Abstract
The mechanisms that regulate T cell quiescence are poorly understood. We report that tuberous sclerosis complex 1 (Tsc1) establishes a quiescence program in naive T cells by controlling cell size, cell cycle entry, and responses to T cell receptor stimulation. Loss of quiescence predisposed Tsc1-deficient T cells to apoptosis that resulted in loss of conventional T cells and invariant natural killer T cells. Loss of Tsc1 function dampened in vivo immune responses to bacterial infection. Tsc1-deficient T cells exhibited increased mTORC1 but diminished mTORC2 activities, with mTORC1 activation essential for the disruption of immune homeostasis. Therefore, Tsc1-dependent control of mTOR is crucial in establishing naive T cell quiescence to facilitate adaptive immune function.
Keywords: naive T cells, quiescence, apoptosis, immune response, mTOR
T lymphocytes are pivotal in adaptive immunity. Mature T cells circulate through the blood and peripheral lymphoid organs in a quiescent state (G0) characterized by decreased cell size and metabolic activity. Survival of naive T cells relies on the engagement of T cell receptors (TCRs) by self-peptide MHC complexes and the interaction between interleukin-7 (IL-7) and interleukin-7 receptor (IL-7R)1–3. As these signals can potentially result in inappropriate activation, T cell quiescence must be actively maintained4–6. Transcription factors Foxo and Klf2 were thought to regulate T cell quiescence by inducing the expression of inhibitors of cellular activation7–9. However, recent evidence suggests that they might not directly maintain the quiescent state; instead they contribute to T cell homeostasis by regulating T cell survival and trafficking10–15. Therefore, it remains poorly defined how quiescence in T cells is established, and whether it is coordinately regulated with T cell survival.
When naive T cells encounter cognate antigen, they undergo extensive clonal expansion and switch their metabolic program from catabolism to anabolism. Despite adequate amounts of oxygen in the environment, activated T cells employ glycolysis to generate energy, a phenomenon known as the Warburg effect16, 17. The mammalian target of rapamycin (mTOR), a conserved Ser-Thr kinase, is a central regulator of glycolysis and cellular metabolism18. mTOR exists in two multi-protein complexes in metazoans, mTORC1 and mTORC2. mTORC1 contains the scaffolding protein Raptor and is partly sensitive to the immunosuppressant rapamycin. mTORC1 promotes translational initiation by phosphorylating S6 kinase (S6K) and 4E-BP1, and stimulates cell growth and metabolism. mTORC1 activity is stringently controlled by tuberous sclerosis complex 1 and 2 (Tsc1 and 2), the mutations of which are found in hamartoma syndrome that involves tissue overgrowth. Tsc1 and Tsc2 function as an integral complex, and ablation of either Tsc1 or Tsc2 disrupts the complex18. A second mTOR complex, mTORC2, contains a distinct scaffolding protein, Rictor, and is important for activation of Akt by inducing Ser473 phosphorylation and selective Akt targets such as Foxo18. There is a considerable interplay between the two mTOR complexes, and activated mTORC1 institutes negative feedback loops to inhibit Akt18. An essential role for mTOR in both the innate and adaptive immune systems is emerging19–21. In T cells, mTOR is an important determinant of lineage differentiation after antigen stimulation, but whether it is involved in the homeostasis of naive T cells is unclear.
Using a Tsc1-deficient murine model, we report here that Tsc1 is a central regulator of naive T cell quiescence and homeostasis. Loss of quiescence predisposed Tsc1-deficient T cells to apoptotic cell death and prevented effective generation of an adaptive immune response. These data point to a novel checkpoint active in naive cell quiescence that affects immune homeostasis and function. Tsc1 deficiency resulted in increased mTORC1 but reduced mTORC2-Akt activities. Combined use of genetic models and pharmacological approaches indicated that Tsc1 regulates T cell homeostasis and function mainly by negative control of mTORC1 activity.
RESULTS
Role of Tsc1 in regulating peripheral T cell populations
mTOR is induced upon TCR engagement, but how it is regulated in naive T cells is unclear. Tsc1, a modulator of mTOR signaling, was expressed in thymic and peripheral naive T cells (Supplementary Fig. 1a–c). To investigate the function of Tsc1 in T cells, we crossed mice carrying a conditional Tsc1 allele (Tsc1flox/flox)22 with Cd4-Cre transgenic mice to delete the floxed Tsc1 allele specifically in T cells (hereafter referred to as “Tsc1−/− mice”). mRNA and protein analyses indicated efficient deletion of Tsc1 in mature thymocytes and peripheral T cells (Supplementary Fig. 1a–c). Wild-type (WT) and Tsc1−/− mice contained similar numbers of total thymocytes as well as double-negative, double-positive, CD4 single-positive (CD4SP) and CD8 single-positive (CD8SP) subsets (Fig. 1a), and comparable expression of thymocyte maturation markers including CD62L, CD69 and CD24 (Supplementary Fig. 1d). In contrast, Tsc1−/− mice contained drastically reduced T cells in the spleen (Fig. 1b), peripheral blood and lymph nodes and an altered CD4/CD8 ratio, compared to the WT or heterozygous (Tsc1+/−) littermates (Supplementary Fig. 1e–g).
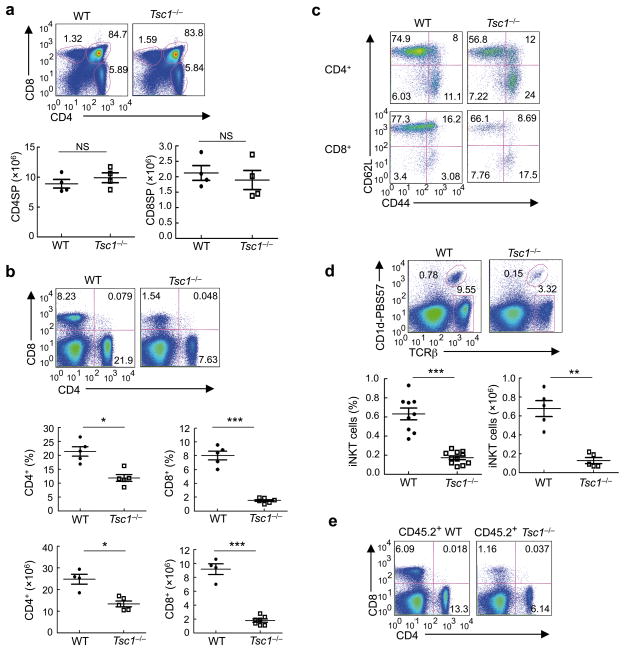
Figure 1. Tsc1 deficiency leads to disrupted homeostasis of peripheral T cell pools.
(a) Flow cytometry of thymocytes in wild-type (WT) and Tsc1−/− mice. Lower panels, numbers of CD4SP and CD8SP thymocytes (n=4–7). (b) Flow cytometry of CD4 and CD8 T cells in the spleens of WT and Tsc1−/− mice. Lower panels, proportions and absolute numbers of CD4 and CD8 T cells in the spleens of WT and Tsc1−/− mice (n=4–6). (c) CD62L and CD44 expression on splenic T cells of WT and Tsc1−/− mice. (d) Flow cytometry of iNKT cells (TCRβ+CD1d-PBS57+) in the spleens of WT and Tsc1−/− mice (n≥5). (e) Flow cytometry of CD4 and CD8 T cells in the spleens of mixed BM chimeras. BM stem cells from wild-type (CD45.1+) and WT or Tsc1−/−mice (CD45.2+) were mixed at 1:1, and transferred into sublethally irradiated Rag1−/− mice to generate mixed BM chimeras, followed by analysis at 8 weeks after reconstitution. NS, not significant; * P < 0.005; ** P < 0.0005; *** P < 0.0001. Data are representative of 4 (a–d) and 2 (e) independent experiments.
We next analyzed the distribution of various T cell subsets in the periphery of Tsc1−/− mice. Among the conventional CD4 and CD8 T cell compartments, Tsc1−/− mice contained an expanded CD62LloCD44hi population with activated or memory phenotypes, with a corresponding reduction of CD62LhiCD44lo naive T cells (Fig. 1c). This alteration was further exacerbated in aged mice (Supplementary Fig. 2a). Tsc1 deficiency did not appear to affect homeostasis of Foxp3-expressing Treg cells (Supplementary Fig. 2b). In contrast, invariant natural killer T (iNKT) cells, as detected by the CD1d-PBS57 tetramer, were markedly reduced in the spleen and liver of Tsc1−/− mice (Fig. 1d and Supplementary Fig. 2c). Tsc1 is thus crucial for maintaining the peripheral populations of conventional T cells and iNKT cells.
