Abstract
The LRRK2 protein has both GTPase and kinase activities and mutation in either enzymatic domain can cause late-onset Parkinson’s disease (PD). Nucleotide binding in the GTPase domain may be required for kinase activity and residues in the GTPase domain are potential sites for autophosphorylation, suggesting a complex mechanism of intrinsic regulation. To further define the effects of LRRK2 autophosphorylation, we applied a technique optimal for detection of protein phosphorylation, electron transfer dissociation (ETD), and identified autophosphorylation events exclusively nearby the nucleotide binding pocket in the GTPase domain. PD-linked mutations alter kinase activity but did not alter autophosphorylation site specificity or sites of phosphorylation in a robust in vitro substrate myelin basic protein. Amino-acid substitutions in the GTPase domain have large effects on kinase activity, as insertion of the GTPase-associated R1441C pathogenic mutation together with the G2019S kinase-domain mutation resulted in a multiplicative increase (~7-fold) in activity. Removal of a conserved autophosphorylation site (T1503) by mutation to an alanine residue resulted in greatly decreased GTP-binding and kinase activity. While autophosphorylation likely serves to potentiate kinase activity, we find that oligomerization and loss of the active dimer species occurs in an ATP and autophosphorylation independent manner. LRRK2 autophosphorylation sites are overall robustly protected from dephosphorylation in vitro, suggesting tight control over activity in vivo. We developed highly specific antibodies targeting pT1503 but failed to detect endogenous autophosphorylation in protein derived from transgenic mice and cell lines. LRRK2 activity in vivo is unlikely to be constitutive but rather refined to specific responses.
Keywords: dardarin, PARK8, Parkinson’s disease, neurodegeneration, serine/threonine kinase
Introduction
The most common known cause of inherited late-onset Parkinson disease (PD) is dominant missense mutations in the leucine-rich repeat kinase 2 (LRRK2) gene 1,2. The 2527 amino acid LRRK2 protein has both a Ras-like GTP-binding domain in addition to a serine/threonine kinase domain, encoded in a ROC (Ras of complex) GTPase domain and an MLK (mixed-lineage kinase)-like kinase domain 3. Most PD-causative LRRK2 mutations localize to these domains, indicating a critical role for LRRK2 enzymatic activities in disease susceptibility, at least in PD cases with LRRK2 mutations 4. LRRK2 is a cytosolic and membrane associated protein that is expressed in many mammalian tissues and cell types 5–7. As yet, the endogenous function of the evolutionarily conserved LRRK2 protein kinase is not clear, with putative functions in regulating protein translation, cytoskeleton architecture, and signaling stress responses in the MAPK pathway 8–14.
Biochemical changes imparted by LRRK2 mutations may reveal molecular aspects of neurodegeneration in PD and therapeutic targets. Increased in vitro kinase activity due to the most prevalent known LRRK2 pathogenic mutation, G2019S, compared to wild-type (WT) activity, is universally observed in a variety of experimental conditions 3,15–18. However, the effects of other mutations on LRRK2 kinase activity, especially those outside of the kinase domain (e.g., the GTPase domain), have not been ubiquitously linked to kinase up-regulation in every study. LRRK2 kinase regulation likely involves a complex mechanism involving intrinsic factors (e.g., the GTPase domain) and extrinsic interacting proteins. For example, GTPase proteins usually require cofactors for enzymatic activity, and these as yet unidentified LRRK2 interacting proteins may be differentially expressed in different experimental conditions. Further delineation of the mechanics of LRRK2 enzymatic activation and downstream function are required to identify pathogenic disease-associated functions and intervening strategies.
An important clue in resolving the mechanism of LRRK2 enzymatic activation (and thus action of PD-associated mutations) may be the recently described autophosphorylation occurring in residues in or nearby the GTPase domain. Protein kinases commonly autophosphorylate and in some cases autophosphorylation is required for initiation of substrate phosphorylation. Conversely, kinase inhibition can result from autophosphorylation, for example the DAPK protein where autophosphorylation of the Ca2+ binding domain results in shut-down 19. Past in vitro mass-spectrometry studies map LRRK2 autophosphorylation sites to its own GTPase domain suggesting possible kinase control over GTPase function 20–22. However, mutations within the GTP-binding pocket are known to potently disrupt kinase activity3,23,24. The effects of LRRK2 autophosphorylation on overall activity, both initial activation, sustained kinase activity, and GTP-binding activity, are not clear.
PD-causative LRRK2 mutations may plausibly alter specificity of substrate interaction in a true gain-of-function mechanism. Without a bona fide kinase substrate, multiple LRRK2 studies have utilized myelin-basic protein (MBP) as a surrogate kinase substrate 18,23,25. Several soluble serine/threonine protein kinases can phosphorylate MBP in vitro on multiple sites and LRRK2 can phosphorylate MBP 3,18,23,26–30. The sites of LRRK2 phosphorylation on MBP or whether specificity is altered by the PD-associated mutation G2019S has not been previously described. Methods to identify LRRK2 mediated phosphorylation sites on MBP may provide insight into possible gain of functions mechanisms in LRRK2 PD associated mutations and help resolve preferred motifs of phosphorylation.
This study further explores the functional effects of LRRK2 autophosphorylation, the relationship between GTP-binding and kinase activity, and gain-of-function mechanisms on phosphorylation site selectivity. We find that the previously tentative link between the GTPase domain and kinase activity, defined largely through mutations that greatly disrupt nucleotide binding in the GTPase domain (i.e., K1347A, T1348N) is further solidified in this study through evaluation of PD-linked mutations and modification of autophosphorylation sites. Analysis of autophosphorylated LRRK2 protein indicates a primary autophosphorylation residue at T1503 that is required for normal kinase activity and GTP-binding. We map the sites of LRRK2 phosphorylation on MBP to determine possible alterations in kinase specificity in PD associated mutants, and find no differences except for the rate of activity. We find no evidence that autophosphorylation results in kinase inactivation. To the contrary, we suggest that LRRK2 autophosphorylation serves to propagate protein kinase activity via control of the GTPase domain.
Results
PD-linked G2019S LRRK2 protein incorporates more phosphates than WT-LRRK2 before oligomerization driven inactivation
LRRK2 may autophosphorylate in a sequential manner over time in a process potentially altered by pathogenic mutations. In order to characterize the prominence of LRRK2 autophosphorylation and ensure saturation of possible autophosphorylation activity, in vitro kinase assays were conducted with highly purified recombinant LRRK2 (Δ1–970) protein and incorporation quantitatively tracked over time (Figure 1A). Phosphate incorporation for both WT and G2019S mutant protein was measured by dissecting LRRK2 bands from SDS-PAGE gels and determining incorporation via liquid scintillation. Within one hour, WT-LRRK2 protein incorporates 0.19 phosphates per LRRK2 molecule compared to the G2019S LRRK2 variant which incorporates approximately 0.5 phosphates per LRRK2 molecule. Since more than a dozen autophosphorylation sites have been proposed (Table 1), these data suggest that the vast majority of LRRK2 protein cannot autophosphorylate and may reside in an inactive state, and the G2019S mutation either increases rates of incorporation before kinase shut-down, enhances the proportion of an active state conformation, or induces additional autophosphorylation sites not present in WT protein.
Fig. 1. Autophosphorylation profile of G2019S mutant and WT-LRRK2.
