Abstract
Despite significant improvements in therapeutic protocols, Head and Neck Squamous Cell Carcinoma (HNSCC) remains a major health problem worldwide. The 5-year post therapeutic survival rate is among the lowest of the major cancers with loco-regional relapse being the main cause of death. Moreover, in most instances, the quality of life of the afflicted patient is severely compromised. The poor prognosis for HNSCC is primarily due to disease detection at advanced stages. Accordingly, development of early detection and preventive strategies are essential. Recent advances in our understanding of the molecular biology and etiology of HNSCC should facilitate development of improved intervention and therapeutic approaches. The present review discusses the potential role of such factors for developing preventive and early diagnostic strategies for HNSCC management.
INTRODUCTION
Head and Neck Squamous Cell Carcinoma (HNSCC) is the fifth leading cause of cancer related deaths with an annual incidence of 500,000 cases worldwide (Dasgupta et al., 2010). Approximately, 40,000 new cases of HNSCC are diagnosed annually in the United States alone (Dasgupta et al., 2010). This cancer involves five major anatomical sites including the oral cavity, oropharynx, nasopharynx, hypopharynx and larynx (Chung and Gillison, 2009). Despite significant improvements in therapeutic modalities, 5-year post therapeutic survival rates are still among the lowest of the major cancers, with loco-regional relapse being the primary cause of death (Chung and Gillison, 2009; Dasgupta et al., 2010). Due to poor survival, improved methods for early disease detection and prevention are clearly warranted.
Etiology and Risk Factors for HNSCC
Several potential risk factors have been identified that associate with HNSCC development and progression. Different modes of tobacco intake such as cigarettes, pipes, cigars, smokeless tobacco and tobacco chewing are implicated in HNSCC development and these mitigating factors have been found to be responsible for 90% of HNSCC related deaths in males (Cinciripini and McClure, 1998). Notably, other than chewing tobacco, different chewing products such as betel nuts, paan, chaalia, gutka, naswar and areca that are widely used in the Indian subcontinent, South East Asia and South Pacific Islands, also increase the risk of HNSCC (Chen et al., 2006). Consumption of alcohol is the second most important correlative risk factor for HNSCC and above 30 grams of alcohol intake per day linearly increases risk of HNSCC (Altieri et al., 2004; Franchi et al., 2002; Rodriguez et al., 2004).
Comprehensive epidemiological studies also demonstrated the synergistic effect of tobacco and alcohol use on the occurrence of HNSCC (Petersen, 2009). Exposure to ultraviolet light, chronic irritation to the lining of the mouth and dental plaque formation have also been linked with an increased risk of some forms of HNSCC (Bloching et al., 2007; Perea-Milla Lopez et al., 2003; Rosenquist et al., 2005). Dietary deficiencies, particularly of vitamin A, vitamin C, vitamin E, iron, selenium, folate and other trace elements, are also linked to an increased risk of HNSCC (Pelucchi et al., 2003; Taghavi and Yazdi, 2007). In addition, reactive oxygen species (ROS), reactive nitrogen species (NOS), diabetes and a family history of HNSCC are also potential risk factors for HNSCC (Bahar et al., 2007; Rasheed et al., 2007).
Recently, a causal association between human papilloma virus (HPV) infection and a subset of oropharyngeal (Ang et al., 2010) HNSCC was established (Chung and Gillison, 2009). HPV is a 7.9-kb non-enveloped; double stranded circular DNA virus with a specific tropism for squamous epithelium (Chung and Gillison, 2009). Although over 320 HPV-subtypes have been identified so far, HPV-16 and HPV-18 were found to be the most important subtypes associated with oropharyngeal HNSCC (Chung and Gillison, 2009). In a recent comprehensive analysis of 323 patients with oropharyngeal HNSCC, HPV infection was detected in 63.8% of cases (Ang et al., 2010) indicating the potential role of HPV in HNSCC development.
Genetic Progression model of HNSCC
A progression model for HNSCC has been proposed and was the basis for identification of several chromosomal and molecular changes involved in HNSCC progression (Califano et al., 1996). A representative model incorporating these genomic changes is presented in Fig. 1. According to this model, benign squamous hyperplastic lesions arise due to the loss of human chromosome 9p followed by the development of dysplasia due to the loss of chromosome 3p and 17p. Potential carcinoma in situ lesions develops subsequently from dysplasia due to the amplification of 11q13 and loss of chromosome 13q and 14q. Finally, invasive carcinoma develops due to the loss of chromosomes 6p, 8 and 4q. The gene alterations affected in this progression pathway include: p16 (9p21), TP53 (17p), CyclinD1 (11q13), Retinoblastoma (13q), the key players in many cancers. Prior to proposing this model, the same laboratory detected clonal TP53 mutation in surgical margins and also established a definitive relationship between tobacco smoking and development of HNSCC (Brennan et al., 1995a; Brennan et al., 1995b).
Fig. 1.
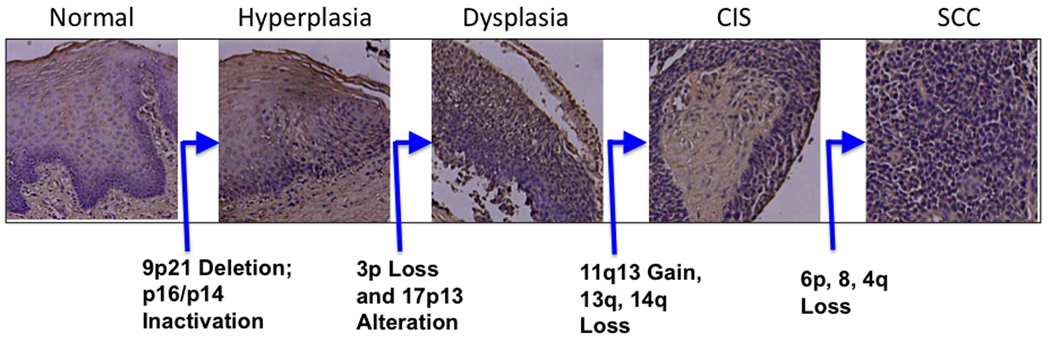
Genetic progression model of HNSCC. Normal epithelium progresses to benign hyperplasia and dysplasia by sequential genetic losses of 9p21, 3p and 17p13. Subsequently, carcinoma in situ (CIS) and frank carcinoma (SCC, squamous cell carcinoma) develops through the alterations of several chromosomal regions. H & E photomicrographs were from unpublished observation in the authors’ laboratory.
