imanishi Y, Kuksa V, Maeda T, Kubota R, Palczewski K. Lecithin:retinol acyltransferase is responsible for amidation of retinylamine, a potent inhibitor of the retinoid cycle. J Biol Chem. 2005;280:42263–42273. doi: 10.1074/jbc.M509351200. [DOI] [PubMed] [Google Scholar]
- 17.Imanishi Y, Batten ML, Piston DW, Baehr W, Palczewski K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J Cell Biol. 2004;164:373–383. doi: 10.1083/jcb.200311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imanishi Y, Gerke V, Palczewski K. Retinosomes: new insights into intracellular managing of hydrophobic substances in lipid bodies. J Cell Biol. 2004;166:447–453. doi: 10.1083/jcb.200405110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palczewska G, Maeda T, Imanishi Y, Sun W, Chen Y, Williams DR, Piston DW, Maeda A, Palczewski K. Noninvasive multiphoton fluorescence microscopy resolves retinol and retinal condensation products in mouse eyes. Nat Med. 2010;16:1444–1449. doi: 10.1038/nm.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orban T, Palczewska G, Palczewski K. Retinyl ester storage particles (retinosomes) from retinal pigmented epithelium are homologous variants of lipid droplets in other tissues. J Biol Chem. 2010 doi: 10.1074/jbc.M110.195198. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda T, Maeda A, Matosky M, Okano K, Roos S, Tang J, Palczewski K. Evaluation of potential therapies for a mouse model of human age-related macular degeneration caused by delayed all-trans-retinal clearance. Invest Ophthalmol Vis Sci. 2009;50:4917–4925. doi: 10.1167/iovs.09-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda T, Maeda A, Leahy P, Saperstein DA, Palczewski K. Effects of long-term administration of 9-cis-retinyl acetate on visual function in mice. Invest Ophthalmol Vis Sci. 2009;50:322–333. doi: 10.1167/iovs.08-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeda T, Maeda A, Casadesus G, Palczewski K, Margaron P. Evaluation of 9-cis-retinyl acetate therapy in Rpe65−/− mice. Invest Ophthalmol Vis Sci. 2009;50:4368–4378. doi: 10.1167/iovs.09-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maeda T, Cideciyan AV, Maeda A, Golczak M, Aleman TS, Jacobson SG, Palczewski K. Loss of cone photoreceptors caused by chromophore depletion is partially prevented by the artificial chromophore pro-drug, 9-cis-retinyl acetate. Hum Mol Genet. 2009;18:2277–2287. doi: 10.1093/hmg/ddp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda A, Maeda T, Golczak M, Chou S, Desai A, Hoppel CL, Matsuyama S, Palczewski K. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem. 2009;284:15173–15183. doi: 10.1074/jbc.M900322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda A, Golczak M, Maeda T, Palczewski K. Limited roles of Rdh8, Rdh12, and Abca4 in all-trans-retinal clearance in mouse retina. Invest Ophthalmol Vis Sci. 2009;50:5435–5443. doi: 10.1167/iovs.09-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci U S A. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang JS, Kefalov VJ. An alternative pathway mediates the mouse and human cone visual cycle. Curr Biol. 2009;19:1665–1669. doi: 10.1016/j.cub.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang JS, Estevez ME, Cornwall MC, Kefalov VJ. Intra-retinal visual cycle required for rapid and complete cone dark adaptation. Nat Neurosci. 2009;12:295–302. doi: 10.1038/nn.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mata NL, Radu RA, Clemmons RC, Travis GH. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schonthaler HB, Lampert JM, Isken A, Rinner O, Mader A, Gesemann M, Oberhauser V, Golczak M, Biehlmaier O, Palczewski K, Neuhauss SC, von Lintig J. Evidence for RPE65-independent vision in the cone-dominated zebrafish retina. Eur J Neurosci. 2007;26:1940–1949. doi: 10.1111/j.1460-9568.2007.05801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleisch VC, Schonthaler HB, von Lintig J, Neuhauss SC. Subfunctionalization of a retinoid-binding protein provides evidence for two parallel visual cycles in the cone-dominant zebrafish retina. J Neurosci. 2008;28:8208–8216. doi: 10.1523/JNEUROSCI.2367-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones GJ, Crouch RK, Wiggert B, Cornwall MC, Chader GJ. Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc Natl Acad Sci U S A. 1989;86:9606–9610. doi: 10.1073/pnas.86.23.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT) J Biol Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu L, Gudas LJ. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J Biol Chem. 2005;280:40226–40234. doi: 10.1074/jbc.M509643200. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald PN, Ong DE. Evidence for a lecithin-retinol acyltransferase activity in the rat small intestine. J Biol Chem. 1988;263:12478–12482. [PubMed] [Google Scholar]
