Abstract
BACKGROUND
We evaluated the efficacy of carboplatin, irinotecan and bevacizumab among recurrent glioblastoma (GBM) patients after prior progression on bevacizumab therapy in a phase 2, open-label, single-arm trial.
METHODS
Eligible patients received carboplatin (area under the plasma curve [AUC] 4 mg/ml-min) on day one, while bevacizumab (10 mg/kg) and irinotecan (340 mg/m2 for patients on CYP3A-enzyme inducing anti-epileptics [EIAEDs] and 125 mg/m2 for patients not on EIAEDs) were administered on days 1 and 14 of every 28-day cycle. Patients were evaluated after each of the first two cycles and then after every other cycle. Treatment continued until progressive disease, unacceptable toxicity, non-compliance or voluntary withdrawal. The primary endpoint was progression-free survival at six months (PFS-6) and secondary endpoints included safety and median overall survival (OS).
RESULTS
All patients had progression on at least one prior bevacizumab regimen and 56% enrolled after either second or third overall progression. The median OS was 5.8 months (95% confidence interval [CI]: 4.0, 7.0 months) and PFS-6 rate was 16% (95% CI: 5.0, 32.5%). The most common grade 3 or 4 events were hematologic and occurred in 29% of cycles. Nine patients (38%) required dose modification. There were no treatment related deaths.
CONCULSION
Carboplatin, irinotecan and bevacizumab is associated with modest activity and adequate safety among recurrent GBM patients who progressed on bevacizumab previously.
Keywords: Glioblastoma, angiogenesis, bevacizumab, vascular endothelial growth factor, irinotecan, carboplatin
INTRODUCTION
Outcome for glioblastoma (GBM), the most common malignant primary brain tumor, remains poor with median overall survival of only 14.6 months following current standard therapy that includes maximum safe resection followed by involved-field radiation with temozolomide and adjuvant temozolomide.1 Salvage therapies following recurrence have had limited activity.2–4 Initial clinical trials with bevacizumab, a humanized monoclonal antibody (MAb) against vascular endothelial growth factor (VEGF), were conducted among recurrent GBM patients because GBM tumors are highly angiogenic5, 6 and secrete high levels of VEGF.7–9 In addition, VEGF targeting MAbs inhibit growth in in vivo orthotopic GBM xenograft models10, 11 and can enhance the anti-tumor activity of cytotoxic therapy.12–14 Furthermore, significant clinical benefit associated with bevacizumab plus chemotherapy had been noted in other aggressive solid tumors.15–20 Encouraging rates of radiographic response and improved survival reported by initial studies in recurrent GBM21, 22 triggered follow-up studies which ultimately led to accelerated approval by the U.S. Food and Drug Administration (FDA) of single-agent bevacizumab based on durable radiographic responses.23–25
However, the survival benefit following bevacizumab with or without other systemic agents is modest with most patients developing progressive disease within 8–10 months and dying from refractory tumor soon thereafter.22–24, 26–30 Thus effective therapy for GBM patients following progression on bevacizumab-based therapy represents a major unmet need.
Carboplatin and irinotecan exhibit modest anti-tumor activity when administered separately among recurrent malignant glioma patients.31–38 In addition, both agents impair DNA replication via potentially complementary mechanisms and are associated with primarily non-overlapping toxicities. We therefore hypothesized that a regimen combining carboplatin and irinotecan may be adequately tolerated and associated with greater anti-tumor benefit than either agent alone. Studies evaluating this regimen in other cancer populations were the basis for the dosing schedule utilized in the current study.{Hermes, 2008 #10764;Jones, 2003 #9143} The rationale for continuing bevacizumab was to avoid rebound, fulminant progressive tumor following bevacizumab discontinuation39 and to potentially normalize tumor vasculature to allow enhanced chemotherapy delivery.40
PATIENTS AND METHODS
Protocol Objectives
Our primary objective was to evaluate the activity, defined by progression-free survival at six months (PFS-6) of carboplatin and irinotecan combined with bevacizumab among adults with recurrent GBM who progressed on prior bevacizumab therapy. In addition, we sought to evaluate the safety of this regimen in this patient population.
Patient Eligibility
Patients were required to have histologic confirmation of WHO grade IV malignant glioma (GBM or gliosarcoma) that progressed after prior bevacizumab based therapy. Patients with prior low-grade glioma were eligible if histologic transformation to grade IV malignant glioma was confirmed. Eligible patients were also: at least 18 years of age; had a KPS ≥ 70, and were on a stable corticosteroid dose for at least 1 week. Additional enrollment criteria included: hematocrit >29%; absolute neutrophil count >1000 cells/µl; platelet count >100,000 cells/µl; and serum creatinine, aspartate aminotransferase and bilirubin within 1.5 times the institutional upper limit of normal. At least 4 weeks between surgical resection or chemotherapy, and at least 12 weeks between radiotherapy and enrollment were required. All patients provided informed consent. There were no limits based on either the number of prior episodes of progression or therapeutic regimens received.
