Abstract
Background
Naltrexone (NTX) is an opioid antagonist indicated for the treatment of alcoholism, which is not universally effective. Thus, identifying individual predictors of NTX’s behavioral effects is critical to optimizing its therapeutic use. Moreover, given the high rate of relapse during treatment for alcoholism, understanding NTX’s behavioral effects when combined with moderate ethanol intake is important. Our previous study of abstinent alcoholics and control subjects showed that a more internal Locus of Control score predicted increased impulsive choice on NTX (Mitchell et al., 2007). Here we tested whether this predictive relationship remains in the context of moderate alcohol intake.
Methods
In the present study we tested the effect of acute NTX (50mg) on impulsive choice, motor inhibition, and attentional bias after ingestion of moderate ethanol (~0.3g/kg, n = 30 subjects). Subjects included those recruited from a pool of ~1200 UC Berkeley undergraduates on the basis of scores on the Barratt Impulsiveness Scale (BIS).
Results
Impulsive choice was positively correlated with breath alcohol concentration in placebo sessions. Locus of Control was again the sole predictor of NTX’s effect on decision-making among subjects with a family history of alcoholism. We also found a weak interaction between BIS scores and NTX’s effect on impulsive choice.
Conclusions
Our results reinforce the predictive relationship between Locus of Control and NTX’s effect on decision-making in those with a family history of alcoholism, suggesting a possible biological basis to this relationship.
Keywords: decision-making, delay-discounting, ethanol, impulsivity, naltrexone
INTRODUCTION
Naltrexone (NTX), an opioid antagonist, is approved by the U.S. Food and Drug Administration for treating alcoholism. While NTX reduces alcohol consumption in both humans (Anton et al., 1999; Davidson et al., 1999; O’Brien et al., 1996); see (Heidbreder, 2005) for a review) and animals (Boyle et al., 1998; Stromberg et al., 1998), likely by blocking the action of endogenous opioids, the neural mechanisms underlying NTX’s therapeutic effect are unknown. One possibility is that NTX reduces the rewarding “high” experienced from drinking alcohol (Sinclair, 2001; Swift et al., 1994; Volpicelli et al., 1995). Another possibility is that, when taken with alcohol, NTX causes aversive effects, such as nausea or dysphoria (Davidson et al., 1999; McCaul et al., 2000; Mitchell et al., 2009; Ortner et al., 2003). NTX reduces alcohol craving and urges in abstinent alcoholics (Anton et al 1999; Monti et al 1999; Rohsenow et al 2000; O’Malley et al 2000). Clinical data and basic studies in animals and humans suggest that NTX may reduce impulsiveness. For example, NTX effectively treats other impulse control disorders, such as pathological gambling (Kim et al., 2001). In addition, rodent studies demonstrate that NTX decreases a morphine-induced preference for small immediate rewards over larger delayed rewards (Kieres et al., 2004). Moreover, in humans, NTX reduces preferences for immediate alcohol consumption versus an equivalent amount of money (O’Malley et al., 2002). A laboratory study in a sample of abstinent alcoholics and healthy moderate drinkers found that NTX had a personality dependent effect on impulsive choice (Mitchell et al., 2007). This could represent one mechanism by which NTX helps to reduce drinking: by helping to favor the long-term benefits of abstinence over the short-term benefits of taking a drink of alcohol. Moreover, the personality-dependence of this effect could contribute to the variability in NTX’s therapeutic efficacy. Here we endeavored to test whether this personality factor, Locus of Control, predicts NTX’s effect on impulsive choice in a larger sample in a clinically relevant context: after consumption of a moderate dose of alcohol. When a patient samples alcohol during NTX treatment, these “slips” less frequently precipitate a full-blown relapse (Volpicelli et al., 1992). Although several factors could underlie this therapeutic benefit, this finding suggests that NTX effects on decision-making may persist in the context of moderate alcohol intake, helping to favor the long-term benefits of stopping after just one or two drinks over the short-term benefits of subsequent drinking.
To determine whether the previously observed effect of NTX on impulsive choice remains in humans under the influence of a moderate alcohol dose, we used a modified delay discounting (DD) task, which allows for separate evaluation of impulsive decision-making and motor impulsiveness. In this task, abstinent alcoholics select the smaller, sooner reward option significantly more often than do moderate drinking control subjects (Boettiger et al., 2007; Mitchell et al., 2005b; Mitchell et al., 2007). This tendency to choose impulsively was positively correlated with trait impulsivity as measured by the Barratt Impulsiveness Scale (BIS) (Mitchell et al., 2005b). Using a double-blind placebo-controlled randomized crossover design, we tested whether impulsive choice was reduced in healthy, young adults by a single acute dose of NTX (50 mg) when subjects were under the influence of moderate alcohol. Our earlier study also found that NTX reduced the “mismatch” of choices in the dominant task condition and inferred choices in a control condition (Mitchell et al., 2007). Our previous results supported the interpretation that this effect was not due to NTX effects on motor inhibition, but rather on attentional bias toward large monetary rewards. Thus, in the present study, we included direct measures of motor control (Go-noGo task) and attentional bias (dot-probe task). This allowed us to determine whether a reduction of mismatch by NTX was attributable to improved motor inhibition or to reduced attentional bias towards large monetary reward stimuli.
MATERIALS AND METHODS
Subjects
Subjects (n=30) were recruited from the community (n=15) and from a University of California, Berkeley (UCB; n=15) undergraduate population prescreened on the basis of scores on the Barratt Impulsiveness Scale (BIS; (Barratt, 1994). Students in the middle, upper, and lower 20th percentiles of BIS scores were targeted for recruitment. Student and community participants did not differ in terms of BIS scores (t28 = 0.49, p = 0.63) and were equally distributed across the high-impulsive and low impulsive groups (χ2(1) = 0.13, p = 1). All subjects were healthy individuals 21-35 years old with no history of alcohol or opiate abuse, neurological disorders, current treatment for any psychological disorders, or current psychoactive drug use, excluding nicotine, caffeine, and moderate alcohol. Subjects provided written, informed consent, as approved by the UCB Committee for the Protection of Human Subjects. Subjects participated in two sessions ≥96 hours apart (mean session separation time: 13.9 days) to allow for elimination of NTX between sessions (Lee et al., 1988; Verebey et al., 1976). Sessions spanned ~5 hrs and subjects received monetary compensation for participating. In addition to the behavioral testing (see “Behavioral Tasks”), during session 1, subjects completed a standard battery of questionnaires (see “Behavioral Inventories”). Subjects were instructed to abstain from alcohol and unnecessary medications for 24 hours prior to each session, and to eat a low fat, light meal approximately one hour before arriving. Upon arrival, subjects were screened for alcohol use via breathalyzer (FC-10, Lifeloc Inc., Wheat Ridge, CO) and for psychoactive drug use via urine screen (Biotechnostix Inc., Markham, ON). A non-zero breath alcohol concentration (BrAC) was grounds for exclusion, as was a sample positive for cocaine, amphetamine, methamphetamine/MDMA, or opiates. Due to the long half-life of THC, urine samples positive for THC (n = 1) were not considered grounds for exclusion.