- 40.MacDonald PN, Ong DE. A lecithin:retinol acyltransferase activity in human and rat liver. Biochem Biophys Res Commun. 1988;156:157–163. doi: 10.1016/s0006-291x(88)80818-0. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz A, Winston A, Lim YH, Gilbert BA, Rando RR, Bok D. Molecular and biochemical characterization of lecithin retinol acyltransferase. J Biol Chem. 1999;274:3834–3841. doi: 10.1074/jbc.274.6.3834. [DOI] [PubMed] [Google Scholar]
- 42.Anantharaman V, Aravind L. Evolutionary history, structural features and biochemical diversity of the NlpC/P60 superfamily of enzymes. Genome Biol. 2003;4:R11. doi: 10.1186/gb-2003-4-2-r11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uyama T, Morishita J, Jin XH, Okamoto Y, Tsuboi K, Ueda N. The tumor suppressor gene H-Rev107 functions as a novel Ca2+-independent cytosolic phospholipase A1/2 of the thiol hydrolase type. J Lipid Res. 2009;50:685–693. doi: 10.1194/jlr.M800453-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han BG, Cho JW, Cho YD, Kim SY, Yoon HJ, Song HK, Cheong HK, Jeon YH, Lee DK, Lee S, Lee BI. Expression, purification and biochemical characterization of the N-terminal regions of human TIG3 and HRASLS3 proteins. Protein Expr Purif. 2010;71:103–107. doi: 10.1016/j.pep.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 45.Duncan RE, Sarkadi-Nagy E, Jaworski K, Ahmadian M, Sul HS. Identification and functional characterization of adipose-specific phospholipase A2 (AdPLA) J Biol Chem. 2008;283:25428–25436. doi: 10.1074/jbc.M804146200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin XH, Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Discovery and characterization of a Ca2+-independent phosphatidylethanolamine N-acyltransferase generating the anandamide precursor and its congeners. J Biol Chem. 2007;282:3614–3623. doi: 10.1074/jbc.M606369200. [DOI] [PubMed] [Google Scholar]
- 47.Ren X, Lin J, Jin C, Xia B. 1H, 13C and 15N resonance assignments of human H-REV107 N-terminal domain. Biomol NMR Assign. 2010;4:175–178. doi: 10.1007/s12104-010-9238-5. [DOI] [PubMed] [Google Scholar]
- 48.Xue L, Rando RR. Roles of cysteine 161 and tyrosine 154 in the lecithin-retinol acyltransferase mechanism. Biochemistry. 2004;43:6120–6126. doi: 10.1021/bi049556f. [DOI] [PubMed] [Google Scholar]
- 49.Mondal MS, Ruiz A, Hu J, Bok D, Rando RR. Two histidine residues are essential for catalysis by lecithin retinol acyl transferase. FEBS Lett. 2001;489:14–18. doi: 10.1016/s0014-5793(00)02428-5. [DOI] [PubMed] [Google Scholar]
- 50.Mondal MS, Ruiz A, Bok D, Rando RR. Lecithin retinol acyltransferase contains cysteine residues essential for catalysis. Biochemistry. 2000;39:5215–5220. doi: 10.1021/bi9929554. [DOI] [PubMed] [Google Scholar]
- 51.Shi YQ, Hubacek I, Rando RR. Kinetic mechanism of lecithin retinol acyl transferase. Biochemistry. 1993;32:1257–1263. doi: 10.1021/bi00056a009. [DOI] [PubMed] [Google Scholar]
- 52.Golczak M, Palczewski K. An acyl-covalent enzyme intermediate of lecithin:retinol acyltransferase. J Biol Chem. 2010;285:29217–29222. doi: 10.1074/jbc.M110.152314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moise AR, Golczak M, Imanishi Y, Palczewski K. Topology and membrane association of lecithin: retinol acyltransferase. J Biol Chem. 2007;282:2081–2090. doi: 10.1074/jbc.M608315200. [DOI] [PubMed] [Google Scholar]
- 54.Mateja A, Szlachcic A, Downing ME, Dobosz M, Mariappan M, Hegde RS, Keenan RJ. The structural basis of tail-anchored membrane protein recognition by Get3. Nature. 2009;461:361–366. doi: 10.1038/nature08319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stefanovic S, Hegde RS. Identification of a targeting factor for posttranslational membrane protein insertion into the ER. Cell. 2007;128:1147–1159. doi: 10.1016/j.cell.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 56.Ghyselinck NB, Bavik C, Sapin V, Mark M, Bonnier D, Hindelang C, Dierich A, Nilsson CB, Hakansson H, Sauvant P, Azais-Braesco V, Frasson M, Picaud S, Chambon P. Cellular retinol-binding protein I is essential for vitamin A homeostasis. EMBO J. 1999;18:4903–4914. doi: 10.1093/emboj/18.18.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saari JC, Nawrot M, Garwin GG, Kennedy MJ, Hurley JB, Ghyselinck NB, Chambon P. Analysis of the visual cycle in cellular retinol-binding protein type I (CRBPI) knockout mice. Invest Ophthalmol Vis Sci. 2002;43:1730–1735. [PubMed] [Google Scholar]
- 58.Zhang XEL, Lu J, Tso P, Blaner WS, Levin MS, Li E. Increased neonatal mortality in mice lacking cellular retinol-binding protein II. J Biol Chem. 2002;277:36617–36623. doi: 10.1074/jbc.M205519200. [DOI] [PubMed] [Google Scholar]
- 59.Piantedosi R, Ghyselinck N, Blaner WS, Vogel S. Cellular retinol-binding protein type III is needed for retinoid incorporation into milk. J Biol Chem. 2005;280:24286–24292. doi: 10.1074/jbc.M503906200. [DOI] [PubMed] [Google Scholar]
- 60.Yen CL, Monetti M, Burri BJ, Farese RV., Jr The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. J Lipid Res. 2005;46:1502–1511. doi: 10.1194/jlr.M500036-JLR200. [DOI] [PubMed] [Google Scholar]
