Abstract
In this article, a theory is presented which assumes that the visual P1 reflects the same cognitive and physiological functionality as alpha (with a frequency of about 10 Hz).Whereas alpha is an ongoing process, the P1 is the manifestation of an event-related process. It is suggested that alpha and the P1 reflect inhibition that is effective during early access to a complex knowledge system (KS). Most importantly, inhibition operates in two different ways. In potentially competing and task irrelevant networks, inhibition is used to block information processing. In task relevant neural networks, however, inhibition is used to increase the signal to noise ratio (SNR) by enabling precisely timed activity in neurons with a high level of excitation but silencing neurons with a comparatively low level of excitation. Inhibition is increased to modulate the SNR when processing complexity and network excitation increases and when certain types of attentional demands – such as top–down control, expectancy or reflexive attention – increase. A variety of findings are reviewed to demonstrate that they can well be interpreted on the basis of the suggested theory. One interesting aspect thereby is that attentional benefits (reflected e.g., by a larger P1 for attended as compared to unattended items at contralateral sites) and costs (reflected e.g., by a larger P1 at ipsilateral sites) can both be interpreted in terms of inhibition. In the former case an increased P1 is associated with a more effective processing of the presented item (due to an inhibition modulated increase in SNR), in the latter case, however, with a suppression of item processing (due to inhibition that blocks information processing).
Keywords: Oscillation, Memory, EEG, Alpha, P1, ERP
Highlights
► The P1 and alpha are considered to reflect functionally similar processes. ► A comprehensive cognitive interpretation of the P1is presented. ► The key argument is that the P1 reflects inhibition.
1. Introduction
The aim of this paper is to present a theory that tries to bridge the gap between ongoing oscillatory brain activity in the alpha frequency range and the generation of early components of the visual event-related potential (ERP). It is suggested that early ERP components – and the P1 in particular – are generated at least in part by oscillations in the alpha frequency range (cf. Klimesch et al. 2007a,b and Sauseng et al. 2007 for an extensive discussion and review of this issue). Thus, we start with a brief outline of the functionality of alpha in this section. Then, in Section 2, we discuss the functionality of the P1 in relation to alpha on the basis of a brief selective literature review. In Section 3, the details of the proposed theory are presented, and its explanatory power and predictions are discussed. The central hypothesis thereby is that the P1 amplitude reflects inhibition that enables the suppression of task irrelevant and potentially competing processes. Finally, in Section 4, we focus on a variety of implications of this theory with respect to cognitive and physiological processes.
1.1. Basic assumptions
The proposed theory is based on two general assumptions about the generation and modulation of the visual P1 component.
-
(1)The first assumption relates the P1 component to alpha oscillations and comprises three aspects:
-
(1a)The P1 is generated and modulated at least in part by alpha oscillations.
-
(1b)The P1 reflects the same type of functionality as alpha does.
-
(1c)The functionality of alpha can be explained on the basis of the inhibition-timing hypothesis (Klimesch et al. 2007a).
-
(1a)
-
(2)
The second assumption refers to the cognitive relevance of the P1 amplitude and states that it is not a sensory-evoked component, but instead a manifestation of early stimulus categorization that operates under top–down control or in a default like mode.
The inhibition-timing hypothesis is the central link between the inferred (physiological and cognitive) functionality of alpha and the P1. Thus, we start with a brief summary of this hypothesis (see Klimesch et al. 2007a for an extensive review).
The central idea is that alpha reflects inhibitory processes (operating under top–down control or in a default like mode) that control cortical activation. Alpha amplitude (or power) is associated with a certain level of inhibition whereas phase reflects the time and direction of a rhythmic change in inhibition (build up of and release from inhibition). For event-related processes and the generation of early ERP components we assume that alpha phase reorganization will be a powerful mechanism for the event-related timing of cortical processes that underlie the generation of the P1 (cf. Klimesch et al., 2007b).
With respect to its cognitive functionality, we have suggested that alpha reflects a basic processing mode that controls the flow of information in the cortex of the human brain (Klimesch et al. 2007a,b). It enables access to a complex long-term storage system, which we have termed knowledge system (KS) in order to emphasize that it comprises not only traditional long-term memory (LTM) – a system closely associated with the storage of declarative information – but any type of knowledge including procedural and implicit-perceptual knowledge.
Traditional theories about the structure of LTM, such as the ACT- and ACT*-model proposed by Anderson (1981, 1983) and Anderson and Pirolli (1984) are characterized by the assumption that memory search can be described by a spreading activation process that is initiated at some point in the storage network (for a review and critical evaluation of traditional spreading activation theories cf. Klimesch, 1994). One important question, these models did not attempt to explain, is the way in which the memory network is accessed. Here, the focus is primarily on those processes that provide access to information, stored in memory. An important assumption here is that perception, encoding, and recognition are processes that are closely related to the access of information in the KS. During perception, the extraction of global stimulus features is an important early stage of encoding that allows to narrow down the search area in memory. This early stage of encoding can be considered an early stage of stimulus categorization that is based on global features. It operates to establish an ‘access field’ which is considered a necessary step for initiating a spreading activation process that underlies stimulus recognition.