Patients were excluded for: grade ≥ 3 toxicity on prior bevacizumab; progressive disease or grade ≥ 3 toxicity on prior carboplatin or irinotecan; uncontrolled hypertension; acute hemorrhage on baseline MRI; urine protein:creatinine ratio > 1; homozygosity for the *28UGT1A1 allele; pregnancy or nursing; active infection requiring intravenous antibiotics; therapeutic anti-coagulation with warfarin; and prior stereotactic radiosurgery, radiation implants, or radiolabeled monoclonal antibody therapy unless there was unequivocal disease progression (such as a new lesion or biopsy-proven recurrence).
Treatment Design
Eligible patients for this open-label phase II study received bevacizumab at 10 mg/kg intravenously every 14 days. Carboplatin was administered at an AUC of 4 on day one of each 28-day cycle. Irinotecan was administered on days 1 and 14 at 340 mg/m2 for patients receiving cytochrome P450 CYP3A enzyme-inducing anti-epileptics (EIAEDs; phenytoin, phenobarbital, carbamazepine, oxcarbazepine and primidone) and at 125 mg/m2 for those not on EIAEDs. Study therapy continued until progressive disease, unacceptable toxicity, non-compliance with study protocol guidelines or withdrawal of consent.
Response Evaluation
Study investigators determined response by neurologic examination and contrast-enhanced MRI after the first two treatment cycles and then prior to every other cycle based on the recently published Response Assessment in Neuro-Oncology criteria.41 A complete response (CR) required disappearance of all enhancing and non-enhancing tumor on consecutive MRIs at least 4 weeks apart, with corticosteroid discontinuation and neurologic stability or improvement. A partial response (PR) required ≥ 50% reduction in size (product of largest perpendicular diameters) of enhancing tumor with stability or improvement of neurologic status and corticosteroids. Progressive disease (PD) included ≥ 25% increase of enhancing tumor, a new enhancing lesion, significant worsening of non-enhancing tumor including that detected by FLAIR or T2 sequences, or clinical decline. Stable disease (SD) was defined as any assessment not meeting CR, PR, or PD criteria. Partial responses and stable disease also required stable or improved signal abnormality on fluid-attenuated inversion recovery (FLAIR) sequences.
Dose Modification and Retreatment Criteria
Chemotherapy doses were held for grade 3 or 4, related, non-hematologic toxicity, grade 3 thrombocytopenia, grade 4 neutropenia, and fever and neutropenia (any grade) until the event resolved to grade 1 or pre-treatment baseline. Thereafter, chemotherapy doses were reduced by 25%. Chemotherapy doses were also reduced by 25% for any related event that required > 2 weeks to satisfy re-treatment criteria. Patients who required more than 3 chemotherapy dose reductions were allowed to remain on study and receive bevacizumab alone. Bevacizumab was discontinued for uncontrollable hypertension, grade 2 or greater hemorrhage, arterial thrombosis, wound dehiscence requiring surgical intervention, intestinal perforation or grade 4 venous thrombosis, proteinuria or congestive heart failure. Bevacizumab was held until other related grade 3 events resolved to grade ≤ 1.
Initiation of each cycle required: an ANC ≥ 1000/mm3; a platelet count ≥ 100,000/mm3; creatinine ≤ 1.5 times the upper limit of normal (× ULN), bilirubin ≤ 2 × ULN and AST ≤ 2.5 × ULN; proteinuria <3+ on urinalysis or urine protein:creatinine ratio ≤ 1.0; and resolution of any related grade ≥ 3 event to grade ≤ 1.
Statistical Considerations
Our primary goal was to evaluate the PFS-6 rate of carboplatin, irinotecan and bevacizumab among recurrent GBM patients who had progressed on prior bevacizumab therapy. At the time this study was designed, benchmarks for outcome among recurrent GBM patients who had progressed on prior bevacizumab therapy had not been established, therefore we chose to use the historical benchmark established with temozolomide at first recurrence. Hence, given a PFS-6 rate of 21% among recurrent GBM patients treated with temozolomide at first recurrence,42 a sample size goal of 24 recurrent GBM patients was chosen to allow 88% power to differentiate between 6-PFS rates of 5% and 25% with a type I error rate of 0.03.
An interim efficacy analysis after 16 patients were accrued to each arm was planned a priori. If 12 or more of these 16 patients progressed or died within 2 months of study initiation, further accrual would be suspended. The interim efficacy threshold was met and therefore the study completed accrual as specified.
For this study among heavily pretreated patients with an extremely poor prognosis, rates of unacceptable toxicity, defined as grade ≥ 2 CNS hemorrhage or grade 4 or 5 non-hematologic toxicity, of 15% or less were considered desirable, while rates of 40% or greater were considered undesirable. Stopping rules for unacceptable toxicity based upon boundaries proposed by Pocock were used to monitor this study after each group of 4 patients.43 Accrual was not suspended to formally assess the toxicity profile unless the following thresholds of unacceptable toxicity were satisfied: ≥3/4; ≥4/8; ≥5/12; ≥6/16; ≥7/20; ≥7/24. The type I and type II errors associated with this monitoring were 0.05 and 0.07, respectively. These guidelines did not adjust for differential length of follow-up of accrued patients.
