Abstract
Lon and ClpXP are the only soluble ATP-dependent proteases within the mammalian mitochondria matrix, which function in protein quality control by selectively degrading misfolded, misassembled or damaged proteins. Chemical tools to study these proteases in biological samples have not been identified, thereby hindering a clear understanding of their respective functions in normal and disease states. In this study, we applied a proteolytic site-directed approach to identify a peptide reporter substrate and a peptide inhibitor that are selective for Lon but not ClpXP. These chemical tools permit quantitative measurements that distinguish Lon-mediated proteolysis from that of ClpXP in biochemical assays with purified proteases, as well as in intact mitochondria and mitochondrial lysates. This chemical biology approach provides needed tools to further our understanding of mitochondrial ATP-dependent proteolysis, and contributes to the future development of diagnostic and pharmacological agents for treating diseases associated with defects in mitochondrial protein quality.
INTRODUCTION
The ATP-dependent proteases Lon and ClpXP are highly conserved from bacteria to eukaryotes. In mammals, Lon and ClpXP are nuclear-encoded and synthesized in the cytosol as precursor proteins, which are translocated into the mitochondrial matrix where their respective targeting pre-sequences are removed (1, 2). Human Lon (hLon) is a single ring-shaped protease complex likely composed of six identical subunits (3). By contrast, human ClpXP (hClpXP) is a two-component protease, which consists of a double-ringed tetradecameric protease component composed of identical ClpP subunits, which is capped at either end by a single ring-shaped ATPase complex of ClpX subunits (1, 4, 5).
The physiological functions of mitochondrial Lon and ClpP have been investigated in cultured mammalian cells and genetic knockouts in yeast or worms. Studies show that mitochondrial hLon (or Pim1 in yeast) plays a significant role in mitochondrial quality control, by selectively degrading incompletely assembled or abnormal proteins (6–9), maintaining mitochondrial genome integrity (7, 10, 11), and removing oxidatively damaged proteins (12–15). Mammalian Lon is induced by hypoxia and unfolded proteins in the endoplasmic reticulum (16, 17). Yeast lack a gene encoding ClpP; thus, Lon/Pim1 is the sole mediator of ATP-dependent proteolysis in the mitochondrial matrix. However, in mammals and worms, ClpP is expressed and is responsible for degrading misfolded proteins in the mitochondrial matrix, and participating in the mitochondrial unfolded protein response pathway (mtUPR) (18–20).
An ongoing challenge in developing reagents to define the physiological functions of Lon and ClpXP in mammalian mitochondria is our lack of understanding of the mechanisms of the two proteases in order to distinguish them. At present, there is no convenient quantitative assay for discriminating the protease activities of Lon and ClpXP in biological samples. For example, the detection of ATP-dependent proteolysis in mammalian mitochondria has been limited to using mitochondrial lysates, and monitoring changes in the ATP-dependent degradation of casein, which is a protein substrate cleaved by most energy-dependent, as well as energy–independent proteases. To address this deficiency, we set out to develop the chemical tools for distinguishing not only purified Lon from ClpXP, but also for measuring Lon-mediated proteolysis in a complex mixture such as mitochondrial lysates where ClpXP is also present. Toward this end, we took advantage of the observation that bacterial Lon and ClpXP have distinct peptide cleavage site specificities. Although bacterial Lon and ClpXP degrade the same protein substrate such as oxidized insulin B (1, 21), they cleave this substrate at uniquely different sites. We speculated that the unique peptide cleavage site specificities of Lon and ClpXP could be exploited to design peptidyl substrates and peptide-based inhibitors that are specific for each respective protease.