The perceptual analysis of more global stimulus features will be strongly influenced by expectancy and is considered a fast process that precedes the actual recognition of a stimulus. Early categorization operates under the top–down control of attentional processes that are guided by specific expectations. In the absence of expectancy, early categorization may operate in a default-like mode that is guided by reflexive attention. This means that those stimulus properties that elicit reflexive attention (such as e.g., color or size) enhance stimulus recognition.
The KS provides us with the basic ability to be continuously semantically orientated in our environment with respect to all kinds of information that represent our knowledge of that environment (Klimesch et al., 2010). Within the visual processing domain, the perception and transient representation of objects and their locations allow us to be continuously oriented in space and time. These processes that control the flow of information into (the KS of) the brain establish transient mental representations but are not (directly) involved in the encoding of new (episodic) information. This distinction is important because access to the knowledge system is considered a continuous process that may modify information stored in this system without creating new episodic memories.
With respect to physiology, the central idea is that those processes that enable and control access to the KS are reflected by alpha oscillations. Thus, alpha is not associated with attention in the sense of a global mechanism (e.g., not including vigilance) as was suggested from the early days of EEG research. We assume a rather specific function for alpha not only with respect to the type of cognitive processes but also with respect to physiology. With respect to physiology one important aspect is that alpha operates to inhibit task irrelevant neural structures and thereby helps to establish a more focused access to the KS. There may be different kinds of attentional processes comprising e.g., also those which rely on excitatory processes only. In addition, there may be different kinds of attention, related to different cognitive processing modes, such as a sustained focus on the encoding of new or alerting information. We consider alpha a specific kind of attention that is related to inhibitory control processes of the KS.
The next section is a selective review about variables that typically modulate P1 amplitude and/or latency. The most important examples are (1) spatial attention, (2) reflexive attention, (3) object based attention, (4) target properties investigated in search paradigms, and (5) perceptual features. The aim of this brief review is to provide evidence for the second assumption stating that the P1 amplitude reflects early categorization processes during access to the KS, which are based on the analysis of global stimulus properties.
2. The P1 component and early categorization processes
2.1. Spatial attention: Location-based selection and early stages of visual processing
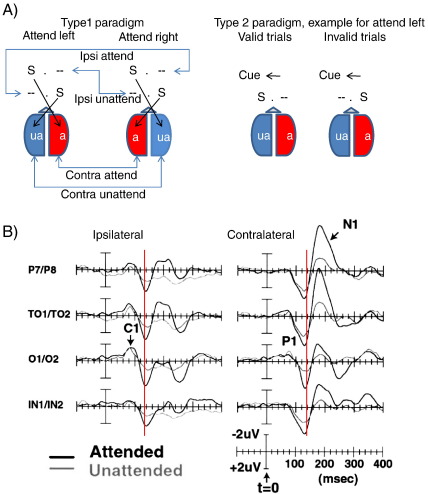
The spatial location of a relevant stimulus or object may be considered an important variable that influences early stages of visual processing and access to the KS. For the investigation of spatial attention, at least three types of paradigms can be distinguished. The first two investigate top–down controlled spatial selection processes. As illustrated in Fig. 1A, type 1 paradigms are designed to direct attention to a specific location – usually to the left or right hemifield – over a run (block) of trials simply by instructing subjects to do so. Type 2 paradigms use a cue to direct attention to a specific location on a trial per trial basis. Type 3 paradigms are used to study reflexive attention, either using a cue or not.
Fig. 1.
(A) Two different designs for the investigation of spatial attention are shown. In a type 1 paradigm subjects attend – over a block of trials – either to stimuli (S) in the left or right hemifield. Thus, one hemisphere is set in a continuous mode to attend (a), the other to unattend (ua). In type 2 paradigms, a cue is used to direct attention to the left or right hemifield on a trial per trial basis. As illustrated for the attend left condition, a cue may be valid (e.g., in 75% of all trials) or invalid. (B) The findings obtained from a type 1 paradigm are shown. Data are from Mangun et al. (2001), the figure is reprinted with permission. The ERPs were collapsed over the scalp sites contralateral and ipsilateral to the presented stimulus. As an example, contralateral occipital scalp sites for attended stimuli were obtained by averaging the waveforms from the left occipital site for attended right stimuli with the waveforms for attended left waveforms recorded from the right occipital site (cf. also the respective arrowheads in Fig. 1A for ‘contra attend’). Note that the P1 is generally larger in the attended hemisphere, regardless of whether this hemisphere received a stimulus (attend contralateral) or not (attend ipsilateral). Also note the latency differences between contra- and ipsilateral recordings. The vertical red line marks a latency of 120 ms. Ipsilateral P1 components are clearly delayed by about 5 ms. Request to reprint is currently requested.