Progression-free survival (PFS) was defined as the time between the cycle one start date and the date of disease progression or death. PFS was censored at the time of last follow-up if the patient remained alive without disease progression, or at the start of non-study treatment if initiated before disease progression. Overall survival (OS) was calculated from the start of therapy until death or last contact if censored. PFS and OS were summarized using Kaplan-Meier estimator including 95% CIs.
RESULTS
Patient Characteristics
Characteristics of the 25 patients who enrolled on this study between September 2009 and July 2010 are summarized in Table 1. Patients were relatively young (median age, 52 years), over half had a KPS of at least 90, and 10 (40%) were on corticosteroids at enrollment. Patients were moderately pretreated with 14 (56%) having 2–3 episodes of prior progression. All patients had progressed after standard therapy with radiation and temozolomide chemotherapy as well as prior bevacizumab. Most patients (96%) had progressed on one prior bevacizumab regimen, while one patient enrolled after progression on two prior bevacizumab regimens. Thirteen patients (52%) enrolled after having progressed on bevacizumab used at recurrence while twelve patients (48%) enrolled after progressing on bevacizumab incorporated into adjuvant therapy for newly diagnosed GBM patients. Sixty percent of patients progressed previously on bevacizumab in combination with chemotherapy, while 36% progressed on bevacizumab monotherapy and one patient progressed on bevacizumab with a targeted agent (sorafenib). Nearly all patients (92%) enrolled while progressing on bevacizumab therapy, however two (8%) progressed on bevacizumab and then received either single agent temozolomide or XL-184, a dual VEGFR-2/met tyrosine kinase inhibitor, for 2–4 months prior to study enrollment. Median duration of initial bevacizumab therapy was nine months with a range of 2–34 months.
Table 1.
Patient Characteristics
| Age (years) | |
| Median | 52 |
| Range | 19 – 76 |
| Gender | |
| Male | 17 (68) |
| Female | 8 (32) |
| KPS | |
| 90–100 | 13 (52) |
| 80 | 10 (40) |
| 70 | 2 (8) |
| Time from Diagnosis (weeks) | |
| Median | 58.1 |
| Range | 29.9–260.9 |
| EIAED | |
| Yes | 1 (4) |
| No | 24 (96) |
| Corticosteroids | |
| Yes | 10 (40) |
| Average Dose (mg/day) | 2.8 |
| Range (mg/day) | 0–16 |
| No | 15 (60) |
| Surgery Prior to Enrollment | |
| None | 22 (88) |
| Biopsy | 2 (8) |
| STR | 1 (4) |
| Number Prior PD | |
| 1 | 11 (44) |
| 2 | 13 (52) |
| 3 | 1 (4) |
| Progression on BV at Enrollment | |
| No | 2 (8) |
| Yes | 23 (92) |
| BV Partner at Prior Progression | |
| None | 9 (36) |
| Chemotherapy | 15 (60) |
| Etoposide | 2 |
| Temozolomide | 13 |
| Sorafenib | 1 (4) |
| Number Prior BV PD | |
| 1 | 24 (96) |
| 2 | 1 (4) |
| Number months on prior BV | |
| Median | 9 |
| Range | 2–34 |
Abbreviations: BV, bevacizumab; EIAED, CYP3A enzyme inducing anti-epileptic drugs; KPS, Karnofsky performance status; mg, milligram; PD, progressive disease; STR, subtotal resection
As of January 1, 2011, one patient continues to receive study therapy in cycle 6 while all other patients have discontinued study therapy. Two patients remain free of progression including one patient who completed 12 cycles of therapy and remains off study and one patient who discontinued study therapy due to recurrent hematologic toxicity after 4 cycles, and is currently receiving her fifth cycle of non-study irinotecan and bevacizumab with stable disease. Eight patients (32%) remain alive including the two described above and six who are progressed on study therapy and are currently receiving additional salvage therapy. Seventeen patients (68%) have died.
Study Drug Administration and Safety
Study drug administration and compliance with treatment for the intent-to-treat study population were excellent. A total of 80 cycles of therapy were administered including a median of 3 cycles (range, 1– 12) per patient. Twenty-one patients (84%) discontinued study therapy due to progressive disease. Single patients discontinued study therapy due to completion of one year of therapy with no evidence of active disease, recurrent grade 3–4 hematologic toxicity, non-compliance and voluntary withdrawal, respectively.
All patients were assessable for toxicity. Table 2 summarizes the frequency of grade ≥ 2 adverse events that were at least possibly related to the study regimen as a percentage of the total number of cycles administered during the study. Most adverse events were grade 2 while the most common grade 3 or 4 events were hematologic. Grade 3 or 4 neutropenia occurred in 6 (24%) and 2 (8%) patients, while grade 3 or 4 thrombocytopenia occurred in 3 patients (13%), and grade 3 anemia occurred in 2 patients (8%). Among non-hematologic events, grade 3 events were limited to fatigue in 3 patients (13%) and hypertension in 2 patients (8%). Nine patients (36%) required one dose reduction of carboplatin and irinotecan, while two patients (8%) required two dose reductions. There were no study related deaths or episodes of intra-cranial hemorrhage.