Nuclear Genetic Alterations in HNSCC- An overview
In the past 14 years, after the genetic progression model of HNSSC was proposed alterations of several nuclear-encoded genes was found to be associated with HNSCC, thereby enhancing our comprehension of the molecular genetic aspects of HNSCC. These genetic changes are discussed below based on their characteristics and functional classification and they are summarized in Tables 1 and 2.
Table 1.
Key Oncogenes implicated in the development and progression of HNSCC
Table 2.
Key tumor suppressor genes implicated in the development and progression of HNSCC
| Gene name | Location | Role in HNSCC Tumorigenesis |
References |
|---|---|---|---|
| TP53 | 17p13 | Initiation and Progression |
Califano et al., 1996; Brennan et al., 1995a; Brennan et al., 1995b; Ku et al., 2007; |
| P16 | 9p21 | Initiation | Califano et al., 1996; Ai et al., 2003; Reed et al., 1996 |
| P14 | 9p21 | Initiation | Califano et al., 1996; Ai et al., 2003; Reed et al., 1996 |
| PTEN | 10q23 | Progression | Lee et al., 2001; Sqarize et al., 2002 |
| MLHI | 3p21 | Initiation and Promotion |
Ha and Califano, 2007 |
| FHIT | 3p14 | Initiation and Progression |
Ha and Califano, 2007 |
| RASSF1 | 3p21 | Initiation and Promotion |
Ha and Califano, 2007 |
| CDH1 | 16q22 | Initiation and Promotion |
Ha and Califano, 2007 |
| DCC | 18q21 | Initiation and Promotion |
Ha and Califano, 2007 |
| MGMT | 10q26 | Initiation and Promotion |
Ha and Califano, 2007 |
| RARB | 3p24 | Initiation and Promotion |
Ha and Califano, 2007 |
| CDKN2B | 9p21 | Initiation and Promotion |
Ha and Califano, 2007 |
| DAPKI | 9q34 | Initiation and Promotion |
Ha and Califano, 2007 |
| OGG1 | 3p26 | Initiation | Paz-Elizur et al., 2006 |
| KERATIN-4 | 12q12–13 | Recurrence | Schaaij-Visser et al., 2009 |
| CORMULIN | 3q23 | Recurrence | Schaaij-Visser et al., 2009 |
| SMAD4 | 18q21.1 | Initiation and Promotion |
Bornstein et al., 2009 |
Oncogenes
The Epidermal Growth Factor Receptor (EGFR) is the most well characterized and extensively studied oncogene in HNSCC (Table 1) (Hynes and Lane, 2005; Kalyankrishna and Grandis; Ang et al., 2002; Grandis and Tweardy, 1993; Shin et al., 1994) and appears to be an attractive potential target for therapeutic intervention. It belongs to the ErbB family of cell surface receptors and when phosphorylated can orchestrate its oncogenic function through MAPK, Akt, ERK and Jak/STAT pathway (Hynes and Lane, 2005; Kalyankrishna and Grandis, 2006, Ang et al., 2002; Grandis and Tweardy, 1993; Shin et al., 1994). These pathways are the drivers of tumor progression as they are related to cellular proliferation, invasion, angiogenesis and metastasis (Hynes and Lane, 2005, Kalyankrishna and Grandis, 2006, Ang et al., 2002; Grandis and Tweardy, 1993; Shin et al., 1994). Although EGFR expression is detected in hyperplastic and normal epithelial cells and in many other tissues, dysregulated expression of this protein and its associated pathways occurs in approximately 80–90% of HNSCC (Grandis, 1993). Moreover, increased EGFR expression has been linked to improved survival of HNSCC patients (Hynes and Lane, 2005; Kalyankrishna and Grandis, 2006; Ang et al., 2002; Grandis and Tweardy, 1993; Shin et al., 1994), as it allowed targeting the tumor cells by EGFR inhibitors. Thus, EGFR could be an important rational-based molecular target for future therapeutic management of HNSCC.
Matrix metalloproteinases (MMPs) are an important family of genes involved in cell adhesion, proliferation and migration (Fig. 2) (Franchi et al., 2002; Kusukawa et al., 1993; Liu et al., 2007; P et al., 2001; Vairaktaris et al., 2007). Several MMPs are frequently upregulated in HNSCC including MMP-1, MMP-3, MMP-7, MMP-10 and MMP-12 (Franchi et al., 2002; Kusukawa et al., 1993; Liu et al., 2007; O-charoenrat et al., 2001; Vairaktaris et al., 2007, Ye et al., 2008; Ziober et al., 2006). Earlier studies reported an association between MMP2 and lymph node metastases, where MMP9 and MMP3 were implicated in infiltrative growth, involving lymph nodes, and anchorage independent growth, respectively (Ye et al., 2008; Ziober et al., 2006).
Fig. 2.
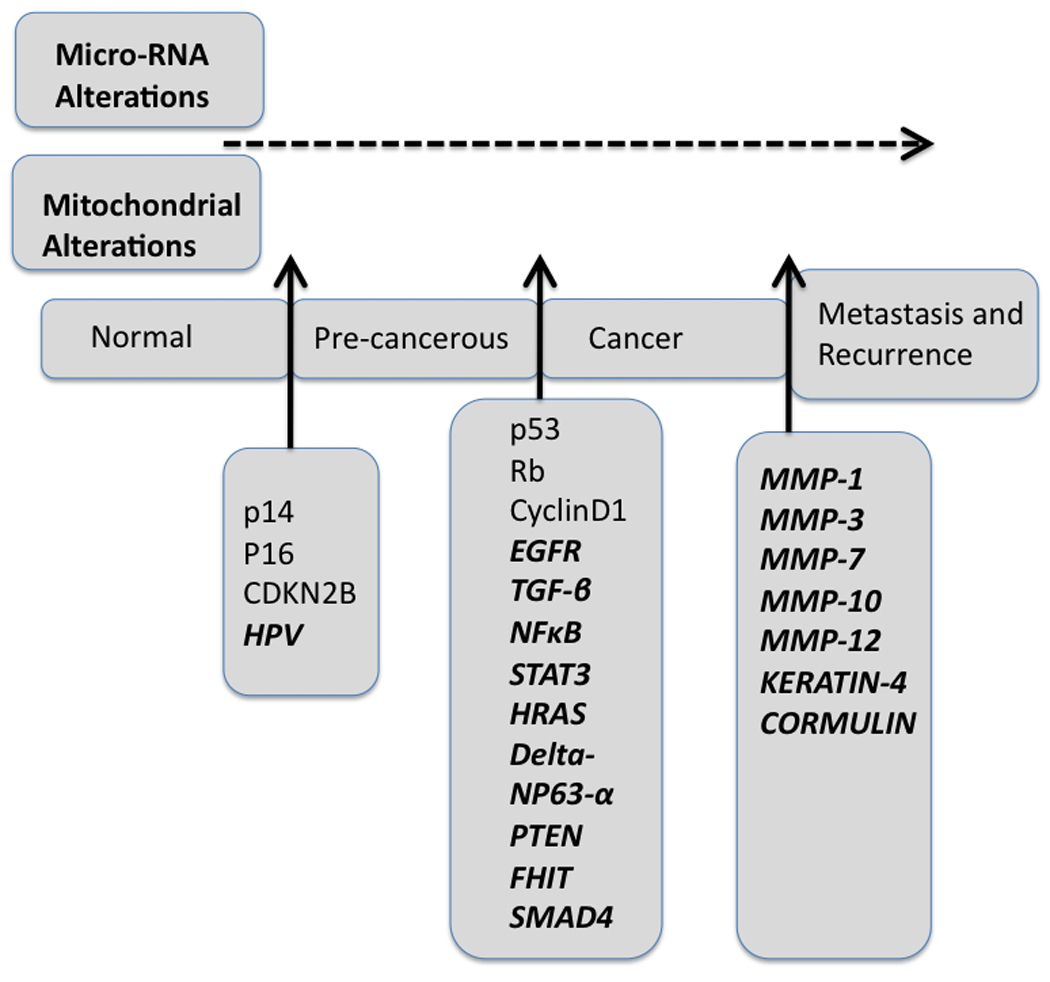
Key genetic alterations in the pathway of HNSCC development and progression. The key genetic changes discovered in the past few decades are represented in bold.