To address whether the reduction of peripheral T cell pools in Tsc1−/− mice is a cell-autonomous defect, we generated mixed bone marrow (BM) chimeras by reconstituting Rag1−/− mice with a 1:1 mixture of WT or Tsc1−/− (CD45.2+) BM cells and wild-type (CD45.1+) BM cells. Compared with WT BM-derived donor T cells, Tsc1−/− donors in the reconstituted chimeras contained reduced CD4 and CD8 T cells and retained the increased CD4/CD8 ratio (Fig. 1e). iNKT cells were also diminished in the mixed chimeras (data not shown). Therefore, Tsc1 exerts a cell-autonomous function in maintaining peripheral T cell populations.
Tsc1 promotes survival of peripheral T cells
The overall size of the peripheral T cell pool is dependent upon T cell survival1–3. We therefore measured caspase activity, a hallmark of apoptotic cell death. Tsc1−/− T cells exhibited higher caspase activity, relative to WT controls, with a more prominent elevation in CD8 T cells (Fig. 2a). In contrast, mature thymocytes from WT and Tsc1−/− mice exhibited similar caspase activity (data not shown). Mitochondrial homeostasis is critical in regulating apoptosis, and loss of mitochondrial membrane potential is a general feature of apoptosis23. Tsc1−/− T cells exhibited decreased mitochondria content and loss of mitochondrial transmembrane potential, as revealed by staining with mitochondria-specific dyes24 (Supplementary Fig. 3). Therefore, deficiency of Tsc1 induces apoptosis of peripheral T cells.
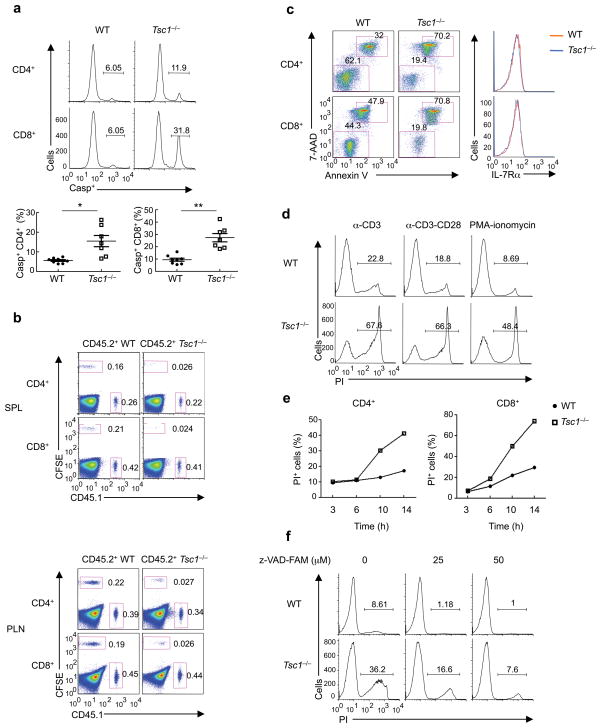
Figure 2. Tsc1 deletion results in markedly elevated apoptosis of T cells.
(a) Caspase activity by FITC-VAD-FMK staining in freshly isolated WT and Tsc1−/− T cells. Lower panels, proportions of caspase-positive (Casp+) T cells (n=7–8). * P <0.005; ** P < 0.0005. (b) Flow cytometry of spleen (upper) and lymph node (lower) cells in the recipient mice (CD45.2+) 6 days after adoptive transfer of equal numbers of CD45.1+ (spike) cells and WT or Tsc1−/− donor cells (CFSE-labeled). SPL, spleen; PLN, peripheral lymph nodes. (c) Annexin V and 7-AAD staining in naive WT or Tsc1−/− T cells cultured with IL-7 in vitro for 3days (left), and expression of IL-7Rα on freshly isolated cells (right). (d) Detection of PI+ apoptotic cells after 16 hours of stimulation of CD8 T cells with anti-CD3, anti-CD3-CD28, or PMA-ionomycin. (e) Kinetics of apoptosis (measured by frequency of PI+ apoptotic cells) after anti-CD3-CD28 stimulation of CD4 and CD8 T cells. (f) Detection of PI+ apoptotic cells in WT and Tsc1−/− CD8 T cells pretreated with vehicle or caspase inhibitor Z-VAD-FAM for 1 hour and then activated with anti-CD3-CD28 for 10 hours. Although not shown here, similar results were observed in CD4 T cells (d,f). Data are representative of 4 (a,c), 2 (b,e), 5 (d) and 3 (f) independent experiments.
We next evaluated the survival ability of Tsc1-deficient T cells in vivo. We adoptively transferred a mixture of equal numbers of WT or Tsc1−/− (CFSE-labeled donor) and CD45.1+ (spike) T cells into CD45.2+ recipient mice. The spike group served as an internal control for normalization, and sorted naive T cells (CD62LhiCD44loCD25 ) were used to obviate the altered homeostasis developed in Tsc1−/− mice. In contrast to WT donor cells that were well maintained upon transfer, Tsc1-deficient cells were rapidly lost and essentially undetectable after 6 days (Fig. 2b). Similar changes were observed in the spleen, lymph nodes (Fig. 2b), and blood (data not shown), indicating that the disappearance of Tsc1−/− cells was unlikely to be caused by altered trafficking25. The presence of a normal lymphocyte pool in the recipients prevented proliferation of donor cells in the time interval described. Therefore, disappearance of Tsc1-deficient T cells is most likely due to a defect in survival in vivo.
We then determined the intrinsic requirement for Tsc1 in the survival of naive T cells by examining IL-7 signaling2, 3. Sorted naive T cells from WT or Tsc1−/− mice were cultured with IL-7 for 3 days. Compared with WT cells, Tsc1−/− cells were much less responsive to IL-7 triggered survival effects (Fig. 2c). Expression of IL-7Rα was comparable between these two types of cells (Fig. 2c), suggesting that the defective response to IL-7 was not due to impaired IL-7Rα expression. These findings demonstrate that Tsc1−/− cells are defective in response to homeostatic cytokine signals in vitro.
To further understand the peripheral T cell deficiency observed in Tsc1−/− mice, we stimulated naive WT and Tsc1−/− T cells with anti-CD3, anti-CD3-CD28, or a combination of phorbol 12-myristate 13-acetate (PMA) and ionomycin. At 16 hours, while only a small subset of WT cells died, as indicated by their positive staining with propidium iodide (PI), the majority of Tsc1−/− T cells became PI-positive (Fig. 2d). Kinetic analysis showed that Tsc1−/− cells exhibited accelerated death after 6 hours of TCR stimulation, with CD8 T cells progressing slightly faster than CD4 T cells (Fig. 2e). T cells can die through both apoptotic and necrotic pathways 1. Treatment with the anti-apoptotic compound Z-VAD attenuated cell death in Tsc1−/− cells (Fig. 2f). In contrast, treatment with necrostatin-1 (Nec-1) to block necrotic death had no noticeable effects (data not shown). Therefore, Tsc1−/− cells respond to TCR signals by undergoing extensive apoptotic death.
Tsc1−/− T cells die via the intrinsic apoptotic pathway
Apoptosis of T cells is mediated by the intrinsic apoptotic pathway (controlled by the balance of pro- and anti-apoptotic Bcl-2 family members) and the extrinsic pathway (controlled by signals delivered from death receptors such as Fas)1–3. Fas expression was comparable between WT and Tsc1−/− T cells (data not shown). We therefore focused on the intrinsic pathway, and found that Bcl-2 expression was downregulated in Tsc1−/− T cells (Fig. 3a). This was further examined by crossing Tsc1−/− mice with mice expressing a Bcl2 transgene in lymphocytes (Bcl2-TG)26. Numbers of CD4 and CD8 T cells as well as iNKT cells were nearly completely restored in Tsc1−/− Bcl2-TG mice (Fig. 3b,c and Supplementary Fig. 4). Moreover, in response to TCR stimulation, the enhanced death of Tsc1−/− naive T cells was considerably reduced in Tsc1−/−Bcl2-TG cells (Fig. 3d,e). These results demonstrate that the exacerbated death of Tsc1−/− T cells is mediated by the intrinsic apoptotic pathway.
