Abstract
There is a tight interaction of the bone and the immune system. However, little is known about the relevance of the complement system, an important part of innate immunity and a crucial trigger for inflammation. The aim of this study was, therefore, to investigate the presence and function of complement in bone cells including osteoblasts, MSC and osteoclasts. qRT-PCR and immunostaining revealed that the central complement receptors C3aR and C5aR, complement C3 and C5, and membrane-bound regulatory proteins CD46, CD55, and CD59 were expressed in human mesenchymal stem cells, osteoblasts, and osteoclasts. Furthermore, osteoblasts and particularly osteoclasts were able to activate complement by cleaving C5 to its active form C5a as measured by ELISA. Both C3a and C5a alone were unable to trigger the release of inflammatory cytokines interleukin (IL)-6 and IL-8 from osteoblasts. However, co-stimulation with the pro-inflammatory cytokine IL-1β significantly induced IL-6 and IL-8 expression as well as the expression of receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoprotegerin (OPG) indicating that complement may modulate the inflammatory response of osteoblastic cells in a pro-inflammatory environment as well as osteoblast-osteoclast interaction. While C3a and C5a did not affect osteogenic differentiation, osteoclastogenesis was significantly induced even in the absence of RANKL and macrophage-colony stimulating factor (M-CSF) suggesting that complement could directly regulate osteoclast formation. It can therefore be proposed that complement may enhance the inflammatory response of osteoblasts and increase osteoclast formation, particularly in a pro-inflammatory environment, for example during bone healing or in inflammatory bone disorders.
Keywords: COMPLEMENT, C3a, C5a, OSTEOBLAST, MESENCHYMAL STEM CELL, OSTEOCLAST, INTERLEUKIN
INTRODUCTION
There is a steadily increasing interest for the interaction of the skeletal and the immune system, a scientific field known as osteoimmunology [Takayanagi, 2007]. In particular, the crucial cytokines for the crosstalk between bone-forming osteoblasts and bone resorbing osteoclasts, macrophage-colony stimulating factor (M-CSF) and receptor activator of nuclear factor-kappaB ligand (RANKL) closely link the bone and the immune system. While M-CSF is a growth factor for macrophages as well as for osteoclast precursors, RANKL plays a role in T-cell activation and also regulates osteoclast activity [Takayanagi, 2007]. Furthermore, inflammatory cytokines, for example interleukin (IL)-1β, IL-6 and tumour necrosis factor (TNF), enhance the activity of osteoclasts and modulate bone regeneration [Bar-Shavit, 2007; Nakashima and Takayanagi, 2009]. Whereas the role of cytokines in bone is being extensively explored, little is known about the relevance of the complement system, a crucial part of innate immunity and the main trigger for local and systemic inflammation.
The functions of the complement system include the opsonization of antigens, the lysis of microorganisms, the support of phagocytosis, and the induction of inflammatory reactions. Following tissue damage or detection of pathogen associated molecular patterns by immune cells, the enzymatic cascade of the complement system consisting of over 30 proteins can be activated by four pathways: classical; lectin; alternative; extrinsic [Ehrnthaller et al., 2011; Ricklin et al., 2010]. All four pathways lead to the production of the central anaphylatoxins C3a and C5a, which are small polypeptides generated by proteolytic cleavage by C3/5 convertases from their respective precursor proteins C3 or C5. C3a and C5a can act via their corresponding receptors C3aR and C5aR, respectively, inducing the migration of phagocytes, degranulation of mast cells, cytokine release, and respiratory burst reaction, as well as regulating apoptosis in inflammatory cells [Ehrnthaller et al., 2011; Flierl et al., 2006]. The excessive activation of complement can also provoke detrimental side effects. Therefore, to strictly control its activation and to protect healthy cells against a complement attack there are many fluid phase complement regulators as well as membrane-bound regulatory proteins (complement receptor 1 / CR1 / CD35; membrane cofactor protein / MCP / CD46; decay accelerating factor / DAF/ CD55, protectin / CD59) [Kim and Song, 2006; Zipfel and Skerka, 2009].
There is evidence for complement playing a regulative role in bone biology. The starting enzyme of the classical pathway, C1s, which, after activation by C1r, cleaves C2 and C4 to form C3 convertase, was found in hypertrophic chondrocytes in the primary ossification centre of human and hamster femurs. Due to its potency in degrading collagen type I and II, C1s was suggested to participate in cell disintegration and matrix degradation during enchondral bone formation [Sakiyama et al., 1994; Sakiyama et al., 1997]. Andrades et al. reported the presence of C3, C5, C9, and factor B in the growth plate of rats and reasoned that cartilage-bone transformation might be influenced by the alternative pathway [Andrades et al., 1996]. It was shown that osteoblasts generate C3 in response to 1α,25-dihydroxyvitamin D3 [Sato et al., 1991] and that C3 could potentiate osteoclast differentiation, indicating a regulatory function in osteoblast-osteoclast interaction [Sato et al., 1993; Tu et al., 2010]. Furthermore, the key receptor C5aR was expressed in osteoblast-like osteosarcoma cells (MG-63) and modulated the expression of IL-6 [Pobanz et al., 2000]. Recently, we demonstrated that C5aR was expressed in a distinct spatial and temporal pattern by osteoblasts, osteoclasts and chondroblasts in the fracture callus during intramembranous and enchondral ossification [Ignatius et al., 2011]. We found that C5aR was strongly up regulated during osteogenic differentiation and that its activation by C5a induced osteoblast migration suggesting that complement might regulate their recruitment during bone healing [Ignatius et al., 2011]. These findings support the hypothesis of complement playing a crucial role in bone formation.
To further elucidate the role of complement in bone cells, we investigated the expression of receptors (C3aR, C5aR), anaphylatoxin precursors (C3, C5), and complement regulatory proteins (CD46, CD55, CD59) in human mesenchymal stem cells (MSC), in osteoblasts, as well as in osteoclasts. We studied whether osteoblasts and osteoclasts could directly activate complement by cleaving C3 and C5 and whether C3a and C5a could induce an inflammatory response in these cells by inducing the production and release of inflammatory cytokines. Furthermore, we asked whether C3a and C5a could induce osteoclast formation directly or indirectly via modulation of RANKL and osteoprotegerin (OPG) expression in osteoblasts.
MATERIAL AND METHODS
Cultivation of human MSC, osteoblasts, and osteoclasts
The isolation of human primary cells was approved by the Ethical Committee of the University of Ulm, Germany (01/08).
MSC were isolated from bone marrow aspirates obtained from surgical procedures on 5 male, donors (aged 20–38 years) by density gradient centrifugation and adhesion to tissue culture plastic material [Pittenger et al., 2000]. The cells were cultured in a basal medium consisting of Dulbecco’s Modified Eagle Medium (DMEM, Biochrom, Germany) containing 10% fetal calf serum (FCS, Cambrex, Belgium), 4 mM L-glutamine, 100 IU/ml penicillin and 0,1 mg/ml streptomycin (Biochrom, Germany). The isolation procedure is specific for adherent MSC and maintains their progenitor phenotype [Dominici et al., 2006; Fickert et al., 2004; Pittenger et al., 1999]. MSC of passages 2 or 3 were used for the experiments. To promote osteogenic differentiation 1×104 MSC/cm2 were seeded in 24-well tissue culture plates or in 175 cm2 flasks (Nunc, Germany) in basal medium supplemented with 0.1 µM dexamethasone, 10 mM β-glycerophosphate and 0.2 mM ascorbate-2-phospate (all from Sigma-Aldrich, Germany). Von Kossa staining of calcium deposition and alkaline phosphatase staining (86R-1KT, Sigma-Aldrich, Germany) were used to confirm osteogenic differentiation. To investigate if osteogenic differentiation was influenced by the presence of anaphylatoxins, 1 µg/ml C3a or 0,1 µg/ml C5a were added to the differentiation medium. The concentrations were chosen according serum levels in patients with posttraumatic systemic inflammation [Gebhard et al., 2000].
Osteoblasts were isolated according to Robey & Termine [Robey and Termine, 1985] from bone samples (femur or tibia) of 4 male and 1 female patients (aged 23–70 years) undergoing surgery for fracture repair. Briefly, bone pieces were subjected to collagenase digestion and cell isolation was performed in calcium-free DMEM to inhibit fibroblast growth. At sub-confluence, cells were sub-cultured by 0.05% trypsin/0.02% EDTA (Biochrom, Germany) or Accutase™ (PAA, Germany) treatment. For expansion 5,000 cells/cm2 were seeded in DMEM supplemented with 10% FCS (Biochrom, Germany), 4 mM L-glutamine, 100 U/ml penicillin and 0.1 mg/ml streptomycin at 37°C, 8.5% CO2 and saturated humidity. Cells of passages 3 or 4 were used for the experiments following culture for 14 days in osteogenic medium described above.