Table 2.
Frequency of grade ≥ 2 adverse events at least possibly related to the study regimen as a percentage of total cycles administered (n=80).
| Event | Grade | ||
|---|---|---|---|
| 2 | 3 | 4 | |
| Anemia | 7 (9%) | 2 (3%) | - |
| Anorexia | 2(3%) | - | - |
| Dehydration | - | 1 (1%) | - |
| Diarrhea | 2 (3%) | - | - |
| Fatigue | 4 (5%) | 3 (4%) | - |
| Hypertension | 2(3%) | 2 (3%) | - |
| Hyponatremia | - | 1 (1%) | - |
| Hypophosphatemia | - | 1(1%) | - |
| Infection | 1(1%) | - | |
| Mucositis | - | 1 (1%) | - |
| Nausea/emesis | 4 (5%) | 1(1%) | - |
| Neutropenia | 14 (18%) | 10 (13%) | 3 (4%) |
| Proteinuria | 5 (6%) | - | - |
| Thrombocytopenia | 13 (16%) | 7 (9%) | 3(4%) |
| Thrombosis | - | 1 (1%) | - |
| Transaminase elevation | 2 (3%) | 1 (1%) | - |
| Weight loss | 2 (3%) | - | - |
Outcome
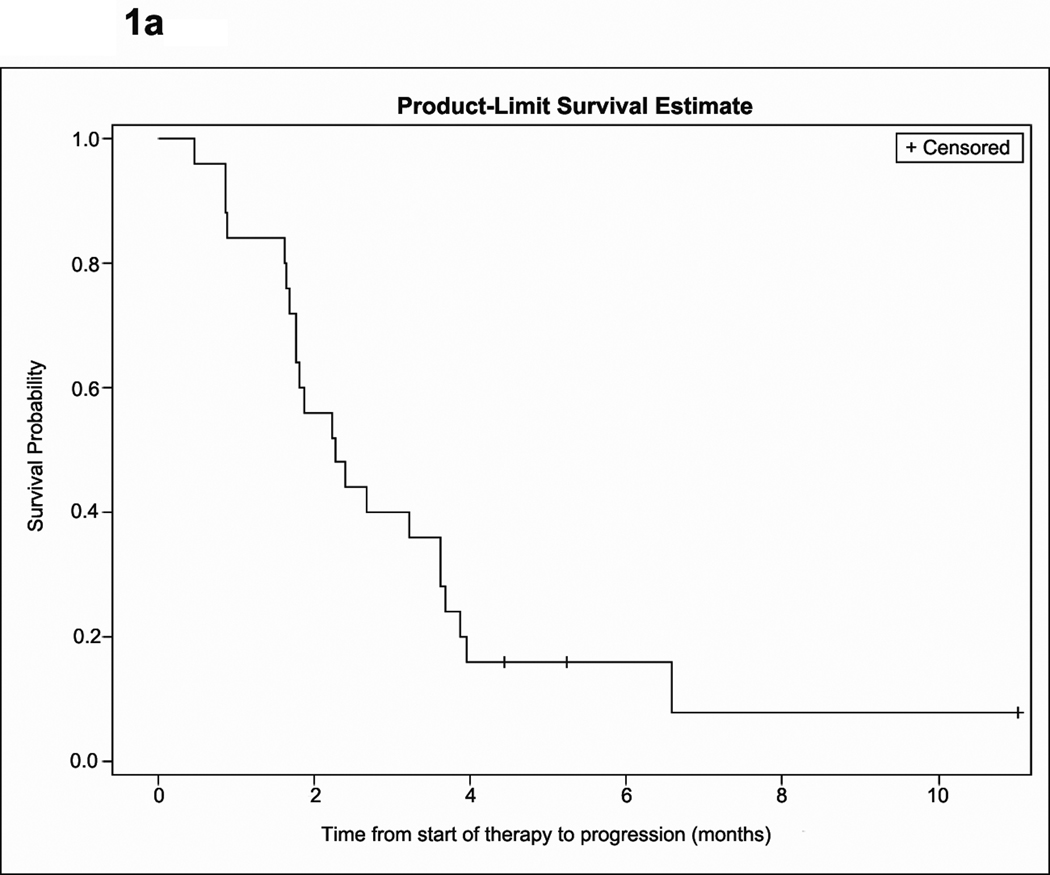
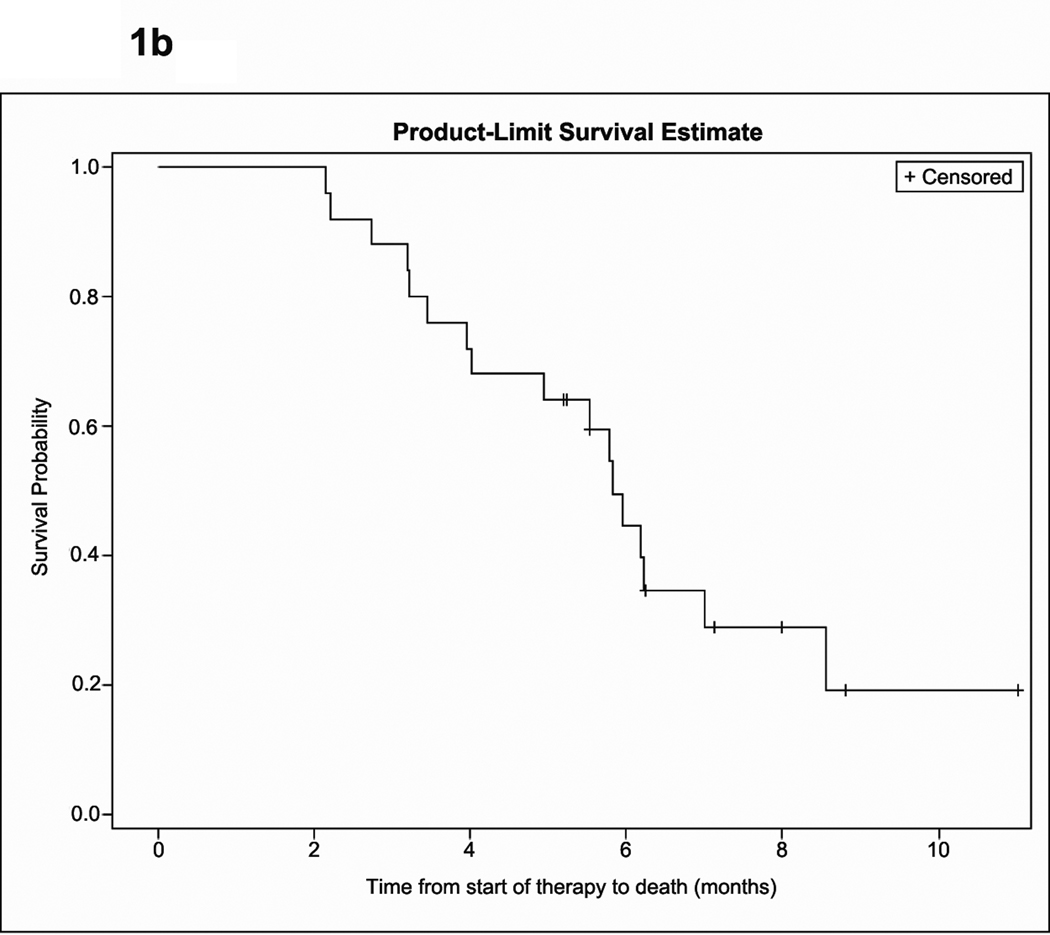
The median follow-up for all patients was 7.99 months (95% CI, 6.25, 11.02). Outcome analysis was based on the intent-to-treat population and includes one patient who discontinued study therapy on day 2 of cycle 1 due to voluntary withdrawal. Median OS, PFS and PFS-6 rate are summarized in Table 3 and Figure 1. Best radiographic response was stable disease in twenty patients (80%), and progressive disease in four (16%) while one patient was not evaluable. None of the patients met criteria for radiographic response. Among 10 patients who were on dexamethasone at study initiation, five were able to taper by an average of 4.6 mg per day, including two patients who were able to completely discontinue dexamethasone. There was no difference in either PFS or OS among patients who received adjuvant bevacizumab compared to those who received bevacizumab at recurrence. nor was there a difference in OS for patients who progressed on bevacizumab