- 61.Orland MD, Anwar K, Cromley D, Chu CH, Chen L, Billheimer JT, Hussain MM, Cheng D. Acyl coenzyme A dependent retinol esterification by acyl coenzyme A: diacylglycerol acyltransferase 1. Biochim Biophys Acta. 2005;1737:76–82. doi: 10.1016/j.bbalip.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Isken A, Holzschuh J, Lampert JM, Fischer L, Oberhauser V, Palczewski K, von Lintig J. Sequestration of retinyl esters is essential for retinoid signaling in the zebrafish embryo. J Biol Chem. 2007;282:1144–1151. doi: 10.1074/jbc.M609109200. [DOI] [PubMed] [Google Scholar]
- 63.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 64.Gollapalli DR, Rando RR. All-trans-retinyl esters are the substrates for isomerization in the vertebrate visual cycle. Biochemistry. 2003;42:5809–5818. doi: 10.1021/bi0341004. [DOI] [PubMed] [Google Scholar]
- 65.Isken A, Golczak M, Oberhauser V, Hunzelmann S, Driever W, Imanishi Y, Palczewski K, von Lintig J. RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell Metab. 2008;7:258–268. doi: 10.1016/j.cmet.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Golczak M, Kuksa V, Maeda T, Moise AR, Palczewski K. Positively charged retinoids are potent and selective inhibitors of the trans-cis isomerization in the retinoid (visual) cycle. Proc Natl Acad Sci U S A. 2005;102:8162–8167. doi: 10.1073/pnas.0503318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maeda A, Maeda T, Golczak M, Imanishi Y, Leahy P, Kubota R, Palczewski K. Effects of potent inhibitors of the retinoid cycle on visual function and photoreceptor protection from light damage in mice. Mol Pharmacol. 2006;70:1220–1229. doi: 10.1124/mol.106.026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Golczak M, Maeda A, Bereta G, Maeda T, Kiser PD, Hunzelmann S, von Lintig J, Blaner WS, Palczewski K. Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J Biol Chem. 2008;283:9543–9554. doi: 10.1074/jbc.M708982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oppermann U, Filling C, Hult M, Shafqat N, Wu X, Lindh M, Shafqat J, Nordling E, Kallberg Y, Persson B, Jornvall H. Short-chain dehydrogenases/reductases (SDR): the 2002 update. Chem Biol Interact. 2003;143–144:247–253. doi: 10.1016/s0009-2797(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 70.Rattner A, Smallwood PM, Nathans J. Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J Biol Chem. 2000;275:11034–11043. doi: 10.1074/jbc.275.15.11034. [DOI] [PubMed] [Google Scholar]
- 71.Palczewski K, Jager S, Buczylko J, Crouch RK, Bredberg DL, Hofmann KP, Asson-Batres MA, Saari JC. Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction. Biochemistry. 1994;33:13741–13750. doi: 10.1021/bi00250a027. [DOI] [PubMed] [Google Scholar]
- 72.Maeda A, Maeda T, Imanishi Y, Kuksa V, Alekseev A, Bronson JD, Zhang H, Zhu L, Sun W, Saperstein DA, Rieke F, Baehr W, Palczewski K. Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J Biol Chem. 2005;280:18822–18832. doi: 10.1074/jbc.M501757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haeseleer F, Jang GF, Imanishi Y, Driessen CA, Matsumura M, Nelson PS, Palczewski K. Dual-substrate specificity short chain retinol dehydrogenases from the vertebrate retina. J Biol Chem. 2002;277:45537–45546. doi: 10.1074/jbc.M208882200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Belyaeva OV, Korkina OV, Stetsenko AV, Kim T, Nelson PS, Kedishvili NY. Biochemical properties of purified human retinol dehydrogenase 12 (RDH12): catalytic efficiency toward retinoids and C9 aldehydes and effects of cellular retinol-binding protein type I (CRBPI) and cellular retinaldehyde-binding protein (CRALBP) on the oxidation and reduction of retinoids. Biochemistry. 2005;44:7035–7047. doi: 10.1021/bi050226k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maeda A, Maeda T, Imanishi Y, Sun W, Jastrzebska B, Hatala DA, Winkens HJ, Hofmann KP, Janssen JJ, Baehr W, Driessen CA, Palczewski K. Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. J Biol Chem. 2006;281:37697–37704. doi: 10.1074/jbc.M608375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kurth I, Thompson DA, Ruther K, Feathers KL, Chrispell JD, Schroth J, McHenry CL, Schweizer M, Skosyrski S, Gal A, Hubner CA. Targeted disruption of the murine retinal dehydrogenase gene Rdh12 does not limit visual cycle function. Mol Cell Biol. 2007;27:1370–1379. doi: 10.1128/MCB.01486-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Janecke AR, Thompson DA, Utermann G, Becker C, Hubner CA, Schmid E, McHenry CL, Nair AR, Ruschendorf F, Heckenlively J, Wissinger B, Nurnberg P, Gal A. Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy. Nat Genet. 2004;36:850–854. doi: 10.1038/ng1394. [DOI] [PubMed] [Google Scholar]