Naltrexone administration
Following screening for contraindications for NTX and ethanol, including a urine pregnancy test for females, subjects were administered either a 50mg NTX capsule or an identical placebo capsule. Capsule order was counter balanced across subjects and double blinded. During session one, participants filled out a series of questionnaires, and then relaxed until the alcoholic drink was administered. Following the protocol of (Mitchell et al., 2007), administration of behavioral testing began approximately 3 hours following capsule ingestion. This interval was selected to minimize acute physiological effects of NTX during testing, while still achieving significant opioid receptor blockade (Atkinson, 1984; King et al., 1997; Swift et al., 1994).
Ethanol administration
Two and a half hours following capsule ingestion, subjects commenced a 15 minute alcohol drinking interval. The alcohol drink was prepared immediately prior to consumption and consisted of 190 proof U.S.P. ethyl alcohol (0.3g/kg of body weight) diluted 1:5 in fruit juice (Capri Sun, Kraft Foods, Northfield, IL). The drink was consumed in 3 equal parts, and subjects were allowed 5 minutes to consume each third, although in practice most took only 1 of the 5 allotted minutes. BrAC values were measured via breathalyzer 30 minutes after the onset of the drinking interval, and behavioral testing commenced thereafter.
Behavioral Inventories
We administered a number of standard questionnaires to quantify personal history and behavioral traits that could impact our results. We quantified alcohol use behavior with the Alcohol Use and Disorders Identification Test (AUDIT; (Saunders et al., 1993) and drug and alcohol use behavior with the Drug Use Screening Inventory, Domain I (DUSI-I; (Tarter, 1990). DUSI-I scores are reported in terms of the percent of affirmative answers from Domain I, part B. We calculated density of familial alcohol abuse using the Family Tree Questionnaire (FTQ; (Mann et al., 1985). Gambling habits were assessed with the South Oaks Gambling Screen (SOGS; (Lesieur and Blume, 1987). Neuropsychological questionnaires included the Barratt Impulsiveness Scale-11 (BIS; (Barratt, 1994), the Beck Depression Inventory (BDI; (Beck and Steer, 1987), Rotter’s Locus of Control Scale (LOC; (Rotter, 1966), the Future Time Perspective Inventory (FTPI; (Wallace, 1956), the State-Trait Anxiety Inventory (STAI; (Spielberger, 1985), and the Antisocial Practices Scale (ASP) of the Minnesota Multiphasic Personality Inventory 2 (MMPI-2; (Butcher JN, 1990). Education and occupation were quantified with the Hollingshead Socioeconomic Status (SES) score (Hollingshead, 1975). We estimated general intellectual function with the Shipley Institute of Living Scale (SILS; (Zachary, 1991).
Behavioral Tasks
Delay Discounting Task
The paradigm was based on a previously described task (Mitchell et al., 2005a; Mitchell et al., 2007). Briefly, in each session, subjects completed a short (~4 min) practice run and then 8 full runs of approximately 42 or 43 trials each (~7 min). There were four trial types or cues: WANT (W), DON’T WANT (DW), SOONER, and LARGER (together: CON for control conditions; see Figure 1A). Trials were randomly ordered and weighted so that half of trials were the W condition and the rest were evenly split between the other three conditions. Each trial began with an instruction cue, followed by two options (see Figure 1B), each consisting of a monetary value and a time. Subjects were asked to evaluate the choices as if they would actually receive the specified amounts at the corresponding times. In each trial, one of the options consisted of one of five “full” amounts ($2, $5, $10, $20, or $100) and one of five future delays (1 week, 2 weeks, 1 month, 3 months, or 6 months). The other option included a discounted amount (70, 85, 90, or 95% of the “full” amount) always offered with no delay (”TODAY”).
Figure 1.
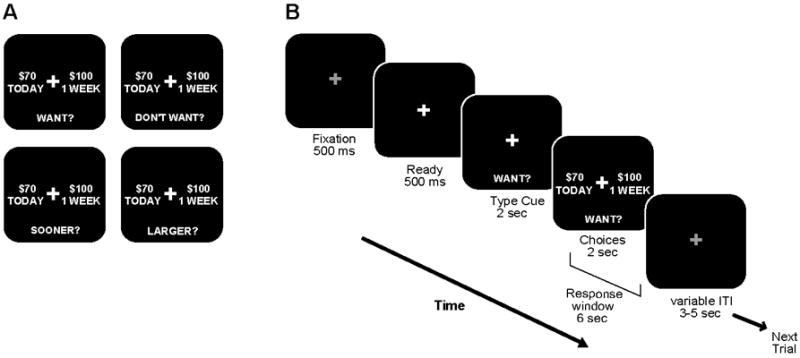
Illustration of delay discounting paradigm. A, Depiction of the four trial types. The four trial types included WANT (W), DON’T WANT (DW), and two controls (CON): SOONER and LARGER. Trial ratio was 1/2 for the W condition and 1/6 each for the other three trial types. B, The temporal sequence of events are shown for one example W trial. Illumination of a fixation cross (“Ready”) indicated the start of each trial. The instruction cue was then displayed for 2 s, informing the subject of the upcoming trial type. The two options were then presented while the instruction cue remained on the screen. The choices remained on the screen for 2 s, however subjects had a total of 6 s to indicate their choice following the appearance of the two options.
Subjects were instructed to make a choice in each trial, according to the trial type. They were to select their preferred option on W trials, their non-preferred option on the DW trials, and the side with the sooner time or larger amount of money for SOONER and LARGER (CON) trials, respectively. These control trials verified that subjects understood the task and maintained attention. The order of trial types was the same for all subjects; however, the delayed amount, delay time, and discount were selected from a randomly ordered list. Hypothetical rewards were used rather than actual money based on results from comparison studies (Critchfield and Kollins, 2001; Johnson and Bickel, 2002; Lagorio and Madden, 2005; Madden et al., 2003; Madden et al., 2004).
Go-NoGo Task
Subjects also completed a Go-NoGo task, which allowed direct measurement of the effect of NTX on motor inhibition. The “Go” stimulus was “$70”, in the same font and size as the stimuli used in the delay discounting task. The “NoGo” stimulus was an identically formatted and positioned “$100”. On each trial, stimuli appeared on the screen for 700 ms followed by a blank screen for 300 ms. Subjects completed two runs of 80 trials each, with 15% NoGo trials, presented in pseudorandom order.
Dot-probe Task
To measure NTX’s effect on attentional bias, subjects performed a dot-probe type task (MacLeod et al., 1986). The stimuli mimicked the options screen of the delay discounting task (excluding the instruction cue). For each trial, the two stimuli appeared on either side of a fixation cross for 500 ms. The stimuli then disappeared and each was replaced by a target (two dots) or non-target (one dot). Subjects pressed a button to indicate the side of the target stimulus. To quantify attentional bias towards the larger amount we calculated the following reaction time (RT) bias index from correct trials: RTS – RTL, where RTS indicates the mean RT for trials in which the target appears on the side with the smaller amount, and RTL indicates the mean RT for trials in which the target appeared on the side with the larger amount. Using this measure, positive values indicate faster responses to targets cued by the larger amounts, which reflects attentional bias to the larger amounts. During each session, subjects completed one run of 80 trials. For all but four subjects, the delay discounting task preceded the dot probe task.