A) In vitro kinase assays were conducted with the indicated LRRK2 (Δ1–970) enzyme at 100 nM concentration and 100 μM ATP at 30°C and reactions resolved by SDS-PAGE. After exposure to autoradiography film, bands were excised from dried gels. B) Incorporation of 32P into LRRK2 protein bands in A was measured by liquid scintillation with correction for counting efficiency and normalized to a standard curve, and average phosphates incorporated per LRRK2 at each time point were determined. Error bars represent ±S.E.M. No significant additional incorporation was noted after 30 minutes. C) Recombinant LRRK2 Δ1–970 and full-length (FL) proteins were treated with 100 μM LRRKtide peptide for the indicated interval in kinase assays. 32P incorporation into peptides was determined via liquid scintillation. Activity during the denoted intervals was normalized to the activity in the first 20 min interval for each condition. ‘Free’ denotes the condition where LRRK2 was included in soluble form; ‘Solid Surface’ denotes reactions where LRRK2 protein was bound to agarose beads. Error bars represent ±S.E.M. D) Kinase reactions were conducted over the indicated time and analyzed by silver stained SDS-PAGE gels and native-PAGE Blue gels.
Table 1.
| Residue | Peptide (reference)* | Enzyme | Method |
|---|---|---|---|
| S4/S5 | M.ApSGpSCQGCEEDEETLKKLIVRL.N21 | Trypsin | CID |
| T424 | F.QASANALSTpLLEQNVNF.R21 | Chymotrypsin | CID |
| T524 | F.IVPGMPEESREDTpEF.H21 L.HFIVPGMPEESREDpTEF.H21 |
Chymotrypsin Chymotrypsin |
CID CID |
| T776 | L.pTISIGKGDSQIISLLL.R21 | Chymotrypsin | CID |
| T826 | K.VEPSWLGPLFPDKpTSNLR.K21 | Trypsin | CID |
| T833 | L.RKQpTNIASTL.A21 | Chymotrypsin | CID |
| T838 | N.IASpTLARM.V21 | Chymotrypsin | CID |
| S1124 | K.ISGICpSPLR.L21 | Trypsin | CID |
| S1292 | R.SFPNEMGKLpSK.I21 | Trypsin | CID |
| T1343 |
L.MIVGNpTGSGKTTLL.Q21,22 L.MIVGNpTGSGKTTL.L K.LMIVGNpTGSGK.T21 |
Chymotrypsin Lys-C Trypsin, Lys-C |
CID ETD CID/ETD |
| S1345 | L.MIVGNTGpSGKTTLLQQL.M21 | Trypsin | CID |
| T1348 |
K.pTTLLQQLMK.T K.LMIVGNTGSGKpTTLLQQLMK.T21 |
Lys-C Trypsin, Lys-C |
ETD CID/ETD |
| T1357 | K.TTLLQQLMKpTK.K | Trypsin | ETD |
| T1368 |
K.KSDLGMQSApTVGIDVK.D K.SDLGMQSApTVGIDVK.D21 |
Lys-C Trypsin, Lys-C |
ETD ETD |
| T1410 | F.YSTHPHFMpTQRALY.L21 Y.STHPHFMpTQRALY.L21 EFYSTHPHFMpTQRA22 |
Chymotrypsin Chymotrypsin Trypsin |
CID CID CID |
| T1452 | R.ASSSPVILVGpTHLDVSDEK.Q21,22 | Trypsin | CID |
| 1491 | RGFPAIRDYHFVNApTEESDALAK20 Y.HFVNApTEESDALAKL.R21,22 R.DYHFVNApTEESDALAK.L20,21 |
Lysyl endopeptidase Chymotrypsin, Trypsin Trypsin |
CID CID CID/ETD |
| T1503 |
L.RKpTIINESLNF.K21 K.pTIINESLNFK.I21 R.KpTIINESLNFK.I K.LRKpTIINESLNFK.I22 |
Chymotrypsin Lys-C, Trypsin Trypsin Trypsin |
CID CID/ETD CID CID/ETD |
| T1967 | ASLTRTLQHR20 | Trypsin | CID |
| T1969 | ASLTRTLQHR20 | Trypsin | CID |
| T2031 | R.MGIKpTSEGTPGFR.A22 | Trypsin | CID |
| T2483 | Y.NRKNTEGpTQKQKEIQSCL.T21 | Chymotrypsin | CID |
Shaded areas highlight autophosphorylation sites identified by more than one group. Bolded Text represents peptides identified in this study.
Alternative fragmentations for indicated peptides were excluded.
Both mutant and WT-LRRK2 protein autophosphorylation saturates within one hour (Figure 1A and B) and is accompanied by a precipitous decrease in kinase activity. Figure 1C indicates that after 40 minutes of incubation in a kinase reaction buffer, the WT-LRRK2 fragment in solution possesses just ~30% of original activity relative to activity in the first 20 min, whereas WT-LRRK2 Δ1–970 protein retains significantly more activity over the same duration when bound to affinity agarose beads (solid surface). Significant differences in loss of activity were also observed for incubation of full-length protein purified from Flag BAC transgenic mouse tissue compared to full-length protein bound to affinity agarose beads (Figure 1C). Incubation of soluble WT-LRRK2 Δ1–970 for the same time interval without ATP resulted in similar diminishments in activity when ATP is later added, suggesting that kinase inactivation is independent of kinase activity.
Because linkage of LRRK2 to a solid surface greatly protects from kinase activity shut-down, we hypothesized that LRRK2 oligomerizes in an ATP and kinase-independent manner at temperatures compatible with enzymatic activity. In kinase assay incubations greater than one hour, LRRK2 protein partially accumulates as insoluble protein that does not successfully resolve by denaturing SDS-PAGE (Figure 1D). While aggregation is not observed at time points early than one hour on SDS-PAGE gels, native-PAGE analysis reveals a precipitous decline in the quantity of the kinase-active LRRK2 dimer (Figure 1D). These data suggest that LRRK2 kinase inactivation is not related to autophosphorylation but may be associated with progressive LRRK2 oligomerization.
The G2019S mutation does not alter autophosphorylation site specificity in the GTPase domain
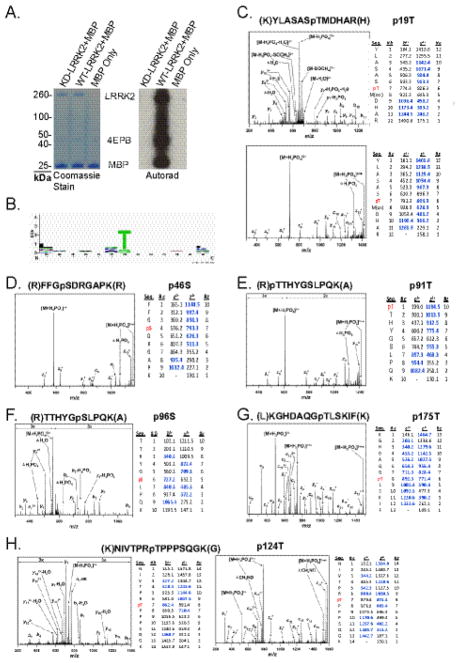
The G2019S-LRRK2 protein may demonstrate higher levels of autophosphorylation due to additional sites of autophosphorylation caused by altered kinase specificity, or due to increased activity prior to enzyme inactivation, or some combination. In order to identify the specific residues of LRRK2 autophosphorylation, in vitro kinase assays were conducted with LRRK2 WT, G2019S and kinase dead D1994A (KD) variants and autophosphorylation was visualized by Pro-Q diamond staining prior to band dissection (Figure 2A). Notably, the D1994A LRRK2 protein has no detectable phosphorylation either in nascent protein (recombinant protein derived from baculovirus infected SF-9 cells) or post-kinase reaction. Nascent phosphorylation at any residue could not be detected in WT and G2019S LRRK2 derived from SF-9 cells when probed with phospho-threonine specific antibodies (Figure 2B).
Fig. 2. LRRK2 autophosphorylation specificity in the ROCO domain is unchanged by the pathogenic G2019S mutation.