Convergent evidence from type 1 and 2 paradigms indicates that stimuli flashed at an attended location elicit a larger P1 than stimuli flashed at unattended locations (e.g., Heinze et al. 1994; Heinze and Mangun, 1995; Mangun et al., 1997; Mangun and Buck, 1998 for reviews cf. Mangun, 2003; Hillyard et al. 1998; Hillyard and Anllo-Vento, 1998). As a first example, let us consider the findings from a type 1 paradigm (attend left vs. right hemifield) used in a study by Mangun et al. (2001). Stimuli were gratings of vertically oriented black and white stripes and were presented for 100 ms. Targets were slightly shorter than standards and appeared in 25% of all trials. All stimuli were randomly presented to the left or right hemifield. Subjects were instructed to respond to a target in the attended hemifield only. The results for standard stimuli are depicted in Fig. 1B and show that the P1 is larger for attended as compared to unattended (= ignored) stimuli. Most interestingly, however, this finding not only is obtained for recording sites over the contralateral, but also for the ipsilateral hemisphere. As is evident from Fig. 1B, the P1 is primarily modulated by attention and not by stimulus presentation. The attended hemisphere (see the ‘attend’ condition in Fig. 1B) shows a general larger P1, regardless whether this hemisphere receives a stimulus (contralateral, attend) or not (ipsilateral, attend). This fact is also manifested statistically as a main effect for attention (at temporo-parietal sites) with an absence of significant interactions with hemifield of presentation and hemisphere of recording for the P1.
The important finding here is the large ipsilateral P1 and the fact that the P1 is modulated by attention in the ipsilateral hemisphere in the same way as in the contralateral hemispheres. We argue that this finding suggests an inhibitory function of the P1 and conflicts with traditional interpretations. For the sake of clarity, we distinguish between three different hypotheses, which we term the (i) baseline, (ii) stimulus enhancement (or evoked), and (iii) inhibition hypothesis. The baseline hypothesis was suggested by Hillyard et al. (1998), Luck et al. (1994), and Luck and Hillyard (1995). The idea here is that relative to a neutral baseline (e.g., relative to a neutral cue) the P1 is not increased by attention, but suppressed in the unattended condition. This interpretation is interesting because it also assumes an inhibitory function of the P1 but in the sense that inhibition reduces the P1 amplitude. Or in other words, if irrelevant information must be suppressed, the P1 will be smaller as compared to a case where attention is focused on relevant information. The stimulus enhancement or evoked hypothesis predicts that the P1 is enlarged if the processing of a stimulus (which evokes an ERP-component) is enhanced by attention. If a stimulus is not attended, it still will elicit an evoked component, but the component will be smaller as compared to attended stimuli. The inhibition hypothesis – which will be introduced below – assumes that the P1 reflects inhibitory processes that have different functions in task relevant and task irrelevant neural structures. In the former, inhibition operates to increase the signal to noise ratio (SNR), in the latter inhibition operates to block information processing. The central prediction here is that inhibition increases the P1. The question, in which way inhibition shapes the P1 amplitude in task relevant and irrelevant neuronal structures is discussed in detail in Section 3.
The critical point now is the claim that the baseline and enhancement hypotheses will not be able to explain why a large P1 is generated at ipsilateral recording sites. Both interpretations appear plausible to explain the findings for the contra- but not those observed for the ipsilateral hemisphere. Because the ipsilateral side is not (directly) involved in the processing of the stimulus, the appearance of a large ipsilateral P1 in the unattended hemisphere is difficult to interpret in terms of the baseline and enhancement hypotheses. Both hypotheses would clearly have to assume that the ipsilateral P1 should always be smaller than the contralateral P1.
It could be objected, however, that the large ipsilateral P1 simply is an artifact which is due to volume conduction. Depending on the location and spatial orientation of a dipole, ERP components on the scalp will vary in amplitude size and/or polarity. Because volume conduction is extremely fast (operating at the speed of light), the peak latencies of the components must be identical for all recording sites. Inspection of Fig. 1B, however, clearly indicates that all ipsilateral P1 components are shifted in latency by about 5 ms. The extent of the latency shift is even more pronounced in the examples shown in Figs. 2 and 4, where the ipsilateral P1 components are delayed by about 20 ms or more. These findings are in good agreement with other studies showing that the delayed ipsilateral P1 must be modeled by a separate dipole that is clearly distinct from that which is used to model the contralateral P1 (cf. Di Russo et al., 2002).