Osteoclasts were generated from peripheral blood mononuclear cells (PBMNC) isolated from blood samples of 4 male, healthy donors (aged 29–35 years) by density gradient centrifugation as previously described [Kreja et al., 2010]. Briefly, 5×105 PBMNC/cm2 were seeded in 96-well tissue culture plastic plates (Nunc, Germany) in α-Medium (Biochrom, Germany) supplemented with 10% selected fetal calf serum (Gibco, Germany), 4 mM L-Glutamine, 100 IU/ml penicillin, 0,1 mg/ml streptomycin (Biochrom, Germany), 20 ng/ml human recombinant human (rh) RANKL and 10 ng/ml rh M-CSF (both Chemicon International, USA). The medium was changed twice a week. On day 21 cells were fixed with 4% buffered formaldehyde and stained for tartrate-resistant acid phosphatase (TRAP) using the Acid Phosphatase Leukocyte Kit (Sigma-Aldrich, 387A, Germany). Osteoclasts were identified as TRAP-positive multinucleated cells containing at least 3 or more nuclei by light microscopy at 400-fold magnification [Buckley et al., 2005].
For the investigation of osteoclast resorption activity 5×105 PBMNC/cm2 were cultured in the combined presence of 40 ng/ml RANKL and 20 ng/ml M-CSF in calcium phosphate coated 16-well slides (BD BioCoat™ Osteologic™ Bone Cell Culture System; Becton Dickinson, Germany). After 28 days the wells were filled with pure water for 24 h to detach the cells and the remaining mineralized surface was visualized via von Kossa staining according to manufacturer instructions.
Stimulation of osteoblasts with C3a and C5a
Osteoblasts were treated with C3a (1 µg/ml medium, Calbiochem, Germany) or C5a (0.1 µg/ml medium, Sigma-Aldrich, Germany) as well as with the pro-inflammatory cytokine IL-1β (0.1 ng/ml medium, Sigma-Aldrich, Germany) to investigate their ability to respond to inflammatory stimuli. In these experiments osteoblasts were seeded at 100.000 cells/cm2 in DMEM supplemented with 10% FCS. After 24 h cells were stimulated for 24 h with the different factors in medium containing 2% heat inactivated FCS (56°C for 30 min). The concentrations used were within the range of the patho-physiological concentrations measured in the sera of patients with polytrauma [Gebhard et al., 2000]. As a positive control cells were stimulated with 0.1 µg/ml lipopolysaccharide (LPS; E. coli serotype 055:B5; Sigma-Aldrich, Germany).
After 24 h supernatants were collected, centrifuged at 800 g for 5 min and stored at −80 °C. IL-6 and IL-8 release was assessed using enzyme-linked immunosorbent assay (ELISA; R&D Systems, United Kingdom) according to the manufacturer’s instructions. Cell lysates were harvested to determine mRNA expression of IL-6, RANKL, M-CSF and osteoprotegerin (OPG).
Analysis of mRNA expression
Effects on gene expression were examined by RT-PCR as previously described [Liedert et al., 2006]. Briefly, total RNA was isolated from cells using the RNeasy Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. 1 µg RNA was transcribed into cDNA using the Omniscript RT Kit (Qiagen, Germany). Specific primer pairs (Table 1) were designed using published gene sequences (PubMed, NCBI Entrez Nucleotide database) and were synthesized by Thermo Electron Ulm (Germany). Amplification products were cloned and used as standards for RT-PCR (StepOnePlus™ Real-Time PCR System, Applied Biosystems, Germany). The amount of the respective amplification product was determined relative to the housekeeping gene GAPDH. Normalized values of stimulated cells were compared to the respective control group. Amplification products were separated on 2% agarose gel (Life Technologies, USA) and visualized by ethidium bromide staining.
Table 1.
Primer sequences used for real-time RT-PCR.
| mRNA | Forward primer (5’-3’) | Reverse primer (5’-3’) | PCR product size (bp) |
|---|---|---|---|
| C3 | ACC AGC AGA CCG TAA CCA TC | GCA GCC TTG ACT TCC ACT TC | 100 |
| C5 | TGT CGT CGC AAG CCA GCT CC | TGC CAA TGC CTT GAA TTT CCC AGG | 215 |
| C3aR | GCA GGT TCC TAT GCA AGC TC | GAA GAT TTC CCG GTA CAC GA | 221 |
| C5aR | CTC AAC ATG TAC GCC AGC AT | CAG GAA GGA GGG TAT GGT CA | 168 |
| CD46 | GTG AGG AGC CAC CAA CAT TT | GCG GTC ATC TGA GAC AGG T | 177 |
| CD55 | CAG CAC CACCAC AAA TTG AC | CTG AAC TGT TGG TGG GAC CT | 215 |
| CD59 | CCG CTT GAG GGA AAA TGA G | CAG AAA TGG AGT CAC CAG CA | 130 |
| IL-6 | AGG AGA CTT GCC TGG TGA AA | CAG GGG TGG TTA TTG CAT CT | 180 |
| M-CSF | GGA GAC CTC GTG CCA AAT TA | GGC CTT GTC ATG CTC TTC AT | 223 |
| OPG | AGG AAA TGC AAC ACA CGA CA | TAC TTT GGT GCC AGG CAA AT | 168 |
| RANKL | CCA GCA TCA AAA TCC CAA GT | CCC CAA AGT ATG TTG CAT CCT G | 194 |
| AP | GAA CGT ATT TCT CCA GAC CCA GA | GTG GTC TTG GAG TGA GTG AGT GA | 224 |
| BSP | CGA GGG GGA GTA CGA ATA CA | AGG TTC CCC GTT CTC ACT TT | 79 |
| OP | CTC AGG CCA GTT GCA GCC | GCC ACA GCA TCT GGG TAT TT | 177 |
Immunostaining of MSC, osteoblasts and osteoclasts
MSC and osteoblasts were seeded on 13 mm Ø microscope cover glasses or on 8 well Lab-Tek chamber slides (Nunc, Wiesbaden, Germany) with 4000 cells/cm2 2–3 days before staining. Osteoclasts were cultured for 21 days in Lab-Tek chamber slides. Cells were fixed with 4 % phosphate-buffered formalin. Visualization of complement regulatory proteins and receptors was performed by detection of the primary antibodies: mouse-anti-human-CD88 (C5aR), -CD46, -CD55, and -CD59 (all from AbD Serotec, Germany), rabbit-anti-human-C3aR (Santa Cruz Biotechnology Inc., Germany) by the binding of a fluorescence-labeled secondary antibody (Rabbit anti-mouse IgG-Texas Red or goat anti-rabbit IgG-Texas Red, Abcam, UK). Cell nuclei were counterstained with Hoechst 33342 (Invitrogen, Germany). For negative controls the primary antibody was substituted with mouse immunoglobulin (mouse IgG1, AbD Serotec, Germany) or rabbit immunoglobulin (IgG: I5006, Sigma Aldrich, Germany). Unspecific binding was blocked with 2% BSA in PBS.
To investigate C3aR and C5aR localization after stimulation with the respective ligands, osteoblasts were incubated with C3a and C5a respectively for 15 or 45 min before immunostaining. To detect the internalization of the receptors the cells were fixed, and incubated with the respective primary antibody for 60 min followed by 60 min incubation with a green fluorescence-labeled secondary antibody (Rabbit anti-mouse IgG-FITC or goat anti-rabbit IgG-FITC; Abcam, UK). Cells were permeabilized with 0.1 % Triton X-100 in PBS for 5 minutes and the procedure was repeated with the same primary antibody and a red fluorescence-labeled secondary antibody. Images were taken with a Zeiss Cell Observer miroscope using a Photometrics CoolSNAP EZ camera and version 7.5.5.0 of the metamorph software.
C3 and C5 cleavage by osteoclasts and osteoblasts
PBMNC cultured for 14 days in 96-well plates in medium with 20 ng/ml RANKL and 10 ng/ml rh M-CSF were incubated for 4 h with 2.5 µg of purified human complement protein C5 or C3 (Quidel®, USA) in 50 µl FCS-free medium per well. Osteoblasts were seeded at 10.000 cells/cm2 in 24-well plates and cultured in osteogenic medium for 14 days. Cells were then incubated for 4 h with 5 µg C3 or C5 in 250 µl FCS-free medium per well. In parallel cultures, cells were treated with C3 or C5 in the presence of 25 ng/ml phorbol 12-myristate 13-acetate (PMA) as a stimulus (Sigma Aldrich, Germany). PMA, which activates the protein kinase C (PKC) is often used as an activator for neutrophils and macrophages. PMA was shown to activate human neutrophils and rat alveolar macrophages which then where able to effectively cleave C5 to C5a [Huber-Lang et al., 2002]. Supernatants were centrifuged at 800 g for 5 min and stored at −80 °C until use. The cleavage of C3 and C5 was analysed using a C3a ELISA Kit (Quidel®, USA) or a C5a ELISA Kit (DRG Diagnostics, Germany), respectively, according to the manufacturer’s instructions.