LRRK2 in vitro kinase assays were conducted for 30 min with 100 nM G2019S (kinase-overactive), WT or D1994A (kinase-dead) LRRK2 protein (Δ1–970) in the presence of 100 μM ATP. A) Reactions were resolved by SDS-PAGE, and Pro-Q diamond staining highlights phosphorylation of LRRK2 protein after a 30 min in vitro kinase assay. Fluorescence was detected using a Typhoon Trio scanner. B) ATP treated and naïve LRRK2 proteins were run on the same gel and phosphorylation at threonine residues measured C–H) Kinase reactions were resolved by SDS-PAGE, and coomassie stained bands were digested with LysC, or chymotrypsin, or trypsin. Minimum spectra required to make an assignment for a phosphorylated residue are represented. In most cases multiple high quality peptides (Table 1) corroborate the assignment. Blue text highlights matched values from expected. Identical phosphorylated residues were detected in both G2019S and WT-LRRK2 protein, but no phosphorylated residues were detected in D1994A-LRRK2 protein. C) Representative CID spectra from 1343 phosphorylated peptide. D–H) Representative ETD spectra from other identified LRRK2 phosphorylated peptides.
Autophosphorylation-saturated LRRK2 protein bands were digested with trypsin, or chymotrypsin, or LysC protease, and analyzed by mass spectrometry. In order to maximize the number of peptide-spectrum matches and detection of phosphorylation modifications, both Collision Induced Dissociation (CID) and Electron Transfer Dissociation (ETD) fragmentation techniques were implemented on two different platforms: an LTQ-FTICR and an LTQ-XL equipped with ETD. ETD methods are often superior for the identification of phosphorylation and large peptides because fragmentation is restricted to the peptide backbone; thus, the modification remains intact31. As opposed to previous mass spectrometry studies to identify LRRK2 autophosphorylation sites, steps to enrich or select for phosphorylated peptides were omitted to avoid biases in the types of peptides isolated (e.g., acidic peptides versus the omission of phosphorylated basic peptides).
Representative high-confidence spectra map autophosphorylation exclusively within or nearby the GTPase domain, despite excellent and near-complete coverage across the kinase domain and other putative areas for autophosphorylation (Figure 2C–H). Table 1 highlights sites of autophosphorylation from this study and others, identified in multiple mass spectrometry studies that use different approaches and sources of recombinant protein. Autophosphorylation sites associated with multiple peptides derived from diverse sources of proteins and alternative mass spectrometry techniques with different proteolytic enzymes provide very strong evidence against artifactual assignment. Notably, most sites replicated in more than one study reside in or nearby the GTPase domain. Mass spectrometry analysis of both WT and G2019S LRRK2 using ETD and CID sources indicated that the same residues were phosphorylated suggesting that the preferred intra-protein motifs are likely the same between mutant and WT protein, consistent with other reports using different assay conditions 21.
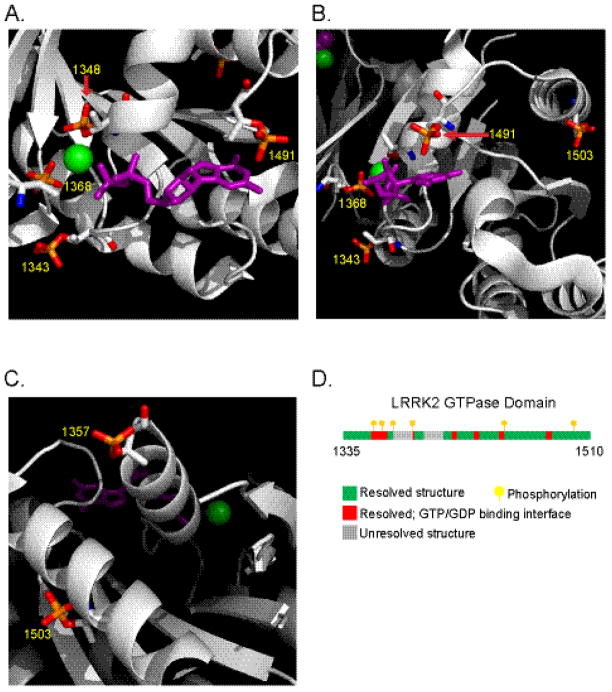
To determine the possible effect phosphorylation would have on the GTPase domain structure, we inserted phosphorylation modifications to the crystal structure of the GTPase domain. The p1343, p1368 and p1348 phosphoryl moieties are predicted to closely interact with the magnesium ion (Figure 3A). In this model, steric hindrance would be unlikely to reduce binding affinity of magnesium. Rather, stabilizing ionic interactions should form between bound magnesium and these phospho groups. The autophosphorylation at 1491 would likely interact with the purine ring of bound nucleotide, whereas phosphorylation at 1503 occurs in a flanking α-helix coil (Figure 3B). A previously undiscovered phosphorylated residue in the GTP-binding pocket, pT1357, was detected but localized to a position where the surrounding structure is only partially resolved (Figure 3C). Remarkably, all phosphorylation sites with the exception of 1503 and 1357 can be considered as occurring in amino acids that comprise the nucleotide binding pocket and interface of the GTPase domain (Figure 3D).
Fig. 3. LRRK2 autophosphorylation occurs primarily in the GTP binding pocket.
A–C) Cartoon models of the LRRK2 GTPase domain with autophosphorylation at detected sites. N=Blue, O=Red, P=Orange and the bound GDP (Purple) is shown in stick format and the Mg2+ ion (Green) shown as a sphere. Pictures were created with PyMOL with domain structure 2ZEJ (Protein Data Bank) D) Linear representation of the LRRK2 GTPase domain illustrates the location of phosphorylations (yellow flags) and residues lining the binding pocket (colored in red) and those unresolved in the crystal structure (gray).
LRRK2 autophosphorylation of residue T1503 is reversible and can be detected with phosphorylation specific antibodies
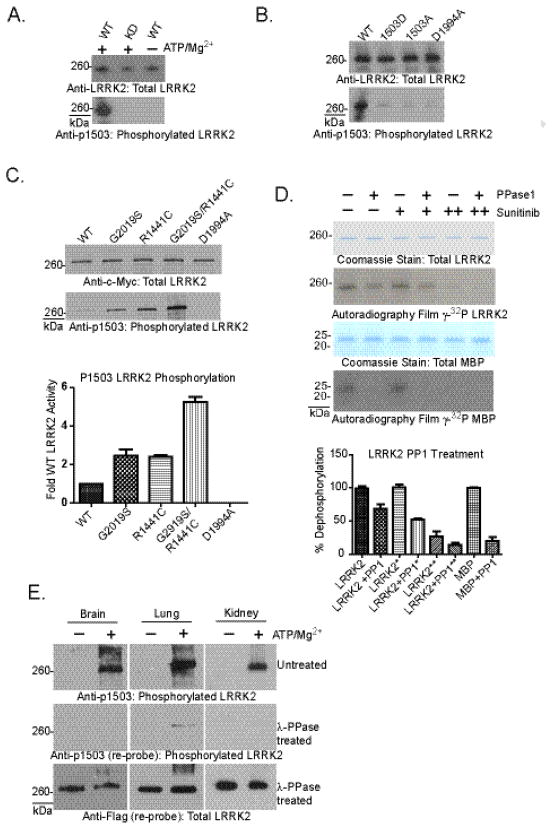
Antibodies directed to specific sites of autophosphorylation may provide insight into LRRK2 activity in vivo. Most of the autophosphorylation residues detected in this study reside in kinked primary peptide structures that comprise the GTP-binding pocket and make poor choices for synthetic peptide generation and antibody production. The threonine at position 1503 is well replicated as a genuine site of autophosphorylation (Table 1) and localizes to an ordered and exposed α-helix that flanks the pocket and serves as a better site to direct an antigenic response. Affinity purified peptide-derived polyclonal antibodies were specific to phosphorylated forms of peptide and did not significantly bind to non-phosphorylated peptide as determined by ELISA analysis (data not shown). Application of pT1503 antibody to LRRK2 protein derived from transfected HEK-293FT cells was unable to detect signal by western blot whether total lysate or immunoprecipitated protein was examined (data not shown). However, robust signal is detected after in vitro autophosphorylation in kinase active but not kinase dead LRRK2 protein (Figure 4A). Mutation of the 1503 site to either alanine or aspartic acid likewise ablates the ability of the antibody to bind (Figure 4B). Similar to the effects of pathogenic mutations that enhance overall autophosphorylation levels (Figure 1 and 5), these mutations also increase staining at pT1503 post-autophosphorylation reactions in vitro (Figure 4C).