For proof of principle, we described the development of chemical probes that can be used to monitor ATP-dependent activity in mitochondria lysate and an inhibitor that can specifically inhibit the proteolytic but not ATPase activity of hLon in isolated mitochondria of HeLa cells. Our results suggest that these probes will be useful in determining the physiological roles of Lon versus ClpXP in isolated mitochondria, and potentially in intact cells, during diverse metabolic or disease states. As mitochondrial protein aggregation and misfolding are associated with variety of diseases, it is likely that changes in mitochondrial ATP-dependent proteolysis will likely be either up- or down- regulated. Thus, the chemical probes described here will be valuable in determining the protease activity of Lon versus ClpXP in normal and dysfunctional mitochondria, and may have applications in determining the potential of these ATP-dependent proteases as diagnostic markers and/or therapeutic targets.
RESULTS and DISCUSSION
Fluorescent peptide reporter of ATP-dependent proteolysis
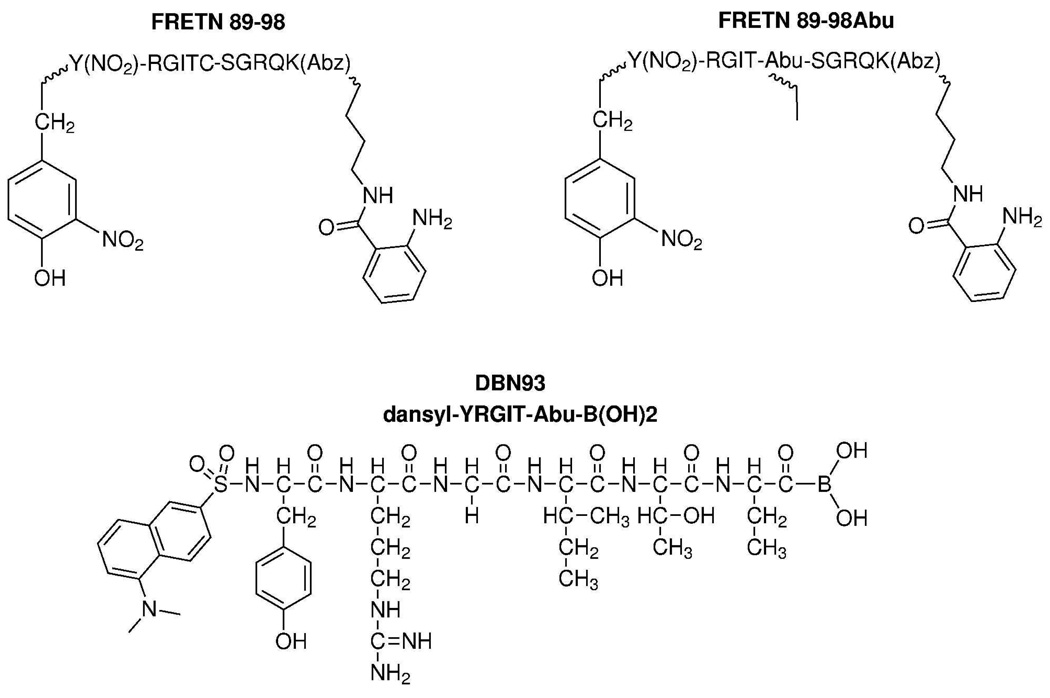
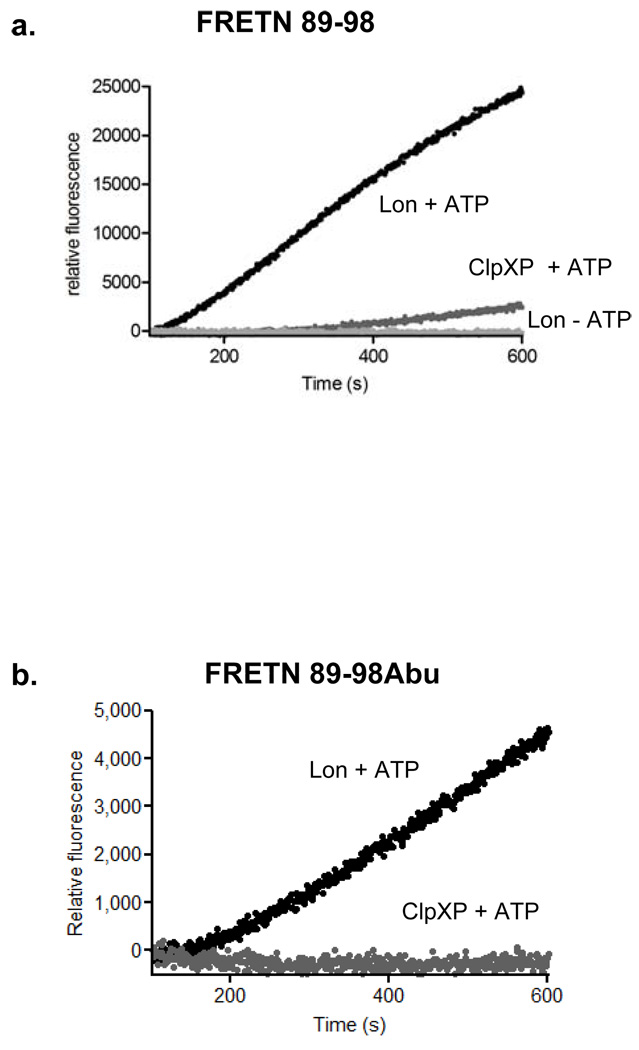
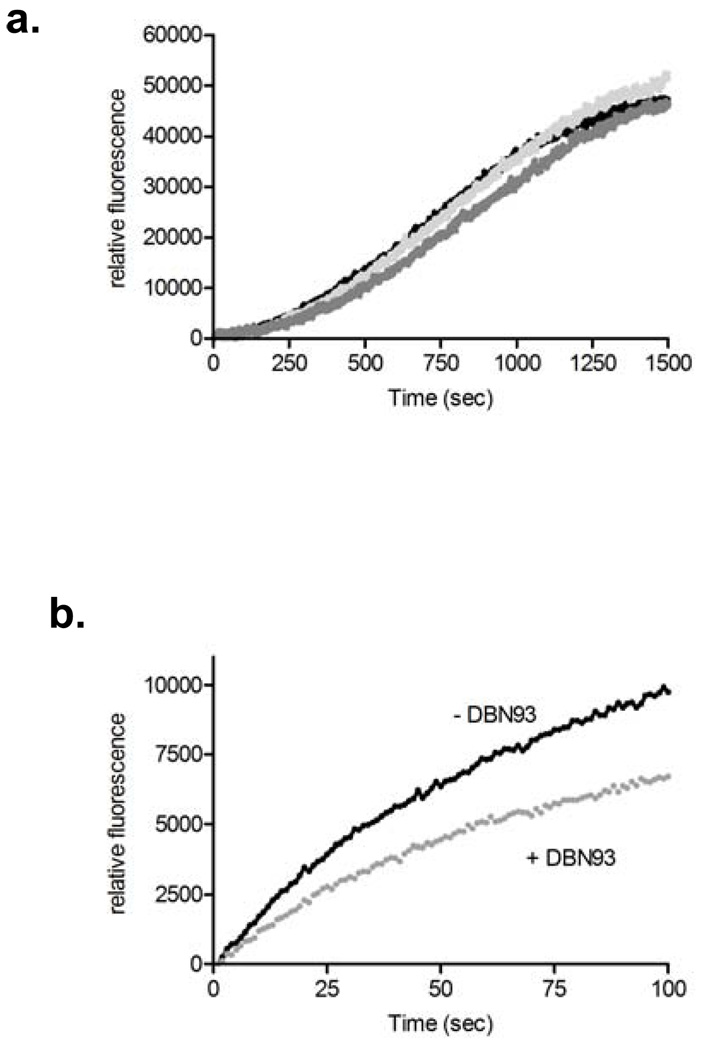
The different cleavage profiles of oxidized insulin B by Lon and ClpXP reported by Maurizi and others (1, 21) lends the possibility of generating specific peptide substrate reporters for the proteases. As such, we hypothesize that the peptide reporter FRETN 89–98 (Figure 1), which has been shown to be degraded by bacterial and human Lon (22) will be at least a preferred, if not specific, substrate of hLon, but not hClpXP. Indeed, under identical conditions, the rate of hLon meditated ATP-dependent FRETN89–98 cleavage was 5-fold faster than that of hClpXP (Figure 2, panel a). By contrast, the FRETN 89–98Abu (Figure 1), where the Cys at the cleavage site of FRETN 89–98 is replaced with the non-natural amino acid aminobutyric acid (Abu), was only cleaved by hLon and not hClpXP (Figure 2, panel b). These results demonstrate that specific probes for monitoring ATP-dependent protease activity can be generated by exploiting the differences in their peptide cleavage specificities, a task that is not easily accomplished with protein substrates as reporters. Furthermore, the peptide FRETN 89–98 could be used to monitor the activities of purified hLon or hClpXP individually in vitro or the total contribution of ATP-dependent protease activity in a mitochondrial matrix sample, whereas FRETN 89–98Abu can be used to selectively monitor the activity of hLon in the presence of hClpXP.
Figure 1. Structures of peptide-based substrates and inhibitor used in these experiments.
FRETN 89–98 contains an anthranilamide fluorescent donor at the carboxy terminus and a nitrotyrosine quencher at the amino terminus. Upon cleavage of the peptide at Cys-Ser, the donor and quencher separate and an increase in fluorescence emission can be monitored over time. FRETN 89–98Abu contains a substitution of Abu for Cys at the cleavage site. Inhibitor DBN93 was designed from a product of FRETN 89–98 peptide hydrolysis by Lon with a boronic acid moiety on the carboxy end to interact with the active site of the protease.
Figure 2. Peptide-based substrates are used to monitor activity of human Lon and human ClpXP.
(a) FRETN 89–98 is cleaved by both human Lon (black) and human ClpXP (dark gray) in the presence of ATP with different efficacies. The time course for hLon cleavage of FRETN 89–98 in the absence of ATP is shown in light gray. (b) FRETN 89–98Abu is a specific substrate for human Lon (black) and is not cleaved by human ClpXP (gray).
Generation of selective inhibitor, DBN93
The preferential cleavage of FRETN 89–98Abu peptide by hLon suggests that the hydrolyzed peptide product YRGIT(Abu) would be bound to the proteolytic site of hLon but not hClpP. Therefore, derivatizing the carboxyl terminal of YRGIT(Abu) with a boronic acid moiety, which acts as an electrophile to sequester the proteolytic site Ser in Lon, should generate a mechanism-based inhibitor that blocks proteolysis. This chemical tool will aid in evaluating the contribution of hLon in protein quality control in mammalian mitochondria. The peptidyl boronic acid dansyl-YRGIT-Abu-B(OH)2, abbreviated as DBN93 (Figure 1), contains part of the sequence derived from a hydrolyzed peptide product generated from λN protein, as well as FRETN 89–98, degradation by Lon (23). The amino terminal of DBN93 is also derivatized with a dansyl group to offer the opportunity to probe its mechanism of interaction with Lon if needed (24).