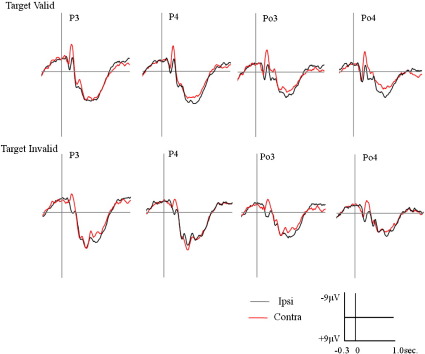
Fig. 2.
Results from a type 2 spatial cuing paradigm from Freunberger et al. 2008a. At the beginning of each trial, an arrow, pointing to either the right or the left, was foveally presented. Subjects were instructed to focus their attention on the cued hemifield. After an interval with a duration ranging between 600 and 800 ms (jittered between trials), a small or large white bar on black background was presented for 50 ms. Subjects had to indicate by button press whether the bar was small or large. Frequencies for small and large targets were 50%, and were equally distributed between the different experimental conditions. The intertrial interval was 2300 ms. A total of 1024 trials were run. In half of them, attention was cued to the right hemifield, and in the other half, attention was cued to the left hemifield. In 75% of the trials, cue and target location were congruent (valid condition), and in the remaining 25%, they were incongruent (invalid condition). Reprinted with permission.
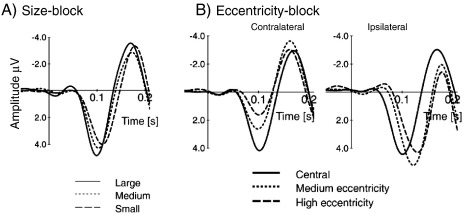
Fig. 4.
The P1 component (recorded at representative recording sites) is modulated by stimulus size (A) and eccentricity (B). Data are from Busch et al. (2004), and the figure is reprinted with permission. In the size-block the P1 amplitude decreases with decreasing stimulus size. In the eccentricity-block the P1 is larger for central (solid line) than for eccentric stimuli (dotted line: medium eccentric; dashed line: highly eccentric stimuli). Note large latency differences at ipsilateral recordings.
This remarkable finding of a large ipsilateral P1 appears to be even more pronounced in type 2 paradigms. The reason for this may be seen in the fact that in type 2 paradigms a cue directs attention to different locations on a trial per trial basis. Thus, the attentional top–down control may be more effortful (and require more inhibitory control) than in type 1 paradigms where over an entire run of trials attention remains focused on the same location. As an example for a type 2 paradigm, Freunberger et al. (2008a) found that P1 amplitudes are actually larger (and delayed) over ipsi- as compared to contralateral recording sites (cf. Fig. 2). In this experiment, targets were white bars on black background presented either right or left from the center of the computer monitor. Subjects had to indicate by a button press, whether the bar was small or large. Frequencies for small and large targets were 50% and were equally distributed to the different experimental conditions. In half of them attention was cued to the right and in the other half attention was cued to the left hemifield. In 75% of the trials, cue and target locations were congruent (valid condition) and the remaining 25% were incongruent (invalid condition).
2.2. Reflexive attention
Cue predictability is closely related to top–down control. High predictability enables focused, top–down controlled attention, whereas low predictability is associated with unfocused attention. If a cue is non-predictive, the P1 for cued and uncued locations is of equal magnitude (e.g., Hopf and Mangun, 2000) which means that top–down controlled attention is unfocused and equally distributed to cued and uncued locations. This phenomenon is used in experiments studying reflexive spatial attention where a non predictive cue is used to avoid top–down controlled attentional focus and to induce reflexive attention e.g., by the presentation of a target at the same location where the cue was presented. In these paradigms, the critical factor is the cue-to-target interstimulus (ISI) interval as e.g., a study by Hopfinger and Mangun (1998) revealed. At short ISIs (between about 50–250 ms) a target presented at the same location as the cue elicits a larger P1 than a target presented at the uncued location. At long ISIs (between about 550–750 ms), however, the opposite finding is observed: The P1 is smaller at the cued location.
Reflexive non-spatial attention can be studied by using targets with pop-out stimulus properties (e.g., color targets). Research reviewed by Taylor (2002) shows that pop-out targets generally elicit a larger P1 than non-pop out targets. In a similar way, Busch, Herrmann and colleagues have shown that stimulus size and eccentricity elicit a larger P1 (cf. Section 2.5). Hemifield preferences for object features may also be considered a special type of reflexive attention (cf. Section 2.3.1).