- 78.Perrault I, Hanein S, Gerber S, Barbet F, Ducroq D, Dollfus H, Hamel C, Dufier JL, Munnich A, Kaplan J, Rozet JM. Retinal dehydrogenase 12 (RDH12) mutations in leber congenital amaurosis. Am J Hum Genet. 2004;75:639–646. doi: 10.1086/424889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maeda A, Maeda T, Sun W, Zhang H, Baehr W, Palczewski K. Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc Natl Acad Sci U S A. 2007;104:19565–19570. doi: 10.1073/pnas.0707477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiao M, Hendrickson A. Spatial and temporal expression of short, long/medium, or both opsins in human fetal cones. J Comp Neurol. 2000;425:545–559. [PubMed] [Google Scholar]
- 82.Sun W, Gerth C, Maeda A, Lodowski DT, Van Der Kraak L, Saperstein DA, Heon E, Palczewski K. Novel RDH12 mutations associated with Leber congenital amaurosis and cone-rod dystrophy: biochemical and clinical evaluations. Vision Res. 2007;47:2055–2066. doi: 10.1016/j.visres.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schuster A, Janecke AR, Wilke R, Schmid E, Thompson DA, Utermann G, Wissinger B, Zrenner E, Gal A. The phenotype of early-onset retinal degeneration in persons with RDH12 mutations. Invest Ophthalmol Vis Sci. 2007;48:1824–1831. doi: 10.1167/iovs.06-0628. [DOI] [PubMed] [Google Scholar]
- 84.Marchette LD, Thompson DA, Kravtsova M, Ngansop TN, Mandal MN, Kasus-Jacobi A. Retinol dehydrogenase 12 detoxifies 4-hydroxynonenal in photoreceptor cells. Free Radic Biol Med. 2010;48:16–25. doi: 10.1016/j.freeradbiomed.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haeseleer F, Huang J, Lebioda L, Saari JC, Palczewski K. Molecular characterization of a novel short-chain dehydrogenase/reductase that reduces all-trans-retinal. J Biol Chem. 1998;273:21790–21799. doi: 10.1074/jbc.273.34.21790. [DOI] [PubMed] [Google Scholar]
- 86.Simon A, Hellman U, Wernstedt C, Eriksson U. The retinal pigment epithelial-specific 11-cis retinol dehydrogenase belongs to the family of short chain alcohol dehydrogenases. J Biol Chem. 1995;270:1107–1112. [PubMed] [Google Scholar]
- 87.Golczak M, Kiser PD, Lodowski DT, Maeda A, Palczewski K. Importance of membrane structural integrity for RPE65 retinoid isomerization activity. J Biol Chem. 2010;285:9667–9682. doi: 10.1074/jbc.M109.063941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamamoto H, Simon A, Eriksson U, Harris E, Berson EL, Dryja TP. Mutations in the gene encoding 11-cis retinol dehydrogenase cause delayed dark adaptation and fundus albipunctatus. Nat Genet. 1999;22:188–191. doi: 10.1038/9707. [DOI] [PubMed] [Google Scholar]
- 89.Marmor MF. Fundus albipunctatus: a clinical study of the fundus lesions, the physiologic deficit, and the vitamin A metabolism. Doc Ophthalmol. 1977;43:277–302. doi: 10.1007/BF01569200. [DOI] [PubMed] [Google Scholar]
- 90.Nakamura M, Hotta Y, Tanikawa A, Terasaki H, Miyake Y. A high association with cone dystrophy in Fundus albipunctatus caused by mutations of the RDH5 gene. Invest Ophthalmol Vis Sci. 2000;41:3925–3932. [PubMed] [Google Scholar]
- 91.Cideciyan AV, Haeseleer F, Fariss RN, Aleman TS, Jang GF, Verlinde CL, Marmor MF, Jacobson SG, Palczewski K. Rod and cone visual cycle consequences of a null mutation in the 11-cis-retinol dehydrogenase gene in man. Vis Neurosci. 2000;17:667–678. doi: 10.1017/s0952523800175029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Driessen CA, Winkens HJ, Hoffmann K, Kuhlmann LD, Janssen BP, Van Vugt AH, Van Hooser JP, Wieringa BE, Deutman AF, Palczewski K, Ruether K, Janssen JJ. Disruption of the 11-cis-retinol dehydrogenase gene leads to accumulation of cis-retinols and cis-retinyl esters. Mol Cell Biol. 2000;20:4275–4287. doi: 10.1128/mcb.20.12.4275-4287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jang GF, Van Hooser JP, Kuksa V, McBee JK, He YG, Janssen JJ, Driessen CA, Palczewski K. Characterization of a dehydrogenase activity responsible for oxidation of 11-cis-retinol in the retinal pigment epithelium of mice with a disrupted RDH5 gene. A model for the human hereditary disease fundus albipunctatus. J Biol Chem. 2001;276:32456–32465. doi: 10.1074/jbc.M104949200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stecher H, Gelb MH, Saari JC, Palczewski K. Preferential release of 11-cis-retinol from retinal pigment epithelial cells in the presence of cellular retinaldehyde-binding protein. J Biol Chem. 1999;274:8577–8585. doi: 10.1074/jbc.274.13.8577. [DOI] [PubMed] [Google Scholar]
- 95.Redmond TM, Poliakov E, Kuo S, Chander P, Gentleman S. RPE65, visual cycle retinol isomerase, is not inherently 11-cis-specific: support for a carbocation mechanism of retinol isomerization. J Biol Chem. 2010;285:1919–1927. doi: 10.1074/jbc.M109.027458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin B, White JT, Ferguson C, Wang S, Vessella R, Bumgarner R, True LD, Hood L, Nelson PS. Prostate short-chain dehydrogenase reductase 1 (PSDR1): a new member of the short-chain steroid dehydrogenase/reductase family highly expressed in normal and neoplastic prostate epithelium. Cancer Res. 2001;61:1611–1618. [PubMed] [Google Scholar]