Apart from the MMPs, aberrant expression of Ras, NFκB, and STAT has been implicated in HNSCC (Mao et al., 2004; Das et al., 2000; Clark et al., 1993; Saranath et al., 1991; Karin et al., 2005; Ondrey et al., 1999; Mishra et al., 2006; Sawhney et al., 2007, Bindhu et al., 2006; Bromberg et al., 2002; Grandsi et al., 1998; Grandis et al., 2000a; Grandis et al 2000b; Masuda et al., 2002; Kar et al., 2006; Xi et al., 2003). Activating gain-of-function (GOF) mutation of H-ras and other ras activated genes were detected in varying degrees in HNSCC (Clark et al., 1993; Das et al., 2000; Mao et al., 2004). A high frequency of ras mutation was detected among Asian populations in association with areca nut chewing (Saranath et al., 1991).
The transcription factor NFκB is known to play a central role in regulating cell proliferation and survival and contributes to different pathological processes including cancer (Karin and Greten, 2005). It is composed of 5 family members including p50 (NFκB1), p52 (NFκB2), p65 (RELA), cRel and and RelB (Karin and Greten, 2005). The constitutive activation of NFκB family members is a frequent event in the etiology and progression of multiple cancers including HNSCC (Table 1) (Ondrey et al., 1999). A number of studies demonstrated elevated levels of NFκB among the progressive stages of HNSCC development, thereby suggesting a key role of NFκB in the development of HNSCC phenotype (Bindhu et al., 2006; Mishra et al., 2006; Ondrey et al., 1999; Sawhney et al., 2007).
Aberrant expression of Signal Transducer and Activator of Transcription (STAT) family of proteins can contribute to a number of human diseases, including cancer as GOF of the STAT pathway is found to be frequently associated with cellular transformation and oncogenesis (Bromberg, 2002). Upregulation of phosphorylated STAT3 in progressive stages of HNSCC patients as well as in HNSCC cell lines has been reported (Table 1) (Grandis et al., 1998). Moreover, inhibiting STAT3 activity culminates in suppression of tumor growth in HNSCC (Grandis et al., 1998; Grandis et al., 2000b). Activated STAT3 also correlates with lymph node metastasis and poor prognosis in HNSCC (Grandis et al., 1998; Masuda et al., 2002). Other than STAT3, constitutively active STAT5, STST5A and STST5B are detected in HNSCC (Kar and Supakar, 2006; Xi et al., 2003) and inhibition of STAT5B promotes growth arrest in HNSCC cells (Xi et al., 2003). Thus, persistent activation of the STAT family of proteins could be a contributing factor in HNSCC tumorigenesis.
In an interesting study, delta NP63-alpha, a TP53 homologue was overexpressed in HNSCC and it promoted a potential tumor growth favoring effect (Table 1) (Hibi et al., 2000). It was suggested that such overexpression leads to the accumulation of Beta-catenin, and eventually activates the Wnt signaling pathway. In an earlier study, over expression of Amphiregulin (AREG), Cadherin3 (CDH3), Kalikarin 10 (KLK10), Neuromedin U (NmU) and secretory leukocyte protease inhibitor (SLPI) was reported at both mRNA and protein levels in HNSCC tumors compared to corresponding normal cellular counterparts (Dasgupta et al., 2006b).
Tumor Suppressor Genes (TSGs)
Although a number of oncogenes have been reported to be overexpressed or activated in HNSCC, only a few tumor suppressor genes (TSGs) were found to strongly associate with HNSCC tumorigenesis (Table 2). The tumor suppressors are potential growth regulatory genes that can be deregulated by several mechanisms including hemizygous deletion (LOH, loss of heterozygosity), homozygous deletion (HD), inactivating mutations, epigenetic alterations (promoter hypermethylation, chromatin remodeling, microRNA alterations, etc.) during tumorigenesis. The apoptosis and cell cycle regulatory and guardian gene TP53 located on human chromosome 17p13 is one of the most crucial molecules involved in the development and progression of HNSCC (Brennan et al., 1995a; Brennan et al., 1995b; Califano et al., 1996). Recent comprehensive studies showed that disruptive TP53 mutations are associated with reduced survival of HNSCC patients (Poeta et al., 2007). On the other hand, complete silencing of TP53 in a mouse model of HNSCC led to the development of metastatic SCC (Ku et al., 2007). Thus, TP53 could also be a crucial molecule in the developmental cascade of HNSCC (Fig. 2).