2.3. Object based attention
The recognition of an object is a fast process. It can be accomplished within a few hundred milliseconds. As an example, complex pictures (such as e.g., natural scenes) can be categorized with a median reaction time (RT) of about 380 ms (e.g., VanRullen and Thorpe, 2001a). As RT is a measure that comprises also the motor response, one interesting question is, when an object can be identified. This question can be investigated by determining the time, when the ERP waveforms for targets and non targets start to differ. Research by Thorpe et al. (1996) and VanRullen and Thorpe (2001b) have shown that differences between targets and non targets can be found reliably at around 150 ms. Other studies, however, found very early differences starting already about 50–80 ms (cf. the review in Rousselet et al., 2007). At least two factors are of importance here, object category and type of comparison. As an example, faces represent a category that may be processed particularly fast (cf. Thorpe et al., 1996). But also the type of comparison plays an important role. If targets and non targets are compared one has to consider the possibility that stimuli of the target and non target category (e.g. human faces vs. animal faces) may differ with respect to ‘low level’ physical properties. One way to tackle only object specific effects is to change the target status of the stimulus category. As an example, in counterbalanced blocks subjects are asked to respond to human faces (and to ignore animal faces) and then to respond to animal faces (and to ignore human faces). The calculation of task related differences between e.g., human faces as targets vs. non targets will now show differences that are object specific. By using such an approach, Rousselet et al. (2007) were able to demonstrate that for faces, object specific differences emerged already around 100 ms. Differences that may be due to low-level properties were observed even earlier, starting at about 60–80 ms. These findings are well in line with the hypothesis that early categorization takes place in the (extended) time window of the P1 component.
It should also be emphasized that the typical sequence of ERP components that can be observed for visual stimuli allows to make a similar conclusion. It is well documented that the P1 is not the first component in the visual ERP. It is preceded by the C1 component (with a latency of about 80 ms) that can be observed reliably when stimuli are flashed in different quadrants of the visual field and if a large number of trials are used for averaging. Source analyses and its strict retinotopic relationship indicate that the C1 is generated in the striate cortex around the calcarine fissure (Di Russo et al., 2002). This indicates that the P1 with a latency of about 100 ms is preceded by sensory specific processes (see also Foxe and Simpson, 2002). The P1 usually is followed by a negative component, the N1, with a latency of about 160 ms. Source analyses have indicated that the P1 is generated in extrastriate regions (e.g., Di Russo et al. 2002; Mangun et al. 1997) whereas the N1 (or N1-like components, such as the N200) which is associated with stimulus recognition or identification is generated at more anterior regions of the ventral pathway (e.g., Allison et al. 1999, 2002). Thus, the temporal sequence of the three ERP components is well in line with the hypothesis that the P1 reflects early stimulus categorization that precedes stimulus recognition or identification (reflected by N1-like or even later components; e.g., Doniger et al. 2000) but follows sensory processes (reflected by the C1).
In summarizing, the time course of processing visual information may be characterized by three consecutive time windows that are associated with different ERP components, sensory encoding (around about 80 ms), early categorization (around about 100 ms) and stimulus recognition (around about 150 ms). With respect to terminology, we will distinguish primarily between early categorization and recognition (or identification) in the sense that early categorization is a process that precedes and enables recognition (or identification). The meaning of recognition or identification depends strongly on the type of task. In a categorization task (e.g., in a go/no go task requiring the distinction of targets and non targets on the basis of global features) the terms categorization and recognition can be used synonymously because recognition may already be possible on the basis of global features. If, however, the analysis of very specific features is required, we will use the term stimulus identification instead of recognition.
2.3.1. Artificial and natural objects
Because spatially based attention effects on P1 amplitude are large and well documented, the experimental demonstration of object (feature) based attention effects requires a careful control of spatial location with respect to object information. In an elegant study with artificial objects by Valdes-Sosa et al. (1998) subjects had to judge one of two superimposed transparent stimuli, covering the same location in space. Thus, the ‘attentional spotlight’ (Posner, 1980) is focused at a single location where stimulus properties are varied. If attentional effects on the P1 amplitude can still be observed, these must be due to object based attention. The authors used two sets of dots with different colors that were varied with respect to coherent movement. In the 2-object condition both sets of dots were rotating, creating the impression of two moving, transparent surfaces, sliding across each other. In the 1-object condition one set was rotating, the other was stationary. Subjects had to perform an oddball task in which the target was defined by color of the dots and the direction of a linear, simultaneous displacement of one set of dots. At random intervals the rotating dots of one color were displaced in different directions for 150 ms. ERPs were recorded in synchrony with the onset of the displacement. The results showed a significant increase in P1 amplitude for attended (as compared to unattended) stimuli in the two (but not single) object condition, thereby demonstrating that P1 amplitude is modulated by object based attention.