Statistical analysis
Experiments were performed independently 3–7 times in triplicate or quadruplicate cultures. The expression of complement components during osteogenic differentiation was evaluated using a non-parametric Wilcoxon signed rank test for paired samples. A non-parametric Mann-Whitney U-test for non-paired samples was performed to evaluate differences between control and cells treated with anaphylatoxins and IL-1β (SPSS Version 18.0.0, SPSS Inc., Chicago, Illinois, USA). To evaluate C3 and C5 cleavage activity of osteoclasts a Student’s t-test was performed. Statistical differences of p<0.05 were considered significant. Boxplots depict median, lower and upper quartiles as well as minimum and maximum; outlier values are indicated as single data points.
RESULTS
Expression of complement regulatory proteins
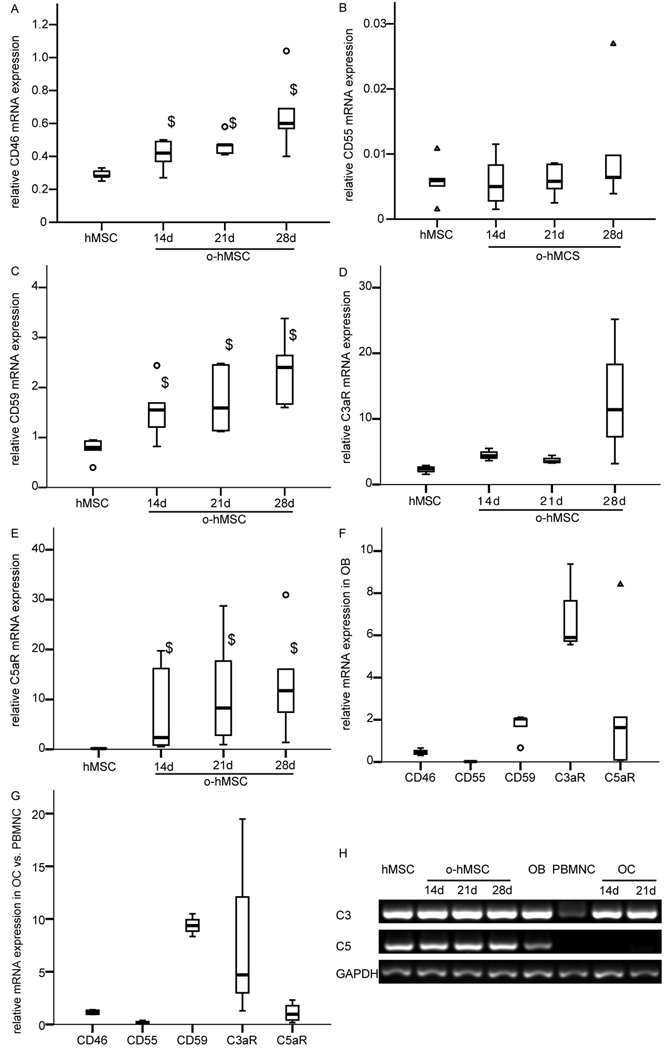
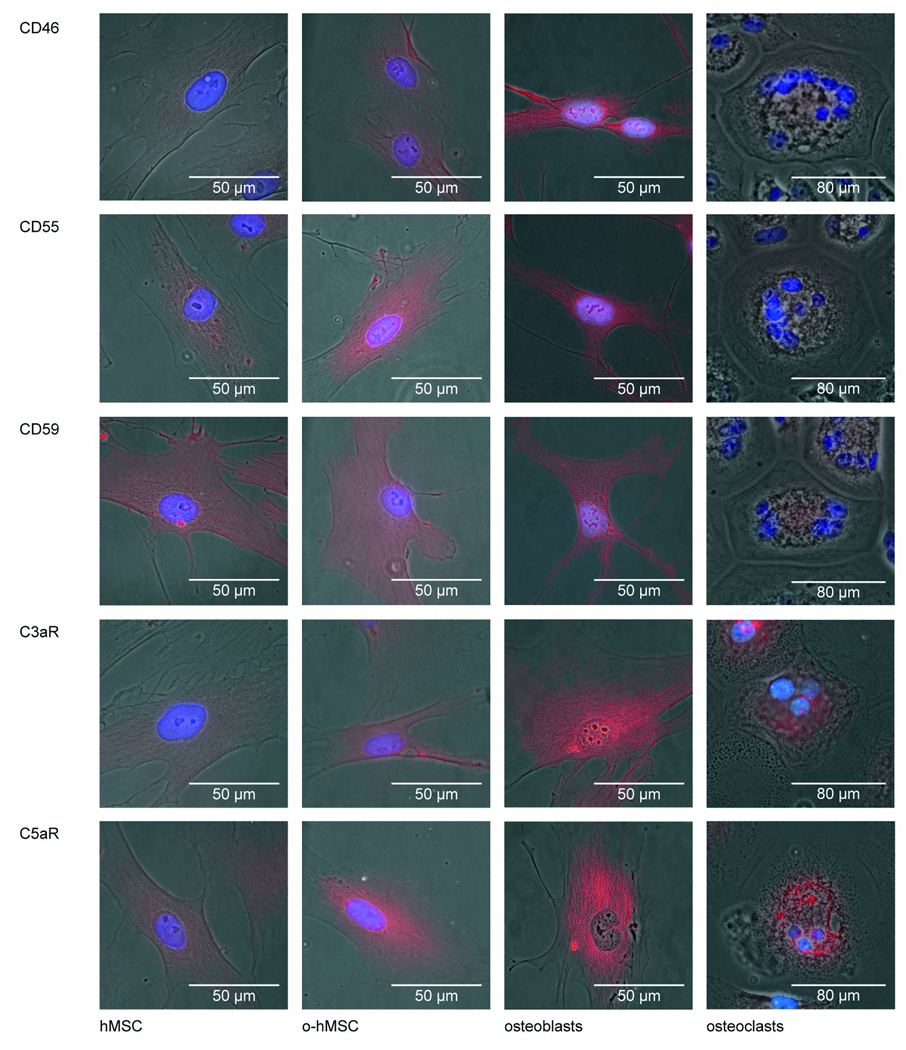
The cell bound complement regulatory proteins CD46, CD55, and CD59 were expressed by MSC and osteoblasts at the mRNA as well as the protein level as revealed by RT-PCR and immunostaining (Fig. 1 A, B, C, F; Fig. 2). CD46 and CD59 mRNA expression was significantly up-regulated during osteogenic differentiation compared to undifferentiated MSC (Fig. 1 A, C). Mature osteoblasts were intensively stained for CD46, CD55, and CD59 (Fig. 2). Osteoclasts also expressed all regulatory proteins but the staining was weaker compared to osteoblasts (Fig. 1G, Fig. 2).
Figure 1.
Expression of complement regulatory proteins (A, B, C, F), complement receptors (D, E, F), and C3 and C5 (H) in bone cells: hMSC, o-hMSC, OB and OC. mRNA expression of the respective gene was related to the housekeeping gene GAPDH. (G) Increase in mRNA expression after OC formation from PBMNC. (H) Representative agarose gels, C3, and C5 expression in hMSC, o-hMSC, OB, PBMNC, and OC. Experiments were performed independently 3–7 times in triplicate or quadruplicate cultures; $ p<0.05
Abbreviations:
hMSC: human mesenchymal stem cells; o-hMSC: hMSC cultivated under osteogenic conditions for 14, 21, and 28 days; OB: primary osteoblasts; OC: osteoclasts, multinucleated tartrate-resistant acid phosphatase (TRAP)-positive cells generated from peripheral blood mononuclear cells (PBMNC)
Figure 2.
Fluorescence images of immunostained complement regulators proteins (CD46, CD55, CD59) and receptors (C3aR, C5aR) in bone cells.
Expression and localization of complement receptors
C5aR and C3aR were expressed by undifferentiated and differentiated MSC (Fig. 1D, E; Fig.2). The mRNA expression of the complement receptor C5aR was significantly up-regulated during osteogenic differentiation (Fig. 1E). C3aR mRNA expression was slightly but not significantly increased after 28 days of osteogenic differentiation (Fig. 1D). Immunostaining of C3aR revealed no considerable difference between differentiated and undifferentiated MSC whereas the staining for C5aR was stronger on differentiated MSC. The receptors were strongly expressed in primary osteoblasts (Fig. 1F, Fig. 2) and osteoclasts (Fig. 1G, Fig. 2). PBMNC expressed both receptors. The C5aR mRNA expression was in tendency up-regulated up to 2.3 fold, the C3aR mRNA expression up to 20 fold during the formation of osteoclast-like cells (Fig. 1G).