Data Analysis
Our index of temporal discounting was the proportion of earlier choices, which we have termed the impulsive choice ratio (ICR). This value was calculated across all W trials, as well as separated according to delay time and delayed amount. On DW trials, inferred ICR (iICR) was calculated based on the non-selected value for each delay time. As a gross index of motor control, “motor mismatch” (MM) was calculated as the absolute difference between ICR and iICR for each delay time, averaged across all delay times.
To test the significance of across group comparisons, we used unpaired two-tailed t-tests for continuous measures and χ2 tests for categorical measures. For multi-factorial comparisons, mixed repeated measures ANOVAs in SPSS were used, with group as a between subjects factor. When necessary, a Greenhouse-Geisser non-sphericity correction was applied. Post-hoc paired comparisons were performed where indicated using two-tailed t-tests. When data were not normally distributed, appropriate arcsine-root transformations were applied in Excel prior to making statistical comparisons to ensure the validity of parametric statistical tests. Simple regression analysis and analyses of covariance (ANCOVA) were performed in SPSS. To estimate which continuous variables had the greatest predictive value for the NTX effects on ICR and MM, linear multiple regression analyses were carried out using SPSS. For each multiple regression analysis, we entered variables stepwise, divided into five blocks. The blocks were as follows: block 1 - age, gender, ethnic group, years of education, HH-SES-self, HH-SES-parent, IQ, body mass index (BMI); block 2 – AUDIT, FTQ density, DUSI; block 3 – BIS, BDI, SOGS, LOC, APS, FTPI-I-mean extension, FTPI-I-max extension, FTPI-2-mean extension, STAI-State, STAI-Trait; block 4 – peak BrAC or change in peak BrAC; EtOH metabolism rate or change in EtOH metabolism rate; block 5 – WANT (or DW) trial RT or RT change, Go-NoGo false alarms (FA) or FA change, dot probe RT difference or RT difference change.
RESULTS
Demographic and psychometric data
We previously found that BIS scores positively correlate with ICR (Mitchell et al., 2005b) and that abstinent alcoholics score significantly higher on the BIS relative to controls (Mitchell et al., 2005b; Mitchell et al., 2007). Here we tested whether BIS scores predicted ICR differences in a sample with high variance in BIS scores, but no history of substance use disorders. We divided subjects into two groups based on a median split of Barratt Impulsiveness Scale (BIS) scores. There were no significant differences between the two groups in terms of age, education, socioeconomic status (SES), gender, ethnicity, family history of alcohol abuse (FTQ density), orientation towards the future (FTPI), locus of control (LOC) or gambling history (SOGS; see Table 1). The more impulsive group (High Imp) did however, report slightly greater depression (BDI), trait anxiety (STAI-T), alcohol use (AUDIT), and endorsed more positive answers on the Drug Use Screening Inventory, part IB (DUSI-I B) than did the low impulsive group (Low Imp).
Table 1.
Demographic and psychometric data
| Low Imp (n = 15) | High Imp (n = 15) | t(28) | p value | |
|---|---|---|---|---|
| General | ||||
| Age (yrs) | 23 ± 4 | 23 ± 3 | 0.55 | ns |
| Education (yrs) | 16 ± 2 | 16 ± 2 | 0.00 | ns |
| Hollingshead SES | 41 ± 9 | 41 ± 9 | 0.12 | ns |
| Gender (# female) | 10 | 10 | ns† | |
| Ethnicity (# non-white) | 8 | 10 | ns† | |
| Alcohol-related | ||||
| AUDIT | 4 ± 3 | 7 ± 4 | 2.22 | 0.034 |
| DUSI-I (B) | 1 ± 1 | 3 ± 2 | 2.98 | 0.006 |
| FTQ density (%) | 15 ± 16 | 15 ± 16 | 0.25 | ns |
| Psychometric | ||||
| Depression (BDI) | 3 ± 4 | 7 ± 5 | 2.39 | 0.024 |
| Impulsivity (BIS-11) | 55 ± 7 | 74 ± 8 | 7.19 | <0.001 |
| Future orientation (FTPI) | 33 ± 22 | 38 ± 17 | 0.72 | ns |
| STAI – State Anxiety | 29 ± 4 | 32 ± 8 | 1.33 | ns |
| STAI – Trait Anxiety | 34 ± 7 | 43 ± 8 | 3.08 | 0.005 |
| Internal-External control (LOC) | 11 ± 3 | 11 ± 4 | 0.39 | ns |
| Antisocial Practices (ASP) | 7 ± 4 | 8 ± 4 | 0.61 | ns |
| Gambling (SOGS) | 0.2 ± 0.6 | 0.2 ± 0.4 | 0.00 | ns |
Values are reported as mean ± standard deviation. Reported p-values reflect the results of unpaired two-tailed comparison between groups. Exact p-values reported unless p < 0.001. SES, socioeconomic status; AUDIT, Alcohol Use Disorders Identification Test; DUSI-I, Drug Use Screening Inventory, Domain I; FTQ, Family Tree Questionnaire; BDI, Beck Depression Inventory; BIS, Barratt Impulsiveness Scale-11; FTPI, Future Time Perspective Inventory (Maximum Extension, part I); STAI, State-Trait Anxiety Inventory; LOC, Rotter’s Locus of Control Scale; ASP, Antisocial Practices Scale of the MMPI-2; SOGS, South Oaks Gambling Screen.
p-value represents results of χ2 test.
Factors predicting impulsive choice tendency in the presence of alcohol
In the placebo session, the High Imp group tended to select the smaller, sooner reward more frequently (ICR: 0.40 ± .33) than did the Low Imp group (0.39 ± .38); however, this difference did not reach statistical significance (p = 0.79). A simple regression analysis also failed to find a significant relationship between BIS scores and ICR (p = 0.63). To identify factors that did predict ICR in the context of moderate alcohol consumption, we performed a multiple regression analysis of placebo session ICR data that identified two factors that predicted impulsive choice tendencies: future orientation (as measured by FTPI part 1 max) and peak BrAC (Table 2; Fig. 2A,B). ICR was negatively correlated with future-orientation and positively associated with BrAC. This latter correlation indicates that moderate EtOH intake increases impulsive choices, although the mean placebo ICR we observed here (0.39) was similar to that observed in other samples with no history of substance use disorders and not under the influence of EtOH (0.3-0.4) (Boettiger et al., 2007; Mitchell et al., 2005b; Mitchell et al., 2007).
Table 2.
Multiple regression analyses: factors predicting ICR under the influence of moderate alcohol (placebo sessions)
| B | SE B | β | |
|---|---|---|---|
| Step 1 | |||
| Constant | 1.00 | 1.67 | |
| FTPI-MAX | -0.01 | 0.004 | -0.44** |
| Step 2 | |||
| Constant | 0.44 | 0.24 | |
| FTPI-MAX | -0.01 | 0.004 | -0.48** |
| Peak BrAC | 18.55 | 6.19 | 0.46** |
Results from Multiple Linear Regression analysis of predictors of ICR after consuming a moderate dose of EtOH (placebo sessions). B: beta value; SE B: beta value standard error; β: standardized beta; FTPI-MAX, Future Time Perspective Inventory (part 1), maximum extension; BrAC, Breath alcohol concentration;
p < .01.
Figure 2.