The attentional ‘spotlight’ may be moved by top–down control processes but also by reflexive attention. This issue was investigated in a study by Handy et al. (2003) in order to demonstrate object-based attention effects on P1 amplitude. The logic of the experimental design refers to findings showing that the right (as well as the lower) visual field is dominant for the processing of (visual) object features that elicit object specific motor programs (object-specific motor features). Objects belonging to the tool category (as compared to non-tools) are particularly likely to activate this type of features (e.g., Danckert and Goodale, 2001; Kenemans et al., 2000). The basic prediction is the following: If a picture of a tool is presented at the right visual field, object-specific motor features direct the attentional spotlight also to the right hemifield. The validity of this prediction can be tested, e.g., by flashing a target stimulus to the same or opposite hemifield. If the spotlight is focused to the dominant hemifield, the target flashed in that hemifield should elicit a larger P1 than a target flashed to the opposite hemifield. To avoid the influence of top–down processes, the authors used an ‘incidental encoding’ paradigm. Subjects were presented a pair of objects (one in each hemifield) and were told to ignore these objects and to wait until a target (square wave grating) appears superimposed on one of the objects. They had to respond with a left or right button press indicating the spatial location of the target. The results clearly document the influence of object based attention on the P1 and show that amplitudes were larger for targets in the dominant (as compared to the non-dominant) hemifield, but only when the target was preceded by a tool in that hemifield.
These and related findings (cf. Nobre et al., 2006) are consistent with the hypothesis that the P1 reflects early stimulus categorization but not object identification or recognition (cf. e.g, Debruille et al., 1998). During this early stage of categorization global features are probably more important than specific features (such as e.g. verbal-linguistic features) that are analyzed in subsequent time windows (see e.g., the findings reported by Cristescu and Nobre, 2008, Ruz and Nobre, 2008).
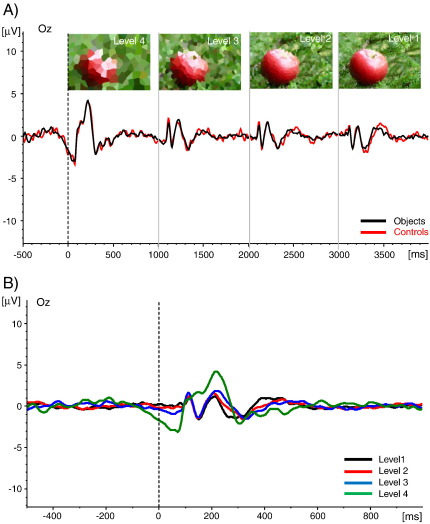
Finally, there is evidence that the appearance of a P1 is associated with the ability to recognize a stimulus. As an example, in a study by Freunberger et al. (2008b) a series of 4 pictures with decreasing levels of distortion (high, medium, low, and no distortion) was presented in each trial. Subjects had to indicate by a button press, when they recognized the object. The interesting finding, depicted in Fig. 3, was that the first of the four pictures (with high distortion) which never could be recognized did not elicit a P1. The P1 emerged, when object features were less distorted, thus, enabling early categorization and object recognition. Very similar – although non-significant – effects were obtained in a study with fragmented pictures by Doniger et al. (2000). The rather weak effects of this study are most likely due to the fact that subjects had to give a recognition response to each of the 8 pictures in a trial. Thus, subjects were probably not able to establish a continuous process mode that enhances the detection of gradually emerging stimulus features. In contrast, the study by Freunberger et al. (2008b) favored focus on early categorization because subjects were asked to respond as soon as possible during the stream of picture presentation.
Fig. 3.
(A) The experimental procedure of an object recognition paradigm and the ERPs elicited by each of 4 pictures presented in a single trial (Freunberger et al. 2008b). The pictures were obtained by distorting images from real objects or from meaningless objects (controls). Each picture was presented for 1 s. The subjects had to indicate, by a button press, whether they could recognize an object or not. The trains of pictures were presented from high to low levels of distortion. It was assured that subjects could recognize an object within level 2 but not earlier. (B) Same data as in (A) for the object condition, but ERPs for each level of distortion are shown superimposed. Note the lack of a P1 component for level 4 pictures which never could be recognized. The ERPs shown here represent unpublished data that were obtained from the study by Freunberger et al. (2008b). Reprinted with permission.
2.3.2. Faces
For the encoding of faces there is clear evidence that early categorization can be observed in the P1-latency range. As an example, Allison et al. (1999) observed larger P1-amplitude differences at occipital sites between different categories such as scrambled faces, checkerboards, butterflies or flowers. Most interestingly, these intracranial recordings demonstrated that the P1 is absent in areas of the fusiform gyrus, where the largest face specific N200 components were found (cf. Allison et al., 2002). These findings suggest again that early categorization is reflected by the P1-component (which is confined to occipital regions), and show in addition that object recognition takes place at a later time window and at more anterior regions of the ventral pathway.