Fig. 4. Autophosphorylation-specific LRRK2 antibodies identify pT1503 in LRRK2 protein derived from cell lines and tissue.
A) Recombinant LRRK2 Δ1–970 derived from SF-9 cells was probed for phosphorylated T1503, with or without ATP treatment. Specificity of the pT1503 antibody is demonstrated through lack of reactivity with kinase dead (KD, D1994) and naïve LRRK2. B and C) Full-length LRRK2 protein purified from HEK-293FT cells was probed for total LRRK2 protein and phosphorylated T1503. Specificity of the pT1503 antibody is demonstrated through minimal reactivity with kinase dead (KD, D1994), phosphomimetic T1503D and unphosphorylatable T1503A LRRK2. D) In vitro kinase assays were conducted with LRRK2 recombinant protein prior to treatment with PPase1. Treatment of autophosphorylated LRRK2 with PPase1 (2.5 U, where 0.1 units removes 0.5 nmol serine/threonine phosphates from 10 μM phospho-MBP) and sunitinib (500nM) after in vitro kinase assays. (++) denotes that LRRK2 was treated with sunitinib both during the kinase assay and phosphatase treatment. E) Western blots for LRRK2 protein derived from BAC LRRK2-Flag G2019S mouse tissue. Western blots for pT1503 were conducted before and after treating the membrane with λ-phosphatase. Total LRRK2 was determined with anti-Flag. Quantifications depicted are from 3 independent experiments.
Fig. 5. Effect of variation in the ROC domain on LRRK2 enzymatic activity.
A and C) Full length LRRK2 protein conjugated to agarose beads was incubated in kinase reaction buffer containing 100 μM LRRKtide substrate for 30 min. Beads were removed from the reaction, LRRK2 removed from the beads, denatured and resolved by SDS-PAGE, with LRRK2 protein highlighted by coomassie stain and accompanying autoradiography exposure. B and D) Kinase reaction supernatant from A and C containing phosphorylated LRRKtide was spotted on P-81 cellulose paper and 32P incorporation was measured by liquid scintillation. Results from three experiments are given, and error bars represent ±S.E.M., n.s. is not significant and * indicates p<.001 compared to WT-LRRK2, determined by one-way ANOVA with Tukey’s post hoc test. E) Lysate from HEK-293FT cells transiently transfected with full length LRRK2 were combined with γ-S-GTP sepharose beads. Input fractions and GTP pull-down fractions were resolved by SDS-PAGE and western blotting. F) Raw luminescence values were determined on an Alpha-Innotech Flurochem HD, background signal removed and values normalized to T1348N LRRK2 across experiments after correction for total protein levels (Input fraction) in 4 independent experiments. Error bars are ±S.E.M. and * indicates p<.05 and *** indicates p<.001 relative to WT, determined by one-way ANOVA and Tukey’s post hoc test.
Treatment of recombinant WT LRRK2 protein with PPase1 removes a proportion of phosphorylation modification at T1503 (data not shown). Despite using a vast excess of 3-orders of magnitude more enzyme than should be necessary to dephosphorylate LRRK2 and inclusion of a LRRK2 kinase inhibitor at ten-times IC50 (SU11248, 500nM) concentrations post-kinase reaction, PPase1 is only able to remove ~50% of autophosphorylation incorporation (Figure 4D). To validate that the kinase inhibitor is preventing LRRK2 activity during phosphatase treatment, we also included this compound initially in the reaction and autophosphorylation was effectively prevented. In contrast, PPase1 treatment of LRRK2-phosphorylated MBP in the presence of the LRRK2 kinase inhibitor effectively removes the phosphorylation modification. Other robust phosphatases commonly used in vitro including lambda phosphatase and alkaline phosphatase were similarly ineffective despite vast excesses of enzyme in the reaction under favored buffer conditions (data not shown), suggesting that LRRK2 displays a very unusual property in inducing autophosphorylation that is not readily reversible to protein phosphatases; this is consistent with phosphorylation of a relatively inaccessible binding pocket of the GTPase domain. To determine whether phosphorylation of LRRK2 residue 1503 occurs in vivo, LRRK2 protein from BAC Flag-G2019S LRRK2 transgenic mouse brain, lung, and kidney tissue was immunoprecipitated from rapidly dissected tissues in the presence of phosphatase inhibitors, and protein treated with ATP and magnesium or directly eluted to evaluate the endogenous unstimulated state of LRRK2. LRRK2 protein was subjected to SDS-PAGE, probed for p1503, stripped and treated with λ-phosphatase and reprobed with the p1503 antibody, and finally stripped and reprobed with anti-Flag antibody. Phosphorylation at the 1503 residue using protein derived from transgenic mouse organs can be detected readily and at very high levels after treatment of the protein with ATP and magnesium (Figure 4E). However, all efforts to detect autophosphorylation in naive protein preparations failed to detect phosphorylation at residue 1503. Likewise, endogenous phosphorylation of LRRK2 at residue 1503 was not detected above background in human fibroblast cells that express high levels of LRRK2 (data not shown). We conclude that LRRK2 may only be active under specific conditions in vivo that have yet to be elucidated, or autophosphorylation of 1503 is not likely to occur in cells, or activate LRRK2 may not be associated with fractions that are soluble under conditions conducive to immunoprecipitation.
Phosphorylation at 1503 alters kinase and GTP binding activity
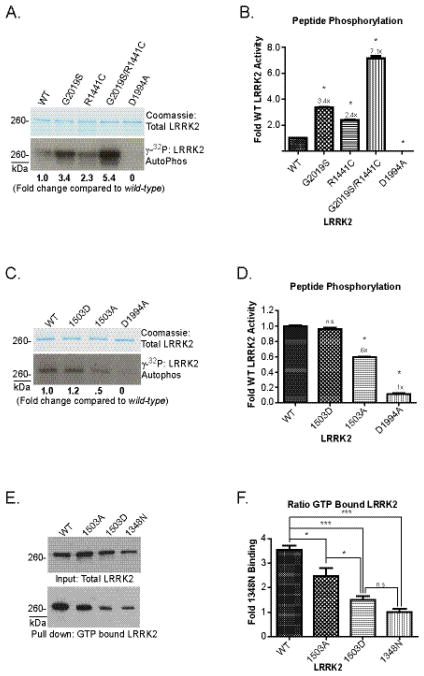
LRRK2 autophosphorylation in the GTPase domain may suggest a reciprocal regulation of the two enzymatic activities. The mutations K1347A and T1348N in the GTP-binding pocket of the GTPase domain have been studied in the past and ablate kinase activity, but may also induce deleterious effects on LRRK2 structure which may lead to the spurious conclusion that GTP-binding regulates LRRK2 kinase activity. Indeed, the T1348N LRRK2 protein significantly under-expresses compared to WT-protein (Figure 5E) and the K1347A is less stable than T1348N in our experience. In order to better define the relationship between GTPase and kinase activities, and the effects of autophosphorylation, we explored additional mutations in the GTPase domain and measured kinase activity. To gain further insight into the relationship of the pathogenic mutation R1441C and kinase activity, R1441C and the kinase domain G2019S mutation were combined into a single full-length LRRK2 construct. The combination induced a multiplicative increase in activity (~7-fold against a peptide substrate, Figure 5A, B), supporting a model of kinase activation for GTPase-linked pathogenic mutations.