The human chromosomal region 9p21 is frequently deleted and appears to be a very interesting region in HNSCC (Ai et al., 2003; Califano et al., 1996; Reed et al., 1996). From this region, the CDKN2A locus containing the putative tumor suppressors p16 and p14 was identified (Califano et al., 1996; Ai et al., 2003; Reed et al., 1996). Both of these genes are involved in cell cycle regulation and stabilize TP53 by regulating MDM2 (Ai et al., 2003; Reed et al., 1996). Frequent loss of the lipid phosphatase PTEN located on human chromosome 10q23.3, was also reported in about 30% of HNSCC patients (Table 2) (Lee et al., 2001; Squarize et al., 2002). The loss of PTEN expression was also suggested as an independent prognostic indicator for poor clinical outcome in HNSCC (Lee et al., 2001; Squarize et al., 2002). Several other potential tumor suppressor and cancer modulating genes were reported to have alterations resulting from promoter hypermethylation in HNSCC including: E-Cadherin 1 (CDH1) involved in cell-cell adhesion; O-6-methylguanine-DNA methyltransferase (MGMT), a DNA adduct detoxifying agent; death associated protein kinase 1 (DAPK1) involved in apoptosis; the retinoic acid receptor beta (RARB) involved in cellular immortalization; CDKN2B involved in cell cycle regulatory retinoblastoma pathway; the Ras homologue 1 (RASSF1); the mismatch repair gene homologue 1 (MLHI); the FHIT gene for maintaining genetic stability; serpin peptidase inhibitor, member 5 (SERPINB5) involved in regulating cell motility and invasion; and early transformation regulatory gene known as deleted in colon cancer (DCC) (Ha and Califano, 2006).
Loss of enzyme activity of the DNA repair enzyme 8-oxoguanine DNA glycosylase 1 (OGG1) has been found to be associated with increased risk of HNSCC (Table 2) (Paz-Elizur et al., 2006). In a recent interesting study, markedly lower expression of two proteins, keratin-4 and cornulin, was evident in surgical margins and was found to be associated with the development of local relapse of HNSCC patients leading to the suggestion that these proteins may provide potential biomarkers for HNSCC monitoring (Fig. 2) (Schaaij-Visser et al., 2009).
Other Regulatory Genes
The transforming growth factor beta (TGFB) gene family is very large and consists of more than 35 secreted polypeptides (Siegel and Massague, 2003). Although, loss of TGFB-RII was reported in HNSCC (Lu et al., 2006), the precise role of TGF beta alterations in HNSCC is not fully understood. In an orthotopic mouse model of HNSCC, concomitant upregulation of active TGFB1 and MMP1, MMP2, MMP9 was found to be associated with increased invasion and metastasis (Dasgupta et al., 2006a). Moreover, in the same model, active TGFB1 was found to be involved in the inactivation of immune cells particularly Natural Killer (NK) cells by downregulation of NKG2D receptors on NK cells (Dasgupta et al., 2005). Thus, TGFB might play a multitude of functions in the pathogenesis of HNSCC. In a recent study, conditional knock out of SMAD4, a downstream signal transducer of the TGFB pathway was shown to cause HNSCC development in mice (Fig. 2) (Bornstein et al., 2009).
Activation of the beta-catenin pathway is a frequent event in different human cancers including colon, kidney, prostate and thyroid. Although little is known about the role of this signaling pathway in HNSCC, several components of the wnt-pathway such as wnt receptors, frizzleds and their downstream targets, dishevelled and wnt14 are highly expressed in HNSCC tumors (Baker et al., 2005; Leethanakul et al., 2000). Accordingly, the wnt-signaling pathway may play a role in HNSCC, however the precise contribution of this change to HNSCC progression and pathogenesis remains to be defined.
Micro-RNAs
Micro RNA (mir) are small 18–25 nucleotide, non-coding RNA molecules shown to regulate post-translational gene expression (Das et al., 2010; Ramdas et al., 2009; Das et al., 2010). Although recent studies demonstrated alterations of Micro RNAs in different tumor types, their alteration patterns and contributions in HNSCC is an emerging and incompletely studied area (Avissar et al., 2009). Several investigations identified upregulation of mir-21, mir-155, let-7i, mir-142-3p, mir-423, mir-106b, mir-20a, and mir-16, and downregulation of mir-125b, mir-375 and mir-10a in HNSCC (Avissar et al., 2009; Hui et al., 2010). Some of these miRNAs are already known to have potential for driving and orchestrating tumorigenesis by targeting different genes regulating cell growth and proliferation. For example, mir-21 was demonstrated to interact with PTEN and STAT3 in epigenetic alterations linking inflammation to cancer (Iliopoulos et al., 2010) and overexpression of mir-375 in HNSCC cell lines reduced cell growth and proliferation (Hui et al., 2010). Since these miRNAs can crosstalk and regulate the expression of a number of potential oncogenes and tumor suppressor genes, they could also be key players in HNSCC tumorigenesis.
Mitochondrial Genetic Alterations in HNSCC
Other than nuclear genetic alterations, mitochondrial DNA (mtDNA) alterations including somatic mtDNA mutations and altered DNA copy numbers were reported in several cancers including HNSCC (Chatterjee et al., 2006). Mitochondria are unique organelles by having their own DNA, inherited maternally, replicated and transcribed simultaneously (Dasgupta et al., 2010). Human mtDNA is a 16.5-kb double stranded closed circular molecule that encodes for 12S and 16S rRNAs, 22 tRNAs and 13 polypetides (Total 37 proteins) required for the oxidative phosphorylation system (OXPHOS) (Dasgupta et al., 2010). Recent studies reported somatic mtDNA sequence variation and copy number changes in HNSCC (Dasgupta et al., 2010; Kim et al., 2004; Zhou et al., 2007 ). In a comprehensive analysis using MitoChip V2.0, the whole mitochondrial genome was sequenced in 83 primary HNSCC patients. Forty-one of 83 (49%) tumors exhibited mtDNA mutations, including both non-coding and coding regions of the mtDNA (Zhou et al., 2007). In another study, 50 recurrent HNSCC patients were analyzed for mtDNA mutations and 48% (24/50) of cases showed somatic mtDNA mutations mainly affecting the cytochrome c oxidase of the respiratory complex-IV (Dasgupta et al., 2010). Interestingly, 46% of the tumor-derived mtDNA mutations were readily detectable in the corresponding histologically normal margins (Dasgupta et al., 2010). Functional impact of somatic mtDNA mutation on tumor growth was shown in prostate, cervical and bladder cancers (Dasgupta et al., 2008; Kim et al., 2004; Petros et al., 2005; Zhou et al., 2007), however, their precise role in HNSCC development and progression remains to be determined. Thus, mtDNA alterations may also be an attractive target for HNSCC management (Fig. 2).
Preventive Measures for HNSCC
From the above findings, it is evident that substantial contribution was made in the past few decades in terms of novel gene discovery and other genetic pathways involved in the HNSCC development and progression (Tables 1 and 2; Fig. 2). This could potentially be helpful for developing novel strategies for HNSCC management. A number of definitive risk factors such as cigarette smoking, HPV infection and key genetic alterations including EGFR, TP53, p16, p14 were identified and many more will be discovered in the coming years. In the current context, by quitting cigarette smoking, limiting alcohol drinking, avoiding tobacco chewing, preventing exposure to second hand tobacco smoke, environmental carcinogens, screening for HPV, maintaining good oral health, nutritional habits and managing stress could be good primary measures for preventing or delaying HNSCC development (Fig. 3A). Timely identification and subsequent surgical removal of erythroplakia or leukoplakia lesions could also be beneficial for preventing HNSCC development.