Site-directed inhibition of human Lon by DBN93
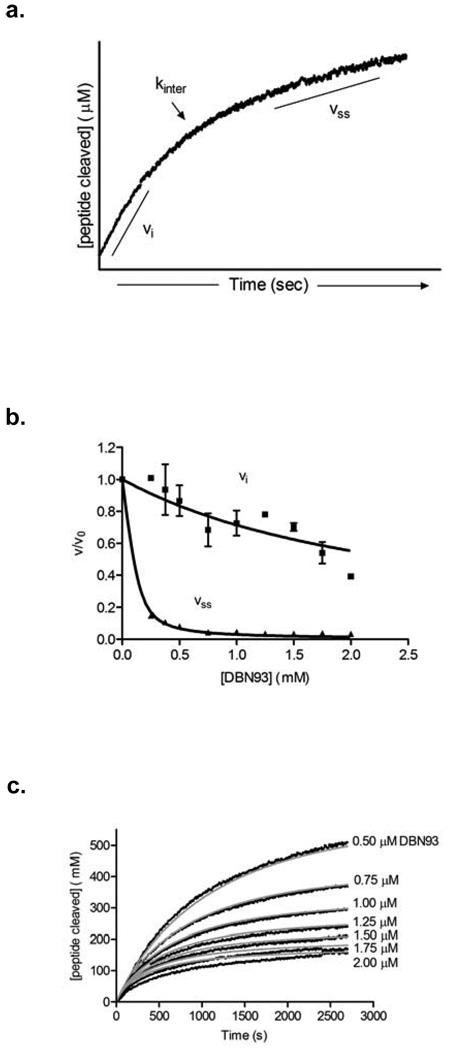
To further evaluate the potency of DBN93 towards the inhibition of hLon, the kinetic parameters of inhibition were determined using the method previously developed for bacterial Lon using FRETN 89–98 as the reporter probe (23). As with S. Typhimurium Lon, the peptidase activity of hLon was inhibited by DBN93 in a time-dependent manner, illustrated by the two distinct rates of peptide cleavage in the representative time course shown in Figure 3, panel a: vi for the initial rate and vss for the final rate before substrate depletion. The rate constant for the interconversion of the two rates, kinter, can also be quantified (25). The vi and vss rates decrease to different degrees with the increase in [DBN93] (Figure 3, panel b), suggesting a two-step mechanism of inhibition. Analysis of this data yielded the following inhibition constants: Ki = 1.35 ± 0.19 µM and Ki*= 0.014 ± 0.001 µM. To support the two-step mechanism, the experimental time courses were globally fitted to both a one- and a two-step inhibition mechanism using the nonlinear fitting program Dynafit (Biokin) (26) (Figure 3, panel c). The experimental data fit best to the two-step mechanism and the resulting kinetic parameters presented in Table 1 agree closely with those obtained by analyzing the individual plots shown in Figure 3 as done previously for S. Typhimurium Lon (23). The Ki* value, which reflects the overall inhibitor potency for hLon, is comparable to the Ki* determined previously for bacterial Lon. However, it is interesting to note that the Ki values, which reflect binding of the inhibitor to the enzyme, of hLon and bacterial Lon exhibit a similar difference as the previously reported Km values of FRETN 89–98 in the two enzymes (~4–6-fold higher for hLon): 300 µM and 1300 µM for bacterial and human Lon, respectively (27). Taken together, these results suggest that even a peptidyl sequence that weakly interacts with the proteolytic active site of Lon is sufficient to confer inhibition by the boronic acid functionality. As suggested in Figure 2, panel b and earlier discussion, the peptide sequence of FRETN 89–98Abu is not a substrate of hClpXP and thus DBN93 does not inhibit this protease because of its negligible interaction with the proteolytic site of hClpXP.
Figure 3. DBN93 inhibits human Lon by a two-step mechanism.
(a) Representative time course of peptide cleavage by human Lon in the presence of ATP and DBN93. Two rates of cleavage, vi and vss, and a rate constant of interconversion between the two, kinter, can be quantified from the experimental time course. (b) Reactions containing 150 nM human Lon were preincubated with 1 mM FRETN 89–98 prior to the addition of 1 mM ATP. After 90 s, varying amounts of DBN93 (0–2 µM) were added and peptide hydrolysis was monitored over 45 min. All experiments were done in at least duplicate and the vi and vss values determined by fitting the time courses as done previously for bacterial Lon (23). The averaged vi (■), vss (▲) in the presence of inhibitor normalized to 1 with the average velocity in the absence of inhibitor were plotted against corresponding inhibitor concentrations. The solid lines represent the best fit of the data, as described in Materials and Methods. (c) Experimental time courses were fit to both one-step and two-step inhibition mechanisms using the global non-linear fitting program, DynaFit (Biokin, Ltd.). Black lines represent the averaged experimental time courses at 150 nM hLon, 1 mM ATP, 1 mM peptide and varying concentrations of DBN93. Gray lines represent the best fit of the data to the two-step time-dependent mechanism which was most consistent with the experimental data. The kinetic parameters yielded from this fit are summarized and compared to those previously determined for bacterial Lon (23) in Table 1.
Table 1.
Kinetic parameters of inhibition of human Lon peptidase activity by DBN93 as determined by global fitting of experimental data using DynaFit. The resultant values are compared to those previously determined for bacterial Lon. Reported errors are s.e.m.