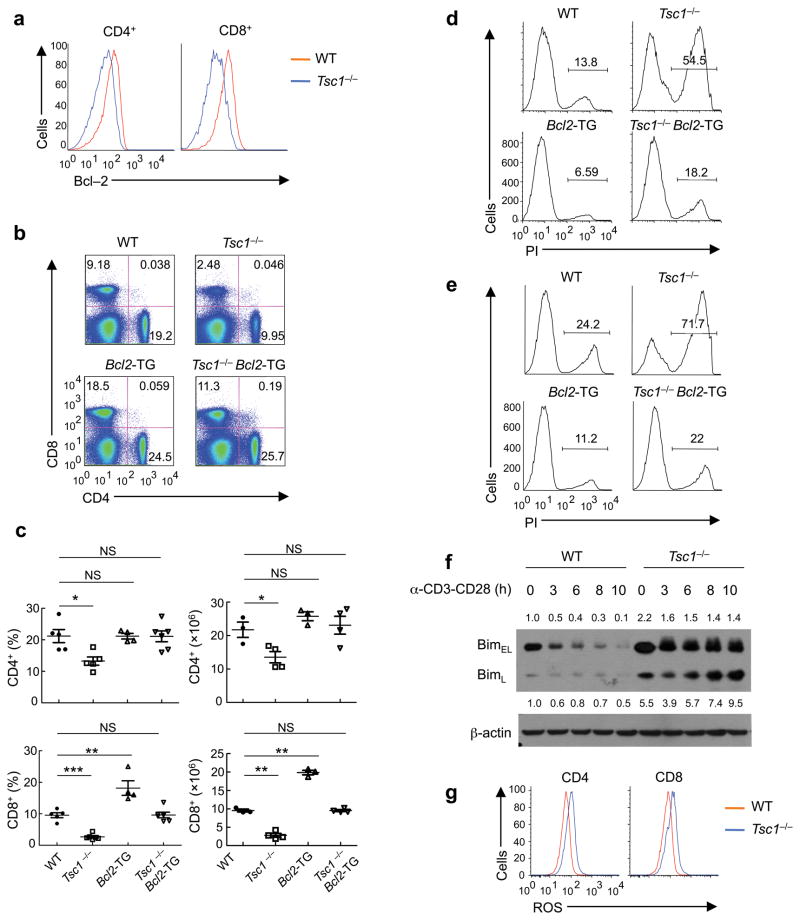
Figure 3. Tsc1-deficient T cells die via the Bcl-2 family-dependent intrinsic apoptotic pathway.
(a) Intracellular Bcl-2 expression in WT and Tsc1−/− T cells. (b) Flow cytometry of CD4 and CD8 T cells in the spleens of WT, Tsc1−/−, Bcl2-TG, and Tsc1−/− Bcl2-TG mice. (c) Proportions and absolute numbers of CD4 and CD8 T cells in WT, Tsc1−/−, Bcl2-TG, and Tsc1−/−Bcl2-TG spleens (n=3–6). NS, not significant; * P <0.05; ** P < 0.005; *** P< 0.0001. (d,e) Detection of PI+ apoptotic cells after 16 hours of stimulation of CD4 (d) and CD8 (e) cells with anti-CD3-CD28. (f) Expression of BimEL and BimL in naive and anti-CD3-CD28 activated WT and Tsc1−/− CD4 T cells. Numbers above (BimEL) and below (BimL) lanes indicate band intensity relative to that of the loading control-actin. (g) ROS production in freshly isolated WT and Tsc1−/− T cells. Data are representative of 3 (a,d–g) and 4 (b,c) independent experiments.
The pro-survival function of Bcl-2 is dependent upon its ability to antagonize the pro-apoptotic Bcl-2 family members. Among them, the BH3-only protein Bim is important to initiate lymphocyte apoptosis1. Tsc1−/− naive T cells showed elevated Bim expression, and upon TCR stimulation, they retained high levels of Bim while WT cells downregulated Bim expression (Fig. 3f and Supplementary Fig. 5a). To determine whether Bim-dependent apoptosis accounted for the survival defect, we crossed Tsc1−/− mice to the Bim-deficient (Bcl2l11−/−) background. The frequency of CD8 T cells in Tsc1−/− Bcl2l11−/− mice was elevated by more than two fold compared with Tsc1−/− mice (Supplementary Fig. 5b). Although CD4 T cell percentage in Tsc1−/− mice was not strongly affected by Bim deficiency in vivo, TCR-mediated apoptosis of Tsc1−/− Bcl2l11−/− double knockout CD4 T cells was less pronounced compared with that of Tsc1−/− cells (Supplementary Fig. 5c). Therefore, Bim-dependent and -independent mechanisms trigger apoptosis when Tsc1 is absent.
We next assessed whether the survival defect of Tsc1−/− T cells was associated with increased production of reactive oxygen species (ROS), which have been implicated in T cell apoptosis27. Tsc1−/− T cells exhibited increased amounts of ROS (Fig. 3g). To test whether elevation of ROS in Tsc1−/− T cells contributes to their extensive apoptosis, we used N-acetylcysteine (NAC), a widely used antioxidant. Following treatment with NAC, the reduced frequencies of CD4 and CD8 T cells in Tsc1−/− mice were modestly but significantly restored (Supplementary Fig. 6a). Consistent with these findings, the excessive TCR-mediated apoptosis in Tsc1−/− T cells was partially suppressed in those from NAC-treated mice (Supplementary Fig. 6b). Collectively, both dysregulated Bcl-2 family proteins and elevated oxidative stress contribute to apoptotic cell death in Tsc1−/− cells.
Loss of quiescence and hyper-activation of Tsc1−/− cells
Homeostasis of naive T cells is believed to be contingent upon the maintenance of a quiescent phenotype4–6. Freshly isolated Tsc1−/− T cells consistently exhibited an increased cell size (Fig. 4a), suggesting a probable increase in cell growth. The cell growth event precedes DNA synthesis and is a prerequisite for cell cycle entry 28. We therefore measured in vivo cycling of T cells using bromodeoxyuridine (BrdU) incorporation. Compared with WT cells, a greater percentage of Tsc1−/− T cells incorporated BrdU under steady-state conditions (Fig. 4b), indicative of an exit from quiescence.
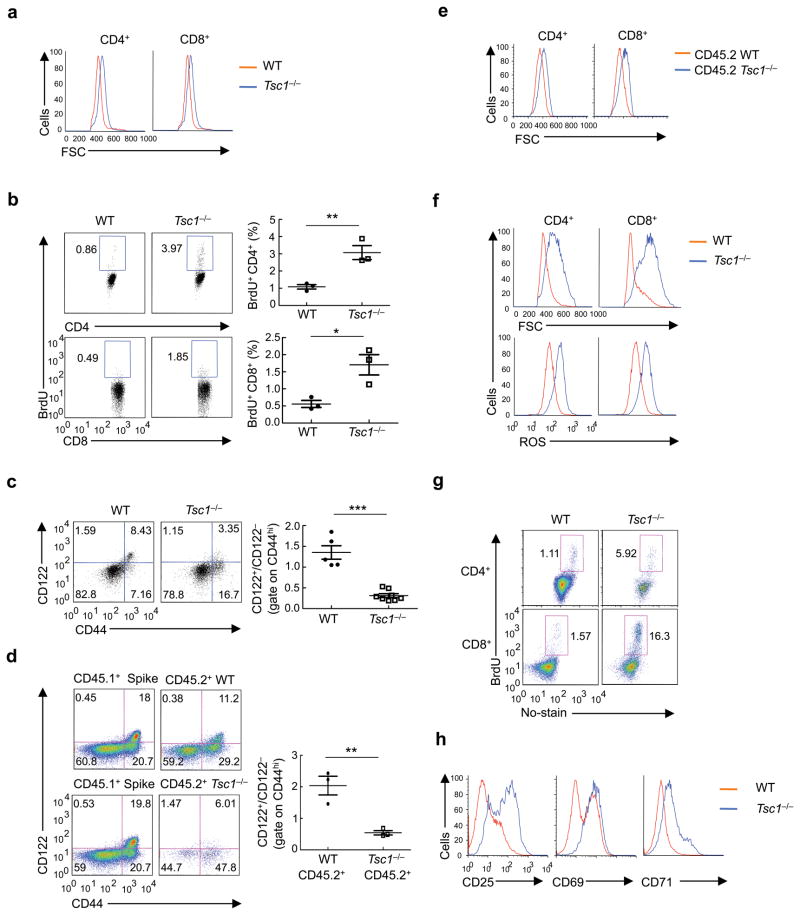
Figure 4. Tsc1 deficiency causes cell-autonomous loss of quiescence in vivo and hyperactive responses to TCR stimulation.