monotherapy compared to bevacizumab in combination with chemotherapy. However, PFS was improved for patients who progressed on bevacizumab monotherapy compared to those who progressed on bevacizumab plus chemotherapy (p=0.0356). Specifically, 44.4% of patients who were treated after bevacizumab monotherapy remained progression-free after 6 months, compared to 0% of patients who were treated after progression on bevacizumab plus chemotherapy.
Table 3.
Summary of outcome in the current study relative to previously reported series of GBM patients treated with bevacizumab therapy after progression on prior bevacizumab-based treatment.
| Study Design | Regimen | Number patients |
Median PFS, months (%% CI) |
PFS-6, % (95% CI) |
Median OS, months (95% CI) |
Citation |
|---|---|---|---|---|---|---|
| Prospective | BV + carboplatin + CPT-11 | 25 | 2.3 (1.8, 3.6) | 16 (5.0, 32.5) | 5.8 (4.0, 7.0) | Current Study |
| Prospective | BV + CPT-11 | 19 | 1.1 (CI – NR) | NR | NR | Kreisl24 |
| Prospective | BV + daily TMZ or VP-16 | 23 | 1.8 (1.0, 2.1) | 4.4 (3.1, 18.2) | 4.1 (2.8, 5.8) | Reardon, in press46 |
| Retrospective | BV + miscellaneous agents | 54 (35 GBM) | 1.3 (1.2, 1.5) | 2 (CI – NR) | 2.9 (CI – NR) | Quant27 |
| Retrospective | BV + miscellaneous agents | 19 | 2 (1.2, 3.3) | 0 | 5.2 (3.3, 8.4) | Iwamoto48 |
| Retrospective | BV + miscellaneous agents | 23 | 1.8 (CI - NR) | NR | NR | Norden26 |
| Retrospective | BV + SRS + chemotherapy | 23 | 2.6 (CI – NR) | NR | 7.2 (CI – NR) | Torcuator47 |
| Retrospective | BV + chemotherapy | 23 | 1.7 (CI- NR) | NR | 3.3 (CI – NR) | Torcuator47 |
Abbreviations: BV, bevacizumab; CI, confidence interval; CPT-11, irinotecan; GBM, glioblastoma; NR, not reported; OS, overall survival; PFS, progression-free survival; SRS, stereotactic radiosurgery; TMZ, temozolomide; VP-16, etoposide
Figure 1.