| Parameter | Human Lon |
S. Typhimurium Lon† |
|---|---|---|
| k3 (× 105 M−1s−1) | 4.34 ± 0.16 | 1.0 ± 0.3 |
| k4 (s−1) | 0.0597 ± 0.0022 | 0.022 ± 0.007 |
| Ki (µM) | 1.37 | 0.216 |
| k5 (s−1) | 0.0090 ± 0.0004 | 0.0032 ± 0.0003 |
| k6 (s−1) | 0.00014 ± 0.00001 | 0.00028 ± 0.00002 |
| Ki* (µM) | 0.021 | 0.017 |
Data previously published (23)
Specific inhibition of Lon protease activity
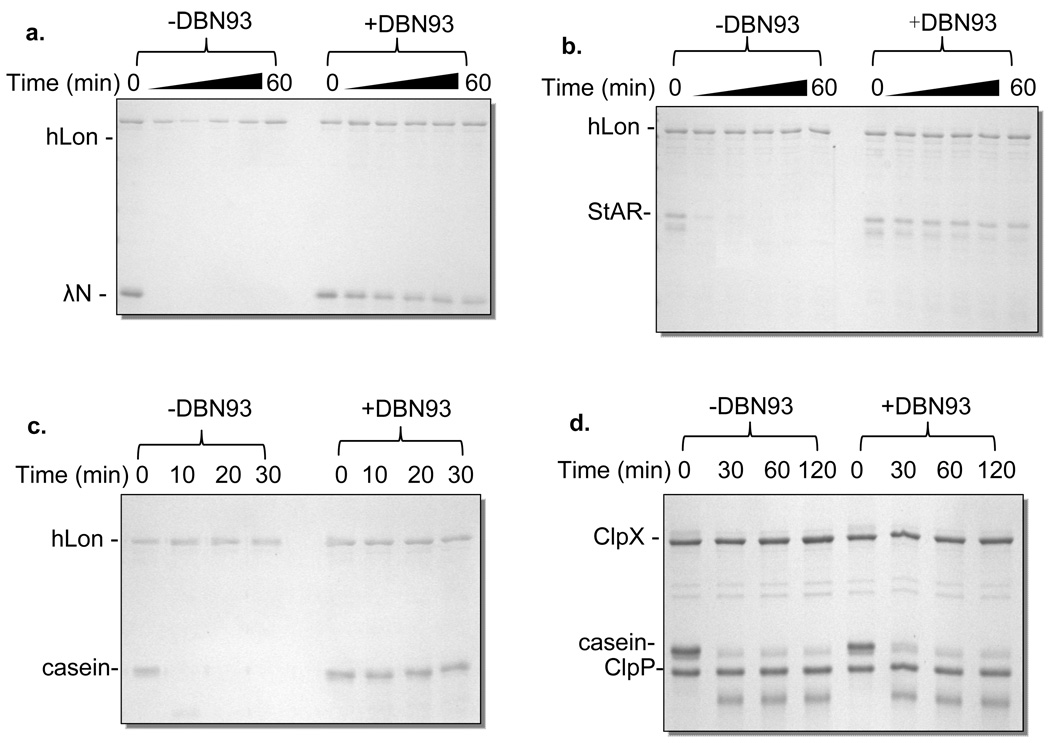
To evaluate the efficacy of DBN93 inhibiting the proteolytic activity of hLon, the ATP-dependent degradation of three protein substrates: λN; Steroidogenic Acute Regulatory (StAR) protein, an endogenous substrate of hLon (6, 28); and α-casein, an unstructured protein degraded by a wide range of proteases, were examined in the presence and absence of the inhibitor. As the peptide sequence in DBN93 is found in the Lon-specific substrate FRETN 89–98Abu and λN protein, we predicted that the protease activity of hLon, but not hClpXP, would be inhibited. As shown in Figure 4, Lon degrades both λN (panel a) and StAR (panel b) within 20 min in the presence of ATP. However, in the presence of 10 µM DBN93, Lon-mediated degradation of λN and StAR is not observed even after 60 min. The effect of DBN93 on the degradation of α-casein was also tested. Under identical experimental conditions, DBN93 inhibited α-casein degradation by hLon (Figure 4, panel c), but not by hClpXP (Figure 4, panel d), as predicted. While the inhibition of λN degradation is expected as the peptide sequence of DBN93 is derived from one of the products, it is not found in StAR or casein. However, the interaction of YRGIT-Abu in DBN93 with Lon likely directs the electrophilic boronic acid functionality to react with the proteolytic site Ser. The specificity of DBN93 towards hLon is further confirmed by the observation that the cleavage of FRETN 89–98 by purified hClpXP was not affected by up to 4 µM DBN93 (Figure 5, panel a), a condition that would have completely inhibited Lon (23). These results therefore collectively highlight the usefulness of exploiting the peptide sequence of substrates and inhibitors to develop specific compounds which can be used to determine the physiological functions of ATP-dependent proteases. Despite the promiscuity of protein substrate specificity and lack of defined peptide cleavage specificity in Lon, a proteolytic site-specific inhibitor can be generated from the hydrolyzed peptide product.
Figure 4. DBN93 inhibits degradation of proteins by human Lon, but not by ClpXP, as resolved by SDS-PAGE.
Reactions containing 10 µM λN (a) or StAR (b) protein, 1 µM human Lon and 5 mM ATP were analyzed by SDS-PAGE in the absence and presence of 10 µM DBN93 inhibitor. The band beneath StAR corresponds to an unidentified product. Casein (10 µM) digests with 1 µM hLon (c) or 2 µM ClpX and 2 µM ClpP (d) with 5 mM ATP in the absence and presence of 10 µM DBN93 were analyzed by SDS-PAGE.
Figure 5. DBN93 inhibits 20S, but not ClpXP peptidase activity.
(a) Cleavage of FRETN 89–98 by ClpXP was monitored in the absence (black) and presence of 1 µM (light gray) or 4 µM (dark gray) DBN93. (b) In the presence of 500 µM ATP, 200 nM human 20S proteasome cleaves 100 µM FRETN 89–98 peptide (black). The addition of 2 µM DBN93 inhibited the cleavage (gray). This illustrates the need to isolate mitochondria from cytosol so Lon can be preferentially inhibited by DBN93.
Although the proteasome belongs to the same protein degradation machine family of Lon and ClpXP, it is absent in the mitochondria. To further assess the specificity of the two fluorogenic peptides as activity probes for hLon, we evaluated the cleavage of FRETN 89–98 by the 20S proteasome, which contains only the proteolytic component of the protease machine. As shown in Figure 5, panel b, an increase in fluorescence attributing to the cleavage of the fluorogenic peptide is detected. Since the ATPase component of the protease is absent in the peptidase reaction, we conclude that unlike Lon, the proteasome degrades FRETN 89–98 in an ATP-independent manner. Since FRETN 89–98 is cleaved by the 20S proteasome, it is conceivable that this protease would be inhibited by DBN93 if the hydrolyzed peptide product of FRETN 89–98 will bind to the proteasome and deliver the boronic acid moiety to inactivate the proteolytic site. Figure 5, panel b also shows that DBN93 inhibits the 20S proteasome catalyzed cleavage of FRETN 89–98, indicating that although hLon and the 20S proteasome are Ser and Thr proteases respectively, they share more similar peptide cleavage specificity than Lon does with ClpXP, which is also Ser protease. Since many proteasome inhibitors also inhibit Lon activity (27, 28), and the proteasome is a therapeutic target, further studies will be needed to evaluate if the inhibition of hLon in the mitochondria provides a beneficial or detrimental effect on the therapeutic value of drugs targeting the proteolytic activity of the 20S proteasome. As FRETN 89–98 is a substrate of hLon and the proteasome in vitro, it should allow the direct comparison of the potency and specificity of a specific drug against the respective proteases.
Inhibition of mitochondrial protein mixture
Because the proteasome degrades the FRETN 89–98 peptide and is inhibited by the peptide boronic acid DBN93 (Figure 5, panel b), the activity of hLon cannot be directly detected in cell lysate or in live cells at this time. However, hClpXP is not inhibited by DBN93, and it is significantly less efficient in cleaving the FRETN 89–98 peptide in a non-ATP-dependent manner (Figure 2). Therefore we propose that DBN93 should selectively inhibit hLon in isolated mitochondrial lysate and could be useful as chemical genetic tool to probe the physiological functions of Lon at a post-translational level. The ATP-dependent cleavage of FRETN 89–98 could be used as a reporter assay to track the activity of Lon in isolated mitochondria lysate, which will allow direct assessment on the contribution of Lon in the cardiac ischemia/reperfusion model study reported by Bulteau et al. (29).