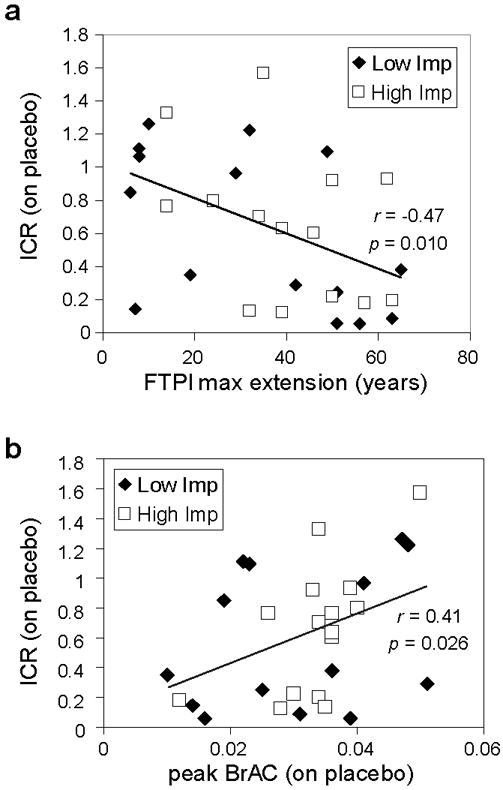
Factors predicting choice behavior while under the influence of alcohol. (a) Arcsine-root transformed impulsive choice ratio (ICR) versus maximum extension time on the Future Time Perspective Inventory (FTPI; part I). (b) ICR (arcsine-root transformed) versus breath alcohol concentration (BrAC) at the onset of behavioral testing.
Acute effects of NTX after moderate alcohol intake
In the delay discounting task, a mixed-model ANOVA revealed a significant main effect of trial type on RT, but no significant main effects of trait impulsiveness group or drug condition, nor any significant interaction between these factors (Table 3). These data are consistent with those found previously (Mitchell et al., 2005b; Mitchell et al., 2007), and indicate that NTX effects on RT are not altered by the presence of moderate alcohol. We found no main effect of NTX on ICR, however there was a significant drug × group interaction (F1,28 = 5.314, p=0.029; Fig. 3). Post hoc tests suggest that this effect was driven by a trend for less impulsive decisions in the Low Imp group on NTX (t14 = 1.93, p = 0.07). These results indicate that NTX does not have uniform effects on decision-making behavior in the context of moderate alcohol intake.
Table 3.
Reaction time data
| PBO | NTX | |
|---|---|---|
| Low Impulsive (n=15) | ||
| CON | 1554 ± 241 | 1620 ± 231 |
| W | 1679 ± 279 | 1758 ± 310 |
| DW | 1716 ± 299 | 1793 ± 323 |
| High Impulsive (n=15) | ||
| CON | 1639 ± 387 | 1617 ± 317 |
| W | 1815 ± 548 | 1809 ± 523 |
| DW | 1852 ± 562 | 1842 ± 547 |
Reaction times (in ms) for each trial type in the delay discounting task following placebo (PBO) or 50mg naltrexone (NTX; mean ± standard deviation). There was a significant effect of Trial type on RT (F1,1.01, 28.4 = 22.3; p <0.001), but no significant effect of group or pill and no significant interactions between factors (maximum F = 2.12). W, WANT trials; DW, DON’T WANT trials; CON, Control trials (SOONER & LARGER).
Figure 3.
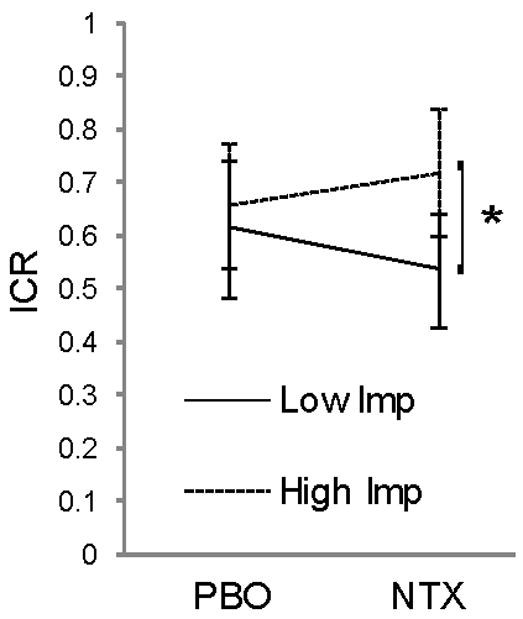
Effect of NTX on decision-making while under the influence of alcohol: interaction between personality and drug. Average ICR (arcsine-root transformed) during placebo (PBO) and naltrexone (NTX) sessions for low and high impulsive groups. * p < .001.
In our previous laboratory study, we found NTX to significantly reduce MM in our task (Mitchell et al., 2007). Contrary to our expectation, we found that under the influence of moderate alcohol, NTX failed to reduce MM (F1,28 = 0.05, p=0.82). We also found no significant drug × impulsivity group interaction (F1,28 = 0.22, p=0.64). Thus, moderate alcohol appears to occlude NTX’s effect on MM. The Go-NoGo and dot-probe tasks were included as a means to dissociate expected NTX effects on MM into motor control and attentional bias factors. Given that we did not detect a significant reduction in MM on NTX, this need was eliminated. We also found that NTX had no effect on motor inhibition in the Go-NoGo task or attentional bias in the dot-probe task (maximum F = 3.1).
Factors predicting NTX effect on impulsive choice in the presence of alcohol
In our previous laboratory study of the effects of NTX on delay discounting, we found that a single factor held significant predictive value: Rotter’s Locus of Control (LOC) scores; this relationship was particularly strong in subjects with a history of alcohol use disorder (Mitchell et al., 2007). Here, we again used multiple linear regression to identify any demographic or psychometric measures that had significant predictive value in terms of the NTX effect on ICR. No variable held significant predictive value in the whole sample. However, our previous finding was derived from a sample in which 83% of participants reported a positive family history of alcohol abuse (FHP) (Mitchell et al., 2007). Thus, we repeated the multiple linear regression procedure including only data from FHP subjects (n=15). Within the FHP group, we found that a single variable, LOC score, again significantly predicted NTX effects on ICR (p = 0.001, Figure 4; see Table 4 for complete model). Lower LOC scores, which reflect a more internal attribution style, again predicted an increase in ICR on NTX, whereas a more external attribution style predicted reduced ICR on NTX.
Figure 4.
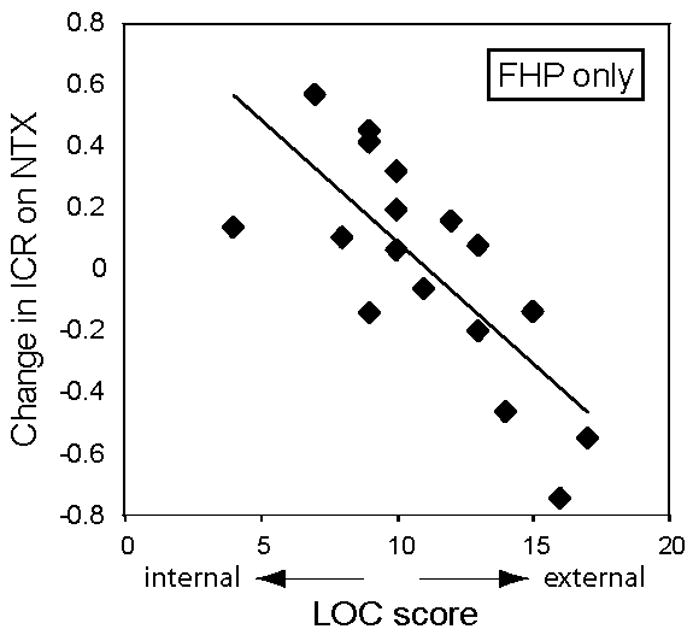
Factors predicting NTX effect on choice behavior. Change in impulsive choice ratio (ICR; arcsine-root transformed) versus locus of control (LOC) score for (a) all subjects and (b) only subjects with a family history positive (FHP) for alcohol abuse. Linear fits are shown for each set of subjects. A negative change in ICR reflects a decrease in impulsive choice when on NTX compared to PBO.