One of the most robust findings is that scrambled and/or inverted faces (as compared to upright faces) elicit a larger P1 (e.g., Allison et al., 1999; Itier and Taylor, 2004; Linkenkaer-Hansen et al., 1998; Sagiv and Bentin, 2001) that in addition tends to be longer in latency (Linkenkaer-Hansen et al., 1998; Sagiv and Bentin, 2001; Taylor et al. 2001c). Object-based attentional effects (larger P1 for attended as compared to unattended faces) are also reported for faces (e.g., Gazzaley et al., 2008).
2.3.3. Words
Lexical decision tasks (requiring a word vs. non-word decision) allow the investigation of sensory-, syntactic- and semantic categorization processes. With respect to the P1 component, several studies have reported increased amplitudes with increasing orthographic neighborhood size (N), increasing word length, but decreasing word frequency, and decreasing orthographic typicality (e.g., Hauk and Pulvermüller, 2004; Hauk et al. 2006a,b; Segalowitz and Zheng, 2009; for a review, cf. Dien, 2009). According to Coltheart et al. (1977), N is a variable reflecting the orthographic relatedness of a letter string with words stored in memory. A large N indicates that many related words are stored in lexical memory. This most likely elicits competition/inhibition which increases processing complexity during early categorization of a letter string. This seems to be indeed the case as e.g., the results from Hauk et al. (2009) show. A very similar interpretation applies for the effects of word length, because it is plausible to assume that long words increase processing complexity. In a study where the effects of word length were studied by controlling for the negative correlation with word frequency, Hauk and Pulvermüller (2004) observed that long words produced a larger P1 than short words. An interesting aspect of the findings of Hauk and Pulvermüller (2004) is that the latency of the P1-word length effect was shorter than that for word frequency. This finding suggests that word length affects early graphemic search/categorization processes that precede those related to accessing the lexicon.
Thus, it appears that processing complexity affects the amplitude of the P1. If early categorization is difficult because processing complexity is high (for a large N and long words a large number of similar memory entries or features must be processed), the P1 tends to be large. A similar interpretation holds true for infrequent words and low orthographic typicality.
Another interesting finding is that the P1 for words and pseudowords usually is of similar magnitude (e.g. Hauk et al. 2006a; Khateb et al. 2002). This is not surprising, if we consider the fact that pseudowords are constructed to exhibit a similar orthographic ‘surface characteristic’ as real words and that the P1 reflects early categorization (related to graphemic–phonetic features) that precedes access to lexical memory.
2.4. Target properties investigated in search paradigms
Target-search paradigms clearly show that the P1 to the target stimulus is larger than the P1 to non-target stimuli (cf. the data reviewed by Taylor, 2002). This not only holds true for artificial stimuli (such as geometric forms, line-patterns or letters) but also for stimuli depicting natural objects (e.g., Batty and Taylor, 2002). Most interestingly the largest P1 is elicited for targets requiring a conjunction search as compared to targets that are characterized by single features, including color pop-out features (e.g. Taylor and Khan, 2000). Furthermore, the latency of the P1 for a conjunction search tends to be longer than those for single features (cf. Taylor et al. 1999, 2001a). Most surprisingly, however, is the finding that array size increases P1 amplitude but decreases latency. In a search paradigm in which array size was varied between 5, 9 and 17 items, the largest P1 and the shortest P1 latencies were found for the largest array with 17 items (Taylor et al., 2001b).
2.5. Perceptual features
Although the C1 component is primarily associated with perceptual encoding, research by Herrmann seems to imply that the P1 component also is a sensory component (Busch et al., 2004; 2006a,b; Fründ et al., 2007). We try to show here that the P1 is not modulated by physical properties per se but only if they are relevant for early categorization or if they elicit reflexive attention.
One important physical property that affects sensory processes is stimulus size. Due to the retinotopic architecture of V1, large stimuli are processed in large cortical areas and small stimuli in small areas. If an electrophysiological parameter is directly affected by stimulus size, it appears save to conclude that it is directly modulated by physical stimulus properties. As an example, let us consider the study by Busch et al. (2006b) who used abstract stimuli that consisted of two areas, a small circular center and a large surrounding. In keeping global stimulus size identical (the center area plus the surrounding area is of the same size for all stimuli), two experimental conditions with a small target (consisting of the center area) and a large target (consisting of the surround area) were compared. In different blocks of trials either the center or the surround area was the target stimulus. In both conditions targets were defined by the orientation of one of two possible gratings. Thus, in both conditions, targets were defined by the same physical property. Busch et al. (2006b) observed that target size did not affect P1 amplitude size. Large and small targets elicited P1 amplitudes of identical magnitude.