- 97.Kasus-Jacobi A, Ou J, Bashmakov YK, Shelton JM, Richardson JA, Goldstein JL, Brown MS. Characterization of mouse short-chain aldehyde reductase (SCALD), an enzyme regulated by sterol regulatory element-binding proteins. J Biol Chem. 2003;278:32380–32389. doi: 10.1074/jbc.M304969200. [DOI] [PubMed] [Google Scholar]
- 98.Kasus-Jacobi A, Ou J, Birch DG, Locke KG, Shelton JM, Richardson JA, Murphy AJ, Valenzuela DM, Yancopoulos GD, Edwards AO. Functional characterization of mouse RDH11 as a retinol dehydrogenase involved in dark adaptation in vivo. J Biol Chem. 2005;280:20413–20420. doi: 10.1074/jbc.M413789200. [DOI] [PubMed] [Google Scholar]
- 99.Kedishvili NY, Chumakova OV, Chetyrkin SV, Belyaeva OV, Lapshina EA, Lin DW, Matsumura M, Nelson PS. Evidence that the human gene for prostate short-chain dehydrogenase/reductase (PSDR1) encodes a novel retinal reductase (RalR1) J Biol Chem. 2002;277:28909–28915. doi: 10.1074/jbc.M202588200. [DOI] [PubMed] [Google Scholar]
- 100.Belyaeva OV, Stetsenko AV, Nelson P, Kedishvili NY. Properties of short-chain dehydrogenase/reductase RalR1: characterization of purified enzyme, its orientation in the microsomal membrane, and distribution in human tissues and cell lines. Biochemistry. 2003;42:14838–14845. doi: 10.1021/bi035288u. [DOI] [PubMed] [Google Scholar]
- 101.Kim TS, Maeda A, Maeda T, Heinlein C, Kedishvili N, Palczewski K, Nelson PS. Delayed dark adaptation in 11-cis-retinol dehydrogenase-deficient mice: a role of RDH11 in visual processes in vivo. J Biol Chem. 2005;280:8694–8704. doi: 10.1074/jbc.M413172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu BX, Chen Y, Fan J, Rohrer B, Crouch RK, Ma JX. Cloning and characterization of a novel all-trans retinol short-chain dehydrogenase/reductase from the RPE. Invest Ophthalmol Vis Sci. 2002;43:3365–3372. [PubMed] [Google Scholar]
- 103.Wu BX, Moiseyev G, Chen Y, Rohrer B, Crouch RK, Ma JX. Identification of RDH10, an All-trans Retinol Dehydrogenase, in Retinal Muller Cells. Invest Ophthalmol Vis Sci. 2004;45:3857–3862. doi: 10.1167/iovs.03-1302. [DOI] [PubMed] [Google Scholar]
- 104.Picozzi P, Marozzi A, Fornasari D, Benfante R, Barisani D, Meneveri R, Ginelli E. Genomic organization and transcription of the human retinol dehydrogenase 10 (RDH10) gene. FEBS Lett. 2003;554:59–66. doi: 10.1016/s0014-5793(03)01089-5. [DOI] [PubMed] [Google Scholar]
- 105.Sandell LL, Sanderson BW, Moiseyev G, Johnson T, Mushegian A, Young K, Rey JP, Ma JX, Staehling-Hampton K, Trainor PA. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007;21:1113–1124. doi: 10.1101/gad.1533407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Farjo KM, Moiseyev G, Takahashi Y, Crouch RK, Ma JX. The 11-cis-retinol dehydrogenase activity of RDH10 and its interaction with visual cycle proteins. Invest Ophthalmol Vis Sci. 2009;50:5089–5097. doi: 10.1167/iovs.09-3797. [DOI] [PubMed] [Google Scholar]
- 107.Belyaeva OV, Korkina OV, Stetsenko AV, Kedishvili NY. Human retinol dehydrogenase 13 (RDH13) is a mitochondrial short-chain dehydrogenase/reductase with a retinaldehyde reductase activity. FEBS J. 2008;275:138–147. doi: 10.1111/j.1742-4658.2007.06184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sagara H, Hirosawa K. Monoclonal antibodies which recognize endoplasmic reticulum in the retinal pigment epithelium. Exp Eye Res. 1991;53:765–771. doi: 10.1016/0014-4835(91)90112-r. [DOI] [PubMed] [Google Scholar]
- 109.Hamel CP, Tsilou E, Harris E, Pfeffer BA, Hooks JJ, Detrick B, Redmond TM. A developmentally regulated microsomal protein specific for the pigment epithelium of the vertebrate retina. J Neurosci Res. 1993;34:414–425. doi: 10.1002/jnr.490340406. [DOI] [PubMed] [Google Scholar]
- 110.Hamel CP, Tsilou E, Pfeffer BA, Hooks JJ, Detrick B, Redmond TM. Molecular cloning and expression of RPE65, a novel retinal pigment epithelium-specific microsomal protein that is post-transcriptionally regulated in vitro. J Biol Chem. 1993;268:15751–15757. [PubMed] [Google Scholar]
- 111.Bavik CO, Eriksson U, Allen RA, Peterson PA. Identification and partial characterization of a retinal pigment epithelial membrane receptor for plasma retinol-binding protein. J Biol Chem. 1991;266:14978–14985. [PubMed] [Google Scholar]
- 112.Bavik CO, Levy F, Hellman U, Wernstedt C, Eriksson U. The retinal pigment epithelial membrane receptor for plasma retinol-binding protein. Isolation and cDNA cloning of the 63-kDa protein. J Biol Chem. 1993;268:20540–20546. [PubMed] [Google Scholar]
- 113.Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR. Specific oxidative cleavage of carotenoids by VP14 of maize. Science. 1997;276:1872–1874. doi: 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]