Fig. 3.

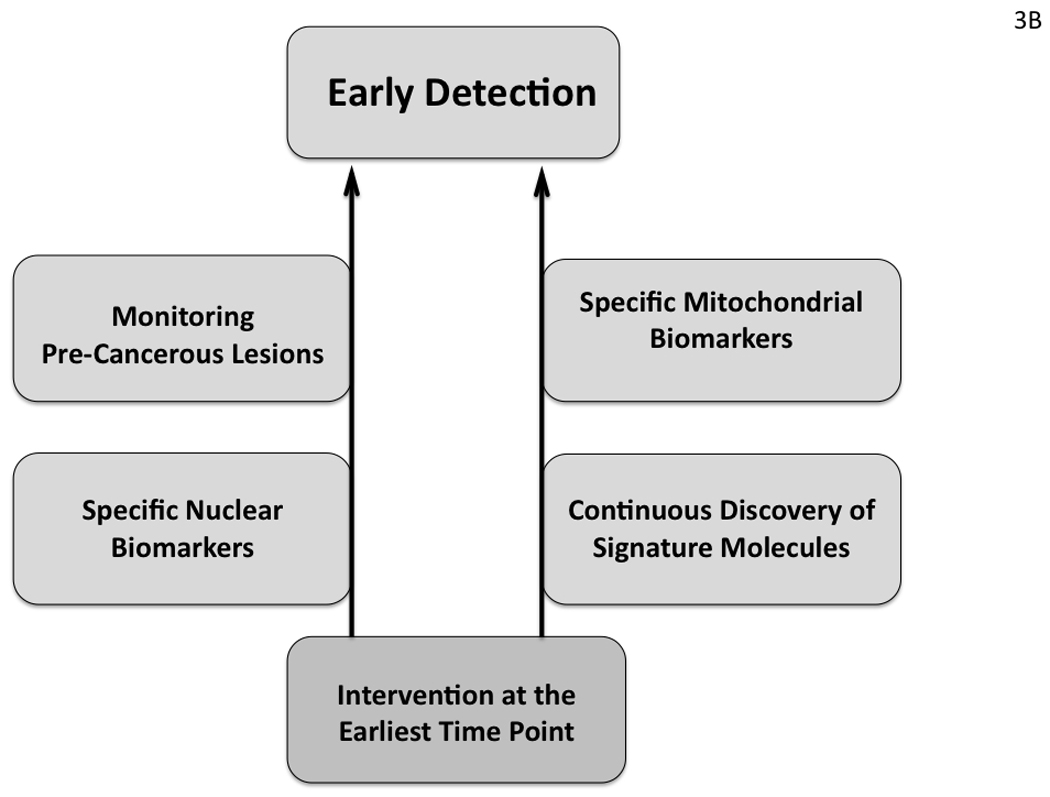
Potential preventive (A) and early detection (B) strategies for HNSCC management based on recent advances in our understanding of HNSCC development and progression.
Early Detection of HNSCC
Early detection is an extremely important component of cancer management and a strategic flow chart is outlined in Fig. 3B based on our current understanding of HNSCC. It is well established that HNSCC develops through progressive histological stages starting from benign hyperplastic to dysplastic changes in the epithelium (Fig. 1) (Califano et al., 1996). In some cases, non-malignant epithelial cells with hyperplastic or dysplastic changes often diagnosed as erythroplakia or leukoplakia can be surgically removed thus preventing HNSCC development. But detection of these lesions at the earliest time point may not always be possible. Moreover, HNSCC may develop without identification of the non-malignant counterparts and can progress very rapidly, particularly in smokers. Therefore, identification of the sequential genetic alterations at the earliest time point of disease development combined with the knowledge already acquired such as the status of EGFR and TP53 alterations would be an ideal framework for developing early detection strategies in HNSCC (Tables 1 and 2). In a recent study using this approach, histological and molecular changes were simultaneously assessed in premalignant margin specimens by combined autoflourescence imaging and LOH analysis (Roblyer et al., 2009). This study clearly demonstrates the value of applying our knowledge on the molecular biology of HNSCC development for monitoring the progression of this cancer. In this early detection strategy, mitochondrial DNA alterations could also be an attractive tool for the development of early diagnostics and risk-assessment, as supported in recent studies (Dasgupta et al., 2008; He et al., 2010; Kim et al., 2004; Petros et al., 2005; Zhou et al., 2007). Moreover, mtDNA detection is more advantageous over nuclear DNA (nDNA) due to high copy number of mutated mtDNA in cancer cells (Dasgupta et al., 2010). For detection purposes, oral rinse, serum/plasma/margin samples could be useful as was shown to be promising for detection of TP53 mutations, mtDNA alterations and other genetic changes in HNSCC (Brennan et al., 1995a; Chatterjee et al., 2006; Dasgupta et al., 2010; Zhou et al., 2007).
Future Perspectives
With enhanced understanding of the molecular pathogenesis and the key regulatory elements controlling the development and progression of HNSCC, new vistas will open for prevention, early detection and therapy. Recent innovative approaches using nuclear genetic as well as mitochondrial genetic changes holds promise for both early detection and monitoring progression of HNSCC. We are rapidly approaching a time when there will be a finite and manageable number of signature molecules available that will not only allow better management of HNSCC, but will also permit sensitive screening of high-risk populations such as smokers and those with genetic predisposition to developing HNSCC.
Acknowledgement
Research support was provided in part by National Institutes of Health Grants R01 CA093812, R01 CA127641, R01 CA134721, R01 CA138540 and P01 CA104177, the National Foundation for Cancer Research and the A D Williams fund. DS is a Harrison Research Scholar and PBF holds the Thelma Newmeyer Corman Chair in Cancer Research in the VCU Massey Cancer Center.
Contract grant sponsor: National Institutes of Health; Contract grants R01 CA093812, R01 CA127641, R01 CA134721 and P01 CA104177, and the National Foundation for Cancer Research to P.B.F.; National Institutes of Health; Contract grant R01 CA138540, the Dana Foundation and the McDonnell Foundation to D.S.; and a grant from the A D Williams fund to S.D.