Apparent from PD-linked mutations in the GTPase domain, nearby autophosphorylation modifications may alter kinase activity; therefore, we determined whether mutations mimicking or preventing phosphorylation in the GTPase domain affected LRRK2 enzymatic function. We created LRRK2 protein deficient in phosphorylation at 1503 with a T1503A alteration, and found a ~40% reduction in kinase activity compared to WT LRRK2 protein (Figure 5C, D). The T1503A alteration also significantly reduced the proportion of LRRK2 bound to GTP (Figure 5E, F). Unexpectedly, the T1503D substitution likewise reduced GTP-binding in LRRK2 protein, but recovered kinase activity to WT levels. A plausible explanation is that T1503D has structural effects on the GTPase domain that partially mimics a GTP-bound state allowing for continued kinase activity as though bound to GTP but reducing actual GTP-binding. We created additional substitutions at other autophosphorylation residues such as T1343A and T1348A, but these alterations greatly decreased protein expression suggesting destabilization of the pocket and stability of LRRK2. It is possible that autophosphorylation may help structurally organize the GTPase domain into a configuration favorable for continued and full kinase activity although this could not be confirmed in all phosphomimetic mutations due to poor protein stability and expression, and the inability to induce these alterations in protein that has already folded.
Phosphorylation of the in vitro LRRK2 substrate MBP demonstrates unaltered specificity in the G2019S mutation of LRRK2 and a lack of consensus targeting sequences in native proteins
A plausible explanation for the increase in LRRK2 kinase activity in the G2019S PD associated mutation occurring in the kinase domain is that substrate specificity is altered, even in the event autophosphorylation is the same between pathogenic and WT protein. To explore possible changes in substrate site preference, we examine the LRRK2 in vitro substrate, MBP, by mass spectrometry. LRRK2 phosphorylates MBP with high efficiency that dwarves signal generated by autophosphorylation (Figure 6A). LRRK2 phosphorylated the predominant MBP monomer in addition to SDS-resistant MBP oligomers as resolved by SDS-PAGE. To discover the sites of LRRK2 phosphorylation on MBP and provide insight into target site preference and activity of mutant LRRK2, dephosphorylated MBP protein (Upstate) was treated with kinase-active LRRK2 or kinase-dead LRRK2 prior to digestion and analysis by CID and ETD mass spectrometry (Figure 6). Six phosphorylated residues were detected with very high certainty as sites of LRRK2-phosphorylation (Figure 6C–H). No phosphorylated residues were detected in MBP treated with kinase-dead LRRK2.
Fig. 6. Identification of LRRK2 sites of phosphorylation on MBP.
A) In vitro kinase assay with recombinant dephosphorylated MBP with WT or kinase dead (D1994A) LRRK2 (Δ1–970) analyzed by SDS-PAGE and protein bands resolved with coomassie stain. Exposure to autoradiography film indicates comparative phosphorylation of substrate proteins. B) Web logo identified no significant trends in peptide sequence using the 13mer peptide sequence around the 12 phosphorylated residues identified by mass spectrometry in this study. C–H) In vitro kinase assays composed of 50 nM LRRK2 (Δ1–970) and 1 μM dephosphorylated MBP were resolved by SDS-PAGE, stained with coomassie and the predominate MBP isoform excised and digested with LysC, trypsin or chymotrypsin. Detected peptides and corresponding spectra required to make an assignment are given along with the identity of the phosphorylated residue. In all cases multiple high quality peptides (not shown) corroborate the assignment.
Unexpectedly, a preferred phosphorylation motif could not be identified from these sequences when ±6 residues are evaluated from the position of the phosphorylation in substrate MBP and LRRK2 autophosphorylation (Figure 6B). However, most of the phosphorylated peptides identified from both MBP and LRRK2 have very high iso-electric points similar to the previously identified peptide substrate LRRKtide 16,32. Of the twelve peptides sequences described here (Figure 2 and 6), only four fell below pI 8.5 (ProtParam), indicating LRRK2 may prefer to phosphorylate highly basic sequences with an unusual tolerance and flexibility for target site specificity.
Discussion
The effect that pathogenic mutations in LRRK2 impart on protein function may provide insight into the molecular basis of PD. As LRRK2 is under investigation as a potential drug target for PD, clear understanding of enzyme mechanics will aid in identifying critical targets of activity. Past studies have noted enhanced autophosphorylation in G2019S-LRRK2 relative to WT protein under conditions where saturation of autophosphorylation sites might be expected 3,18. We have excluded kinase shut down due to autophosphorylation and its inactivation coincides with a rapid reduction in the soluble fraction of LRRK2 leading to SDS-resistant aggregates at later time points. It is unclear whether this phenomenon occurs in vivo, although LRRK2 aggregate formation in cell culture conditions have been reported 33.
Autophosphorylation often induces functional changes in kinase activity in feed-forward or feed-back loops. The LRRK2 regulatory system likely combines GTP-binding activity and autophosphorylation-regulated mediation of GTPase dependent activities in controlling kinase activity. The paradigm of GTPase control over kinase activity is well established as in the Ras/Raf pathway. Ras family GTPase undergoes a conformational change upon GTP binding to achieve an active state that mediates downstream protein kinases 34,35. The LRRK2 GTPase domain may control conformations such as dimerization that expose the kinase domain to substrates and autophosphorylation sites. This study identifies autophosphorylation occurring in the GTPase domain using mass spectrometry and affinity purified phosphorylation specific antibodies. Interestingly, most autophosphorylation sites identified in more than one mass spectrometry study occur in or proximal to the GTPase domain (table 1).
Alignments of the LRRK2 GTPase domain with other GTPase proteins like H-Ras suggest that LRRK2 GTPase hydrolytic activity should be exceedingly low and the pocket may have very poor affinity for GTP (i.e., a high exchange rate), predictions which appear to hold experimentally 23,36. Physiologically, a low activation state of the GTPase domain may balance a more active downstream kinase activity, suggesting that GTPase modifiers may be the primary mechanism regulating LRRK2 kinase activity. The R1441C mutation causes altered folding in the ROC domain and reduces GTP-hydrolysis activity 23,36,37. Combination of R1441C together with G2019S has a multiplicative effect on enhanced kinase activity, which is difficult to explain unless the GTPase domain affects some aspect of initial kinase activation, increased proportion of active protein, or increased enzyme kinetics. The R1441C/G2019S combination induces a ~7 fold increase in kinase activity relative to WT protein that may be useful in the generation of animal models that seek to produce kinase dependent phenotypes.
While specific inhibitors that target LRRK2 GTPase activities are not likely to be developed in the short term in light of poor success with developing specific inhibitors of Ras and other GTPase proteins, interpretation of experimental measurements of LRRK2 GTPase activity are aided through the utilization of mutations in the GTP-binding pocket that hinder GTP-binding. These mutations, such as T1348N, help delineate activities from co-purifying GTPase proteins. However, as previously noted, these mutations potentially destabilize overall protein folding. In addition, it is now known that the T1348N mutation removes an autophosphorylation site and therefore associated activities are difficult to interpret. We have found that aspartic acid substitutions to autophosphorylation sites within the GTP-binding pocket generally destabilized LRRK2 and were very poorly expressed by mammalian cells, potentially because the GTP-binding pocket cannot fold properly with an acidic residue at these positions. In contrast, substitution at the 1503 residue is well tolerated as it flanks the pocket in an adjacent conserved α-helix loop. The T1503A unphosphorylatable LRRK2 construct has a significant reduction in kinase activity in both autophosphorylation and against a peptide substrate, indicating that autophosphorylation may be required for full kinase activity. Unexpectedly, the T1503D phosphomimetic similarly reduced GTP-binding levels as T1503A but rescued kinase levels back to WT activity. We hypothesize that the aspartic acid substitution at 1503 may make an active state conformation more favorable by partially mimicking a GTP-bound state. Indeed, if LRRK2 possess a high GTP exchange rate as predicted, autophosphorylation may serve overall to stabilize an active state conformation and bound nucleotide.