- 114.Tan BC, Schwartz SH, Zeevaart JA, McCarty DR. Genetic control of abscisic acid biosynthesis in maize. Proc Natl Acad Sci U S A. 1997;94:12235–12240. doi: 10.1073/pnas.94.22.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, Gal A. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 116.Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, Claustres M, Hamel CP. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet. 1997;17:139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- 117.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 118.Choo DW, Cheung E, Rando RR. Lack of effect of RPE65 removal on the enzymatic processing of all-trans-retinol into 11-cis-retinol in vitro. FEBS Lett. 1998;440:195–198. doi: 10.1016/s0014-5793(98)01459-8. [DOI] [PubMed] [Google Scholar]
- 119.Gollapalli DR, Maiti P, Rando RR. RPE65 operates in the vertebrate visual cycle by stereospecifically binding all-trans-retinyl esters. Biochemistry. 2003;42:11824–11830. doi: 10.1021/bi035227w. [DOI] [PubMed] [Google Scholar]
- 120.Xue L, Gollapalli DR, Maiti P, Jahng WJ, Rando RR. A palmitoylation switch mechanism in the regulation of the visual cycle. Cell. 2004;117:761–771. doi: 10.1016/j.cell.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 121.Maiti P, Gollapalli D, Rando RR. Specificity of binding of all-trans-retinyl ester to RPE65. Biochemistry. 2005;44:14463–14469. doi: 10.1021/bi0510779. [DOI] [PubMed] [Google Scholar]
- 122.Mata NL, Moghrabi WN, Lee JS, Bui TV, Radu RA, Horwitz J, Travis GH. Rpe65 is a retinyl ester binding protein that presents insoluble substrate to the isomerase in retinal pigment epithelial cells. J Biol Chem. 2004;279:635–643. doi: 10.1074/jbc.M310042200. [DOI] [PubMed] [Google Scholar]
- 123.Nikolaeva O, Takahashi Y, Moiseyev G, Ma JX. Purified RPE65 shows isomerohydrolase activity after reassociation with a phospholipid membrane. FEBS J. 2009;276:3020–3030. doi: 10.1111/j.1742-4658.2009.07021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Giuliano G, Al-Babili S, von Lintig J. Carotenoid oxygenases: cleave it or leave it. Trends Plant Sci. 2003;8:145–149. doi: 10.1016/S1360-1385(03)00053-0. [DOI] [PubMed] [Google Scholar]
- 125.Kloer DP, Schulz GE. Structural and biological aspects of carotenoid cleavage. Cell Mol Life Sci. 2006;63:2291–2303. doi: 10.1007/s00018-006-6176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kamoda S, Habu N, Samejima M, Yoshimoto T. Purification and Some Properties of Lignostilbene-Alpha-Beta-Dioxygenase Responsible for the C-Alpha-C-Beta Cleavage of a Diarylpropane Type Lignin Model-Compound from Pseudomonas Sp Tmy1009. Agric Biol Chem. 1989;53:2757–2761. [Google Scholar]
- 127.Kamoda S, Saburi Y. Cloning, Expression, and Sequence-Analysis of a Lignostilbene-Alpha,Beta-Dioxygenase Gene from Pseudomonas-Paucimobilis Tmy1009. Biosci Biotechnol Biochem. 1993;57:926–930. doi: 10.1271/bbb.57.926. [DOI] [PubMed] [Google Scholar]
- 128.Kamoda S, Samejima M. Cloning of a Lignostilbene-Alpha,Beta-Dioxygenase Gene from Pseudomonas-Paucimobilis Tmy1009. Agric Biol Chem. 1991;55:1411–1412. [PubMed] [Google Scholar]
- 129.von Lintig J, Vogt K. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem. 2000;275:11915–11920. doi: 10.1074/jbc.275.16.11915. [DOI] [PubMed] [Google Scholar]
- 130.Oberhauser V, Voolstra O, Bangert A, von Lintig J, Vogt K. NinaB combines carotenoid oxygenase and retinoid isomerase activity in a single polypeptide. Proc Natl Acad Sci U S A. 2008;105:19000–19005. doi: 10.1073/pnas.0807805105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hamel CP, Jenkins NA, Gilbert DJ, Copeland NG, Redmond TM. The gene for the retinal pigment epithelium-specific protein RPE65 is localized to human 1p31 and mouse 3. Genomics. 1994;20:509–512. doi: 10.1006/geno.1994.1212. [DOI] [PubMed] [Google Scholar]
- 132.Nicoletti A, Wong DJ, Kawase K, Gibson LH, Yang-Feng TL, Richards JE, Thompson DA. Molecular characterization of the human gene encoding an abundant 61 kDa protein specific to the retinal pigment epithelium. Hum Mol Genet. 1995;4:641–649. doi: 10.1093/hmg/4.4.641. [DOI] [PubMed] [Google Scholar]
- 133.Ma J, Xu L, Othersen DK, Redmond TM, Crouch RK. Cloning and localization of RPE65 mRNA in salamander cone photoreceptor cells1. Biochim Biophys Acta. 1998;1443:255–261. doi: 10.1016/s0167-4781(98)00221-8. [DOI] [PubMed] [Google Scholar]
- 134.Znoiko SL, Crouch RK, Moiseyev G, Ma JX. Identification of the RPE65 protein in mammalian cone photoreceptors. Invest Ophthalmol Vis Sci. 2002;43:1604–1609. [PubMed] [Google Scholar]
- 135.Nicoletti A, Kawase K, Thompson DA. Promoter analysis of RPE65, the gene encoding a 61-kDa retinal pigment epithelium-specific protein. Invest Ophthalmol Vis Sci. 1998;39:637–644. [PubMed] [Google Scholar]
- 136.Liu SY, Redmond TM. Role of the 3′-untranslated region of RPE65 mRNA in the translational regulation of the RPE65 gene: identification of a specific translation inhibitory element. Arch Biochem Biophys. 1998;357:37–44. doi: 10.1006/abbi.1998.0817. [DOI] [PubMed] [Google Scholar]