Literature Cited
- Ai L, Stephenson KK, Ling W, Zuo C, Mukunyadzi P, Suen JY, Hanna E, Fan CY. The p16 (CDKN2a/INK4a) tumor-suppressor gene in head and neck squamous cell carcinoma: a promoter methylation and protein expression study in 100 cases. Mod Pathol. 2003;16:944–950. doi: 10.1097/01.MP.0000085760.74313.DD. [DOI] [PubMed] [Google Scholar]
- Altieri A, Bosetti C, Gallus S, Franceschi S, Dal Maso L, Talamini R, Levi F, Negri E, Rodriguez T, La Vecchia C. Wine, beer and spirits and risk of oral and pharyngeal cancer: a case-control study from Italy and Switzerland. Oral Oncol. 2004;40:904–909. doi: 10.1016/j.oraloncology.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, Fu KK, Milas L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. New England J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar M, McClean MD, Kelsey KT, Marsit CJ. MicroRNA expression in head and neck cancer associates with alcohol consumption and survival. Carcinogenesis. 2009;30:2059–2063. doi: 10.1093/carcin/bgp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar G, Feinmesser R, Shpitzer T, Popovtzer A, Nagler RM. Salivary analysis in oral cancer patients: DNA and protein oxidation, reactive nitrogen species, and antioxidant profile. Cancer. 2007;109:54–59. doi: 10.1002/cncr.22386. [DOI] [PubMed] [Google Scholar]
- Baker H, Patel V, Molinolo AA, Shillitoe EJ, Ensley JF, Yoo GH, Meneses-Garcia A, Myers JN, El-Naggar AK, Gutkind JS, Hancock WS. Proteome-wide analysis of head and neck squamous cell carcinomas using laser-capture microdissection and tandem mass spectrometry. Oral Oncol. 2005;41:183–199. doi: 10.1016/j.oraloncology.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Bindhu OS, Ramadas K, Sebastian P, Pillai MR. High expression levels of nuclear factor kappa B and gelatinases in the tumorigenesis of oral squamous cell carcinoma. Head & Neck. 2006;28:916–925. doi: 10.1002/hed.20437. [DOI] [PubMed] [Google Scholar]
- Bloching M, Reich W, Schubert J, Grummt T, Sandner A. The influence of oral hygiene on salivary quality in the Ames Test, as a marker for genotoxic effects. Oral Oncol. 2007;43:933–939. doi: 10.1016/j.oraloncology.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Brennan JA, Boyle JO, Koch WM, Goodman SN, Hruban RH, Eby YJ, Couch MJ, Forastiere AA, Sidransky D. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. New England J Med. 1995a;332:712–717. doi: 10.1056/NEJM199503163321104. [DOI] [PubMed] [Google Scholar]
- Brennan JA, Mao L, Hruban RH, Boyle JO, Eby YJ, Koch WM, Goodman SN, Sidransky D. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. New England J Med. 1995b;332:429–435. doi: 10.1056/NEJM199502163320704. [DOI] [PubMed] [Google Scholar]
- Bornstein S, White R, Malkoski S, Oka M, Han G, Cleaver T, Reh D, Andersen P, Gross N, Olson S, Deng C, Lu SL, Wang XJ. Smad4 loss in mice causes spontaneous head and neck cancer with increased genomic instability and inflammation. J Clin Invest. 2009;119:3408–3419. doi: 10.1172/JCI38854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, Corio R, Lee D, Greenberg B, Koch W, Sidransky D. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996;56:2488–2492. [PubMed] [Google Scholar]
- Chatterjee A, Mambo E, Sidransky D. Mitochondrial DNA mutations in human cancer. Oncogene. 2006;25:4663–4674. doi: 10.1038/sj.onc.1209604. [DOI] [PubMed] [Google Scholar]
- Chen PC, Pan CC, Kuo C, Lin CP. Risk of oral nonmalignant lesions associated with human papillomavirus infection, betel quid chewing, and cigarette smoking in Taiwan: an integrated molecular and epidemiologic study. Arch Pathol & Lab Med. 2006;130:57–61. doi: 10.5858/2006-130-57-ROONLA. [DOI] [PubMed] [Google Scholar]
- Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15:6758–6762. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, McClure JB. Smoking cessation: recent developments in behavioral and pharmacologic interventions. Oncology. 1998;12:249–256. [PubMed] [Google Scholar]
- Clark LJ, Edington K, Swan IRC, McLay KA, Newlands WJ, Wills LC, Young HA, Johnston PW, Mitchell R, Robertson G, Soutar D, Parkinson EK, Birnie GD. The absence of Harvey ras mutations during development and progression of squamous cell carcinomas of the head and neck. British J Cancer. 1993;68:617–620. doi: 10.1038/bjc.1993.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N, Majumder J, DasGupta UB. ras gene mutations in oral cancer in eastern India. Oral Oncology. 2000;36:76–80. doi: 10.1016/s1368-8375(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Das SK, Sokhi UK, Bhutia SK, Azab B, Su ZZ, Sarkar D, Fisher PB. Human polynucleotide phosphorylase selectively and preferentially degrades microRNA-221 in human melanoma cells. Proc Natl Acad Sci USA. 2010;107:11948–11953. doi: 10.1073/pnas.0914143107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Bhattacharya-Chatterjee M, O'Malley BW, Jr, Chatterjee SK. Inhibition of NK cell activity through TGF-beta 1 by down-regulation of NKG2D in a murine model of head and neck cancer. J Immunol. 2005;175:5541–5550. doi: 10.4049/jimmunol.175.8.5541. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Bhattacharya-Chatterjee M, O'Malley BW, Jr, Chatterjee SK. Tumor metastasis in an orthotopic murine model of head and neck cancer: possible role of TGF-beta 1 secreted by the tumor cells. J Cellular Biochem. 2006a;97:1036–1051. doi: 10.1002/jcb.20647. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Hoque MO, Upadhyay S, Sidransky D. Mitochondrial cytochrome B gene mutation promotes tumor growth in bladder cancer. Cancer Res. 2008;68:700–706. doi: 10.1158/0008-5472.CAN-07-5532. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Koch R, Westra WH, Califano JA, Ha PK, Sidransky D, Koch WM. Mitochondrial DNA mutation in normal margins and tumors of recurrent head and neck squamous cell carcinoma patients. Cancer Prevent Res. 2010;3:1205–1211. doi: 10.1158/1940-6207.CAPR-10-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Tripathi PK, Qin H, Bhattacharya-Chatterjee M, Valentino J, Chatterjee SK. Identification of molecular targets for immunotherapy of patients with head and neck squamous cell carcinoma. Oral Oncol. 2006b;42:306–316. doi: 10.1016/j.oraloncology.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Franchi A, Santucci M, Masini E, Sardi I, Paglierani M, Gallo O. Expression of matrix metalloproteinase 1, matrix metalloproteinase 2, and matrix metalloproteinase 9 in carcinoma of the head and neck. Cancer. 2002;95:1902–1910. doi: 10.1002/cncr.10916. [DOI] [PubMed] [Google Scholar]