Autophosphorylation regulates LRRK2 kinase activity as implicated by the functional impact of unphosphorylatable mutants. Phosphorylation is usually considered a highly reversible post-translational modification. However, the existence of autophosphorylation at a protected site has previously been described in protein kinase C (PKC), where phosphorylation required for LTP that persists for up to an hour 38. PKC dephosphorylation requires activating cofactors and is dependent on accessibility granted by the active conformation of the protein. Since LRRK2 activating co-factors are not known it is difficult to test this model. G2019S LRRK2 has increased kinase activity but was not more susceptible to PP1 treatment in vitro indicating that cofactors may be necessary to activate LRRK2 to achieve similar dephosphorylation to PKC.
Pathological mutations in the LRRK2 protein often cause phenotypes similar to the more prevalent idiopathic late-onset PD, suggesting a shared etiology between the two diseases 39. The most straightforward approach to hone in on the pathogenic activity associated with LRRK2 may lie in successful identification of the difference between mutant and WT protein. The simplistic expectation is that all the pathogenic mutations affect a common biochemical property of LRRK2. Such a biochemical activity has been proposed as kinase activity against as yet unknown protein substrates and pathways.
Materials and methods
Plasmids
Previously described LRRK2 cDNA constructs cloned into the pcDNA3.1-myc/his vector backbone 18 were provided by Ted Dawson (Johns Hopkins University, Baltimore, MD). Plasmids were purified with the Qiagen Hi-Speed Maxiprep Kit. Exogenous LRRK2 protein levels in transfected cell lines were assessed by SDS-PAGE western blots (described below) to confirm equal levels of expression. Mutations in the LRRK2 pcDNA3.1-myc/his plasmids were generated with the QuikChange II Site-Directed Mutagenesis Kit (Stratagene). WT and mutant plasmid constructs were verified by Sanger sequencing of the open reading frame (primer sequences available upon request).
Cell culture and transfection
HEK-293FT cells were purchased from Invitrogen. Cells were maintained in Opti-MEM supplemented with 10% fetal bovine serum (FBS). 10 cm plates were transfected with 10 μg LRRK2 plasmid described above in serum-free Opti-MEM with FuGene HD transfection reagent (Roche) according to the manufacturers protocol. 24 hours after transfection media was changed to 2% FBS serum Opti-MEM. Cells were collected at 48 hours post-transfection.
LRRK2 Kinase Assays
Recombinant commercially purified human LRRK2 (Δ1–970), kinase-over active G2019S and kinase dead (KD) D1994A LRRK2 proteins were purchased from Invitrogen for use in some experiments. Recombinant proteins were assessed for equal purity (>95% by coomassie SDS-PAGE) and protein concentration as determined by BCA assay (Pierce). For kinase inactivation experiments, LRRK2 Δ1–970 binding was conducted with 4 μg of protein and 20 μl of magnetic bead slurry conjugated to glutathione (Pierce) for 2 hours at 4°C for determining the kinase inactivation on a solid surface. Full-length LRRK2 protein was purified from transfected HEK-293FT cells or Flag G2019S-LRRK2 BAC transgenic mouse tissue as described below. Elution of flag epitope tagged LRRK2 was conducted in 150 ng/ml triple flag peptide, 50% glycerol and 2mM DTT for 3 hours at 4°C. Reactions were incubated without peptide, with or without ATP, until the indicated time interval. After the completion of the time interval, reactions were removed to ice to halt activity. Reactions were conducted at 30°C and detection of kinase activity was determined by liquid scintillation from P-81 cellulose paper as described below. All values were normalized to the initial 20 min signal obtained for each condition to determine the percentage of activity remaining in subsequent reactions.
Recombinant human full-length LRRK2 protein derived from mammalian cells was isolated from HEK-293FT cells (Invitrogen) transfected with LRRK2 plasmid. Cells were collected 48 hours post-transfection and centrifuged at 500 × g for 5 min. Pelleted cells were re-suspended in lysis buffer (0.5% Triton-X 100, 1 × protease inhibitor (Roche) and 1× PhoStop (Roche) in phosphate-buffered saline without Ca2+ or Mg2+, pH 7.4, [PBS]) and rotated at 4°C for 1 hour. Lysates were clarified by centrifugation at 20,000 × g for 10 min. Supernatant was incubated with anti-c-myc antibody (clone 9E10, Roche) conjugated Dynabeads G (Invitrogen) for 16 hours. Supernatants were discarded and beads washed 3 × in 500 mM NaCl supplemented PBS, 3 × in PBS and resuspended into kinase buffer (5 mM EGTA and 20 mM β-glycerol phosphate in PBS). Reactions were initiated by addition of activation buffer to final concentrations that includes 0.1 mM ATP and 20 mM MgCl2 and incubation at 30°C with shaking for the specified time. Reactions were terminated by placing the tubes in ice, removing supernatant to P-81 Whatman paper for scintillation counting in LRRK2 peptide phosphorylation experiments or suspension of reactions to 2 × Laemmli buffer. Reactions were then heated at 75°C for 10 min and Laemmli buffer diluted to 1 × with PBS and reactions electrophoresed on tris-acetate SDS-PAGE gels. Immunoprecipitated proteins were stained using coomassie G-250 (Bio-Rad), silver stain (Pierce), or Pro-Q diamond stain (Invitrogen) according to the manufacturer’s protocol. Whatman P-81 discs were washed in phosphoric acid buffer 5 × or with additional washes until no radioactivity could be detected in wash buffer. For comparisons in levels of activity in autophosphorylation or kinase activity against peptide substrates, one-way ANOVA and Tukey’s post-hoc test were used to determine significance.
Mass Spectrometry
Recombinant LRRK2 protein bands or MBP bands from LRRK2 kinase reactions were excised from SDS acrylamide gels and equilibrated in 100 mM ammonium bicarbonate (AmBc). Slices were reduced for 15 min (2.8 mM DTT in 100 mM AmBc) at 50°C then carbidomethylated at room temperature in the dark for 15 min (5.9 mM 2-iodoacetamide). Gel bands were then dehydrated in 50% acetonitrile and 50 mM AmBc for 15 min and 100% acetonitrile for 5 min prior to desiccation via speed vac centrifugation. In-gel digests were conducted individually with Trypsin (Promega), or LysC (Roche), or Chymotrypsin (Roche) at 37°C for Trypsin and LysC and room temp for Chymotrypsin. After digestion, peptides were extracted from the gel using 50 percent acetonitrile and 5 percent formic acid. Peptides were lyophilized in a speed vac and resolubilized in 0.1% formic acid.
Digests of LRRK2 and MBP were loaded onto both a linear ion trap-Fourier transform ion cyclotron resonance hybrid mass spectrometer (LTQ–FTICR, Thermo Fisher Scientific) or a linear ion trap with electron transfer dissociation capabilities (LTQ-XL-ETD, Thermo Fisher Scientific). The LTQ-FTICR LC-MS system was composed of a MicroAS autosampler and a 2D LC nanopump (Eksigent) and the LTQ-XL-ETD system is composed of a split flow Surveyor auto sampler and Surveyor pump (Thermo). Peptides were separated using a 100 μm (inner diameter) 11 cm pulled packed tip with Jupiter 5 μm C18 reversed phase beads (Phenomenex) with an acetonitrile gradient spanning from 5% to 40% over 50 min at 650 nl min−1 in 0.1% formic acid. The Xcalibur settings for the FTICR were set to normal mass range with a 50,000 resolution MS scan followed by four data dependent MS/MS scans per cycle in profile mode. Peptides were analyzed on the LTQ-XL ETD through a parent scan, three secondary ultra zoom scans at ± 2.5 da, and three CID or ETD MS/MS scans corresponding to the ion from the prior ultra zoom scan per cycle in profile mode. The dynamic exclusion settings for both instruments were set to exclude ions for 2 min after a repeat count of three within a 45 sec duration.