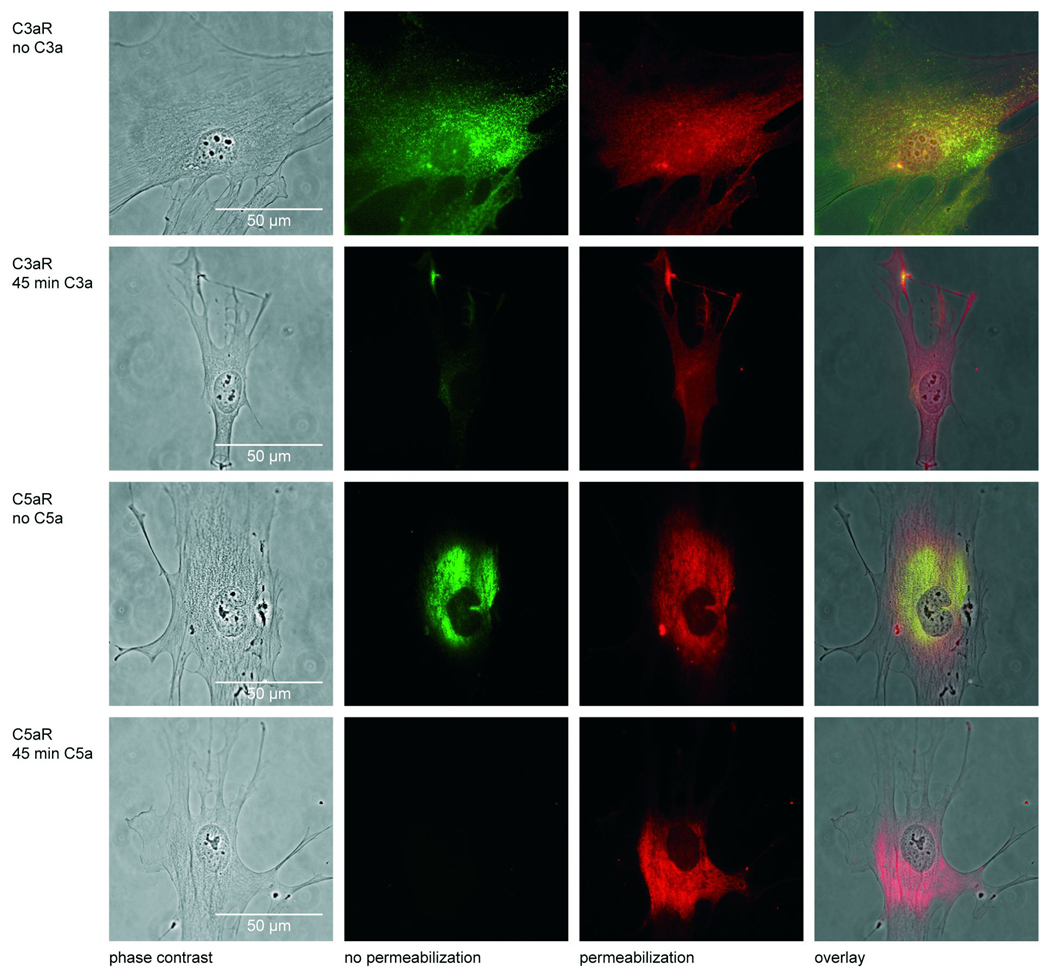
Figure 3 demonstrates the presence and localization of C3aR and C5aR in osteoblasts without and after a 45 min treatment with C3a and C5a, respectively. The second column of Figure 3 shows the green labelling of the receptors before membrane permeabilisation. Only membrane-bound receptors are stained, because the antibody cannot penetrate the cell membrane. The third column demonstrates the staining after cell permeabilisation; because the antibody could permeate the cell, extra- as well as intracellularly located receptors are visible. In the overlay (fourth column) the yellow colour indicates the membrane bound receptor, the red the intracellular receptor. In unstimulated osteoblasts C3aR was mainly localized on the cell surface but was also detected in the nucleus after membrane permeabilisation (first row of Fig. 3). C5aR was found extra- as well as intracellularly in the cytoplasm but not in the nucleus (third row). After stimulation with C3a the majority of C3aR was internalized into the cells (second row). Stimulation with C5a led to complete internalization of C5aR (forth row). C5aR was completely internalized even after 15 min of stimulation with C5a (data not shown). The nuclei were not stained suggesting that C5aR was not translocated to the nucleus.
Figure 3.
Phase contrast and fluorescence images of immunostained C3aR and C5aR in osteoblasts without and after 45 min stimulation with 1µg/ml C3a or 0.1µg/ml C5a; first column: phase contrast images; second column: for the detection of membrane-bound receptors cells were fixed and the respective receptors were detected by a green fluorescent antibody as described in Materials and Methods; third column: for the detection of membrane-bound as well as internalized receptors cells were permeabilized before labelling with a red fluorescent antibody; fourth column: in the overlay the yellow colour indicates the membrane-bound receptors, whereas the internalized receptors are stained red.
Expression of the complement factors C3 and C5
MSC as well as primary osteoblasts expressed C3 and C5 mRNA (Fig. 1 H). The expression of both molecules did not change during osteogenic differentiation of MSC. PBMNC expressed no C5 but some C3 (Fig. 1H). Osteoclasts lacked C5 expression whereas C3 expression was increased in comparison to their precursor cells (PBMNC).
Cleavage of C3 and C5
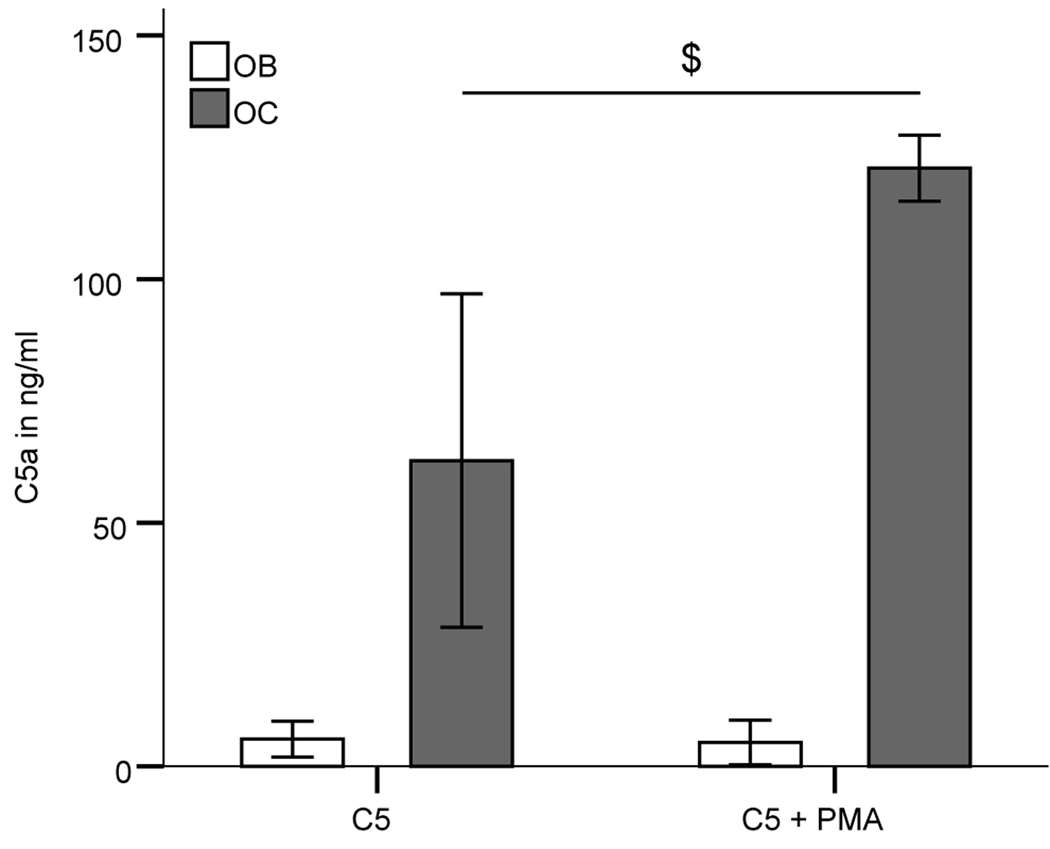
Both osteoblasts and osteoclasts were able to actively cleave native C5, which was added to the cell culture medium osteoclasts being more effective (Fig. 4). C5a could be detected in supernatants of both cultures incubated for 4 h with C5. In osteoclasts but not in osteoblasts C5 cleavage was significantly increased in the presence of a PMA stimulus. There was no C5a found in cultures lacking C5. In contrast, no C3 cleavage was evident either in osteoblasts or in osteoclasts as no C3a was detected in the supernatants.