Table 4.
Multiple regression analyses: factors predicting NTX effect on ICR
| B | SE B | β | |
|---|---|---|---|
| FHP group only: | |||
| Step 1 | |||
| Constant | 0.89 | 0.22 | |
| LOC | -0.08 | 0.02 | -0.75** |
Results from Multiple Linear Regression analysis of predictors of NTX effect on impulsive choice ratio (ICR; arcsine-root transformed). B: beta value; SE B: beta value standard error; β: standardized beta; LOC, Rotter’s Locus of Control Scale; FHP, family history positive for alcohol abuse;
p < .01.
NTX may retard ethanol metabolism
A surprising finding in this study was a trend toward slower ethanol metabolism in NTX than in placebo sessions (F1,26 = 3.901, p=0.059). This effect has not been reported previously; however NTX has been reported to alter the subjective effects of alcohol, decreasing the stimulating effects and increasing the sedating effects of alcohol compared to placebo (Swift et al 1994). A slowing of ethanol metabolism by NTX could contribute to changes in perceived subjective effects of ethanol. Moreover, published reports have been based primarily on Caucasian subjects, while our subject pool was only 40% Caucasian.
DISCUSSION
Trait impulsivity, impulsive choice, and moderate alcohol
Previous research has found a correlation between delay discounting behavior and subjective measures of trait impulsivity (Mitchell et al., 2005b; Swann et al., 2002), although this finding is not universal (Lane et al., 2003; Mitchell, 1999). This variability may have several causes. First, self-report measures are prone to numerous limitations (Nisbett and Wilson, 1977; Wilson and Dunn, 2004) such that these may not accurately reflect actual behavior. That said, the measure of trait impulsivity used here, BIS score, has shown substantial heritability (>40%) in twin studies (Seroczynski et al., 1999), supporting its validity as a phenotypic measure of at least certain aspects of the multifaceted construct of impulsivity (Congdon and Canli, 2005; Evenden, 1999). Second, different methods have been used to measure delay discounting. The one used here shows high test-re-test reliability (Smith et al., personal communication), and demonstrated significant correlation with BIS scores in a previous study (Mitchell et al., 2005b). Thus, we expected to replicate that finding here. However, while BIS scores were collected when subjects were sober, delay discounting was measured following moderate alcohol intake. Although impulsiveness under the influence of moderate ethanol has not been extensively studied, some studies have found increased impulsive choice (Petry, 2001), while others have not (Ortner et al., 2003; Richards et al., 1999). Here we found that the BrAC achieved by the single alcohol dose was positively correlated with impulsive choice, supporting the idea that ethanol concentration influences promotes the tendency to choose immediate over delayed rewards. This effect may have occluded the relationship between trait impulsivity and delay-discounting observed under sober conditions.
Motor mismatch: occlusion of NTX effect by moderate ethanol
Based on our earlier finding that NTX significantly reduced MM, we were surprised to find a lack of effect here. The average MM scores in placebo sessions (~0.1) were nearly identical to those seen in control subjects in two previous studies (Mitchell et al., 2005b; Mitchell et al., 2007), and the range of MM scores was also the same. The simplest explanation is that alcohol is occluding the effect of NTX on MM, but the question arises: Which system is mediating this effect? Two conditions must be met. First, the function of a candidate system must be altered by NTX. Second, alcohol intake must independently alter this same system. Although alcohol elicits endogenous opioid release (Gianoulakis, 1993), which would be the first system to consider, NTX would block any effect of such release (Herz, 1997). Thus, MM is not likely to result from elevated endogenous opioid signaling. A second candidate system regulating MM is the dopamine (DA) system. NTX can indirectly reduce DA release in striatal targets (Fields et al., 2007; Herz, 1997; Herz, 1995; Spanagel et al., 1992), a possible mechanism underlying its ability to reduce MM. In contrast, acute ethanol potently enhances DA release in the striatum (Herz, 1997). Thus, these two opposing actions on the DA system could underlie the lack of an effect of NTX on MM observed in the present study. One hypothesis generated by this interpretation is that high levels of baseline DA signaling should be associated with higher levels of MM. Support for this hypothesis comes from our previous data showing significantly less MM in people with a history of alcoholism (Mitchell et al., 2007), a group reported to exhibit depressed levels of striatal DA (Volkow et al., 2007). Future investigations using genetic methods to address this question may prove illuminating.
Individual differences in NTX effects on impulsive choice: possible explanations for the interaction between family history of alcoholism and LOC
Dopamine system
In our previous study of subjects with or without a personal history of alcoholism, we found that LOC scores were the sole predictor of NTX’s effect on immediate reward bias (Mitchell et al., 2007). LOC is a personality measure reflecting one’s perception of control over life events (Rotter, 1966), and LOC scores show substantial heritability (Miller and Rose, 1982; Pedersen et al., 1989), suggesting a biological basis. Based on evidence that LOC scores reflect tonic frontal DA transmission (Declerck et al., 2006) and that acute elevation of DA may reduce delay discounting (de Wit et al., 2002; Wade et al., 2000), we reasoned that NTX may alter impulsive choice by altering the level of tonic DA transmission in the frontal cortex (Herz, 1995; Margolis et al., 2006; Spanagel et al., 1992). It is important to point out that NTX is an antagonist at both mu and kappa opioid receptors, albeit with approximately 2.5-fold greater affinity for mu-opioid receptors (Emmerson et al., 1994). A 50 mg oral dose of NTX will achieve nearly complete blockade of mu-opioid binding sites in the human brain (Lee et al., 1988; Weerts et al., 2008). The current lack of suitable kappa-opioid PET ligands prevents us from knowing how completely this NTX dose blocks kappa receptors in the human brain, but we do know that this dose blocks ~20% of delta-opioid receptors in the human brain, for which NTX has approximately 25-fold lower affinity, relative to kappa receptors (Emmerson et al., 1994). Thus, a 50 mg oral dose of NTX is certainly achieving very significant blockade of kappa opioid receptor sites, which are comparable in abundance to mu-opioid receptors in the brain (Pfeiffer et al., 1982). Furthermore, there is evidence from rodent studies that whereas mu agonists raise dopamine levels in prefrontal cortex, kappa agonists have the opposite effect (Herz, 1995; Margolis et al., 2006; Spanagel et al., 1992). People with a personal or family history of alcoholism are reported to have relatively low levels of circulating endogenous mu-opioid agonists (Dai et al., 2005; del Arbol et al., 1995; Gianoulakis et al., 1989; Govoni et al., 1983; Vescovi et al., 1992). Therefore, we previously speculated that such individuals would experience more of a dopamine elevating effect of NTX, due to relatively enhanced kappa-opioid blockade effects of NTX (Mitchell et al., 2007). That speculation was supported by our observation that the predictive relationship between LOC and NTX effects on impulsive choice was stronger among the abstinent alcoholic subjects in our previous study. This hypothesis is further supported by the present data showing that LOC most strongly predicts NTX’s effect on decision-making among subjects with a family history of alcoholism. We now expand upon our previously hypothesized model for dopaminergically mediated effects of NTX on decision-making. First, we propose that due to opposing effects of mu- and kappa-opioid receptors on forebrain DA release, individuals with no personal or family history of alcoholism, NTX likely has mixed, opposing effects on frontal DA release (Fig. 5A), with some subjects experiencing DA increases, others, DA decreases, and still others, no net effects on DA signaling. In contrast, individuals with a family history of alcoholism would be expected to experience relatively greater effects of kappa opioid receptor blockade; thus, such individuals would tend to experience an elevation in frontal DA levels in response to NTX (Fig. 5B). Dopaminergic modulation of frontal functions often follows a U-shaped curve, where too little or too much DA causes inefficiencies in frontal functioning (Arnsten, 1997; Williams and Castner, 2006; Zahrt et al., 1997). We propose a U-shaped relationship between impulsive choice and frontal DA levels (Fig. 5C). Such a model fits our experimental data (shown in Fig. 4) well, in that NTX’s effects on ICR are reliably predicted for FHP subjects, but not for FHN subjects. One prediction of this model is that the effects of direct elevation of frontal DA on impulsive decision-making should depend on baseline frontal DA levels, and effects are not expected to interact with family history of alcoholism. Future studies will address this hypothesis.