(a) Cell size of freshly isolated WT and Tsc1−/− T cells. FSC, forward scattering. (b) BrdU staining in splenocytes of WT and Tsc1−/− mice 16 hours after injection with BrdU (n=3). (c) CD122 and CD44 expression on gated CD8 splenocytes of WT and Tsc1−/− mice. Right, ratios of CD122+/CD122− among CD44hi populations (n=5–8). (d) CD122 and CD44 expression on gated CD8 splenocytes in the mixed BM chimeras generated as in Fig. 1e. Right, ratios of CD122+/CD122− cells among CD44hi populations of the CD45.2+ cells (n=3). (e) Cell size of CD45.2+ cells in the mixed BM chimeras. (f) Flow cytometry of WT and Tsc1−/− naive T cells after stimulation with anti-CD3-CD28 for 16 hours for the measurements of cell size (upper) and ROS production (lower). (g) BrdU staining in naive T cells activated with anti-CD3-CD28 for 20 hours, followed by pulsing with BrdU for 90 min. (h) Expression of activation markers in WT and Tsc1−/− naive T cells after stimulation with anti-CD3-CD28 for 16 hours. * P <0.05; ** P < 0.01; *** P< 0.0001. Data are representative of 5 (a,c,f), 3 (b,d,e,g), and 4 (h) independent experiments.
Within the CD44hi population, recently activated and memory-phenotype cells can be discriminated on the basis of expression of the IL-2 receptor β-chain (CD122) that is selectively expressed on memory-phenotype cells6, 29. In WT spleen, a large proportion of CD44hi CD8 T cells expressed CD122, but in Tsc1−/− spleen, most CD44hi CD8 T cells were negative for CD122 (Fig. 4c). Expansion of the CD44hiCD122 population also occurred in CD4 T cells, although to a lesser extent (data not shown). In contrast, other T cell activation markers including CD69, CD25 and CD5 were not altered by Tsc1 deficiency (data not shown). Tsc1−/− T cells thus exist in a semiactivated state in vivo.
Since Tsc1−/− mice possess a lymphopenic environment, it remains possible that the increased T cell activation was caused by homeostatic proliferation. To test this possibility, we used the reconstituted BM chimeras described above (Fig. 1e) to correct the lymphopenia. The elevation of the total CD44hi population observed in Tsc1−/− mice was less pronounced in the mixed BM chimeras (Supplementary Fig. 7a), suggesting that lymphopenia-induced expansion contributes to such a phenotype. In contrast, the CD44hiCD122− population was selectively expanded in donor cells derived from Tsc1−/− BM cells, but not from WT or the co-transferred CD45.1+ cells (Fig. 4d). Moreover, Tsc1−/− cells in the chimeras retained the increases in cell size and cycling (Fig. 4e and Supplementary Fig. 7b). Therefore, the in vivo semiactivated state is not primarily driven by the lymphopenic environment but is intrinsic to Tsc1−/− T cells, indicating a key role for Tsc1 to maintain T cell quiescence.
The loss of quiescence observed in Tsc1−/− naive cells in vivo suggests that they may have a greater response to acute ex vivo TCR signals. To test this hypothesis, we stimulated naive Tsc1−/− cells with TCR ligation and measured various parameters associated with T cell activation. Tsc1−/− cells showed much larger increases in cell size, ROS production and cycling than WT cells (Fig. 4f,g). Further, upregulation of the activation markers CD25, CD69 and CD71 was more prominent in Tsc1−/− cells (Fig. 4h). Tsc1 deficiency thus promotes TCR-mediated activation of T cells.
Tsc1 establishes a quiescent gene expression program
We next used functional genomics to analyze the Tsc1-dependent gene signature in naive T cells. The expression profiles of Tsc1−/− CD4 and CD8 T cells showed similar changes, with both showing more upregulated than downregulated genes (Supplementary Fig. 8a). For these coregulated genes (Supplementary Table 1), gene ontology analysis revealed that one of the significantly regulated groups (P =8.04×10−5) by Tsc1 in naive T cells is the cell cycle pathway. Tsc1−/− naive T cells had higher expression of cell cycle positive regulators including Cyclin A2, Cyclin B2, and E2F2 (Supplementary Fig. 8b), which would serve to synergistically promote cycling. To identify key networks regulated by Tsc1, we performed gene set enrichment analysis (GESA) on Tsc1−/− naive CD4 T cells 30. Tsc1−/− T cells showed significant enrichment of 20 upregulated gene sets (with nominal P values equal to zero) that included a number of metabolic pathways. In addition, genes sets for cell cycle and apoptotic pathways were also upregulated in Tsc1−/− naive cells (Supplementary Fig. 8c). Therefore, Tsc1 deficiency upregulates the metabolic and cell cycle pathways.
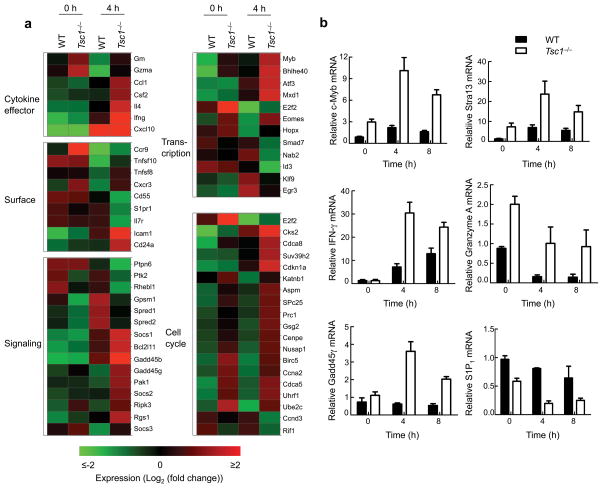
We next determined the dynamics of the response of Tsc1−/− naive T cells to TCR stimulation at the genome-wide level, by comparing gene expression profiles between WT and Tsc1−/− CD4 T cells stimulated with anti-CD3-CD28 for 4 hours (Supplementary Table 2). Gene ontology analysis showed that stimulated Tsc1−/− CD4 T cells upregulated a number of genes associated with T cell activation that encode cytokines and effector molecules (Ifng, Il4, and Gzma), cell surface molecules (Cd24 and Icam1), signaling molecules (Gadd45b and Gadd45g), transcription factors such as Eomes, Myb (c-Myb), Stra13 and cell cycle regulators. Conversely, genes known to be suppressed after TCR stimulation, especially Il7ra and S1pr1, were more profoundly downregulated in Tsc1−/− cells (Fig. 5a). Real-time PCR analyses confirmed altered expression of Myb, Stra13, Ifng, Gzma, Gadd45g and S1pr1 (Fig. 5b). Thus Tsc1 establishes a quiescence gene expression program by linking cell cycle and metabolic machineries and expression of immune response genes in T cells.
Figure 5. Tsc1-dependent gene expression programs.
(a) A subset of genes differentially regulated in WT and Tsc1−/− CD4 T cells activated with TCR for 4 hours (≥ 2-fold difference with false discovery rate <0.1). Triplicate samples were used in the analysis. (b) Real-time PCR analysis of selected genes in WT and Tsc1−/− CD4 T cells activated for various times. Data are representative of 2 independent experiments.