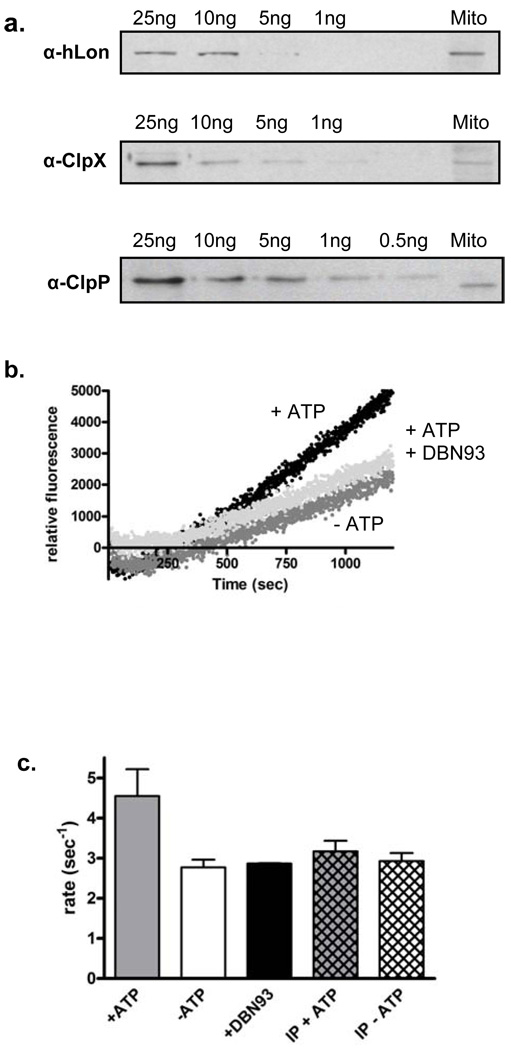
To assess the ability of our peptide reporter to detect ATP-dependent protease activity, and the efficacy of DBN93 to selectively inhibit hLon in biological samples containing hClpXP, we evaluated the cleavage of FRETN 89–98 in mitochondrial matrix proteins isolated from HeLa cell cultures. These proteins were isolated using a method that removed mitochondrial membranes and were thus free of membrane bound ATP-dependent protease contamination. The presence of hLon and hClpXP in the isolated protein mixture was confirmed by Western Blot analysis using antibodies against each enzyme (Figure 6, panel a). The lower molecular weight of ClpP in the mitochondrial matrix protein mixture might be due to autoprocessing of ClpP as reported previously (30) or due to the fact that our purified ClpP protein contains a 6x His-tag which changes the molecular weight.
Figure 6. Monitoring ATP-dependent peptide cleavage of FRETN 89–98 by isolated mitochondria.
(a) Western blots of mitochondria isolated from HeLa cells visualized with antibodies against known amounts of human Lon, ClpX, or ClpP as indicated to approximate amount of enzyme in the isolated mitochondria. (b) Cleavage of 100 µM FRETN 89–98 by 5 µg of the purified mitochondrial matrix protein mixture was monitored in the absence (black) and presence (dark gray) of 1 mM ATP. The addition of 10 µM DBN93 inhibited ATP-dependent peptidase activity (light gray) to intrinsic levels. (c) In the presence of ATP (black), there is an increase in the rate of peptide cleavage by mitochondria containing Lon over that in the absence of ATP (dark gray). In mitochondria immunodepleted of Lon (hatch marks), there is no increase in peptide cleavage in the presence of ATP. Upon addition of 10 µM DBN93 (light gray), ATP-dependent peptide cleavage was brought back down to the background rate in the mitochondria containing Lon and no decrease in peptide cleavage was seen in the immunodepleted mitochondria. Error bars indicate the standard error from least three trials.
To monitor peptidase activity, 5 µg of the isolated matrix protein mixture was incubated with 100 µM FRETN 89–98 with and without the addition of 1 mM ATP. As shown in Figure 6, panel b, an increase in fluorescence emission signal attributed to FRETN 89–98 cleavage over time is detected in the 1 mM ATP time course. By comparison, there is less change in fluorescence intensity over the same time period when no additional ATP is present. This observation confirms that ATP-dependent cleavage of FRETN 89–98 is detectable in mitochondrial protein mixture isolated from cell culture. When DBN93, which inhibits hLon and not hClpXP, was added to the FRETN 89–98 cleavage reaction containing 1 mM ATP, the peptidase signal was reverted to the background level, where ATP was omitted (Figure 6, panel b). This result suggests that we were only detecting hLon cleavage of FRETN 89–98 in the protein mixture. As discovered earlier, purified hClpXP is at least 5-fold less efficient in mediating the ATP-dependent cleavage of FRETN 89–98 (Figure 2, panel a); therefore, the lack of detectable ClpXP activity in the isolated HeLa mitochondrial matrix proteins could be attributed to the presence of a relatively low concentration of functional hClpXP complex. This is supported by the immunoblot analysis showing that the theoretical concentration of hLon in the mitochondria exceeds that of hClpXP by ~ 2.5-fold.
To confirm that we are monitoring only hLon cleavage of the peptide, the isolated mitochondria were immunodepleted of Lon and tested for ATP-dependent peptidase activity. As shown in Figure 6, panel c, we were able to abolish ATP-dependent peptidase activity in two ways: the addition of Lon-specific inhibitor, DBN93, and by immunodepleting Lon from the isolated mitochondria. These results provide further evidence that we are only able to detect hLon cleavage of the FRETN 89–98 peptide. Taken together, these results indicate that the peptidase assay accurately measures the respective ATP-dependent protease activity based on their specific concentrations in the sample.