Kaplan-Meier estimate of time to progression (A) and overall survival (B).
DISCUSSION
Bevacizumab is indicated for the treatment of recurrent GBM patients in the United States, Canada and a growing number of countries worldwide.25 In addition, bevacizumab is currently being evaluated for newly diagnosed GBM patients in two multinational, blinded placebo-controlled randomized phase III studies as well as additional phase II studies.44, 45 Thus a high percentage of GBM patients are currently receiving bevacizumab either at recurrence or following initial diagnosis. Although bevacizumab substantially improves rates of radiographic response and PFS, all patients ultimately progress. Furthermore, survival after bevacizumab progression is poor.
To date, effective treatment after bevacizumab progression has not been identified (Table 3). Two prospective studies have been reported. Among 19 patients with recurrent GBM who progressed on single-agent bevacizumab, median PFS following initiation of bevacizumab plus irinotecan was only 1.1 months. Overall survival was not reported for these patients.24 Similarly, among 23 recurrent GBM patients treated with bevacizumab plus protracted daily (metronomic) chemotherapy using either temozolomide or etoposide after bevacizumab failure, the median OS was 4.1 months (95% CI: 2.8, 5.8) and PFS-6 was 4.4% (95% CI, 3.1,18.2).46 Several relatively small retrospective series (range of evaluated patients, 19–35) report median OS and PFS-6 probability of 2.2–4.1 months and 0–4.4%, respectively.26, 27, 48{Torcuator, 2010 #10972; Lu-Emerson 2010} Of note, a retrospective review reported that patients who received stereotactic radiosurgery with bevacizumab and chemotherapy achieved a median PFS of 2.6 months and a median OS of 7.2 months after prior bevacizumab failure; in comparison, patients who received bevacizumab and chemotherapy alone, without a radiosurgical boost, had a median PFS of 1.7 months and a median OS of 3.3 months.{Torcuator, 2010 #10972} These results suggest that stereotactic radiosurgery may improve outcome for some patients following progression on bevacizumab; however, these findings require prospective evaluation.
Our phase II study demonstrates that continuation of bevacizumab with carboplatin and irinotecan is associated with a modest improvement in outcome compared to historical data for recurrent GBM patients who have progressed on bevacizumab therapy. Although prospective, this small series enrolled patients with relatively favorable prognostic factors including younger age and good performance status, thus further evaluation of this regimen should be considered in a randomized, controlled trial. Another concern is that our study design precludes determination of whether therapeutic benefit required combination of all three study agents or if a subset of the study regimen may have been sufficient to render therapeutic benefit. Specifically, we did not evaluate whether either carboplatin alone or carboplatin and irinotecan without bevacizumab may have improved outcome on our study. However, it is unlikely that irinotecan alone, irinotecan plus bevacizumab or carboplatin plus bevacizumab were responsible for therapeutic benefit in our study based on the lack of benefit observed with these regimens in previously reported series.24, 26, 27, 48
The rationale for this study regimen was based on several considerations. Bevacizumab was included to avoid fulminant “rebound” tumor re-growth that has been reported upon abrupt bevacizumab discontinuation,39 and because anti-VEGF therapy can normalize tumor vasculature and improve chemotherapy delivery.49 Furthermore, a retrospective registry series reported that bevacizumab continuation after progression on a bevacizumab regimen improves survival in patients with metastatic colorectal cancer.50 The combination of carboplatin and irinotecan was considered attractive for several reasons. First each of these agents has modest anti-tumor activity when administered separately among recurrent malignant glioma patients.31–36, 38, 51 Second, the mechanisms of cytotoxicity of each agent are potentially complementary. Specifically, irinotecan inhibits DNA replication by blocking topoisomerase 1 while carboplatin induces DNA cross-links and adducts. Third, the primary toxicities of each agent, gastrointestinal for irinotecan and hematologic for carboplatin, are non-overlapping; thus combining the two agents is expected to be associated with acceptable toxicity. Fourth, pharmacologic metabolism paths of each agent differ suggesting that detrimental pharmacologic interactions associated with a combined regimen are not expected. Finally, studies using carboplatin plus irinotecan for patients with other aggressive solid tumors have generated encouraging evidence of anti-tumor activity and overall adequate tolerance.52–54
Our study demonstrates that the combination of carboplatin, irinotecan and bevacizumab is associated with adequate safety, despite enrolling patients who were moderately pretreated. The type, frequency and severity of encountered toxicity was similar to that which has been reported for each agent when administered separately,31–36, 38, 51 thus the combination did not cause unexpected events. One factor that may have contributed to the acceptable safety profile we observed was the exclusion of patients homozygous for the *28 UGT1A1 allele since such patients are predicted to be at increased risk of irinotecan toxicity.55, 56
Treatment of GBM patients who progress on bevacizumab therapy is currently a dire unmet need in neuro-oncology. We demonstrate that carboplatin, irinotecan and bevacizumab is associated with modest anti-tumor benefit and adequate safety in moderately pre-treated GBM patients who have progressed on bevacizumab. Further evaluation of this regimen is warranted and the identification of effective therapies for GBM patients who progress on bevacizumab should be highly prioritized.