Acute deletion of Tsc1 abrogates T cell quiescence
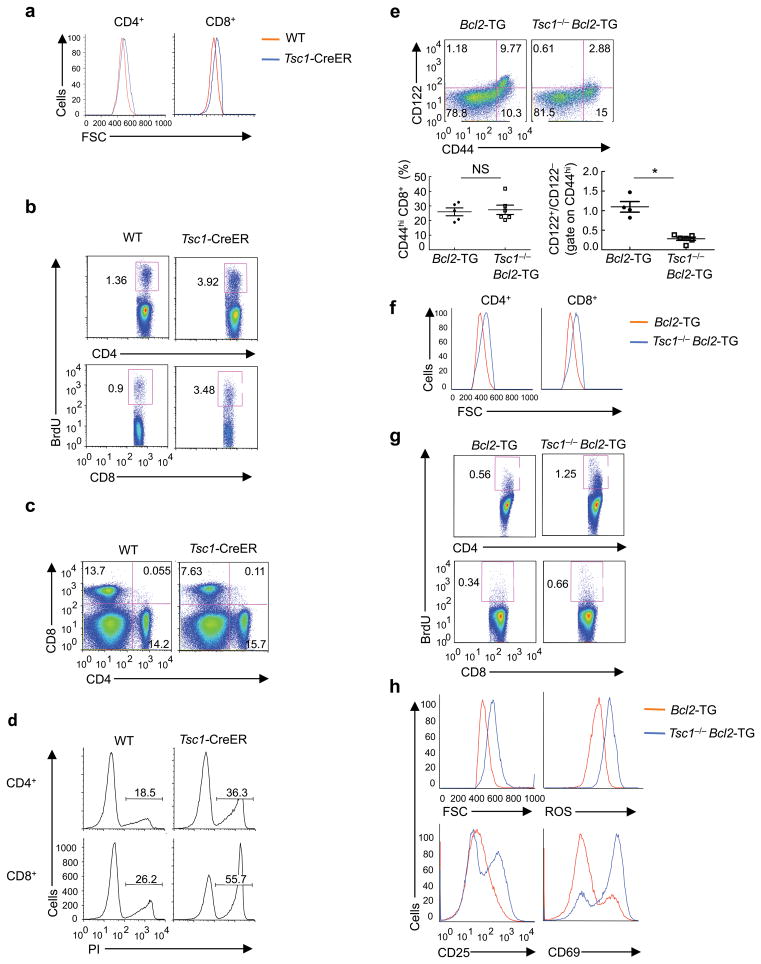
To directly investigate the effects of Tsc1 on T cell quiescence, we crossed Tsc1flox/flox mice with Rosa26-Cre-ERT2 mice (a Cre-ER fusion gene was recombined into the ubiquitously expressed Rosa26 locus) to generate Tsc1flox/flox Rosa26-Cre-ERT2 mice (“Tsc1-CreER mice” hereafter). Tamoxifen-mediated acute deletion of Tsc1 allowed us to exclude the contribution of potential secondary effects introduced by extensive T cell apoptosis, a lymphopenic environment, or continuous loss of Tsc1 throughout the lifespan of T cells. We treated WT and Tsc1-CreER mice with tamoxifen daily for a total of 4 days, and then rested them for 2 weeks. Tsc1 was efficiently deleted in T cells (data not shown), associated with enlarged cell size (Fig. 6a) and more rapid cycling in vivo (Fig. 6b). After TCR stimulation, naive T cells from these animals upregulated cell size and CD25 more extensively than control cells (Supplementary Fig. 9a, b). Moreover, the short-term deficiency of Tsc1 modestly affected peripheral CD8 T cell pools in vivo (Fig. 6c). In response to ex vivo TCR signals, Tsc1-deficient T cells were more susceptible to apoptosis than Tsc1-sufficient controls (Fig. 6d). A more sustained loss of Tsc1, induced by tamoxifen treatment followed by resting for 5 weeks, resulted in greater upregulation of cell size and reduction of peripheral CD4 and CD8 T cells, as well as the development of semi-activation phenotypes in vivo (Supplementary Fig. 9c–e). Therefore, inducible deletion of Tsc1 in adult mice disrupts T cell quiescence.
Figure 6. Loss of T cell quiescence results from inducible deletion of Tsc1 and is independent of cell survival.
(a–d) Analyses of WT and Tsc1-CreER mice at 2 weeks after tamoxifen injection, for cell size of freshly isolated T cells (a), BrdU staining in splenocytes of mice 16 hours after injection with BrdU (b), splenic T cell populations (c), and PI+ apoptotic cells after 16 hours of anti-CD3-CD28 stimulation of naive T cells (d). (e–h) Analyses of WT and Tsc1−/− mice expressing the Bcl2 transgene, for expression of CD122 and CD44 on gated CD8 splenocytes (lower left, proportions of total CD44hi populations; lower right, ratios of CD122+/CD122− populations among CD44hi cells, n=4–6) (e), cell size of freshly isolated T cells (f), and BrdU staining in splenocytes of mice 16 hours after injection with BrdU (g). (h) Flow cytometry of naive T cells after stimulation with anti-CD3-CD28 for 16 hours for the measurements of cell size, ROS production, and expression of CD25 and CD69. NS, not significant; * P = 0.0001. Data are representative of 2 (a–d,h), 4 (e,f), and 3 (g) independent experiments.
Tsc1 enforces quiescence independently of cell survival
The results with inducible Tsc1 deletion suggest that Tsc1 deficiency abrogates T cell quiescence in the absence of overt apoptosis. To further explore the relationship between T cell quiescence and survival, we analyzed Tsc1−/− mice expressing the Bcl2 transgene. Compared with Bcl2-TG mice, Tsc1−/− Bcl2-TG mice contained an expanded population of semiactivated (CD44hiCD122 ) T cells (Fig. 6e). Also, T cells in these mice exhibited increases in cell size and cycling (Fig. 6f,g). After TCR stimulation, Tsc1−/− Bcl2-TG cells retained the phenotypes of increased cell size, hyper-production of ROS and greater upregulation of activation markers relative to the control Bcl2-TG cells (Fig. 6h). Therefore, defective survival and quiescence phenotypes in Tsc1−/− cells were uncoupled upon blockade of apoptosis. These findings, together with the results above (Fig. 6a–d), indicate that loss of quiescence is intrinsic to Tsc1−/− T cells and apoptosis is likely a consequence.
mTORC1 activation disrupts immune homeostasis
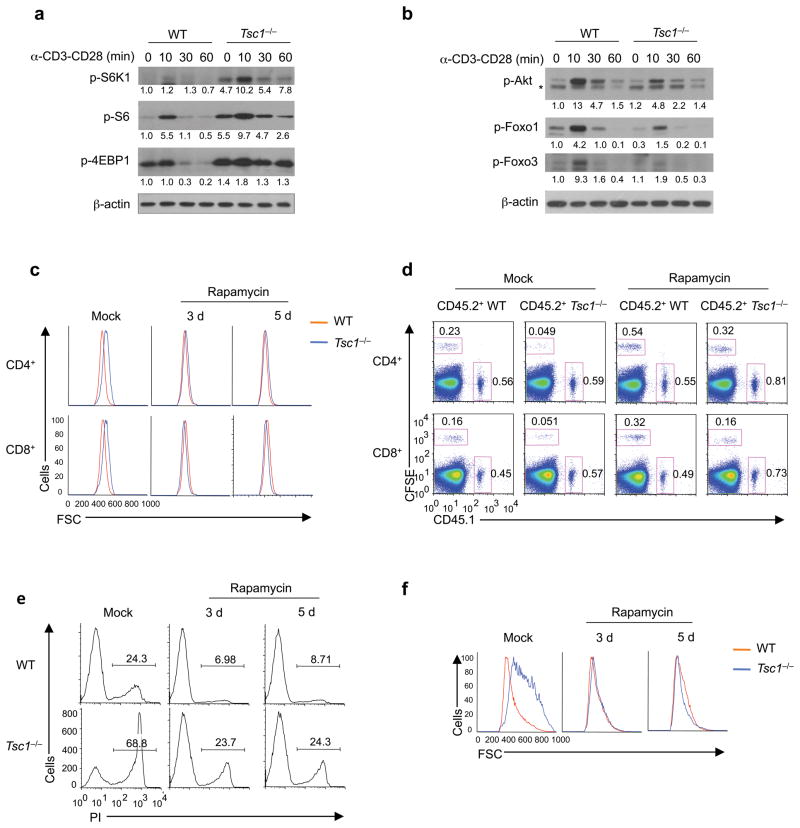
We determined the biochemical mechanisms by which Tsc1 controls T cell homeostasis. We first measured the effects of Tsc1 deficiency on mTORC1 activity by examining S6K1, S6 and 4EBP1 phosphorylation18. Compared with WT naive CD4 T cells, Tsc1−/− cells showed increases in both basal and TCR-induced activities of these conventional mTORC1 targets (Fig. 7a), indicating an inhibitory role of Tsc1 on mTORC1 activity. We next measured mTORC2 activity by analyzing Ser473 phosphorylation of Akt18. Tsc1−/− T cells exhibited decreased phosphorylation of Akt (Ser473) and of Foxo1 and Foxo3, two mTORC2-Akt targets18 (Fig. 7b). Similar alterations in mTORC1 and mTORC2 activities were observed in Tsc1−/− CD8 T cells (Supplementary Fig. 10a). Interestingly, markedly elevated mTORC1 activity was also observed in Tsc1−/− CD4SP thymocytes (Supplementary Fig. 10b), despite the lack of defects in their survival or homeostasis. Thus, the physiological function of Tsc1 may be context dependent, with the effects influenced by the developmental stages of T cells or environmental cues received by T cells.