Inhibition of StAR degradation in organello
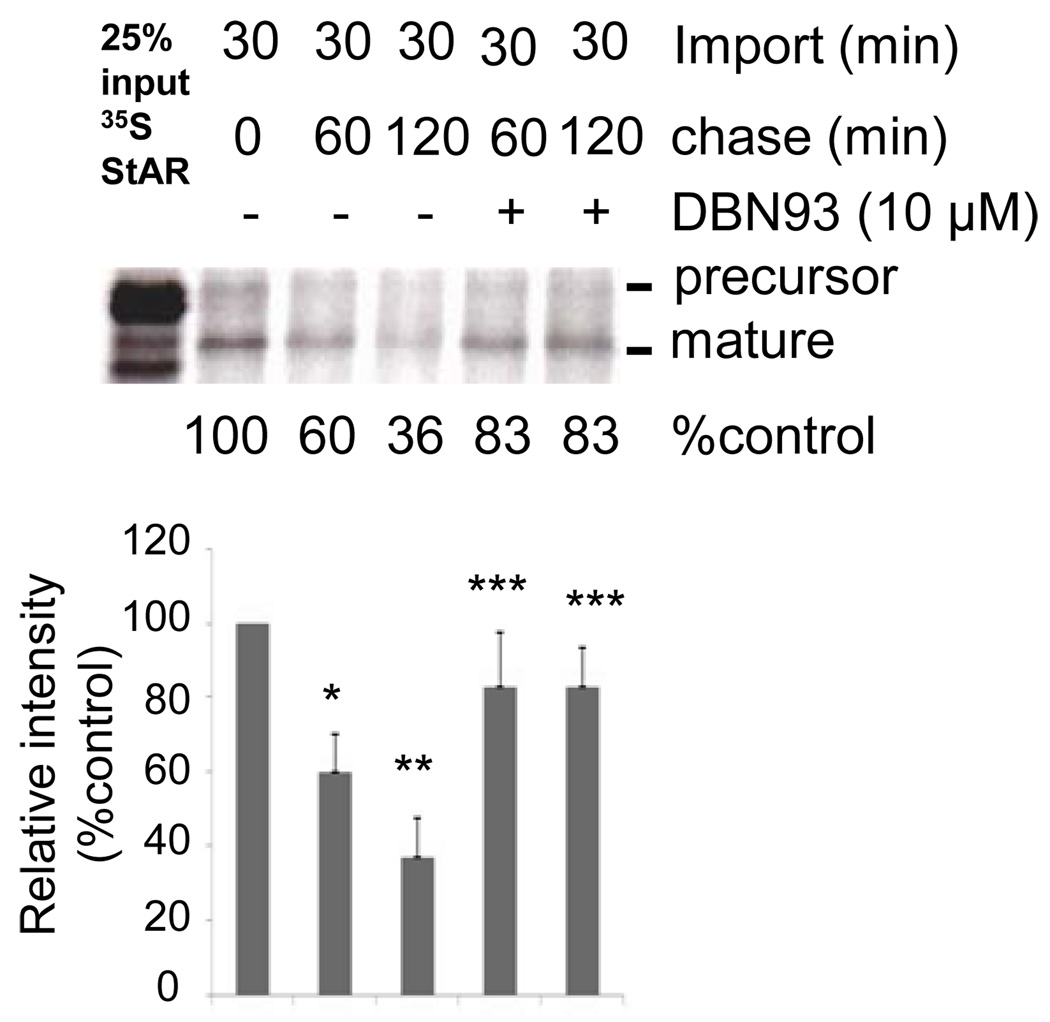
To evaluate the efficacy of DBN93 in inhibiting hLon in isolated mitochondria, the degradation profile of 35S- labeled Lon substrate StAR was monitored in rat mitochondria in the absence and presence of 10 µM DBN93. The 35S Met-labeled StAR precursor containing its mitochondrial targeting sequence was imported into isolated mitochondria and then chased for the indicated time periods (Figure 7). The amount of 35S Met-labeled StAR precursor that was imported into mitochondria and processed to the mature protein was determined by removing the untranslocated precursor by trypsin-treating the mitochondria. The proteolytic turnover of 35S-labeled StAR was determined in the presence and absence of DBN93. In the absence of the inhibitor, 36% of the 35S Met- labeled StAR remained after the 120 min chase. By contrast, in the presence of DBN93, 83% of the 35S Met-labeled StAR was present after the chase. These data indicate that DBN93 can penetrate the mitochondrial membranes to inhibit Lon in the matrix. Taken together, these results demonstrate that in mitochondrial lysates, the FRETN 89–98 reporter substrate can be used to measure the protease activity of Lon, and that in mitochondrial lysates as well as in intact mitochondria, DBN93 can be employed to inactivate Lon-mediated proteolysis.
Figure 7. DBN93 inhibits degradation of StAR in rat mitochondria.
35S-StAR precursor protein was imported into rat mitochondria for either 30 min, or 30 min with a 1 or 2 hr chase, with or without 10 µM DBN93 Lon inhibitor. The mean relative intensities of 3 independent experiments are shown. SEM (n=3); p-values were calculated by Student’s t-test as compared to control. * p < 0.05, ** p <0.01, *** p > 0.05.
Conclusion
In this study, we demonstrate the feasibility of developing a specific fluorogenic peptide substrate (FRETN 89–98 Abu), as well as a peptidyl boronic acid inhibitor (DBN93) to distinguish the ATP-dependent proteolytic activities of hLon and hClpXP. We propose that the proteolytic-site directed approach reported here provides a generalizable strategy for developing protease-specific peptide substrate reporters and peptide inhibitors to measure the respective activities of mitochondrial and non-mitochondrial ATP-dependent proteases. This chemical biology approach depends upon identifying peptides carrying protease-specific cleavage sites that can be exploited in developing these chemical tools. Future work is directed toward identifying peptide reporters and peptide inhibitors to distinguish, for example, the protease activity of mitochondrial Lon versus the 20S proteasome.
The FRETN 89–98 Abu reporter substrate and the DBN93 inhibitor will also permit a quantitative analysis of Lon activity in isolated mitochondria in response to metabolic or environmental changes such as oxygen availability or oxidative stress. Data suggest that mitochondrial ATP-dependent proteolysis functions as a defense mechanism during oxidative stress. In vitro, Lon has been implicated in degrading mildly oxidized aconitase (31, 32). In an animal model of cardiac ischemia, increased levels of oxidized proteins are observed in mitochondrial lysates after cardiac reperfusion, which is followed by increased ATP-dependent proteolysis and a decrease in oxidized proteins (29). Similarly, treatment of isolated mitochondria with an H2O2-generating system stimulates energy-dependent proteolysis (29, 33). Questions remain, however, as to whether Lon and/or ClpXP are activated by oxidative stress and the extent to which each protease is responsible for degrading oxidatively damaged proteins in mammalian mitochondria. Future work employing the chemical tools described here will help to answer these important questions. In addition, we anticipate that the development of new and improved peptide reporters and peptide inhibitors that specifically distinguish the proteasome and mitochondrial ATP-dependent proteases will be invaluable to understanding the role that these enzymes play in diseases linked to the misfolding, damage, and aggregation of cellular proteins.
METHODS
Methods and materials on the synthesis of peptide substrates, inhibitors, expression and purification of human Lon as well as ClpXP; kinetic measurement of DBN93 inhibition as well as data analyses are detailed in the supporting Information available online.
Steady state peptidase activity assay
Reactions containing 50 mM HEPES (pH 8.0), 5 mM Mg(OAc)2, 2 mM DTT, 200 nM hLon, hClpXP or human 20S proteasome, 100 µM FRETN 89–98 or FRETN 89–98Abu, and 500 µM ATP were run on a FluoroMax-3 fluorometer at 37 °C. An increase in fluorescence emission at 420 nm (λex= 320 nm) indicates cleavage of the peptide, the rate of which can be quantified by the slope of the linear phase of the time course. For Lon reactions, HEPES, Mg(OAc)2, DTT, peptide and Lon were incubated for 1 min at 37 °C prior to initiation with ATP. For ClpXP reactions, HEPES, Mg(OAc)2, DTT, ClpX, ClpP, and ATP were incubated for 1 min at 37 °C prior to initiation by peptide. For proteasome reactions, reactions were initiated with the addition of 20S.
Steady-state inhibition of protein degradation
Reactions containing 50 mM Tris (pH 8.1), 15 mM Mg(OAc)2, 5 mM DTT, 10 µM λN or StAR protein, 1 µM hLon or 2 µM ClpX and 2 µM ClpP, and 5 mM ATP were incubated at 37 °C in the absence and presence of 10 µM DBN93. Reaction aliquots were quenched at various time points (0–90 min) with SDS-PAGE loading dye. Each time point was loaded onto a 12.5% SDS-PAGE gel and stained by Coomassie Brilliant Blue.