Abbreviations List
- MG
malignant glioma
- ITT
intent-to treat
- ANC
absolute neutrophil count
- AST
aspartate aminotransferase
- CNS
central nervous system
- CR
complete response
- GBM
glioblastoma
- KPS
Karnofsky performance status
- ORR
overall response rate
- OS
overall survival
- PD
progressive disease
- PFS
progression-free survival
- PR
partial response
- SD
stable disease
- VEGF
vascular endothelial growth factor
Footnotes
This work was supported by NIH Grants 5P50-NS-20023 and 5 R37 CA11898; and a grant from Genentech Pharmaceuticals.
CONFLICT OF INTEREST DISCLOSURES
D. A. Reardon, J. J. Vredenburgh, and H. S. Friedman reported receiving speaker, advisory, and consultant honorarium for Roche/Genentech; A. Desjardins, K. B. Peters, S. Gururangan, J. H. Sampson, R. L. McLendon, J. E. Herndon, II, A. Coan, S. Threatt, and A. H. Friedman made no disclosures.
(ClinicalTrials.gov number NCT00953121)
REFERENCES
- 1.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Ballman KV, Buckner JC, Brown PD, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007;9:29–38. doi: 10.1215/15228517-2006-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: An important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10:162–170. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 5.Brem S, Cotran R, Folkman J. Tumor angiogenesis: a quantitative method for histologic grading. J Natl Cancer Inst. 1972;48:347–356. [PubMed] [Google Scholar]
- 6.Plate KH, Risau W. Angiogenesis in malignant gliomas. Glia. 1995;15:339–347. doi: 10.1002/glia.440150313. [DOI] [PubMed] [Google Scholar]
- 7.Flynn JR, Wang L, Gillespie DL, et al. Hypoxia-regulated protein expression, patient characteristics, and preoperative imaging as predictors of survival in adults with glioblastoma multiforme. Cancer. 2008;113:1032–1042. doi: 10.1002/cncr.23678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt NO, Westphal M, Hagel C, et al. Levels of vascular endothelial growth factor, hepatocyte growth factor/scatter factor and basic fibroblast growth factor in human gliomas and their relation to angiogenesis. Int J Cancer. 1999;84:10–18. doi: 10.1002/(sici)1097-0215(19990219)84:1<10::aid-ijc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Zhou YH, Tan F, Hess KR, Yung WK. The expression of PAX6, PTEN, vascular endothelial growth factor, and epidermal growth factor receptor in gliomas: relationship to tumor grade and survival. Clin Cancer Res. 2003;9:3369–3375. [PubMed] [Google Scholar]
- 10.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 11.Rubenstein JL, Kim J, Ozawa T, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jahnke K, Muldoon LL, Varallyay CG, Lewin SJ, Kraemer DF, Neuwelt EA. Bevacizumab and carboplatin increase survival and asymptomatic tumor volume in a glioma model. Neuro Oncol. 2009;11:142–150. doi: 10.1215/15228517-2008-077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CG, Heijn M, di Tomaso E, et al. Anti-Vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000;60:5565–5570. [PubMed] [Google Scholar]
- 14.Mathieu V, De Neve N, Le Mercier M, et al. Combining bevacizumab with temozolomide increases the antitumor efficacy of temozolomide in a human glioblastoma orthotopic xenograft model. Neoplasia. 2008;10:1383–1392. doi: 10.1593/neo.08928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 16.Kindler HL, Friberg G, Singh DA, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033–8040. doi: 10.1200/JCO.2005.01.9661. [DOI] [PubMed] [Google Scholar]
- 17.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 18.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 19.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 22.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 23.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 24.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14:1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 26.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 27.Quant EC, Norden AD, Drappatz J, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol. 2009;11:550–555. doi: 10.1215/15228517-2009-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reardon DA, Desjardins A, Vredenburgh JJ, et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: a phase II study. Br J Cancer. 2009;101:1986–1994. doi: 10.1038/sj.bjc.6605412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sathornsumetee S, Desjardins A, Vredenburgh JJ, et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro Oncol. 2010;12:1300–1310. doi: 10.1093/neuonc/noq099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 31.Prados MD, Warnick RE, Mack EE, et al. Intravenous carboplatin for recurrent gliomas. A dose-escalating phase II trial. Am J Clin Oncol. 1996;19:609–612. doi: 10.1097/00000421-199612000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Yung WK, Mechtler L, Gleason MJ. Intravenous carboplatin for recurrent malignant glioma: a phase II study. J Clin Oncol. 1991;9:860–864. doi: 10.1200/JCO.1991.9.5.860. [DOI] [PubMed] [Google Scholar]