Figure 7. Tsc1 regulates mTORC1 and mTORC2 activities, with mTORC1 activation essential to disrupt immune quiescence and homeostasis.
(a) Phosphorylation of S6K1, S6 and 4EBP1 in WT and Tsc1−/− naive CD4 T cells after they were stimulated with anti-CD3-CD28 for various times. (b) Phosphorylation of Akt (Ser473), Foxo1 and Foxo3 in WT and Tsc1−/−CD4 T cells after they were stimulated with anti-CD3-CD28 for various times. *, non-specific bands. Numbers below lanes (a,b) indicate band intensity relative to that of the loading control β-actin. (c) Cell size of freshly isolated splenocytes after WT and Tsc1−/− mice were treated with daily injection of rapamycin for a total of 3 or 5 days. (d) Flow cytometry of splenocytes in the recipient mice (CD45.2+) 6 days after adoptive transfer of equal numbers of CD45.1+ (spike) cells and WT or Tsc1−/− donor cells (CFSE-labeled) that were purified from mock or rapamycin-treated mice. Similar results were obtained in the recipient lymph nodes (not shown here). (e,f) Naive T cells from rapamycin or mock treated WT and Tsc1−/− mice were stimulated with anti-CD3-CD28 overnight, followed by measurements of PI+ apoptotic cells (e) and cell size (f). Data are representative of 5 (a,b) and 3 (c–f) independent experiments.
We next dissected the relative contribution of the two mTOR complexes to immune homeostasis. We administered the mTORC1 inhibitor rapamycin to WT and Tsc1−/− mice for 3–5 days. As expected, rapamycin treatment reduced mTORC1 activity (Supplementary Fig. 11a). Although such a short period of treatment was ineffective to affect peripheral T cell pools (Supplementary Fig. 11b), rapamycin blocked the cell size increase of Tsc1−/− T cells (Fig. 7c). When rapamycin-treated Tsc1−/− naive T cells were transferred into WT recipients, their survival was substantially improved (Fig. 7d). Further, upon TCR stimulation, these cells were considerably rescued from the enhanced death (Fig. 7e) and cell size upregulation (Fig. 7f). Therefore, the increased mTORC1 activity in Tsc1−/− cells is required to drive the excessive apoptosis and activation.
To determine the sufficiency and requirement of the diminished mTORC2-Akt activities in Tsc1−/− T cells for immune homeostasis, we used two approaches. First, we analyzed mice deficient in Rictor, the loss of which abrogates mTORC2 activity18. Deletion of Rictor using the Cd4-Cre system (Rictor−/−) ablated Akt phosphorylation at Ser473 and diminished Foxo1 phosphorylation (Supplementary Fig. 12a). Rictor−/− mice contained largely normal peripheral T cell pools except for a slight reduction of CD8 T cells (data not shown), consistent with recent reports31, 32. Deficiency of Rictor did not alter T cell size or responses to TCR, except that Rictor−/− CD8 T cells exhibited slightly increased apoptosis in vitro (Supplementary Fig. 12b–d). Therefore, loss of mTORC2 activity is insufficient to disrupt immune homeostasis. Second, to test whether the diminished mTORC2-Akt activity is required for Tsc1−/− phenotypes, we crossed Tsc1−/− mice with those expressing the active Akt transgene in T cells (Akt-TG)33. As expected, phosphorylation of the Akt target gene, Foxo1, was largely restored in Tsc1−/− Akt-TG mice (Supplementary Fig. 13a). However, the Akt transgene failed to rectify the disrupted T cell homeostasis and survival observed in Tsc1−/− mice (Supplementary Fig. 13b–d). Therefore, mTORC2 is neither necessary nor sufficient to abrogate T cell quiescence or survival. While it remains possible that the impaired Akt activity might contribute to the increased sensitivity of Tsc1−/− cells to apoptosis by accelerating the execution of cell death, ultimately it is not the driving force.
Having established a role for Tsc1 in T cell quiescence via mTORC1 inhibition, we next explored how the activity of the Tsc1 pathway is regulated. We first assessed whether Tsc2 expression was affected by Tsc1 deficiency, as these two molecules function as an integral complex18. Indeed, Tsc1 deficiency in T cells resulted in the loss of Tsc2, thereby ablating the entire Tsc1-Tsc2 complex function (Supplementary Fig. 14a). This finding, although not unexpected, allowed us to dissect how upstream signals were transduced to the complex by examining signal-induced phosphorylation of Tsc218. Notably, Tsc2 activity can be positively and negatively regulated by phosphorylation at Ser1387 and Thr1462, respectively18, 34, 35. Tsc2 phosphorylation at Thr1462 was rapidly induced after T cell activation (Supplementary Fig. 14b), thereby relieving the inhibitory effects of Tsc1-Tsc2 complex on mTORC1 activity. Conversely, Tsc2 phosphorylation at Ser1387 was maintained at a high level in naive T cells but diminished after sustained TCR stimulation (Supplementary Fig. 14b). These results support a model in which the Tsc1-Tsc2 complex is inactivated by TCR signals to facilitate mTORC1 activation, but the complex function is maintained in naive T cells to keep mTORC1 in check.
Tsc1 regulates antigen-specific immune response in vivo
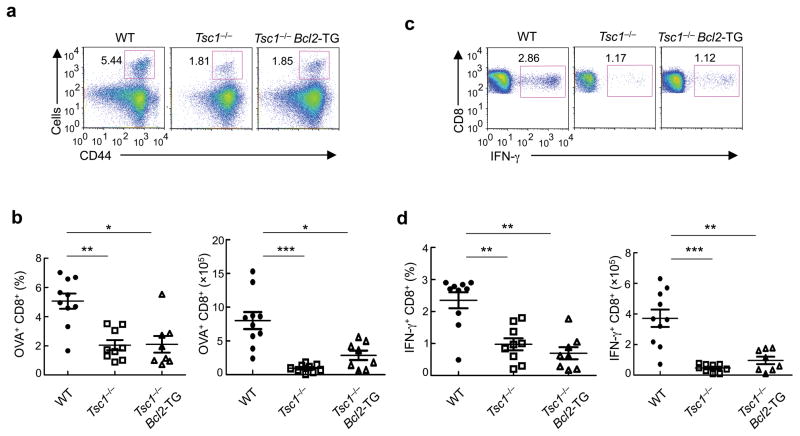
To investigate the roles of Tsc1 in T cell-mediated immune response in vivo, we infected WT and Tsc1−/− mice with recombinant Listeria monocytogenes expressing ovalbumin (LM-OVA). As compared with WT controls, CD8 T cells in the infected Tsc1−/− mice contained significantly reduced frequency and numbers of tetramer-positive, antigen-reactive cells (Fig. 8a,b). Tsc1−/−mice also contained fewer IFN-γ-producing CD8 T cells after ex vivo restimulation with the OVA peptide (Fig. 8c,d). These findings demonstrate that Tsc1 is required for antigen-specific immune response in vivo.
Figure 8. Tsc1 deficiency dampens antibacterial immune response in vivo.