Figure 4.
C5a induction in supernatants of OB and OC after incubation with C5 for 4h. $ indicates a significant difference between osteoclasts treated with C5 alone and with C5 and phorbol 12-myristate 13-acetate (PMA); $ p>0.05; n=3 independent experiments
Stimulation of osteoblasts with C3a and C5a
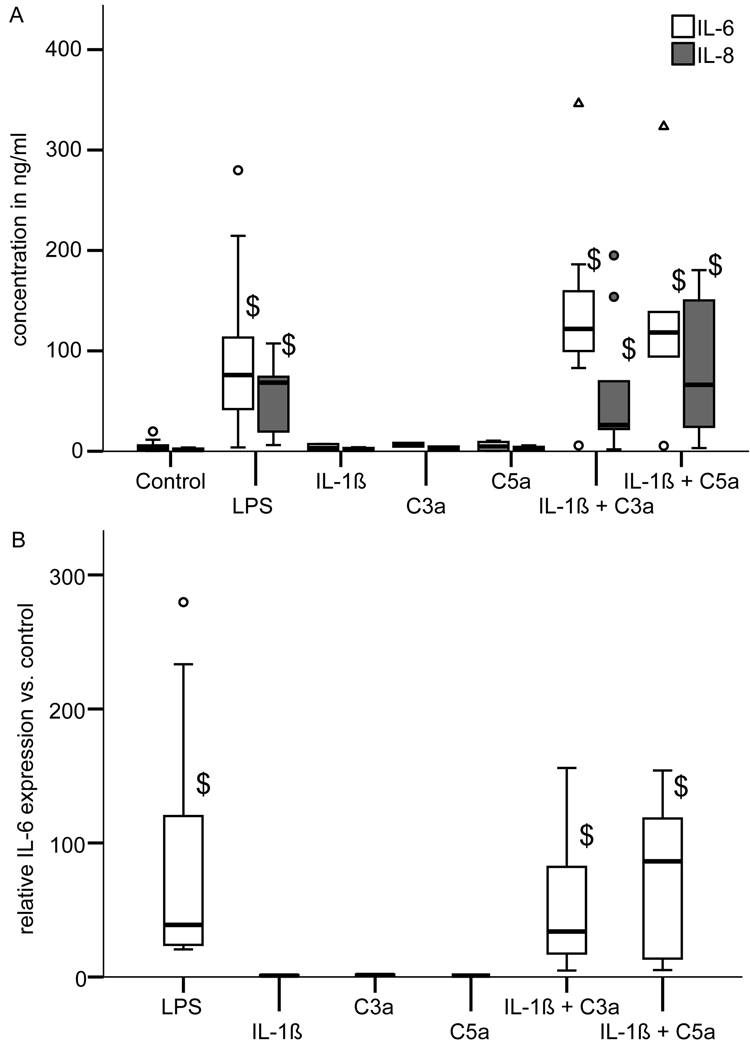
To investigate whether C3a or C5a can act as an inflammatory stimulus osteoblasts were treated with C3a, C5a, or the pro-inflammatory cytokine IL-1β alone or with a combination of IL-1β/C3a and IL-1β/C5a for 24 h. LPS was used as a positive control. Untreated cells were used as a negative control. After 24 h we quantified IL-6 and IL-8 by ELISA (Fig. 5A). The treatment with LPS significantly increased IL-6 and IL-8 release in comparison to untreated cells (Fig. 5A). Stimulation with the individual factors IL-1β, C3a or C5a, respectively, did not significantly induce IL-6 or IL-8 secretion in comparison to untreated cells. In contrast, co-stimulation with IL-1β/C3a or IL-1β/C5a, respectively, significantly up-regulated IL-6 and IL-8 production (Fig. 5A). The data for IL-6 release were confirmed by the results of RT-PCR demonstrating increased IL-6 mRNA expression under these conditions (Fig. 5B).
Figure 5.
Stimulation of osteoblast with lipopolysaccharide (LPS; 0.1 µg/ml), C3a (1 µg/ml), C5a (0.1 µg/ml), IL-1β (0.1 ng/ml) or combinations of IL-1β/C3a and IL-1β/C5a for 24 h. The controls received medium without supplements. (A) IL-6 and IL-8 concentration in the supernatants of osteoblasts measured by ELISA (enzyme-linked immunosorbent assay); (B) IL-6 mRNA expression in stimulated osteoblasts relative to the untreated controls. Experiments were performed independently 3–7 times in triplicate or quadruplicate cultures; $ p<0.05
Influence of C3a and C5a on osteogenic differentiation
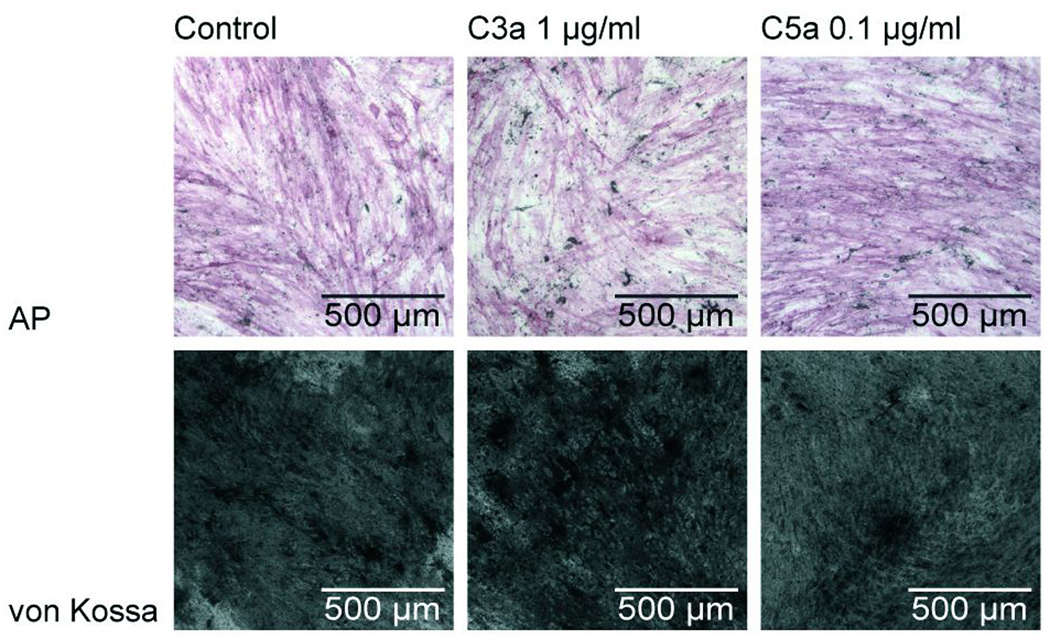
The influence of C3a and C5a on the osteogenic differentiation of MSC was investigated after 21 days of culture in differentiation medium supplemented with 1 µg/ml C3a or 0.1 µg/ml C5a, respectively. The anaphylatoxins did not influence osteogenic differentiation demonstrated by positive von Kossa and AP staining (Fig 6). The mRNA expression of the osteogenic marker genes AP, bone sialoprotein (BSP) and osteopontin did not differ in the presence or absence of C3a and C5a after 28 days of osteogenic differentiation (data not shown).
Figure 6.
Osteogenic differentiation of hMSC after 28 days in differentiation medium containing 1 µg/ml C3a or 0.1 µg/ml C5a. First row: positive staining of alkaline phosphatase; second row: positive von Kossa staining indicating matrix mineralization.