- 137.Boulanger A, Liu S, Henningsgaard AA, Yu S, Redmond TM. The upstream region of the Rpe65 gene confers retinal pigment epithelium-specific expression in vivo and in vitro and contains critical octamer and E-box binding sites. J Biol Chem. 2000;275:31274–31282. doi: 10.1074/jbc.M003441200. [DOI] [PubMed] [Google Scholar]
- 138.Kiser PD, Golczak M, Lodowski DT, Chance MR, Palczewski K. Crystal structure of native RPE65, the retinoid isomerase of the visual cycle. Proc Natl Acad Sci U S A. 2009;106:17325–17330. doi: 10.1073/pnas.0906600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dundas J, Ouyang Z, Tseng J, Binkowski A, Turpaz Y, Liang J. CASTp: computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res. 2006;34:W116–118. doi: 10.1093/nar/gkl282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Picot D, Garavito RM. Prostaglandin H synthase: implications for membrane structure. FEBS Lett. 1994;346:21–25. doi: 10.1016/0014-5793(94)00314-9. [DOI] [PubMed] [Google Scholar]
- 141.Messing SAJ, Gabelli SB, Echeverria I, Vogel JT, Guan JC, Tan BC, Klee HJ, McCarty DR, Amzela LM. Structural Insights into Maize Viviparous14, a Key Enzyme in the Biosynthesis of the Phytohormone Abscisic Acid. Plant Cell. 2010;22:2970–2980. doi: 10.1105/tpc.110.074815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kloer DP, Ruch S, Al-Babili S, Beyer P, Schulz GE. The structure of a retinal-forming carotenoid oxygenase. Science. 2005;308:267–269. doi: 10.1126/science.1108965. [DOI] [PubMed] [Google Scholar]
- 143.Takahashi Y, Moiseyev G, Chen Y, Ma JX. The roles of three palmitoylation sites of RPE65 in its membrane association and isomerohydrolase activity. Invest Ophthalmol Vis Sci. 2006;47:5191–5196. doi: 10.1167/iovs.06-0614. [DOI] [PubMed] [Google Scholar]
- 144.Jin M, Yuan Q, Li S, Travis GH. Role of LRAT on the retinoid isomerase activity and membrane association of Rpe65. J Biol Chem. 2007;282:20915–20924. doi: 10.1074/jbc.M701432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bernstein PS, Law WC, Rando RR. Isomerization of all-trans-retinoids to 11-cis-retinoids in vitro. Proc Natl Acad Sci U S A. 1987;84:1849–1853. doi: 10.1073/pnas.84.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bernstein PS, Law WC, Rando RR. Biochemical characterization of the retinoid isomerase system of the eye. J Biol Chem. 1987;262:16848–16857. [PubMed] [Google Scholar]
- 147.Kiser PD, Palczewski K. Membrane-binding and enzymatic properties of RPE65. Prog Retin Eye Res. 2010;29:428–442. doi: 10.1016/j.preteyeres.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yuan Q, Kaylor JJ, Miu A, Bassilian S, Whitelegge JP, Travis GH. Rpe65 isomerase associates with membranes through an electrostatic interaction with acidic phospholipid headgroups. J Biol Chem. 2010;285:988–999. doi: 10.1074/jbc.M109.025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Deigner PS, Law WC, Canada FJ, Rando RR. Membranes as the energy source in the endergonic transformation of vitamin A to 11-cis-retinol. Science. 1989;244:968–971. doi: 10.1126/science.2727688. [DOI] [PubMed] [Google Scholar]
- 150.Bernstein PS, Rando RR. In vivo isomerization of all-trans- to 11-cis-retinoids in the eye occurs at the alcohol oxidation state. Biochemistry. 1986;25:6473–6478. doi: 10.1021/bi00369a020. [DOI] [PubMed] [Google Scholar]
- 151.McBee JK, Kuksa V, Alvarez R, de Lera AR, Prezhdo O, Haeseleer F, Sokal I, Palczewski K. Isomerization of all-trans-retinol to cis-retinols in bovine retinal pigment epithelial cells: dependence on the specificity of retinoid-binding proteins. Biochemistry. 2000;39:11370–11380. doi: 10.1021/bi001061c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Law WC, Rando RR. Stereochemical inversion at C-15 accompanies the enzymatic isomerization of all-trans- to 11-cis-retinoids. Biochemistry. 1988;27:4147–4152. doi: 10.1021/bi00411a037. [DOI] [PubMed] [Google Scholar]
- 153.Law WC, Rando RR, Canonica S, Derguini F, Nakanishi K. The Necessity of an Intact Polyene for the Biological Isomerization of Vitamin-A. J Am Chem Soc. 1988;110:5915–5917. [Google Scholar]
- 154.Moiseyev G, Takahashi Y, Chen Y, Gentleman S, Redmond TM, Crouch RK, Ma JX. RPE65 is an iron(II)-dependent isomerohydrolase in the retinoid visual cycle. J Biol Chem. 2006;281:2835–2840. doi: 10.1074/jbc.M508903200. [DOI] [PubMed] [Google Scholar]
- 155.Borowski T, Blomberg MR, Siegbahn PE. Reaction mechanism of apocarotenoid oxygenase (ACO): a DFT study. Chemistry. 2008;14:2264–2276. doi: 10.1002/chem.200701344. [DOI] [PubMed] [Google Scholar]
- 156.Hemati N, Feathers KL, Chrispell JD, Reed DM, Carlson TJ, Thompson DA. RPE65 surface epitopes, protein interactions, and expression in rod- and cone-dominant species. Mol Vis. 2005;11:1151–1165. [PubMed] [Google Scholar]
- 157.Wu Z, Bhattacharya SK, Jin Z, Bonilha VL, Liu T, Nawrot M, Teller DC, Saari JC, Crabb JW. CRALBP ligand and protein interactions. Adv Exp Med Biol. 2006;572:477–483. doi: 10.1007/0-387-32442-9_66. [DOI] [PubMed] [Google Scholar]