Isolation of mitochondrial lysate from HeLa cells
Intact mitochondria was isolated from 120 × 106 HeLa cells using the Mitochondria Isolation Kit for Mammalian Cells from Pierce (Rockford, IL) according to the manufacturer’s protocol with the addition of 0.2 mM final EDTA (pH 8). On half of the isolated mitochondria was resuspended in 50 µL 50 mM KPi (pH 7), 20% glycerol, 0.1% Tween 20 and put through a CentriSpin-10 column (Princeton Separations) equilibrated in the same buffer to remove small molecular weight contaminants and stored at 4 °C. The amount of protein present in the isolated mitochondria was quantified using the Bradford assay using BSA as a standard (34). The second half of isolated mitochondria was immunodepleted of Lon as detailed below.
Western Blot analysis of isolated mitochondrial lysate
The presence and relative amount of the respective protease complex components in mitochondria isolated from 120 million HeLa cells suspended in 100 µl 50mM KPi (pH 7), 20% glycerol, 0.1% Tween 20 buffer were extrapolated by Western Blot Analysis. Known amounts of purified hLon, hClpP, or hClpX as reference standards were compared to a sample of partially purified isolated mitochondrial protein mixture. The amount of each enzyme present in the mitochondrial sample was approximated and divided by the volume of sample loaded on the gel to yield ng protein/µl mitochondria. This concentration was then converted to number of copies/cell. This analysis suggests the proteins exist in approximately the following concentrations: 5000 copies of Lon/cell, 5000 copies of ClpX/cell, and 2000 copies of ClpP/cell.
Immunodepletion of human Lon from isolated mitochondrial lysate
Antiserum raised against hLon was purified by incubating production bleed with nitrocellulose containing purified hLon protein and eluting the purified antibody from the nitrocellulose using 100 mM glycine (pH 2.5). Mitochondria isolated from HeLa cells as described above were resuspended in 50 µL 25 mM Tris pH 7.5, 100 mM NaCl, 0.1% Tween 20 immunoprecipitation buffer and incubated overnight at 4 °C with 100 µL of the purified anti-hLon. This mixture was then added to Protein A Agarose (Pierce) that was previously blocked with 1% BSA in TBST and incubated at 4 °C for two hours. After incubation, the mixture was centrifuged at 2500 × g for 2 min to pellet the Protein A agarose bound to antibody and hLon. The supernatant containing isolated mitochondria lysate depleted of Lon was removed and subjected to a Centri-Spin 10 column equilibrated in 50 mM KPi (pH 7), 20% glycerol, 0.1% Tween 20 buffer. The amount of protein was quantified by a Bradford assay, using BSA as a standard (34).
Monitoring specific activity of Lon protease from isolated HeLa mitochondrial lysate
Reactions containing 50 mM HEPES (pH 8), 5 mM Mg(OAc)2, 2 mM DTT, 5 mM imidazole, and 100 µM FRETN 89–98 in the absence and presence of 1 mM ATP were incubated at 37 °C for 1 min. 5 µg of the purified isolated mitochondria was added and cleavage of peptide was observed by monitoring the fluorescent emission at 420 nm (λex=320 nm) for 1 hr in the absence and presence of inhibitor DBN93.
In organello degradation of StAR
The StAR cDNA was sub-cloned into the mammalian expression vector pCDNA3.1, linearized with XhoI and used for in vitro transcription using Ribomax RNA Production System T7 (Promega) according to the manufacturer’s protocol. In vitro translation of radiolabeled StAR precursor protein was carried out using EXPRE35S Protein Labeling Mix (Perkin Elmer) and rabbit reticulocyte lysate (Promega) according to the manufacturer’s protocol. The radiolabeled StAR precursor protein was imported into isolated rat liver mitochondria for 30 min at 30 °C as previously described (28). In brief, isolated mitochondria (600 µg) were incubated in HEPES-Sorbitol-BSA (HSB buffer, 20 mM HEPES, 0.6 M sorbitol, 0.1 mg/ml BSA) supplemented with 0.1 mg/ml BSA, 40 mM KOAc, 10 mM Mg(OAc)2, 5 mM unlabeled methionine, 1 mM dithiothreitol (DTT), 4 mM NADH, 4 mM adenosine triphosphate (ATP), 1 mM guanosine triphosphate (GTP), 20 mM phosphocreatine, and 0.2 mg/ml creatine kinase. Import was initiated by the addition of 35S-Met-labeled precursor protein at 30 °C for 30 min. After the import reaction, unimported precursor polypeptides were digested using 0.2 mg/ml trypsin on ice for 35 min. Trypsin digestion was terminated by adding 5 mg/ml soybean trypsin inhibitor. To determine the stability of imported StAR, mitochondria were pelleted by centrifugation at 15,000 × g at 4 °C for 10 min, and re-suspended in 250 µl of HSB buffer with or without 10 µM Lon inhibitor DBN93, and then incubated at 30 °C for the time periods as indicated. After each time point, 50 µl aliquots were removed, and the mitochondria were centrifuged, washed and then precipitated with 10% trichloroacetic acid. Samples were analyzed by 12% SDS-PAGE and autoradiography. Bands corresponding to the precursor and mature forms of StAR were quantified by ImageJ analysis (NIH) and Excel 2003 (Microsoft) software.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health grants R01 GM067172 (I. Lee) and R01 GM084039 and R21 NS067668 (C. K. Suzuki), the National Science Foundation grant MCB-0919631 (I. Lee), and the Foundation of UMDNJ (C. K. Suzuki).
Footnotes
Supporting Information Available: This material is available free of charge via the Internet.