(a,b) Flow cytometry (a) and proportions and absolute numbers (b) of OVA-reactive tetramer-positive CD8 T cells in WT, Tsc1−/− and Tsc1−/− Bcl2-TG mice infected with LM-OVA. (c,d) Flow cytometry (c) and proportions and absolute numbers (d) of OVA-reactive IFN-γ+ CD8 T cells in LM-OVA infected mice, detected after OVA stimulation and intracellular cytokine staining. * P <0.005; ** P < 0.001; *** P< 0.0001. Data are representative of 3 independent experiments (n=8–10).
Given the excessive apoptosis of Tsc1−/− T cells, we determined whether the requirement for Tsc1 in the immune response was due to its effect on cell survival. To this end, we analyzed the antibacterial immune response in Tsc1−/− Bcl2-TG mice that contained largely normal T cell numbers (Fig. 3b,c). Expansion of OVA-reactive CD8 T cells was impaired in these mice (Fig. 8a–d and Supplementary Fig. 15a), despite that the OVA-reactive cells exhibited a similar extent of apoptosis as Bcl2-TG control cells (Supplementary Fig. 15b). Thus, the effect for Tsc1 to promote immune responses can be largely separated from its role in T cell survival. We then examined whether Tsc1 deficiency was associated with an intrinsic defect in effector T cell differentiation. Under optimal differentiation conditions in vitro, naive CD8 T cells from Tsc1−/− Bcl2-TG cells were able to differentiate into IFN-γ+ cells (with slightly increased expression on a per cell basis as compared with Bcl2-TG controls) (Supplementary Fig. 15c). Deficiency of Tsc1 therefore dampens in vivo immune responses through a mechanism that is largely independent of T cell survival or intrinsic differentiation potential.
DISCUSSION
A long-standing question in immunology is how quiescence of naive T cells is established4, 5. Here we describe that Tsc1-dependent control of mTOR activity is critical to enforce naive cell quiescence. Loss of this control mechanism in Tsc1−/− T cells results in increased cell size, rapid cycling and exuberant TCR activation that predispose these cells to apoptotic cell death, suggesting that Tsc1 represents a bona fide factor to establish T cell quiescence. Furthermore, Tsc1 is important for antigen-specific immune response, a function largely independent of Tsc1-mediated survival effects. At the molecular levels, Tsc1 acts as part of the Tsc1-Tsc2 complex whose activity is dynamically regulated by TCR signals. Tsc1 enforces T cell homeostasis by controlling mTORC1 activity to suppress the metabolic and cell cycle machineries and to establish a quiescent gene expression program. We propose that Tsc1-dependent pathway is a key checkpoint in naive T cells by integrating extracellular signals to control mTOR activity, thereby coordinating T cell quiescence, survival and immune function.
Deficiency of Tsc1 in T cells led to exit from quiescence and excessive apoptosis. Both effects were observed in vivo under lymphopenic and lymphoreplete conditions, and were further recapitulated in highly purified naive T cells in vitro. Are these two effects independent of each other, or causally linked? Tsc1−/− T cells partly relied on Bim-dependent and oxidative stress pathways to trigger apoptosis, and they were protected from death by the Bcl2 transgene. Yet, these T cells retained the phenotypes of disrupted quiescence, as shown by the expansion of semiactivated T cells and increases in cell size, cycling and response to TCR signals. By contrast, short-term rapamycin treatment reduced T cell size and TCR activation and more importantly, restored the resistance of Tsc1−/− cells to apoptosis. Therefore, failure of Tsc1−/−naive T cells to engage the survival machinery stemmed from loss of quiescence, a notion further supported by transcript profiling and analyses of T cells upon acute Tsc1 deletion. We conclude that Tsc1 actively maintains a quiescent program in naive T cells that renders them resistant to apoptosis. Notably, Tsc1 regulates production of innate cytokines36, and Tsc1 deficiency in hematopoietic stem cells results in rapid cycling and expansion but impaired hematopoiesis and self-renewal37, 38. In the thymus, while Tsc1 regulates mTOR activity, no survival defects were noticed in Tsc1−/− thymocytes. Indeed, except for under non-physiological conditions such as nutrient starvation and genome damage39, 40, mTOR activity is generally thought to promote cell growth and survival in diverse cellular contexts18. In T cells, mTOR inhibition by rapamycin in vitro diminishes cell size but not survival41. Therefore, Tsc1 plays a hitherto unappreciated role in naive T cells to link their quiescence and survival, while the activity of the Tsc1-Tsc2 complex is downregulated upon TCR ligation to facilitate mTORC1 activation.
An equally surprising finding is that Tsc1-deficint T cells failed to mount an efficient antigen-specific immune response in vivo, despite their enhanced response to acute TCR stimulation. Such an effect was observed even when the increased apoptosis of Tsc1−/− T cells was largely blocked, suggesting that maintenance of naive T cell quiescence is important for a productive immune response. The precise mechanism by which Tsc1 deficiency dampens the immune response is uncertain, but may involve premature activation of the cell cycle and metabolic machineries and transcriptional responses in Tsc1−/− naive cells before they receive proper TCR signals. While blocking mTOR activity blunts effector T cell differentiation20, our results indicate that hyperactive mTORC1 is also detrimental to antigen-specific responses. These findings highlight the presence of a defined threshold of mTOR activity in naive T cells to engage a productive immune reaction. Notably, mTOR inhibits development of memory CD8 T cells42–44. From a metabolic perspective, both naive and memory cells are quiescent17. It is tempting to speculate that maintenance of a quiescent state is essential for the strength of immune responses mediated by both naive and memory T cells, and further study is required to test this notion.
Recent work on mTOR signaling in the adaptive immune system has centered on its effects on T cell differentiation20. mTOR has been shown to promote the generation of effector cells while inhibiting Treg differentiation26, 31, 32, 45, 46. None of the reported mutants in the mTOR pathway exhibit the phenotypes in T cell quiescence or survival. To our knowledge, the genetic model exhibiting the most extensive similarity as Tsc1−/− mice is electra, an immunodeficient mouse line carrying a mutation in the gene encoding Schlafen-2 (Slfn2), a protein with no clear biochemical functions6. We also noted that memory-phenotype (CD44hiCD122+) cells were greatly decreased in Tsc1−/− mice, a defect resistant to Bcl-2-mediated rescue effects. Therefore, differentiation of Tsc1−/− cells into memory cells is likely blocked, consistent with the effects of rapamycin to promote memory development42–44. In contrast, loss of iNKT cells in Tsc1−/− mice resulted from impaired survival, because the Bcl2 transgene rescued this defect. Tsc1 therefore controls the homeostasis of different T cell populations via distinct mechanisms.
Persistent mTOR activation results in metabolic stress and is associated with diverse pathologies such as cancer and metabolic diseases18. Conversely, inhibition of mTOR by rapamycin prolongs life span and increases quality of life by reducing the incidence of age-related pathologies in various species ranging from worms, fruit flies to mammals47. As a parallel, aberrant mTOR activity due to Tsc1 functional loss in T cells results in exit from quiescence and exacerbated activation and apoptosis, which can be ameliorated by rapamycin treatment. Therefore, the adaptive immune system has evolved to adopt an evolutionarily conserved pathway to regulate the life span and function of naive T cells.
ONLINE METHODS
Mice
Tsc1flox, C57BL/6, CD45.1+, and Rag1−/− mice were purchased from the Jackson Laboratory22. Rictorflox mice were obtained from NIH Mutant Mouse Regional Resource Center48. Cd4-Cre, Bcl2-TG, Akt-TG and Rosa26-Cre-ERT2 mice have been described previously26, 33, 46. Mice at 6–10 weeks old were used unless otherwise noted. BM chimeras were generated by transferring 1×107 T cell-depleted BM cells into sublethally irradiated (5 Gy) Rag1−/− mice, as described previously26, 46. Rapamycin treatment was described previously26. For bacterial infection, mice were injected intravenously with the Listeria monocytogenes strain LM-OVA (3–5×104 cfu/mouse) and analyzed 6–7 days later. For tamoxifen treatment, mice were injected intraperitoneally with tamoxifen (2 mg/mouse) in corn oil daily on 4 consecutive days and analyzed at the indicated times. For NAC treatment, mice were fed with NAC (1 mg/ml; Sigma) in the drinking water with the solution replaced every two days. All mice were kept in specific pathogen-free conditions in Animal Resource Center at St. Jude. Animal protocols were approved by Institutional Animal Care and Use Committee of St. Jude.