- Grandis JR, Drenning SD, Chakraborty A, Zhou MY, Zeng Q, Pitt AS, Tweardy DJ. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth In vitro. J Clin Invest. 1998;102:1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandis JRZQ, Drenning SD. Epidermal growth factor receptor--mediated stat3 signaling blocks apoptosis in head and neck cancer. The Laryngoscope. 2000a;110:868–874. doi: 10.1097/00005537-200005000-00016. [DOI] [PubMed] [Google Scholar]
- Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, Johnson DE, Huang L, He Y, Kim JD. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci USA. 2000b;97:4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53:3579–3584. [PubMed] [Google Scholar]
- Ha PK, Califano JA. Promoter methylation and inactivation of tumour-suppressor genes in oral squamous-cell carcinoma. Lancet Oncol. 2006;7:77–82. doi: 10.1016/S1470-2045(05)70540-4. [DOI] [PubMed] [Google Scholar]
- He Y, Wu J, Dressman DC, Iacobuzio-Donahue C, Markowitz SD, Velculescu VE, Diaz LA, Jr, Kinzler KW, Vogelstein B, Papadopoulos N. Heteroplasmic mitochondrial DNA mutations in normal and tumour cells. Nature. 2010;464:610–614. doi: 10.1038/nature08802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi K, Trink B, Patturajan M, Westra WH, Caballero OL, Hill DE, Ratovitski EA, Jen J, Sidransky D. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci USA. 2000;97:5462–5467. doi: 10.1073/pnas.97.10.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui AB, Lenarduzzi M, Krushel T, Waldron L, Pintilie M, Shi W, Perez-Ordonez B, Jurisica I, O'Sullivan B, Waldron J, Gullane P, Cummings B, Liu FF. Comprehensive MicroRNA profiling for head and neck squamous cell carcinomas. Clin Cancer Res. 2010;16:1129–1139. doi: 10.1158/1078-0432.CCR-09-2166. [DOI] [PubMed] [Google Scholar]
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nature Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24:2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- Kar P, Supakar PC. Expression of Stat5A in tobacco chewing-mediated oral squamous cell carcinoma. Cancer Lett. 2006;240:306–311. doi: 10.1016/j.canlet.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nature Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Kim MM, Clinger JD, Masayesva BG, Ha PK, Zahurak ML, Westra WH, Califano JA. Mitochondrial DNA quantity increases with histopathologic grade in premalignant and malignant head and neck lesions. Clin Cancer Res. 2004;10:8512–8515. doi: 10.1158/1078-0432.CCR-04-0734. [DOI] [PubMed] [Google Scholar]
- Ku TK, Nguyen DC, Karaman M, Gill P, Hacia JG, Crowe DL. Loss of p53 expression correlates with metastatic phenotype and transcriptional profile in a new mouse model of head and neck cancer. Mol Cancer Res. 2007;5:351–362. doi: 10.1158/1541-7786.MCR-06-0238. [DOI] [PubMed] [Google Scholar]
- Kusukawa J, Sasaguri Y, Shima I, Kameyama T, Morimatsu M. Expression of matrix metalloproteinase-2 related to lymph node metastasis of oral squamous cell carcinoma. A clinicopathologic study. Amer J Clin Pathol. 1993;99:18–23. doi: 10.1093/ajcp/99.1.18. [DOI] [PubMed] [Google Scholar]
- Lee JI, Soria JC, Hassan KA, El-Naggar AK, Tang X, Liu DD, Hong WK, Mao L. Loss of PTEN expression as a prognostic marker for tongue cancer. Arch Otolaryngology- Head & Neck Sur. 2001;127:1441–1445. doi: 10.1001/archotol.127.12.1441. [DOI] [PubMed] [Google Scholar]
- Leethanakul C, Patel V, Gillespie J, Pallente M, Ensley JF, Koontongkaew S, Liotta LA, Emmert-Buck M, Gutkind JS. Distinct pattern of expression of differentiation and growth-related genes in squamous cell carcinomas of the head and neck revealed by the use of laser capture microdissection and cDNA arrays. Oncogene. 2000;19:3220–3224. doi: 10.1038/sj.onc.1203703. [DOI] [PubMed] [Google Scholar]
- Liu SY, Liu YC, Huang WT, Huang GC, Su HJ, Lin MH. Requirement of MMP-3 in anchorage-independent growth of oral squamous cell carcinomas. J Oral Path & Med. 2007;36:430–435. doi: 10.1111/j.1600-0714.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- Lu SL, Herrington H, Reh D, Weber S, Bornstein S, Wang D, Li AG, Tang CF, Siddiqui Y, Nord J, Andersen P, Corless CL, Wang XJ. Loss of transforming growth factor-beta type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes & Development. 2006;20:1331–1342. doi: 10.1101/gad.1413306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004;5:311–316. doi: 10.1016/s1535-6108(04)00090-x. [DOI] [PubMed] [Google Scholar]
- Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K, Tomita K, Komiyama S, Weinstein IB. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–3355. [PubMed] [Google Scholar]
- Mishra A, Bharti AC, Varghese P, Saluja D, Das BC. Differential expression and activation of NF-kappaB family proteins during oral carcinogenesis: Role of high risk human papillomavirus infection. Intl J Cancer. 2006;119:2840–2850. doi: 10.1002/ijc.22262. [DOI] [PubMed] [Google Scholar]
- O-charoenrat P, Rhys-Evans P, Eccles SA. Expression of matrix metalloproteinases and their inhibitors correlates with invasion and metastasis in squamous cell carcinoma of the head and neck. Arch Otolaryngology- Head & Neck Sur. 2001;127:813–820. [PubMed] [Google Scholar]
- Ondrey FG, Dong G, Sunwoo J, Chen Z, Wolf JS, Crowl Bancroft CV, Mukaida N, Van Waes C. Constitutive activation of transcription factors NF-(kappa)B, AP-1, and NF-IL6 in human head and neck squamous cell carcinoma cell lines that express pro-inflammatory and pro-angiogenic cytokines. Mol Carcinogenesis. 1999;26:119–129. doi: 10.1002/(sici)1098-2744(199910)26:2<119::aid-mc6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Paz-Elizur T, Ben-Yosef R, Elinger D, Vexler A, Krupsky M, Berrebi A, Shani A, Schechtman E, Freedman L, Livneh Z. Reduced repair of the oxidative 8-oxoguanine DNA damage and risk of head and neck cancer. Cancer Res. 2006;66:11683–11689. doi: 10.1158/0008-5472.CAN-06-2294. [DOI] [PubMed] [Google Scholar]