REFERENCES
- 1.Kang SG, Ortega J, Singh SK, Wang N, Huang NN, Steven AC, Maurizi MR. Functional proteolytic complexes of the human mitochondrial ATP-dependent protease, hClpXP. J Biol Chem. 2002;277:21095–21102. doi: 10.1074/jbc.M201642200. [DOI] [PubMed] [Google Scholar]
- 2.Corydon TJ, Wilsbech M, Jespersgaard C, Andresen BS, Borglum AD, Pedersen S, Bolund L, Gregersen N, Bross P. Human and mouse mitochondrial orthologs of bacterial ClpX. Mamm Genome. 2000;11:899–905. doi: 10.1007/s003350010173. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Nafria J, Ondrovicova G, Blagova E, Levdikov VM, Bauer JA, Suzuki CK, Kutejova E, Wilkinson AJ, Wilson KS. Structure of the catalytic domain of the human mitochondrial Lon protease: proposed relation of oligomer formation and activity. Protein Sci. 19:987–999. doi: 10.1002/pro.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bae MK, Jeong JW, Kim SH, Kim SY, Kang HJ, Kim DM, Bae SK, Yun I, Trentin GA, Rozakis-Adcock M, Kim KW. Tid-1 interacts with the von Hippel-Lindau protein and modulates angiogenesis by destabilization of HIF-1alpha. Cancer Res. 2005;65:2520–2525. doi: 10.1158/0008-5472.CAN-03-2735. [DOI] [PubMed] [Google Scholar]
- 5.Im YJ, Na Y, Kang GB, Rho SH, Kim MK, Lee JH, Chung CH, Eom SH. The active site of a lon protease from Methanococcus jannaschii distinctly differs from the canonical catalytic Dyad of Lon proteases. J Biol Chem. 2004;279:53451–53457. doi: 10.1074/jbc.M410437200. [DOI] [PubMed] [Google Scholar]
- 6.Ondrovicova G, Liu T, Singh K, Tian B, Li H, Gakh O, Perecko D, Janata J, Granot Z, Orly J, Kutejova E, Suzuki CK. Cleavage Site Selection within a Folded Substrate by the ATP-dependent Lon Protease. J. Biol. Chem. 2005;280:25103–25110. doi: 10.1074/jbc.M502796200. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki CK, Suda K, Wang N, Schatz G. Requirement for the yeast gene LON in intramitochondrial proteolysis and maintenance of respiration. Science. 1994;264:273–276. 891. doi: 10.1126/science.8146662. [DOI] [PubMed] [Google Scholar]
- 8.van Dijl JM, Kutejova E, Suda K, Perecko D, Schatz G, Suzuki CK. The ATPase and protease domains of yeast mitochondrial Lon: roles in proteolysis and respiration-dependent growth. Proc Natl Acad Sci U S A. 1998;95:10584–10589. doi: 10.1073/pnas.95.18.10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner I, Arlt H, van Dyck L, Langer T, Neupert W. Molecular chaperones cooperate with PIM1 protease in the degradation of misfolded proteins in mitochondria. EMBO J. 1994;13:5135–5145. doi: 10.1002/j.1460-2075.1994.tb06843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu B, Yadav S, Shah PG, Liu T, Tian B, Pukszta S, Villaluna N, Kutejová E, Newlon CS, Santos JH, Suzuki CK. Roles for the human ATP-dependent Lon protease in mitochondrial DNA maintenance. J. Biol. Chem. 2007;282:17363–17374. doi: 10.1074/jbc.M611540200. [DOI] [PubMed] [Google Scholar]
- 11.van Dyck L, Pearce DA, Sherman F. PIM1 encodes a mitochondrial ATP-dependent protease that is required for mitochondrial function in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1994;269:238–242. [PubMed] [Google Scholar]
- 12.Bayot A, Basse N, Lee I, Gareil M, Pirotte B, Bulteau AL, Friguet B, Reboud-Ravaux M. Towards the control of intracellular protein turnover: Mitochondrial Lon protease inhibitors versus proteasome inhibitors. Biochimie. 2008;90:260–269. doi: 10.1016/j.biochi.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Leidhold C, von Janowsky B, Becker D, Bender T, Voos W. Structure and function of Hsp78, the mitochondrial ClpB homolog. J Struct Biol. 2006;156:149–164. doi: 10.1016/j.jsb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Bota DA, Davies KJA. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat. Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 15.Bulteau AL, Dancis A, Gareil M, Montagne JJ, Camadro JM, Lesuisse E. Oxidative stress and protease dysfunction in the yeast model of Friedreich ataxia. Free Radic Biol Med. 2007;42:1561–1570. doi: 10.1016/j.freeradbiomed.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 17.Hori O, Icinoda F, Tamatani T, Yamaguchi A, Sato N, Ozawa K, Kitao Y, Miyazaki M, Harding HP, Ron D, Tohyama M, Stern DM, Ogawa S. Transmission of cell stress from endoplasmic reticulum to mitochondria: enhanced expression of Lon protease. J. Cell Biol. 2002;157:1151–1160. doi: 10.1083/jcb.200108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aldridge JE, Horibe T, Hoogenraad NJ. Discovery of genes activated by the mitochondrial unfolded protein response (mtUPR) and cognate promoter elements. PLoS ONE. 2007;2:e874. doi: 10.1371/journal.pone.0000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haynes CM, Petrova K, Benedetti C, Yang Y, Ron D. ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev Cell. 2007;13:467–480. doi: 10.1016/j.devcel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002;21:4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maurizi MR. Degradation in vitro of bacteriophage lambda N protein by Lon protease from Escherichia coli. J. Biol. Chem. 1987;262:2696–2703. [PubMed] [Google Scholar]
- 22.Lee I, Berdis AJ. Adenosine triphosphate-dependent degradation of a fluorescent lambda N substrate mimic by Lon protease. Anal. Biochem. 2001;291:74–83. doi: 10.1006/abio.2001.4988. [DOI] [PubMed] [Google Scholar]
- 23.Frase H, Lee I. Peptidyl boronates inhibit Salmonella enterica serovar Typhimurium Lon protease by a competitive ATP-dependent mechanism. Biochemistry. 2007;46:6647–6657. doi: 10.1021/bi7002789. [DOI] [PubMed] [Google Scholar]
- 24.Patterson-Ward J, Huang J, Lee I. Detection and characterization of two ATP-dependent conformational changes in proteolytically inactive Escherichia coli lon mutants by stopped flow kinetic techniques. Biochemistry. 2007;46:13593–13605. doi: 10.1021/bi701649b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Copeland RA. Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists. Hoboken, NJ: John Wiley & Sons; 2005. [PubMed] [Google Scholar]
- 26.Kuzmic P. Program DYNAFIT for the analysis of enzyme kinetic data: application to HIV proteinase. Anal Biochem. 1996;237:260–273. doi: 10.1006/abio.1996.0238. [DOI] [PubMed] [Google Scholar]
- 27.Frase H, Hudak J, Lee I. Identification of the proteasome inhibitor MG262 as a potent ATP-dependent inhibitor of the Salmonella enterica serovar Typhimurium Lon protease. Biochemistry. 2006;45:8264–8274. doi: 10.1021/bi060542e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granot Z, Kobiler O, Melamed-Book N, Eimerl S, Bahat A, Lu B, Braun S, Maurizi MR, Suzuki CK, Oppenheim AB, Orly J. Turnover of mitochondrial steroidogenic acute regulatory (StAR) protein by Lon protease: the unexpected effect of proteasome inhibitors. Mol Endocrinol. 2007;21:2164–2177. doi: 10.1210/me.2005-0458. [DOI] [PubMed] [Google Scholar]
- 29.Bulteau AL, Lundberg KC, Ikeda-Saito M, Isaya G, Szweda LI. Reversible redox-dependent modulation of mitochondrial aconitase and proteolytic activity during in vivo cardiac ischemia/reperfusion. Proc Natl Acad Sci U S A. 2005;102:5987–5991. doi: 10.1073/pnas.0501519102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurizi MR, Clark WP, Kim S-H, Gottesman S. Clp P Represents a Unique Family of Serine Proteases. J Biol Chem. 1990;265:12546–12552. [PubMed] [Google Scholar]
- 31.Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 32.Bota DA, Van Remmen H, Davies KJ. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS Lett. 2002;532:103–106. doi: 10.1016/s0014-5793(02)03638-4. [DOI] [PubMed] [Google Scholar]
- 33.Sweetlove LJ, Heazlewood JL, Herald V, Holtzapffel R, Day DA, Leaver CJ, Millar AH. The impact of oxidative stress on Arabidopsis mitochondria. Plant J. 2002;32:891–904. doi: 10.1046/j.1365-313x.2002.01474.x. [DOI] [PubMed] [Google Scholar]
- 34.Fey PD, Safranek TJ, Rupp ME, Dunne EF, Ribot E, Iwen PC, Bradford PA, Angulo FJ, Hinrichs SH. Ceftriaxone-resistant salmonella infection acquired by a child from cattle. N Engl J Med. 2000;342:1242–1249. doi: 10.1056/NEJM200004273421703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.