Direct and indirect effects of C3a and C5a on osteoclast formation
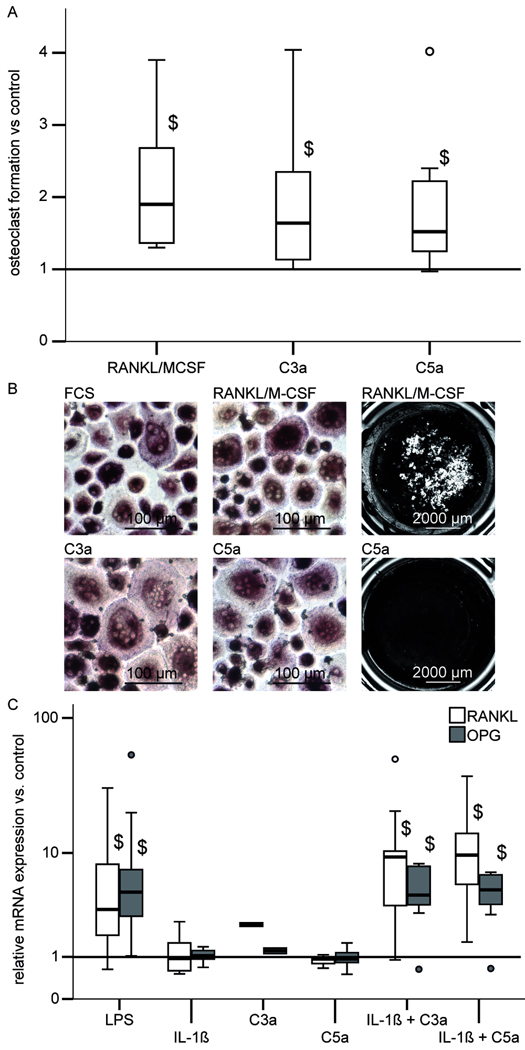
C3a or C5a were added to PBMNC cultures to investigate their effects on osteoclast formation. As a positive control, parallel cultures were treated with 20 ng/ml RANKL and 10 ng/ml M-CSF, which is known to induce osteoclast formation [Boyle et al., 2003]. As a negative control no supplements were added. In the controls the absolute number of multinucleated TRAP-positive cells ranged between 23 and 94 per well (6 independent experiments). As expected the treatment with RANKL/M-CSF significantly increased the number of TRAP-positive multinucleated cells in comparison to untreated cultures (Fig. 7A). Photomicrographs of TRAP-positive multinucleated cell formation in cultures without supplementation and after addition of C3a, C5a or RANKL/M-CSF are presented in Figure 7B. In the absence of RANKL/M-CSF C3a as well as C5a significantly increased formation of TRAP positive multinucleated cells in the same range as the RANKL/M-CSF treatment. If RANKL and M-CSF were present C3a and C5a had no additional effect on formation of TRAP positive multinucleated cells (data not shown). The dissolution of a calcium matrix revealed that the cells resorbed the calcium phosphate coated cell culture surface only after treatment with RANKL/M-CSF (7B). In the absence of RANKL/M-CSF C3a and C5a did not induce resorption activity. If RANKL and M-CSF were present C3a and C5a had no additional effect on osteoclast resorption activity (imaging results not shown).
Figure 7.
(A) Formation of multinucleated TRAP-positive cells from PBMNC after stimulation with RANKL/M-CSF (20 ng/ml / 10 ng/ml), C3a (1 µg/ml) or C5a (0.1 µg/ml). The number of multinucleated TRAP-positive cells in stimulated cultures were related to control cultures without supplements (bar at 1). Experiments were performed independently 6 times in triplicate cultures; $ p<0.05
(B) Photomicrographs of TRAP-stained cells (left and middle column) after 21 days cultivation of PBMNC on plastic and von Kossa-stained 28 days cultures (right column) on calcium phosphate-coated culture vessels.
TRAP-positive multinucleated cells containing at least 3 nuclei were generated in presence of medium with 10% FCS without supplementation with osteoclastogenic factors (FCS), in presence of 20 ng/ml RANKL and 10 ng/ml M-CSF (RANKL/M-CSF), in presence of 0.1 µg/ml C5a (C5a) or 0.1ng/ml C3a (C3a). Osteoclast resorption lacunae in calcium phosphate-coated cell culture surface (gray and white) were observed in presence of 40 ng/ml RANKL and 20 ng/ml M-CSF. After stimuation with 0.1 µg/ml C5a calcium phosphate coating was not resorbed, culture surface was black after von Kossa-staining.
(C) RANKL and OPG mRNA expression in osteoblasts relative to the untreated controls after stimulation with LPS (0.1 µg/ml), C3a (1 µg/ml), C5a (0.1 µg/ml), IL-1β (0.1 ng/ml) or combinations of IL-1β/C3a and IL-1β/C5a for 24 h. The controls received medium without supplements. Experiments were performed independently 3–7 times in triplicate or quadruplicate cultures; $ p<0.05
Abbreviations:
FCS: fetal calf serum; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; M-CSF: macrophage-colony stimulating factor; OPG: osteoprotegerin; RANKL: receptor activator of nuclear factor-kappaB ligand;
To investigate whether C3a or C5a could influence osteoclast formation indirectly via the expression of osteoclast related genes in osteoblasts we investigated the mRNA expression of RANKL and OPG after the cells were stimulated with C3a, C5a, and IL-1β or with a combination of IL-1β/C3a and IL-1β/C5a for 24 h. LPS was used as a positive control. LPS treatment significantly enhanced RANKL and OPG expression in comparison to untreated osteoblasts (Fig. 7C). An isolated treatment with C3a, C5a or IL-1β had no significant effect on RANKL and OPG expression. However, co-stimulation with IL-1β/C3a or IL-1β/C5a significantly increased the mRNA expression of RANKL and OPG (Fig. 7C). In non-stimulated osteoblasts OPG expression was about 100 fold higher compared to RANKL. The RANKL/OPG ratio was not significantly altered.
DISCUSSION
There is evidence that complement plays a role in bone formation and regeneration, but the exact mechanism is poorly understood [Ehrnthaller et al., 2011]. Based on our previous work showing C5aR mediated osteoblast migration and expression during bone healing [Ignatius et al., 2011], this study demonstrated the presence of complement receptors (C3aR, C5aR), complement regulatory proteins (CD46, CD55, CD59), as well as of C3 and C5 in bone cells. We demonstrated that osteoblasts and especially osteoclasts were able to activate complement by cleaving C5 to its active form C5a. Both C3a and C5a alone were unable to trigger the production of inflammatory cytokines in osteoblasts. In contrast, co-stimulation with IL-1β significantly induced the release of IL-6 and IL-8, indicating that complement may modulate the inflammatory response in osteoblastic cells in a pro-inflammatory environment. While C3a and C5a did not affect osteogenic differentiation of MSC, osteoclastogenesis was significantly induced even in the absence of RANKL/M-CSF suggesting that complement is involved in osteoclast formation.
Our data revealed that C3aR and C5aR were present in human MSC, primary osteoblasts and multinucleated osteoclasts confirming our previous results [Ignatius et al., 2011]. Immunostaining of osteoblasts demonstrated that C3aR was located mainly on the cell surface and to a lesser extent in the cytoplasm and the nucleus, whereas C5aR was found both extra- and intracellularly but not in the nucleus. After stimulation with their respective ligands both receptors were completely internalized. Ligand-induced internalization of C3aR and C5aR into the cytoplasm was reported for immune cells [Settmacher et al., 1999; Van Epps et al., 1990] and also for MSC [Schraufstatter et al., 2009]. Our results of nuclear translocation of C3aR are consistent with observations in MSC by Schraufstatter et al.; these authors discussed whether this intriguing result may be restricted to these cells because of their stem cell nature [Schraufstatter et al., 2009]. The data of the present study suggest that nuclear translocation of C3aR could also occur in differentiated cells of mesenchymal origin; its role in intracellular signalling has to be further elucidated.
Our results showed that C5aR mRNA was significantly up-regulated during osteogenic differentiation, indicating an important role in osteoblast function. C3aR and C5aR modulate many cell activities including cell migration, cytokine release and apoptosis [Fernandez et al., 1978; Haas and van Strijp, 2007; Klos et al., 2009; Scholz et al., 1990]. Currently little is known about their function in bone cells. Our results revealed that the presence of anaphylatoxins did not disturb osteoblast function as demonstrated by successful matrix deposition and mineralization. We previously reported that C5a induced the migration of osteoblasts and their precursors via C5aR [Ignatius et al., 2011]. C3a could also act as a chemoattractant for MSC [Schraufstatter et al., 2009]. Because C3a and C5a are locally generated in injured and inflamed tissues it may be suggested that complement contributes to the recruitment of osteoblast precursor cells during bone regeneration. In addition to migration, complement receptors could potentially modulate the inflammatory response of osteoblasts. Other authors reported that C5a in synergy with IL-1β induced the release of the inflammatory cytokine IL-6 from osteoblast-like MG-63 cells [Pobanz et al., 2000]. In the present study we found similar effects in human primary osteoblasts for C5a, and furthermore, also for C3a. Whereas the individual factors were unable to induce a considerable release of IL-6 and IL-8, cytokine secretion was significantly increased after co-stimulation with IL-1β/C3a or IL1β/C5a. The inflammatory response of osteoblasts after co-stimulation was as high as after LPS treatment. These data were confirmed by the results of RT-PCR demonstrating transcriptional IL-6 induction after IL-1β/C3a and IL-1β/C5a treatment. For other cell types it was previously reported that anaphylatoxin effects are complexly interrelated with pro-inflammatory stimuli. IL-1β was shown to increase C5aR expression on mononuclear cells [Takabayashi et al., 2004] while C5a treatment stimulated IL-1β release from PBMNC [Okusawa et al., 1987]. Furthermore C5a and LPS synergistically enhanced IL-6 release from Kupfer cells [Mack et al., 2001]. The mechanism of the synergism of IL-1β and anaphylatoxins might be due to cross talk between the involved intracellular signaling cascades. It is known that IL-1β effects are mediated by MEKK1 activation and proteins of the interleukin receptor associated kinase (IRAK) family, leading to activation of C-JUN or NF-κB [Flannery and Bowie, 2010]. C5aR signalling involves activation of ERK1/2, AKT and MAPK p38 [Rousseau et al., 2006]. C3aR activation was shown to induce ERK1/2 and NF-kB [Li et al., 2008]. It was speculated that the synergistic effects of C5a and IL-1β on IL-6 production in MG63 cells may possibly occur through co-stimulation of NF-kB bindings sites in the IL-6 promoter [Pobanz et al., 2000]. However, experimental data about the underlining mechanisms are presently lacking and should be the object of further studies. In conclusion it might be suggested that C3a and C5a could enhance the inflammatory response of osteoblasts in a pro-inflammatory environment for example during bone healing or in inflammatory bone disorders.