- 33.Friedman HS, Petros WP, Friedman AH, et al. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol. 1999;17:1516–1525. doi: 10.1200/JCO.1999.17.5.1516. [DOI] [PubMed] [Google Scholar]
- 34.Santisteban M, Buckner JC, Reid JM, et al. Phase II trial of two different irinotecan schedules with pharmacokinetic analysis in patients with recurrent glioma: North Central Cancer Treatment Group results. J Neurooncol. 2008 doi: 10.1007/s11060-008-9749-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batchelor TT, Gilbert MR, Supko JG, et al. Phase 2 study of weekly irinotecan in adults with recurrent malignant glioma: final report of NABTT 97-11. Neuro Oncol. 2004;6:21–27. doi: 10.1215/S1152851703000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilbert MR, Supko JG, Batchelor T, et al. Phase I clinical and pharmacokinetic study of irinotecan in adults with recurrent malignant glioma. Clin Cancer Res. 2003;9:2940–2949. [PubMed] [Google Scholar]
- 37.Prados MD, Lamborn K, Yung WK, et al. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro Oncol. 2006;8:189–193. doi: 10.1215/15228517-2005-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raymond E, Fabbro M, Boige V, et al. Multicentre phase II study and pharmacokinetic analysis of irinotecan in chemotherapy-naive patients with glioblastoma. Ann Oncol. 2003;14:603–614. doi: 10.1093/annonc/mdg159. [DOI] [PubMed] [Google Scholar]
- 39.Zuniga RM, Torcuator R, Jain R, et al. Rebound tumour progression after the cessation of bevacizumab therapy in patients with recurrent high-grade glioma. J Neurooncol. 2010;99:237–242. doi: 10.1007/s11060-010-0121-0. [DOI] [PubMed] [Google Scholar]
- 40.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 41.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 42.Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pocock SJ. Interim analyses for randomized clinical trials: the group sequential approach. Biometrics. 1982;38:153–162. [PubMed] [Google Scholar]
- 44.Vredenburgh J, Desjardins A, Reardon D, et al. Bevacizumab (BEV) in combination with temozolomide (TMZ) and radiation therapy (XRT) followed by BEV, TMZ, and irinotecan for newly diagnosed glioblastoma multiforme (GBM) Proc Am Soc Clin Oncol. Chicago, IL. 2010:185s. [Google Scholar]
- 45.Lai A, Tran A, Nghiemphu PL, et al. Phase II Study of Bevacizumab Plus Temozolomide During and After Radiation Therapy for Patients With Newly Diagnosed Glioblastoma Multiforme. J Clin Oncol. 2011;29:142–148. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reardon DA, Desjardins A, Peters K, et al. Phase II study of metronomic chemotherapy with bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. J Neurooncol. 2010 doi: 10.1007/s11060-010-0403-6. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torcuator RG, Thind R, Patel M, et al. The role of salvage reirradiation for malignant gliomas that progress on bevacizumab. J Neurooncol. 2009 doi: 10.1007/s11060-009-0034-y. [DOI] [PubMed] [Google Scholar]
- 48.Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73:1200–1206. doi: 10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26:5326–5334. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- 51.Prados MD, Yung WK, Jaeckle KA, et al. Phase 1 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro Oncol. 2004;6:44–54. doi: 10.1215/S1152851703000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hermes A, Bergman B, Bremnes R, et al. Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small-cell lung cancer: a randomized phase III trial. J Clin Oncol. 2008;26:4261–4267. doi: 10.1200/JCO.2007.15.7545. [DOI] [PubMed] [Google Scholar]
- 53.Chen G, Huynh M, Fehrenbacher L, et al. Phase II trial of irinotecan and carboplatin for extensive or relapsed small-cell lung cancer. J Clin Oncol. 2009;27:1401–1404. doi: 10.1200/JCO.2008.20.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones SF, Burris HA, 3rd, Hainsworth JD, et al. Phase I. Trial of irinotecan plus carboplatin in two dose schedules. Oncology (Williston Park) 2003;17:36–40. [PubMed] [Google Scholar]
- 55.Innocenti F, Undevia SD, Iyer L, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol. 2004;22:1382–1388. doi: 10.1200/JCO.2004.07.173. [DOI] [PubMed] [Google Scholar]
- 56.Cote JF, Kirzin S, Kramar A, et al. UGT1A1 polymorphism can predict hematologic toxicity in patients treated with irinotecan. Clin Cancer Res. 2007;13:3269–3275. doi: 10.1158/1078-0432.CCR-06-2290. [DOI] [PubMed] [Google Scholar]