- 158.Chung DC, Traboulsi EI. Leber congenital amaurosis: clinical correlations with genotypes, gene therapy trials update, and future directions. J AAPOS. 2009;13:587–592. doi: 10.1016/j.jaapos.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 159.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 160.Stieger K, Lorenz B. Gene therapy for vision loss -- recent developments. Discov Med. 2010;10:425–433. [PubMed] [Google Scholar]
- 161.Friedrich MJ. Seeing is believing: gene therapy shows promise for ocular disorders. JAMA. 2010;304:1543–1545. doi: 10.1001/jama.2010.1412. [DOI] [PubMed] [Google Scholar]
- 162.den Hollander AI, Black A, Bennett J, Cremers FP. Lighting a candle in the dark: advances in genetics and gene therapy of recessive retinal dystrophies. J Clin Invest. 2010;120:3042–3053. doi: 10.1172/JCI42258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cideciyan AV. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res. 2010;29:398–427. doi: 10.1016/j.preteyeres.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Revere KE, Chung DC. Recent breakthroughs in gene therapy for inherited retinal degeneration. Discov Med. 2009;8:125–129. [PubMed] [Google Scholar]
- 165.Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 166.Pang JJ, Chang B, Kumar A, Nusinowitz S, Noorwez SM, Li J, Rani A, Foster TC, Chiodo VA, Doyle T, Li H, Malhotra R, Teusner JT, McDowell JH, Min SH, Li Q, Kaushal S, Hauswirth WW. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol Ther. 2006;13:565–572. doi: 10.1016/j.ymthe.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 167.Batten ML, Imanishi Y, Tu DC, Doan T, Zhu L, Pang J, Glushakova L, Moise AR, Baehr W, Van Gelder RN, Hauswirth WW, Rieke F, Palczewski K. Pharmacological and rAAV gene therapy rescue of visual functions in a blind mouse model of Leber congenital amaurosis. PLoS Med. 2005;2:e333. doi: 10.1371/journal.pmed.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jacobson SG, Boye SL, Aleman TS, Conlon TJ, Zeiss CJ, Roman AJ, Cideciyan AV, Schwartz SB, Komaromy AM, Doobrajh M, Cheung AY, Sumaroka A, Pearce-Kelling SE, Aguirre GD, Kaushal S, Maguire AM, Flotte TR, Hauswirth WW. Safety in nonhuman primates of ocular AAV2-RPE65, a candidate treatment for blindness in Leber congenital amaurosis. Hum Gene Ther. 2006;17:845–858. doi: 10.1089/hum.2006.17.845. [DOI] [PubMed] [Google Scholar]
- 169.Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, Dejneka NS, Pearce-Kelling SE, Maguire AM, Palczewski K, Hauswirth WW, Jacobson SG. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 171.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell’Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Katz ML, Redmond TM. Effect of Rpe65 knockout on accumulation of lipofuscin fluorophores in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2001;42:3023–3030. [PubMed] [Google Scholar]
- 173.Bui TV, Han Y, Radu RA, Travis GH, Mata NL. Characterization of native retinal fluorophores involved in biosynthesis of A2E and lipofuscin-associated retinopathies. J Biol Chem. 2006;281:18112–18119. doi: 10.1074/jbc.M601380200. [DOI] [PubMed] [Google Scholar]
- 174.Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:1981–1989. [PubMed] [Google Scholar]
- 175.Sparrow JR, Parish CA, Hashimoto M, Nakanishi K. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest Ophthalmol Vis Sci. 1999;40:2988–2995. [PubMed] [Google Scholar]
- 176.Weleber RG, Denman ST, Hanifin JM, Cunningham WJ. Abnormal retinal function associated with isotretinoin therapy for acne. Arch Ophthalmol. 1986;104:831–837. doi: 10.1001/archopht.1986.01050180065031. [DOI] [PubMed] [Google Scholar]
- 177.Gollapalli DR, Rando RR. The specific binding of retinoic acid to RPE65 and approaches to the treatment of macular degeneration. Proc Natl Acad Sci U S A. 2004;101:10030–10035. doi: 10.1073/pnas.0401936101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Maiti P, Kong J, Kim SR, Sparrow JR, Allikmets R, Rando RR. Small molecule RPE65 antagonists limit the visual cycle and prevent lipofuscin formation. Biochemistry. 2006;45:852–860. doi: 10.1021/bi0518545. [DOI] [PubMed] [Google Scholar]
- 179.Maiti P, Kong J, Kim SR, Sparrow JR, Allikmets R, Rando RR. Small molecule RPE65 antagonists limit the visual cycle and prevent lipofuscin formation. Biochemistry. 2007;46 doi: 10.1021/bi0518545. [DOI] [PubMed] [Google Scholar]
- 180.Palczewski K. Retinoids for treatment of retinal diseases. Trends Pharmacol Sci. 2010;31:284–295. doi: 10.1016/j.tips.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 182.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 183.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 184.Notredame C. Curr Protoc Bioinformatics. Unit 3. Chapter 3. 2010. Computing multiple sequence/structure alignments with the T-coffee package; pp. 8pp. 1–25. [DOI] [PubMed] [Google Scholar]
- 185.Felsenstein J. PHYLIP (Phylogeny Inference Package) version 3.6. 2005. [Google Scholar]