Thermo Xcalibur. RAW files were converted to mzXML files with ReAdW and mzXML files were converted to. MGF files with the trans-proteomic pipeline tools software suite (Seattle Proteome Center). LRRK2 and MBP peptides were identified using the TurboSEQUEST v.27 (rev.12) (Thermo Fisher Scientific), MASCOT 2.2 (Matrix Biosciences) and Protein Prospector v. 5.2.2 (UC-San Francisco) search engines. SEQUEST and MASCOT searches used the latest available UniRef100 database and Protein Prospector searches incorporated the latest Swiss Prot database where all three engines were searched with the appropriate protease specific cleavage site(s) for LysC and Trypsin and no enzymatic restrictions for Chymotrypsin. The parent ion mass accuracy window was set to 10.0 ppm for the LTQ-FTICR and 2.0 Da for the LTQ-XL-ETD with a fragment tolerance of 0.6 Da. Putative phospho-peptides from SEQUEST and MASCOT searches were processed and initially visualized using Scaffold (Proteome software) and Protein Prospector results were visualized within the Protein Prospector software suite (UCSF). Spectra corresponding to peptides modified with a phosphorylation were confirmed and correctly annotated against the unprocessed profile MS/MS data manually and then overlaid using Grapher 8.0 (Golden software).
Antibodies
LRRK2 staining was conducted with anti-c-myc horseradish peroxidase (HRP)(clone 9E10 HRP conjugated, Roche) in transfected cell conditions. Recombinant LRRK2 Δ1–970 was detected with an antibody provided by the Michael J. Fox Foundation, MJFF c-41 monoclonal rabbit anti-LRRK2. Phosphorylated LRRK2 residue was detected with affinity purified rabbit polyclonal antibody (described below) supplemented with 10ug/ml dephospho-1503 peptide ALAKLRKTIINESLNF.
To generate antibodies specific to LRRK2 autophosphorylation sites, rabbits were injected with a mixture of Ac-ALAKLRK[pT]IINESLNF-Ahx-C-amide and C-Ahx-ALAKLRK[pT]IINESLNF-amide for antibody pT1503. Serum was collected and passed over an affinity matrix conjugated to the non-phospho peptide (C-Ahx-ALAKLRKTIINESLNF-amide) and completeness of immunodepletion measured by elution of protein from an affinity column (Pierce), and the process repeated until no additional cross reacting protein could be eluted. Next, depleted serum was passed over a phospho-peptide conjugated affinity column (Pierce), washed, eluted, dialyzed, and concentration measured by BCA assay (Pierce). All peptides were evaluated for purity prior to usage by MALDI-TOF mass spectrometry, and sequences used to generate antibodies are conserved between human and rodent LRRK2.
Anti-rabbit or mouse HRP conjugated secondary antibodies (Jackson Immuno) were used to probe primary antibodies and chemiluminescent substrate (Peirce) applied to detect labeled proteins.
LRRK2 GTP Binding Assays
HEK-293FT cells transfected with LRRK2 constructs were lysed in 0.5% Triton-X 100 PBS lysis buffer with protease inhibitors after 48 hours post-transfection. Supernatants were incubated with γ-S-GTP sepharose beads (Jena Bioscience) at 16 °C for 2 hours with shaking at 1200 RPM and bead suspensions were centrifuged using SigmaPrep spin columns for 30 sec at 200 × g and flow-through was discarded. Sepharose beads were washed 2 times in 500 mM NaCl PBS, and 2 times with PBS. Sepharose gel was re-suspended in 2 × Laemmli buffer and heated at 75 °C for 10 min to remove conjugated proteins. Samples were diluted to 1 × and electrophoresed on a 7% SDS-PAGE gel and proteins transferred to PVDF. Membranes were incubated with anti-c-myc HRP and then ECL reagent, and chemiluminescence measured by an Alpha Innotech Fluorchem HD machine. Bound samples were normalized to total protein in the input and values then compared T1348N LRRK2. One-way ANOVA and Tukey’s post-hoc test were used to determine significance.
Transgenic Mouse Tissue
Mice expressing bacterial artificial chromosome (BAC) N-terminally Flag epitope tagged G2019S mLRRK2 were obtained from Jackson Laboratories. Mouse brain, kidney and lung were harvested and suspended in lysis buffer (2 × Protease and PhoStop inhibitors in PBS). Tissues were dounce homogenized and subsequently sonicated at 10% power for 10 sec on a Branson dismembrator. Lysates were centrifuged at 20,000 × g for 10 min to pellet insoluble material. Immunoprecipitation (IP) of LRRK2 was performed at 4°C for 3 hours while rotating with magnetic beads conjugated with Anti-Flag antibody. IPs were washed 3× in 500mM NaCl PBS and 2× in PBS. After the wash, IPs were split into 2 separate reactions for kinase assay and untreated control. Kinase assays were conducted as described above.
Acknowledgments
*This work was supported by the Michael J. Fox Foundation for Parkinson’s Research, the American Parkinson’s Disease Association, by the National Institutes of Health grant R00 NS058111 and R01 NS064934, and the benevolence of John A. and Ruth R Jurenko. The authors acknowledge Ted and Valina Dawson for providing LRRK2 expression constructs and Krister Wennerberg for helpful discussions.
Abbreviations
- LRRK2
leucine-rich repeat kinase 2
- PD
Parkinson disease
- GTPase
guanosine triphosphatase
- ETD
electron transfer dissociation
- CID
collision induced dissociation
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–7. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 3.West AB, Moore DJ, Choi C, Andrabi SA, Li X, Dikeman D, Biskup S, Zhang Z, Lim KL, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet. 2007;16:223–32. doi: 10.1093/hmg/ddl471. [DOI] [PubMed] [Google Scholar]
- 4.Webber PJ, West AB. LRRK2 in Parkinson’s disease: function in cells and neurodegeneration. Febs J. 2009;276:6436–44. doi: 10.1111/j.1742-4658.2009.07342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biskup S, Moore DJ, Rea A, Lorenz-Deperieux B, Coombes CE, Dawson VL, Dawson TM, West AB. Dynamic and redundant regulation of LRRK2 and LRRK1 expression. BMC Neurosci. 2007;8:102. doi: 10.1186/1471-2202-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, Faull RL, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–69. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 7.Higashi S, Biskup S, West AB, Trinkaus D, Dawson VL, Faull RL, Waldvogel HJ, Arai H, Dawson TM, Moore DJ, Emson PC. Localization of Parkinson’s disease-associated LRRK2 in normal and pathological human brain. Brain Res. 2007;1155:208–19. doi: 10.1016/j.brainres.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 8.Gehrke S, Imai Y, Sokol N, Lu B. Pathogenic LRRK2 negatively regulates microRNA-mediated translational repression. Nature. 466:637–41. doi: 10.1038/nature09191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carballo-Carbajal I, Weber-Endress S, Rovelli G, Chan D, Wolozin B, Klein CL, Patenge N, Gasser T, Kahle PJ. Leucine-rich repeat kinase 2 induces alpha-synuclein expression via the extracellular signal-regulated kinase pathway. Cell Signal. 2010;22:821–7. doi: 10.1016/j.cellsig.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gloeckner CJ, Schumacher A, Boldt K, Ueffing M. The Parkinson disease-associated protein kinase LRRK2 exhibits MAPKKK activity and phosphorylates MKK3/6 and MKK4/7, in vitro. J Neurochem. 2009;109:959–68. doi: 10.1111/j.1471-4159.2009.06024.x. [DOI] [PubMed] [Google Scholar]