It was reported that mouse bone marrow stromal cells produced C3 in response to 1α,25-dihydroxyvitamin D3 and that C3 induced osteoclast formation mediated by C3 receptors (MCR1, MCR2, CR3) on osteoclast precursor cells [Sato et al., 1993; Sato et al., 1991]. These findings were strengthened by a recent paper demonstrating that C3 is activated via the alternative pathway in mouse bone marrow cells and that in addition to C3 receptors, C3aR and C5aR are required for osteoclasts differentiation [Tu et al., 2010]. Whereas these studies used mixed bone marrow cell cultures hampering the clear differentiation between the role of stromal cells and cells of hematopoietic origin, we investigated osteoclast formation from human PBMNC. Our data demonstrated that the interesting results previously obtained in mouse bone marrow cells [Sato et al., 1993; Tu et al., 2010] could be at least partly transferred to human cells. C3 and C5 were strongly expressed in MSC during osteogenic differentiation as well as in primary osteoblasts, whereas osteoclasts generated from PBMNC, unlike mixed mouse bone marrow cultures, only expressed C3. Furthermore, both osteoblasts and osteoclasts could actively cleave C5 to C5a, with osteoclasts being much more effective. C3 was cleaved neither by osteoblasts nor by osteoclasts. The catalytically active center of systemic C3 and C5 convertase complexes is known to reside in serine protease domains [Huber-Lang et al., 2002]. Although several other proteases including various coagulation factors appear to be capable of cleaving both, C3 and C5, present findings revealed a rather distinctive cell-type dependent effect with the lack of a specific C3 cleaving protease in osteoblasts and osteoclasts.
The most intriguing finding is that both anaphylatoxins could significantly enhance the formation of multinucleated TRAP-positive cells from PBMNC even in the absence of RANKL/M-CSF, which are crucial factors for osteoclastogenesis and are needed to be supplemented for efficient osteoclast formation in PBMNC cultures [Boyle et al., 2003; Kreja et al., 2007]. C3a and C5a were as effective as RANKL/M-CSF supplementation. These data suggest that osteoblasts and their precursors as well as osteoclasts may locally produce and activate complement, which could directly increase osteoclast differentiation. However, C3a or C5a were not able to induce resorption activity in PBMNC derived TRAP positive multinucleated cells in the absence of RANKL/M-CSF indicating that the anaphylatoxins might predominantly influence osteoclastogenesis. In vivo, the induction of osteoclast formation might be enhanced by a increased recruitment of osteoclast precursor cells to the site of inflammation due to the chemotactic effects of the anaphylatoxins [Flierl et al., 2006]. We also investigated whether anaphylatoxins could influence osteoclasts indirectly by modulating RANKL and OPG expression in osteoblasts. Neither RANKL nor OPG expression was significantly altered by C3a or C5a treatment. However, co-stimulation with IL-1β/C3a or IL-1β/C5a synergistically increased RANKL and OPG expression. This effect could potentially be mediated by the observed release of IL-6, which is known to influence osteoclastogenesis by modulation of RANKL/OPG expression [Liu et al., 2005; Suzuki et al., 2011]. These results suggest that C3a and C5a might not only influence osteoclast formation directly but also modulate osteoblast/osteoclast interaction via RANKL/OPG.
MSC and osteoblasts as well as osteoclasts expressed membrane-associated complement regulatory proteins CD46 (membrane cofactor protein), CD55 (decay accelerating factor) and CD59 (protectin). These molecules are widely expressed and protect cells against a complement attack [Kim and Song, 2006; Miwa and Song, 2001]. CD46 regulates C3 activation as a cofactor for factor I-mediated cleavage of C3b. CD59 prevents the formation of the terminal lytic membrane attack complex. CD55 inhibits the activation of C3 and C5 by preventing the formation and accelerating the decay of C3/C5 convertases [Kim and Song, 2006; Miwa and Song, 2001]. Confirming our results it was recently reported that MSC express CD46, CD55, and CD59 and were largely resistant to complement mediated cytotoxicity [Komoda et al., 2010]. We found that CD46 and CD59 were significantly up-regulated during osteogenic differentiation possibly indicating that more mature osteoblastic cells may be more efficiently protected against complement attack.
In conclusion this study demonstrated that bone cells express complement components such as C3 and C5, related receptors C3aR and C5aR, and regulatory proteins. Osteoblasts as well as osteoclasts could generate C5a. Both anaphylatoxins directly induced osteoclast formation from PBMNC. In synergism with IL-1β, C3a and C5a induced the release of IL-6 and IL-8, and up-regulated RANKL/OPG expression, suggesting that complement may enhance the inflammatory response of osteoblasts and modulate their interaction with osteoclasts, especially in a pro-inflammatory environment for example during bone healing or in inflammatory bone disorders.
ACKNOWLEDGMENTS
This study was funded by the German Research Council (Deutsche Forschungsgemeinschaft; KFO-200) and by National Institutes of Health grants AI068730 (to JDL). The excellent technical assistance of Helga Bach and Barbara Acker is gratefully acknowledged. We thank Andrea Tautzenberger for supporting osteoclast experiments. Furthermore we would like to thank Prof. Paul Dietl and Dr. Edward Felder for kindly supporting immunofluorescense microscopy.