Figure 5.
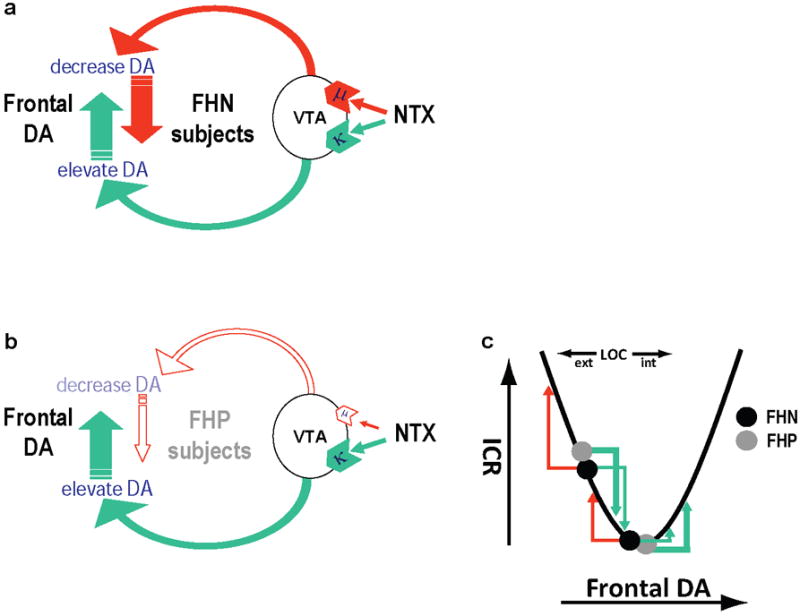
Model depicting opposing actions of naltrexone (NTX) on frontal dopamine (DA) levels. A, In people family history negative (FHN) for alcoholism, mu- and kappa-opioid receptor effects compete, and relative effects likely vary based on individual factors. B, In people with a family history of alcoholism (FHP), kappa-opioid effects receptor effects of NTX are predicted to dominate, elevating frontal dopamine (DA) levels. C, Impulsive choice ratios (ICR) are postulated to depend on baseline frontal DA levels, according to a U-shaped function. For FHP subjects, NTX elevates frontal DA, which reduces ICR in those with external LOC scores, whereas ICR increases in those with internal LOC scores. Effects in FHN subjects are less predictable.
The differential effects of NTX in FHP versus FHN subjects is potentially clinically relevant in light of the fact that NTX appears to be more effective in reducing alcohol intake among FHP alcoholics relative to those that are FHN (Krishnan-Sarin et al., 2007; Monterosso et al., 2001). Here we propose that the differential effects reflect a greater effect of kappa opioid receptor blockade by NTX in FHP subjects. In contrast, O’Malley and colleagues have made the opposite prediction, arguing that NTX effects on alcohol intake are primarily kappa receptor mediated in FHN alcoholics (Krishnan-Sarin et al., 2007). In this regard, animal studies can either increase (Mitchell et al., 2005c) or decrease (Walker et al.) alcohol consumption depending upon the behavioral state of the animal. Direct tests of these opposing hypotheses may be possible in the future via PET imaging of NTX’s actions on each receptor system. The availability of new, receptor-specific ligands would also allow us to directly probe the role of each receptor subtype.
Stress system
Individuals with a family history of alcoholism experience an elevated release of cortisol in response to acute NTX administration (King et al., 2002). Moreover, data suggests that the HPA-axis responds differently to stress based on LOC, with an external LOC predicting a more exaggerated cortisol release in response to stress (Bollini et al., 2004). Thus, cortisol is a hypothetical mediator of the observed interaction between LOC and family history of alcoholism in predicting NTX’s effects on impulsive decision-making. The hyper-release of cortisol in response to NTX of our FHP subjects would be expected to vary with LOC, such that in more internal individuals, cortisol goes up less than it does in external FHP individuals. Some data support the idea that high cortisol levels reduce delay discounting (Takahashi, 2004), which could possibly explain our observed results of reduced discounting on NTX in external FHP subjects and increased discounting on NTX in internal FHP subjects. It is worth noting that one primary mediator of the dysphoric effects of stress is the kappa opioid system (Bruchas et al.). Thus, dysregulation of the stress system in FHP subjects may well be associated with dysregulation in the kappa opioid system as well. An up-regulation of kappa receptor signaling in FHP subjects could thus also contribute to the scenario we propose in Figure 5B.
Summary
In conclusion, in a young, healthy control sample under the influence of moderate alcohol, we did not find a significant correlation between trait impulsivity and the tendency to choose impulsively. Rather, myopia for the future and peak BrAC levels best predicted increased frequency of impulsive choices. In addition, we extend the previous finding that LOC predicts NTX’s effect on impulsive choice, demonstrating that this relationship is also present after moderate ethanol consumption. Again, a lower LOC, reflecting a more internal attribution style, correlates with an increase in impulsive choice on NTX, while a more external attribution style correlates with reduced impulsive choice on NTX. Importantly, we have determined that this predictive relationship is found among individuals with a family history of alcohol abuse. Trait impulsivity showed a weaker but significant interaction of NTX’s effect on impulsive choice, with NTX more effectively reducing impulsive choices in subjects with low trait impulsivity. Consistent with previous findings, moderate acute ethanol intake did not grossly increase impulsive choices (Ortner et al., 2003; Petry, 2001; Richards et al., 1999); however peak BrAC positively correlated with impulsive choice tendency and attentional bias towards smaller, immediate rewards. These data suggest that increasing ethanol intoxication impairs decision-making by biasing decisions toward immediate gratification. We found that NTX fails to reduce mismatch of choices in the DON’T WANT condition in the presence of alcohol. Finally, we made the unexpected finding that NTX significantly slowed ethanol metabolism. Together, the results reported here provide new insights into possible mechanisms for NTX’s ability to reduce total ethanol intake following moderate ethanol ingestion, and suggest several novel lines of research in this area.