- 11.Hsu CH, Chan D, Wolozin B. LRRK2 and the stress response: interaction with MKKs and JNK-interacting proteins. Neurodegener Dis. 2010;7:68–75. doi: 10.1159/000285509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liou AK, Leak RK, Li L, Zigmond MJ. Wild-type LRRK2 but not its mutant attenuates stress-induced cell death via ERK pathway. Neurobiol Dis. 2008;32:116–24. doi: 10.1016/j.nbd.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White LR, Toft M, Kvam SN, Farrer MJ, Aasly JO. MAPK-pathway activity, Lrrk2 G2019S, and Parkinson’s disease. J Neurosci Res. 2007;85:1288–94. doi: 10.1002/jnr.21240. [DOI] [PubMed] [Google Scholar]
- 14.Parisiadou L, Xie C, Cho HJ, Lin X, Gu XL, Long CX, Lobbestael E, Baekelandt V, Taymans JM, Sun L, Cai H. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J Neurosci. 2009;29:13971–80. doi: 10.1523/JNEUROSCI.3799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anand VS, Reichling LJ, Lipinski K, Stochaj W, Duan W, Kelleher K, Pungaliya P, Brown EL, Reinhart PH, Somberg R, Hirst WD, Riddle SM, Braithwaite SP. Investigation of leucine-rich repeat kinase 2: enzymological properties and novel assays. Febs J. 2009;276:466–78. doi: 10.1111/j.1742-4658.2008.06789.x. [DOI] [PubMed] [Google Scholar]
- 16.Jaleel M, Nichols RJ, Deak M, Campbell DG, Gillardon F, Knebel A, Alessi DR. LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson’s disease mutants affect kinase activity. Biochem J. 2007;405:307–17. doi: 10.1042/BJ20070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luzon-Toro B, Rubio de la Torre E, Delgado A, Perez-Tur J, Hilfiker S. Mechanistic insight into the dominant mode of the Parkinson’s disease-associated G2019S LRRK2 mutation. Hum Mol Genet. 2007;16:2031–9. doi: 10.1093/hmg/ddm151. [DOI] [PubMed] [Google Scholar]
- 18.West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102:16842–7. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shohat G, Spivak-Kroizman T, Cohen O, Bialik S, Shani G, Berrisi H, Eisenstein M, Kimchi A. The pro-apoptotic function of death-associated protein kinase is controlled by a unique inhibitory autophosphorylation-based mechanism. J Biol Chem. 2001;276:47460–7. doi: 10.1074/jbc.M105133200. [DOI] [PubMed] [Google Scholar]
- 20.Kamikawaji S, Ito G, Iwatsubo T. Identification of the autophosphorylation sites of LRRK2. Biochemistry. 2009;48:10963–75. doi: 10.1021/bi9011379. [DOI] [PubMed] [Google Scholar]
- 21.Gloeckner CJ, Boldt K, von Zweydorf F, Helm S, Wiesent L, Sarioglu H, Ueffing M. Phosphopeptide analysis reveals two discrete clusters of phosphorylation in the N-terminus and the Roc domain of the Parkinson-disease associated protein kinase LRRK2. J Proteome Res. 2010;9:1738–45. doi: 10.1021/pr9008578. [DOI] [PubMed] [Google Scholar]
- 22.Greggio E, Taymans JM, Zhen EY, Ryder J, Vancraenenbroeck R, Beilina A, Sun P, Deng J, Jaffe H, Baekelandt V, Merchant K, Cookson MR. The Parkinson’s disease kinase LRRK2 autophosphorylates its GTPase domain at multiple sites. Biochem Biophys Res Commun. 2009;389:449–54. doi: 10.1016/j.bbrc.2009.08.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito G, Okai T, Fujino G, Takeda K, Ichijo H, Katada T, Iwatsubo T. GTP binding is essential to the protein kinase activity of LRRK2, a causative gene product for familial Parkinson’s disease. Biochemistry. 2007;46:1380–8. doi: 10.1021/bi061960m. [DOI] [PubMed] [Google Scholar]
- 24.Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci. 2006;9:1231–3. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- 25.Sen S, Webber PJ, West AB. Dependence of leucine-rich repeat kinase 2 (LRRK2) kinase activity on dimerization. J Biol Chem. 2009;284:36346–56. doi: 10.1074/jbc.M109.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erickson AK, Payne DM, Martino PA, Rossomando AJ, Shabanowitz J, Weber MJ, Hunt DF, Sturgill TW. Identification by mass spectrometry of threonine 97 in bovine myelin basic protein as a specific phosphorylation site for mitogen-activated protein kinase. J Biol Chem. 1990;265:19728–35. [PubMed] [Google Scholar]
- 27.Hirschberg D, Radmark O, Jornvall H, Bergman T. Thr94 in bovine myelin basic protein is a second phosphorylation site for 42-kDa mitogen-activated protein kinase (ERK2) J Protein Chem. 2003;22:177–81. doi: 10.1023/a:1023479131488. [DOI] [PubMed] [Google Scholar]
- 28.Kishimoto A, Nishiyama K, Nakanishi H, Uratsuji Y, Nomura H, Takeyama Y, Nishizuka Y. Studies on the phosphorylation of myelin basic protein by protein kinase C and adenosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem. 1985;260:12492–9. [PubMed] [Google Scholar]
- 29.Martenson RE, Law MJ, Deibler GE. Identification of multiple in vivo phosphorylation sites in rabbit myelin basic protein. J Biol Chem. 1983;258:930–7. [PubMed] [Google Scholar]
- 30.Yu JS, Yang SD. Protein kinase FA/glycogen synthase kinase-3 predominantly phosphorylates the in vivo site Thr97-Pro in brain myelin basic protein: evidence for Thr-Pro and Ser-Arg-X-X-Ser as consensus sequence motifs. J Neurochem. 1994;62:1596–603. doi: 10.1046/j.1471-4159.1994.62041596.x. [DOI] [PubMed] [Google Scholar]
- 31.Chi A, Huttenhower C, Geer LY, Coon JJ, Syka JE, Bai DL, Shabanowitz J, Burke DJ, Troyanskaya OG, Hunt DF. Analysis of phosphorylation sites on proteins from Saccharomyces cerevisiae by electron transfer dissociation (ETD) mass spectrometry. Proc Natl Acad Sci U S A. 2007;104:2193–8. doi: 10.1073/pnas.0607084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nichols RJ, Dzamko N, Hutti JE, Cantley LC, Deak M, Moran J, Bamborough P, Reith AD, Alessi DR. Substrate specificity and inhibitors of LRRK2, a protein kinase mutated in Parkinson’s disease. Biochem J. 2009;424:47–60. doi: 10.1042/BJ20091035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waxman EA, Covy JP, Bukh I, Li X, Dawson TM, Giasson BI. Leucine-rich repeat kinase 2 expression leads to aggresome formation that is not associated with alpha-synuclein inclusions. J Neuropathol Exp Neurol. 2009;68:785–96. doi: 10.1097/NEN.0b013e3181aaf4fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349:117–27. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 35.Stokoe D, Macdonald SG, Cadwallader K, Symons M, Hancock JF. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994;264:1463–7. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- 36.Lewis PA, Greggio E, Beilina A, Jain S, Baker A, Cookson MR. The R1441C mutation of LRRK2 disrupts GTP hydrolysis. Biochem Biophys Res Commun. 2007;357:668–71. doi: 10.1016/j.bbrc.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Dunn L, Greggio E, Krumm B, Jackson GS, Cookson MR, Lewis PA, Deng J. The R1441C mutation alters the folding properties of the ROC domain of LRRK2. Biochim Biophys Acta. 2009;1792:1194–7. doi: 10.1016/j.bbadis.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sweatt JD, Atkins CM, Johnson J, English JD, Roberson ED, Chen SJ, Newton A, Klann E. Protected-site phosphorylation of protein kinase C in hippocampal long-term potentiation. J Neurochem. 1998;71:1075–85. doi: 10.1046/j.1471-4159.1998.71031075.x. [DOI] [PubMed] [Google Scholar]
- 39.Sen S, West AB. The therapeutic potential of LRRK2 and alpha-synuclein in Parkinson’s disease. Antioxid Redox Signal. 2009;11:2167–87. doi: 10.1089/ars.2009.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]