Flow cytometry
For analysis of surface markers, cells were stained with antibodies (all from eBioscience) in PBS containing 2% (wt/vol) BSA, unless noted otherwise. PBS57-loaded mCD1d tetramer was obtained form the NIH Tetramer Facility. Foxp3 staining was performed per manufacturer’s instructions (eBioscience). BrdU and Annexin V staining was performed per manufacturer’s instructions (BD Biosciences). Caspase activity was measured using FITC-VAD-FMK per manufacturer’s instructions (Promega). For intracellular cytokine staining, T cells were stimulated for 5 h with PMA/ionomycin or OVA257–264 peptide (SIINFEKL, for LM-OVA infected mice) in the presence of monensin before staining per manufacturer’s instructions (BD Biosciences). To stain mitochondria, lymphocytes were incubated with MitoTracker Green (20 nM; Invitrogen) or TMRM (20 nM; ImmunoChemistry Technologies) at 37°C for 20 min after staining of surface markers. ROS was measured by incubation with CM-H2DCFDA (10 μM; Invitrogen) at 37°C for 30 min after staining of surface markers. Flow cytometry data were acquired on an upgraded 5-color FACScan or LSRII (Becton Dickinson), and analyzed using FlowJo software (Tree Star).
Cell purification and cultures
Lymphocytes were isolated from the lymphoid organs and naive T cells sorted on a MoFlow (Beckman-Coulter) or Reflection (i-Cyt). Sorted naive T cells (CD4+CD62LhiCD44loCD25−) were used for in vitro cultures in Click’s medium (plus β-mercaptoethanol) supplemented with 10% FBS and 1% penicillin-streptomycin as described previously 26, 46. For drug inhibitor treatments, cells were incubated with vehicle or Z-VAD for 1 hour before stimulation.
Gene expression profiling by microarray analysis
RNA samples were analyzed using the Affymetrix HT_MG-430_PM GeneTitan peg array and expression signals were summarized using the RMA algorithm (Affymetrix Expression Console v1.1). Differentially expressed transcripts were identified by ANOVA (Partek Genomics Suite v6.5) and the Benjamini-Hochberg method was used to estimate the false discovery rate. Gene lists were analyzed for enrichment of Gene Ontology and canonical pathways using the DAVID bioinformatics databases 49. Gene set enrichment analysis (GSEA) within canonical pathways was performed as described 30.
RNA and immunoblot analyses
Real time PCR analysis was performed as described previously, using primer and probe sets from Applied Biosystems 26, 46. Immunoblot was performed and quantified as described previously 26, using the following antibodies: p-S6, p-Akt (S473), p-4EBP1, p-S6K1, p-Foxo1, p-Foxo3a, p-Tsc2 (S1387), Tsc1 (all from Cell Signaling Technology), p-Tsc2 (T1462) (Novus Biologicals), and β-actin (Sigma).
Statistical analysis
P values were calculated using Student’s t-test and ANOVA. P values of less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
The authors acknowledge Pamela Ohashi for Akt-TG mice, Thomas Ludwig for Rosa26-Cre-ERT2 mice, Richard Cross, Greig Lennon and Stephanie Morgan for cell sorting, and the NIH Tetramer Facility for providing CD1d-PBS57 tetramer. This work was supported by US National Institutes of Health (K01 AR053573 and administrative supplement, R01 NS064599, and Cancer Center Support Grant CA021765), the Arthritis Foundation, the Lupus Research Institute, and the American Lebanese Syrian Associated Charities (to H.C.).
Footnotes
AUTHOR CONTRIBUTIONS
K.Y. designed and performed cellular, molecular, and biochemical experiments and contributed to writing the manuscript; G.N. performed bioinformatic analyses; D.R.G. contributed genetic models and conceptual insights; W.H. contributed to cell purification; H.C designed experiments, wrote the manuscript, and provided overall direction.
References
- 1.Hedrick SM, Ch’en IL, Alves BN. Intertwined pathways of programmed cell death in immunity. Immunol Rev. 2010;236:41–53. doi: 10.1111/j.1600-065X.2010.00918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 4.Glynne R, Ghandour G, Rayner J, Mack DH, Goodnow CC. B-lymphocyte quiescence, tolerance and activation as viewed by global gene expression profiling on microarrays. Immunol Rev. 2000;176:216–246. doi: 10.1034/j.1600-065x.2000.00614.x. [DOI] [PubMed] [Google Scholar]
- 5.Teague TK, et al. Activation changes the spectrum but not the diversity of genes expressed by T cells. Proc Natl Acad Sci U S A. 1999;96:12691–12696. doi: 10.1073/pnas.96.22.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger M, et al. An Slfn2 mutation causes lymphoid and myeloid immunodeficiency due to loss of immune cell quiescence. Nat Immunol. 2010;11:335–343. doi: 10.1038/ni.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modiano JF, Johnson LD, Bellgrau D. Negative regulators in homeostasis of naive peripheral T cells. Immunol Res. 2008;41:137–153. doi: 10.1007/s12026-008-8017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yusuf I, Fruman DA. Regulation of quiescence in lymphocytes. Trends Immunol. 2003;24:380–386. doi: 10.1016/s1471-4906(03)00141-8. [DOI] [PubMed] [Google Scholar]
- 9.Kuo CT, Veselits ML, Leiden JM. LKLF: A transcriptional regulator of single-positive T cell quiescence and survival. Science. 1997;277:1986–1990. doi: 10.1126/science.277.5334.1986. [DOI] [PubMed] [Google Scholar]
- 10.Kerdiles YM, et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerdiles YM, et al. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity. 2009;30:358–371. doi: 10.1016/j.immuni.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouyang W, et al. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 14.Carlson CM, et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 15.Takada K, et al. Kruppel-like factor 2 is required for trafficking but not quiescence in postactivated T cells. J Immunol. 2011;186:775–783. doi: 10.4049/jimmunol.1000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Pearce EL. Metabolism in T cell activation and differentiation. Curr Opin Immunol. 2010 doi: 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weichhart T, Saemann MD. The multiple facets of mTOR in immunity. Trends Immunol. 2009;30:218–226. doi: 10.1016/j.it.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finlay D, Cantrell DA. Metabolism, migration and memory in cytotoxic T cells. Nature reviews. 2011;11:109–117. doi: 10.1038/nri2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwiatkowski DJ, et al. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 23.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 24.Pendergrass W, Wolf N, Poot M. Efficacy of MitoTracker Green and CMXrosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytometry A. 2004;61:162–169. doi: 10.1002/cyto.a.20033. [DOI] [PubMed] [Google Scholar]
- 25.Sinclair LV, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hildeman DA, et al. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735–744. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- 28.Menon S, et al. COP9 signalosome subunit 8 is essential for peripheral T cell homeostasis and antigen receptor-induced entry into the cell cycle from quiescence. Nat Immunol. 2007;8:1236–1245. doi: 10.1038/ni1514. [DOI] [PubMed] [Google Scholar]
- 29.Boyman O, Cho JH, Tan JT, Surh CD, Sprent J. A major histocompatibility complex class I-dependent subset of memory phenotype CD8+ cells. J Exp Med. 2006;203:1817–1825. doi: 10.1084/jem.20052495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee K, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delgoffe GM, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones RG, et al. Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo. J Exp Med. 2000;191:1721–1734. doi: 10.1084/jem.191.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 35.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 36.Weichhart T, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Gan B, et al. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc Natl Acad Sci U S A. 2008;105:19384–19389. doi: 10.1073/pnas.0810584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen C, et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choo AY, et al. Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol Cell. 2010;38:487–499. doi: 10.1016/j.molcel.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh S, et al. Essential role of tuberous sclerosis genes TSC1 and TSC2 in NF-kappaB activation and cell survival. Cancer Cell. 2006;10:215–226. doi: 10.1016/j.ccr.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 42.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delgoffe GM, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu G, et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Houtkooper RH, Williams RW, Auwerx J. Metabolic networks of longevity. Cell. 2010;142:9–14. doi: 10.1016/j.cell.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shiota C, Woo JT, Lindner J, Shelton KD, Magnuson MA. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev Cell. 2006;11:583–589. doi: 10.1016/j.devcel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 49.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.