- Pelucchi C, Talamini R, Negri E, Levi F, Conti E, Franceschi S, La Vecchia C. Folate intake and risk of oral and pharyngeal cancer. Annals Oncol. 2003;14:1677–1681. doi: 10.1093/annonc/mdg448. [DOI] [PubMed] [Google Scholar]
- Perea-Milla Lopez E, Minarro-Del Moral RM, Martinez-Garcia C, Zanetti R, Rosso S, Serrano S, Aneiros JF, Jimenez-Puente A, Redondo M. Lifestyles, environmental and phenotypic factors associated with lip cancer: a case-control study in southern Spain. British J Cancer. 2003;88:1702–1707. doi: 10.1038/sj.bjc.6600975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen PE. Oral cancer prevention and control--the approach of the World Health Organization. Oral Oncol. 2009;45:454–460. doi: 10.1016/j.oraloncology.2008.05.023. [DOI] [PubMed] [Google Scholar]
- Petros JA, Baumann AK, Ruiz-Pesini E, Amin MB, Sun CQ, Hall J, Lim S, Issa MM, Flanders WD, Hosseini SH, Marshall FF, Wallace DC. mtDNA mutations increase tumorigenicity in prostate cancer. Proc Nat Acad Sci USA. 2005;102:719–724. doi: 10.1073/pnas.0408894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdas L, Giri U, Ashorn CL, Coombes KR, El-Naggar A, Ang KK, Story MD. miRNA expression profiles in head and neck squamous cell carcinoma and adjacent normal tissue. Head & Neck. 2009;31:642–654. doi: 10.1002/hed.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed MH, Beevi SS, Geetha A. Enhanced lipid peroxidation and nitric oxide products with deranged antioxidant status in patients with head and neck squamous cell carcinoma. Oral Oncol. 2007;43:333–338. doi: 10.1016/j.oraloncology.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Reed AL, Califano J, Cairns P, Westra WH, Jones RM, Koch W, Ahrendt S, Eby Y, Sewell D, Nawroz H, Bartek J, Sidransky D. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res. 1996;56:3630–3633. [PubMed] [Google Scholar]
- Roblyer D, Kurachi C, Stepanek V, Williams MD, El-Naggar AK, Lee JJ, Gillenwater AM, Richards-Kortum R. Objective detection and delineation of oral neoplasia using autofluorescence imaging. Cancer Prevent Res. 2009;2:423–431. doi: 10.1158/1940-6207.CAPR-08-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez T, Altieri A, Chatenoud L, Gallus S, Bosetti C, Negri E, Franceschi S, Levi F, Talamini R, La Vecchia C. Risk factors for oral and pharyngeal cancer in young adults. Oral Oncol. 2004;40:207–213. doi: 10.1016/j.oraloncology.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Rosenquist K, Wennerberg J, Schildt EB, Bladstrom A, Goran Hansson B, Andersson G. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Oto-laryngologica. 2005;125:1327–1336. doi: 10.1080/00016480510012273. [DOI] [PubMed] [Google Scholar]
- Saranath D, Chang SE, Bhoite LT, Panchal RG, Kerr IB, Mehta AR, Johnson NW, Deo MG. High frequency mutation in codons 12 and 61 of H-ras oncogene in chewing tobacco-related human oral carcinoma in India. British J Cancer. 1991;63:573–578. doi: 10.1038/bjc.1991.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney M, Rohatgi N, Kaur J, Shishodia S, Sethi G, Gupta SD, Deo SV, Shukla NK, Aggarwal BB, Ralhan R. Expression of NF-kappaB parallels COX-2 expression in oral precancer and cancer: association with smokeless tobacco. Intl J Cancer. 2007;120:2545–2556. doi: 10.1002/ijc.22657. [DOI] [PubMed] [Google Scholar]
- Schaaij-Visser TB, Graveland AP, Gauci S, Braakhuis BJ, Buijze M, Heck AJ, Kuik DJ, Bloemena E, Leemans CR, Slijper M, Brakenhoff RH. Differential proteomics identifies protein biomarkers that predict local relapse of head and neck squamous cell carcinomas. Clinical Cancer Res. 2009;15:7666–7675. doi: 10.1158/1078-0432.CCR-09-2134. [DOI] [PubMed] [Google Scholar]
- Shin DM, Ro JY, Hong WK, Hittelman WN. Dysregulation of epidermal growth factor receptor expression in premalignant lesions during head and neck tumorigenesis. Cancer Res. 1994;54:3153–3159. [PubMed] [Google Scholar]
- Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nature Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- Squarize CH, Castilho RM, Santos Pinto D., Jr Immunohistochemical evidence of PTEN in oral squamous cell carcinoma and its correlation with the histological malignancy grading system. J Oral Path & Med. 2002;31:379–384. doi: 10.1034/j.1600-0714.2002.00142.x. [DOI] [PubMed] [Google Scholar]
- Taghavi N, Yazdi I. Type of food and risk of oral cancer. Arch Iranian Med. 2007;10:227–232. [PubMed] [Google Scholar]
- Vairaktaris E, Serefoglou Z, Yapijakis C, Vylliotis A, Nkenke E, Derka S, Vassiliou S, Avgoustidis D, Neukam FW, Patsouris E. High gene expression of matrix metalloproteinase-7 is associated with early stages of oral cancer. Anticancer Res. 2007;27:2493–2498. [PubMed] [Google Scholar]
- Xi S, Zhang Q, Gooding WE, Smithgall TE, Grandis JR. Constitutive activation of Stat5b contributes to carcinogenesis in vivo. Cancer Res. 2003;63:6763–6771. [PubMed] [Google Scholar]
- Ye H, Yu T, Temam S, Ziober BL, Wang J, Schwartz JL, Mao L, Wong DT, Zhou X. Transcriptomic dissection of tongue squamous cell carcinoma. BMC Genomics. 2008;9:69. doi: 10.1186/1471-2164-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Kachhap S, Sun W, Wu G, Chuang A, Poeta L, Grumbine L, Mithani SK, Chatterjee A, Koch W, Westra WH, Maitra A, Glazer C, Carducci M, Sidransky D, McFate T, Verma A, Califano JA. Frequency and phenotypic implications of mitochondrial DNA mutations in human squamous cell cancers of the head and neck. Proc Natl Acad Sci USA. 2007;104:7540–7545. doi: 10.1073/pnas.0610818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziober AF, Patel KR, Alawi F, Gimotty P, Weber RS, Feldman MM, Chalian AA, Weinstein GS, Hunt J, Ziober BL. Identification of a gene signature for rapid screening of oral squamous cell carcinoma. Clin Cancer Res. 2006;12:5960–5971. doi: 10.1158/1078-0432.CCR-06-0535. [DOI] [PubMed] [Google Scholar]