REFERENCES
- Andrades JA, Nimni ME, Becerra J, Eisenstein R, Davis M, Sorgente N. Complement proteins are present in developing endochondral bone and may mediate cartilage cell death and vascularization. Exp Cell Res. 1996;227:208–213. doi: 10.1006/excr.1996.0269. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit Z. The osteoclast: a multinucleated, hematopoietic-origin, bone-resorbing osteoimmune cell. J Cell Biochem. 2007;102:1130–1139. doi: 10.1002/jcb.21553. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Buckley KA, Chan BY, Fraser WD, Gallagher JA. Human osteoclast culture from peripheral blood monocytes: phenotypic characterization and quantitation of resorption. Methods Mol Med. 2005;107:55–68. doi: 10.1385/1-59259-861-7:055. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Ehrnthaller C, Ignatius A, Gebhard F, Huber-Lang M. New Insights of an Old Defense System: Structure, Function, and Clinical Relevance of the Complement System. Mol Med. 2011 doi: 10.2119/molmed.2010.00149. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez HN, Henson PM, Otani A, Hugli TE. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978;120:109–115. [PubMed] [Google Scholar]
- Fickert S, Fiedler J, Brenner RE. Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res Ther. 2004;6:R422–R432. doi: 10.1186/ar1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery S, Bowie AG. The interleukin-1 receptor-associated kinases: critical regulators of innate immune signalling. Biochem Pharmacol. 2010;80:1981–1991. doi: 10.1016/j.bcp.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Schreiber H, Huber-Lang MS. The role of complement, C5a and its receptors in sepsis and multiorgan dysfunction syndrome. J Invest Surg. 2006;19:255–265. doi: 10.1080/08941930600778263. [DOI] [PubMed] [Google Scholar]
- Gebhard F, Strecker W, Brückner U, Kinzl L. Untersuchungen zur systemischen posttraumatischen Inflammation in der Frühphase nach Trauma. Der Unfallchirurg. 2000:1–96. [Google Scholar]
- Haas PJ, van Strijp J. Anaphylatoxins: their role in bacterial infection and inflammation. Immunol Res. 2007;37:161–175. doi: 10.1007/BF02697367. [DOI] [PubMed] [Google Scholar]
- Huber-Lang M, Younkin EM, Sarma JV, Riedemann N, McGuire SR, Lu KT, Kunkel R, Younger JG, Zetoune FS, Ward PA. Generation of C5a by phagocytic cells. Am J Pathol. 2002;161:1849–1859. doi: 10.1016/S0002-9440(10)64461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius A, Ernthaller C, Brenner RE, Kreja L, Schoengraf P, Lisson P, Blakytny R, Recknagel S, Claes L, Gebhard F, Lambris JD, Huber-Lang M. The anaphylatoxin receptor C5aR is present during fracture healing in rats and mediates osteoblast migration in vitro. J Trauma. 2011 doi: 10.1097/TA.0b013e3181f8aa2d. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DD, Song WC. Membrane complement regulatory proteins. Clin Immunol. 2006;118:127–136. doi: 10.1016/j.clim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753–2766. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoda H, Okura H, Lee CM, Sougawa N, Iwayama T, Hashikawa T, Saga A, Yamamoto-Kakuta A, Ichinose A, Murakami S, Sawa Y, Matsuyama A. Reduction of N-glycolylneuraminic acid xenoantigen on human adipose tissue-derived stromal cells/mesenchymal stem cells leads to safer and more useful cell sources for various stem cell therapies. Tissue Eng Part A. 2010;16:1143–1155. doi: 10.1089/ten.TEA.2009.0386. [DOI] [PubMed] [Google Scholar]
- Kreja L, Brenner RE, Tautzenberger A, Liedert A, Friemert B, Ehrnthaller C, Huber-Lang M, Ignatius A. Non-resorbing osteoclasts induce migration and osteogenic differentiation of mesenchymal stem cells. J Cell Biochem. 2010;109:347–355. doi: 10.1002/jcb.22406. [DOI] [PubMed] [Google Scholar]
- Kreja L, Liedert A, Schmidt C, Claes L, Ignatius A. Influence of receptor activator of nuclear factor (NF)-kappaB ligand (RANKL), macrophage-colony stimulating factor (M-CSF) and fetal calf serum on human osteoclast formation and activity. J Mol Histol. 2007;38:341–345. [PubMed] [Google Scholar]
- Li K, Anderson KJ, Peng Q, Noble A, Lu B, Kelly AP, Wang N, Sacks SH, Zhou W. Cyclic AMP plays a critical role in C3a-receptor-mediated regulation of dendritic cells in antigen uptake and T-cell stimulation. Blood. 2008;112:5084–5094. doi: 10.1182/blood-2008-05-156646. [DOI] [PubMed] [Google Scholar]
- Liedert A, Kaspar D, Claes L, Ignatius A. Signal transduction pathways involved in mechanical regulation of HB-GAM expression in osteoblastic cells. Biochem Biophys Res Commun. 2006;342:1070–1076. doi: 10.1016/j.bbrc.2006.02.063. [DOI] [PubMed] [Google Scholar]
- Liu XH, Kirschenbaum A, Yao S, Levine AC. Cross-talk between the interleukin-6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-{kappa}B (RANK) ligand/RANK system. Endocrinology. 2005;146:1991–1998. doi: 10.1210/en.2004-1167. [DOI] [PubMed] [Google Scholar]
- Mack C, Jungermann K, Gotze O, Schieferdecker HL. Anaphylatoxin C5a actions in rat liver: synergistic enhancement by C5a of lipopolysaccharide-dependent alpha(2)-macroglobulin gene expression in hepatocytes via IL-6 release from Kupffer cells. J Immunol. 2001;167:3972–3979. doi: 10.4049/jimmunol.167.7.3972. [DOI] [PubMed] [Google Scholar]
- Miwa T, Song WC. Membrane complement regulatory proteins: insight from animal studies and relevance to human diseases. Int Immunopharmacol. 2001;1:445–459. doi: 10.1016/s1567-5769(00)00043-6. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Takayanagi H. Osteoclasts and the immune system. J Bone Miner Metab. 2009;27:519–529. doi: 10.1007/s00774-009-0089-z. [DOI] [PubMed] [Google Scholar]
- Okusawa S, Dinarello CA, Yancey KB, Endres S, Lawley TJ, Frank MM, Burke JF, Gelfand JA. C5a induction of human interleukin 1. Synergistic effect with endotoxin or interferon-gamma. J Immunol. 1987;139:2635–2640. [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mosca JD, McIntosh KR. Human mesenchymal stem cells: progenitor cells for cartilage, bone, fat and stroma. Curr Top Microbiol Immunol. 2000;251:3–11. doi: 10.1007/978-3-642-57276-0_1. [DOI] [PubMed] [Google Scholar]
- Pobanz JM, Reinhardt RA, Koka S, Sanderson SD. C5a modulation of interleukin-1 beta-induced interleukin-6 production by human osteoblast-like cells. J Periodontal Res. 2000;35:137–145. doi: 10.1034/j.1600-0765.2000.035003137.x. [DOI] [PubMed] [Google Scholar]
- Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey PG, Termine JD. Human bone cells in vitro. Calcif Tissue Int. 1985;37:453–460. [PubMed] [Google Scholar]
- Rousseau S, Dolado I, Beardmore V, Shpiro N, Marquez R, Nebreda AR, Arthur JS, Case LM, Tessier-Lavigne M, Gaestel M, Cuenda A, Cohen P. CXCL12 and C5a trigger cell migration via a PAK1/2-p38alpha MAPK-MAPKAP-K2-HSP27 pathway. Cell Signal. 2006;18:1897–1905. doi: 10.1016/j.cellsig.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Sakiyama H, Inaba N, Toyoguchi T, Okada Y, Matsumoto M, Moriya H, Ohtsu H. Immunolocalization of complement C1s and matrix metalloproteinase 9 (92kDa gelatinase/type IV collagenase) in the primary ossification center of the human femur. Cell Tissue Res. 1994;277:239–245. [PubMed] [Google Scholar]
- Sakiyama H, Nakagawa K, Kuriiwa K, Imai K, Okada Y, Tsuchida T, Moriya H, Imajoh-Ohmi S. Complement Cls, a classical enzyme with novel functions at the endochondral ossification center: immunohistochemical staining of activated Cls with a neoantigen-specific antibody. Cell Tissue Res. 1997;288:557–565. doi: 10.1007/s004410050841. [DOI] [PubMed] [Google Scholar]
- Sato T, Abe E, Jin CH, Hong MH, Katagiri T, Kinoshita T, Amizuka N, Ozawa H, Suda T. The biological roles of the third component of complement in osteoclast formation. Endocrinology. 1993;133:397–404. doi: 10.1210/endo.133.1.8319587. [DOI] [PubMed] [Google Scholar]
- Sato T, Hong MH, Jin CH, Ishimi Y, Udagawa N, Shinki T, Abe E, Suda T. The specific production of the third component of complement by osteoblastic cells treated with 1 alpha,25-dihydroxyvitamin D3. FEBS Lett. 1991;285:21–24. doi: 10.1016/0014-5793(91)80715-f. [DOI] [PubMed] [Google Scholar]
- cholz W, McClurg MR, Cardenas GJ, Smith M, Noonan DJ, Hugli TE, Morgan EL. C5a-mediated release of interleukin-6 by human monocytes. Clin Immunol Immunopathol. 1990;57:297–307. doi: 10.1016/0090-1229(90)90043-p. [DOI] [PubMed] [Google Scholar]
- Schraufstatter IU, Discipio RG, Zhao M, Khaldoyanidi SK. C3a and C5a are chemotactic factors for human mesenchymal stem cells, which cause prolonged ERK1/2 phosphorylation. J Immunol. 2009;182:3827–3836. doi: 10.4049/jimmunol.0803055. [DOI] [PubMed] [Google Scholar]
- Settmacher B, Bock D, Saad H, Gartner S, Rheinheimer C, Kohl J, Bautsch W, Klos A. Modulation of C3a activity: internalization of the human C3a receptor and its inhibition by C5a. J Immunol. 1999;162:7409–7416. [PubMed] [Google Scholar]
- Suzuki M, Hashizume M, Yoshida H, Shiina M, Mihara M. Intercellular adhesion molecule-1 on synovial cells attenuated interleukin-6-induced inhibition of osteoclastogenesis induced by receptor activator for nuclear factor kappaB ligand. Clin Exp Immunol. 2011;163:88–95. doi: 10.1111/j.1365-2249.2010.04276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabayashi T, Shimizu S, Clark BD, Beinborn M, Burke JF, Gelfand JA. Interleukin-1 upregulates anaphylatoxin receptors on mononuclear cells. Surgery. 2004;135:544–554. doi: 10.1016/j.surg.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- Tu Z, Bu H, Dennis JE, Lin F. Efficient osteoclast differentiation requires local complement activation. Blood. 2010;116:4456–4463. doi: 10.1182/blood-2010-01-263590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps DE, Simpson S, Bender JG, Chenoweth DE. Regulation of C5a and formyl peptide receptor expression on human polymorphonuclear leukocytes. J Immunol. 1990;144:1062–1068. [PubMed] [Google Scholar]
- Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]