Acknowledgments
We thank Elizabeth Kelley for technical assistance.
Support: This work was supported by the Wheeler Center for the Neurobiology of Addiction and Award Number KL2RR025746 from the National Center for Research Resources.
References
- Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999;156(11):1758–64. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11(2):151–62. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Atkinson RL. Endocrine and metabolic effects of opiate antagonists. J Clin Psychiatry. 1984;45(9 Pt 2):20–4. [PubMed] [Google Scholar]
- Barratt ES. The John D and Catherine T MacArthur Foundation series on mental health and development. University of Chicago Press; Chicago: 1994. Impulsiveness and aggression, in Violence and mental disorder: Developments in risk assessment; pp. 61–79. [Google Scholar]
- Beck AT, Steer RA. Manual for the Revised Beck Depression Inventory. Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D’Esposito M, Fields HL. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci. 2007;27(52):14383–91. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollini AM, Walker EF, Hamann S, Kestler L. The influence of perceived control and locus of control on the cortisol and subjective responses to stress. Biol Psychol. 2004;67(3):245–60. doi: 10.1016/j.biopsycho.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Boyle AE, Stewart RB, Macenski MJ, Spiga R, Johnson BA, Meisch RA. Effects of acute and chronic doses of naltrexone on ethanol self-administration in rhesus monkeys. Alcohol Clin Exp Res. 1998;22(2):359–66. [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Research. 1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher JN, G J, Williams CL, Ben-Porath YS. Development and Use of the MMPI-2 Content Scales. University of Minnesota Press; Minneapolis: 1990. p. 196. [Google Scholar]
- Congdon E, Canli T. The endophenotype of impulsivity: reaching consilience through behavioral, genetic, and neuroimaging approaches. Behav Cogn Neurosci Rev. 2005;4(4):262–81. doi: 10.1177/1534582305285980. [DOI] [PubMed] [Google Scholar]
- Critchfield TS, Kollins SH. Temporal discounting: basic research and the analysis of socially important behavior. J Appl Behav Anal. 2001;34(1):101–22. doi: 10.1901/jaba.2001.34-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Gianoulakis C. Differences in the peripheral levels of beta-endorphin in response to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Alcohol Clin Exp Res. 2005;29(11):1965–75. doi: 10.1097/01.alc.0000187599.17786.4a. [DOI] [PubMed] [Google Scholar]
- Davidson D, Palfai T, Bird C, Swift R. Effects of naltrexone on alcohol self-administration in heavy drinkers. Alcohol Clin Exp Res. 1999;23(2):195–203. [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27(5):813–25. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Declerck CH, Boone C, De Brabander B. On feeling in control: A biological theory for individual differences in control perception. Brain Cogn. 2006 doi: 10.1016/j.bandc.2006.04.004. [DOI] [PubMed] [Google Scholar]
- del Arbol JL, Aguirre JC, Raya J, Rico J, Ruiz-Requena ME, Miranda MT. Plasma concentrations of beta-endorphin, adrenocorticotropic hormone, and cortisol in drinking and abstinent chronic alcoholics. Alcohol. 1995;12(6):525–9. doi: 10.1016/0741-8329(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Emmerson PJ, Liu MR, Woods JH, Medzihradsky F. Binding affinity and selectivity of opioids at mu, delta and kappa receptors in monkey brain membranes. The Journal of pharmacology and experimental therapeutics. 1994;271(3):1630–7. [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146(4):348–61. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and excessive alcohol consumption. J Psychiatry Neurosci. 1993;18(4):148–56. [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C, Beliveau D, Angelogianni P, Meaney M, Thavundayil J, Tawar V, Dumas M. Different pituitary beta-endorphin and adrenal cortisol response to ethanol in individuals with high and low risk for future development of alcoholism. Life Sci. 1989;45(12):1097–109. doi: 10.1016/0024-3205(89)90167-7. [DOI] [PubMed] [Google Scholar]
- Govoni S, Bosio A, Di Monda E, Fazzari G, Spano PF, Trabucchi M. Immunoreactive met-enkephalin plasma concentrations in chronic alcoholics and in children born from alcoholic mothers. Life Sci. 1983;33(16):1581–6. doi: 10.1016/0024-3205(83)90699-9. [DOI] [PubMed] [Google Scholar]
- Heidbreder C. Recent Advances in the Pharmacotherapeutic Management of Drug Dependence and Addiction. Current Psychiatry Reviews. 2005;1:45–67. [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129(2):99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Herz A, Spanagel R. In: Endogenous opioids and addiction, in The Pharmacology of Opioids. Tseng L, editor. Harwood; Germany: 1995. pp. 445–462. [Google Scholar]
- Hollingshead A. Hollingshead’s Four Factor Index of Social Status. Yale University Press; New Haven, CT: 1975. [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 2002;77(2):129–46. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieres AK, Hausknecht KA, Farrar AM, Acheson A, de Wit H, Richards JB. Effects of morphine and naltrexone on impulsive decision making in rats. Psychopharmacology (Berl) 2004;173(1-2):167–74. doi: 10.1007/s00213-003-1697-2. [DOI] [PubMed] [Google Scholar]
- Kim SW, Grant JE, Adson DE, Shin YC. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914–21. doi: 10.1016/s0006-3223(01)01079-4. [DOI] [PubMed] [Google Scholar]
- King AC, Schluger J, Gunduz M, Borg L, Perret G, Ho A, Kreek MJ. Hypothalamic-pituitary-adrenocortical (HPA) axis response and biotransformation of oral naltrexone: preliminary examination of relationship to family history of alcoholism. Neuropsychopharmacology. 2002;26(6):778–88. doi: 10.1016/S0893-133X(01)00416-X. [DOI] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O’Brien CP. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology (Berl) 1997;129(1):15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Krystal JH, Shi J, Pittman B, O’Malley SS. Family history of alcoholism influences naltrexone-induced reduction in alcohol drinking. Biol Psychiatry. 2007;62(6):694–7. doi: 10.1016/j.biopsych.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Lagorio CH, Madden GJ. Delay discounting of real and hypothetical rewards III: steady-state assessments, forced-choice trials, and all real rewards. Behav Processes. 2005;69(2):173–87. doi: 10.1016/j.beproc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Rhoades HM, Pietras CJ, Tcheremissine OV. Relationships among laboratory and psychometric measures of impulsivity: Implications in substance abuse and dependance. Addictive Disorders Treatment. 2003;2:33–40. [Google Scholar]
- Lee MC, Wagner HN, Jr, Tanada S, Frost JJ, Bice AN, Dannals RF. Duration of occupancy of opiate receptors by naltrexone. J Nucl Med. 1988;29(7):1207–11. [PubMed] [Google Scholar]
- Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): a new instrument for the identification of pathological gamblers. Am J Psychiatry. 1987;144(9):1184–8. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. J Abnorm Psychol. 1986;95(1):15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Begotka AM, Raiff BR, Kastern LL. Delay discounting of real and hypothetical rewards. Exp Clin Psychopharmacol. 2003;11(2):139–45. doi: 10.1037/1064-1297.11.2.139. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Raiff BR, Lagorio CH, Begotka AM, Mueller AM, Hehli DJ, Wegener AA. Delay discounting of potentially real and hypothetical rewards: II Between- and within-subject comparisons. Exp Clin Psychopharmacol. 2004;12(4):251–61. doi: 10.1037/1064-1297.12.4.251. [DOI] [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend. 1985;15(1-2):61–7. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A. 2006;103(8):2938–42. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology. 2000;22(5):480–92. doi: 10.1016/S0893-133X(99)00147-5. [DOI] [PubMed] [Google Scholar]
- Miller JZ, Rose RJ. Familial resemblance in locus of control: A twin-family study of the internal–external scale. Journal of Personality and Social Psychology. 1982;42(3):535–540. [Google Scholar]
- Mitchell JM, Bergren LJ, Chen KS, Fields HL. Society for Neuroscinece. Washington, DC: 2005a. Prior ethanol consumption predicts aversion elicited by the opioid antagonist naltrexone in rats. [Google Scholar]
- Mitchell JM, Bergren LJ, Chen KS, Rowbotham MC, Fields HL. Naltrexone aversion and treatment efficacy are greatest in rats and humans that consume high levels of ethanol. Neurobiology of Disease. 2009;33:72–80. doi: 10.1016/j.nbd.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D’Esposito M, Boettiger CA. Impulsive responding in alcoholics. Alcohol Clin Exp Res. 2005b;29(12):2158–69. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Liang MT, Fields HL. A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology (Berl) 2005c;182(3):384–92. doi: 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Tavares VC, Fields HL, D’Esposito M, Boettiger CA. Endogenous opioid blockade and impulsive responding in alcoholics and healthy controls. Neuropsychopharmacology. 2007;32(2):439–49. doi: 10.1038/sj.npp.1301226. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146(4):455–64. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Flannery BA, Pettinati HM, Oslin DW, Rukstalis M, O’Brien CP, Volpicelli JR. Predicting treatment response to naltrexone: the influence of craving and family history. Am J Addict. 2001;10(3):258–68. doi: 10.1080/105504901750532148. [DOI] [PubMed] [Google Scholar]
- Nisbett RE, Wilson TD. Telling more than we can know: Verbal reports on mental processes. Psychological Review. 1977;84(3):231–259. [Google Scholar]
- O’Brien CP, Volpicelli LA, Volpicelli JR. Naltrexone in the treatment of alcoholism: a clinical review. Alcohol. 1996;13(1):35–9. doi: 10.1016/0741-8329(95)02038-1. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160(1):19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Ortner CN, MacDonald TK, Olmstead MC. Alcohol intoxication reduces impulsivity in the delay-discounting paradigm. Alcohol Alcohol. 2003;38(2):151–6. doi: 10.1093/alcalc/agg041. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Gatz M, Plomin R, Nesselroade JR, McClearn GE. Individual differences in locus of control during the second half of the life span for identical and fraternal twins reared apart and reared together. J Gerontol. 1989;44(4):P100–5. doi: 10.1093/geronj/44.4.p100. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl) 2001;154(3):243–50. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Pasi A, Mehraein P, Herz A. Opiate receptor binding sites in human brain. Brain Res Mol Brain Res. 1982;248(1):87–96. doi: 10.1016/0006-8993(82)91150-7. [DOI] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71(2):121–43. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr. 1966;80(1):1–28. [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Seroczynski AD, Bergeman CS, Coccaro EF. Etiology of the impulsivity/aggression relationship: genes or environment? Psychiatry Res. 1999;86(1):41–57. doi: 10.1016/s0165-1781(99)00013-x. [DOI] [PubMed] [Google Scholar]
- Sinclair JD. Evidence about the use of naltrexone and for different ways of using it in the treatment of alcoholism. Alcohol Alcohol. 2001;36(1):2–10. doi: 10.1093/alcalc/36.1.2. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992;89(6):2046–50. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C. Assessment of state and trait anxiety: conceptual and methodological issues. South Psychol. 1985;2:6–16. [Google Scholar]
- Stromberg MF, Casale M, Volpicelli L, Volpicelli JR, O’Brien CP. A comparison of the effects of the opioid antagonists naltrexone, naltrindole, and beta-funaltrexamine on ethanol consumption in the rat. Alcohol. 1998;15(4):281–9. doi: 10.1016/s0741-8329(97)00131-6. [DOI] [PubMed] [Google Scholar]
- Swann AC, Bjork JM, Moeller FG, Dougherty DM. Two models of impulsivity: relationship to personality traits and psychopathology. Biol Psychiatry. 2002;51(12):988–94. doi: 10.1016/s0006-3223(01)01357-9. [DOI] [PubMed] [Google Scholar]
- Swift RM, Whelihan W, Kuznetsov O, Buongiorno G, Hsuing H. Naltrexone-induced alterations in human ethanol intoxication. Am J Psychiatry. 1994;151(10):1463–7. doi: 10.1176/ajp.151.10.1463. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Cortisol levels and time-discounting of monetary gain in humans. Neuroreport. 2004;15(13):2145–7. doi: 10.1097/00001756-200409150-00029. [DOI] [PubMed] [Google Scholar]
- Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1–46. doi: 10.3109/00952999009001570. [DOI] [PubMed] [Google Scholar]
- Verebey K, Volavka J, Mule SJ, Resnick RB. Naltrexone: disposition, metabolism, and effects after acute and chronic dosing. Clinical Pharmacology & Therapeutics. 1976;20(3):315–28. doi: 10.1002/cpt1976203315. [DOI] [PubMed] [Google Scholar]
- Vescovi PP, Coiro V, Volpi R, Giannini A, Passeri M. Plasma beta-endorphin, but not met-enkephalin levels are abnormal in chronic alcoholics. Alcohol Alcohol. 1992;27(5):471–5. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27(46):12700–6. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49(11):876–80. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Watson NT, King AC, Sherman CE, O’Brien CP. Effect of naltrexone on alcohol “high” in alcoholics. Am J Psychiatry. 1995;152(4):613–5. doi: 10.1176/ajp.152.4.613. [DOI] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology (Berl) 2000;150(1):90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF. Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol. doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M. Future time perspective in schizophrenia. J Abnorm Psychol. 1956;52(2):240–5. doi: 10.1037/h0039899. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Kim YK, Wand GS, Dannals RF, Lee JS, Frost JJ, McCaul ME. Differences in delta- and mu-opioid receptor blockade measured by positron emission tomography in naltrexone-treated recently abstinent alcohol-dependent subjects. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(3):653–65. doi: 10.1038/sj.npp.1301440. [DOI] [PubMed] [Google Scholar]
- Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139(1):263–76. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Wilson TD, Dunn EW. Self-knowledge: its limits, value, and potential for improvement. Annu Rev Psychol. 2004;55:493–518. doi: 10.1146/annurev.psych.55.090902.141954. [DOI] [PubMed] [Google Scholar]
- Zachary R. Shipley Institute of Living Scale: Revised Manual. Western Psychological Services; Los Angeles: 1991. [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17(21):8528–35. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