In a simple object (square vs. circle) discrimination task, Busch et al. (2004), however, found that P1 amplitude increases with increasing stimulus size, decreases with increasing eccentricity (stimuli presented more laterally elicit smaller P1 components) but is unaffected by exposure duration. The respective findings – as depicted in Fig. 4 – show in addition that for eccentric stimuli, P1 amplitudes are much larger over ipsilateral recording sites. Is this unequivocal evidence that the P1 is a sensory ERP component? Let us first consider the effect of stimulus size. In contrast to Busch et al. (2006b) overall stimulus size was not kept constant. The size of the targets varied from trial to trial. Stimulus size might not only be considered a pure physical property. A large object (e.g., a large animal) may be more important (and potentially e.g., more dangerous) than a small object. A very similar argument may hold true for eccentricity. A more laterally presented object may tend to elicit an orienting response (e.g., an eye movement toward the object). The argument here is that some global physical stimulus features (such as size, eccentricity and color) may already represent a ‘pop-out’ characteristic that elicits reflexive attention and a larger P1.
Another interesting question is the following: What happens when two stimulus categories are very similar (or even identical) at the level of global stimulus features (such as spatial frequency, size, contrast, orientation and second order image statistics) and differ primarily (or even only) at the level of specific features? As an example let us consider the study by Busch et al. (2006a) who used color pictures of familiar, natural objects and unfamiliar ‘nonsense’ objects as targets and non-targets respectively. Unfamiliar objects were obtained by distorting the images of natural objects in a way that spatial frequencies were matched. This resulted in unfamiliar pictures having a very similar ‘stimulus-surface’ as familiar objects with respect to color and figural elements. The interesting finding of this study was that P1 amplitude differences between familiar and unfamiliar objects were abolished. This finding is consistent with the suggested hypothesis that the P1 reflects early categorization which is based on global stimulus feature. If global stimulus features are very similar between the respective stimulus categories, the P1 amplitudes will also be of similar size. The earlier discussed findings from Busch et al. (2006b) allow for an even more straight forward interpretation. Large and small targets were defined on the bases of the same stimulus property (orientation of the grating). Despite differences in target size, P1 amplitudes were identical in amplitude size.
It should also be emphasized that behaviorally significant changes in P1 can be observed that are independent of stimulus features. As an example, in a speeded reaction time task, Fründ et al. (2007) observed significant changes in P1 amplitude sizes, although the same stimulus (a black square) was presented in all trials. Subjects were instructed to respond with a button press as quickly as possible. To keep them motivated, they received feedback about response latency. Trials were sorted with respect to response speed. P1 amplitude was significantly larger in trials with short response latencies. The interpretation is that fluctuations in attentional top down control during stimulus perception underlie the observed differences in P1 amplitude.
Finally it should be mentioned that hemifield location also is a variable that affects P1 amplitude. It has been shown that stimuli presented in the upper hemifield (above fixation) elicit much larger P1 amplitudes than those presented in the lower hemifield (e.g., Gunter et al. 1994). These and related findings (see also Section 2.3.1 and e.g., Danckert and Goodale, 2001; Handy et al. 2003; Kenemans et al., 2000) suggest that different hemifields are dominant for and interact with the processing of different stimulus features.
2.5.1. The P1 is not a sensory evoked component
In the preceding section, it was argued that the P1 is not affected by stimulus properties per se. In other words, the assumption is that the P1 is not a sensory evoked component. But what are the defining properties of a sensory evoked component? Here, two properties are emphasized. A sensory evoked component is generated in response to a stimulus by a (i) feed-forward, bottom-up process, that is (ii) primarily of excitatory nature. A variety of more recent findings obtained with voltage sensitive dyes emphasize the existence of feed-forward, excitatory processes in V1 and complex feedback activation processes between V1 and ‘higher’ cortical regions. The interesting point here is that feedback to V1 is evident already at (but not before) about 100 ms poststimulus (for a review, cf. Roland, 2010). These findings suggest that in the cortex, excitatory feed-forward processes dominate in a period of up to 100 ms, whereas a complex interplay between feed-forward and feedback activation processes (occurring in parallel) characterizes the time period beyond 100 ms. Based on these findings, we suggest that sensory evoked processes can be considered excitatory neuronal activation processes that dominate in a period of up to about 100 ms poststimulus.
It was already emphasized that the large ipsilateral P1 that is observed in spatial cueing tasks most likely reflects an inhibitory process. A large component appearing over task irrelevant brain regions is not what one would expect for an excitatory, sensory evoked component. In the next sections we will provide further evidence for the assumption that the P1 component reflects inhibitory processes. If this assumption can be validated, this would provide strong evidence against the view that the P1 is sensory evoked. The reason is that an evoked component can hardly be considered inhibitory of nature. As a working hypothesis, it is suggested that the P1 reflects an inhibitory feedback wave from ‘higher’ cortical areas that operates as an inhibitory filter to control feed-forward sensory processes.
3. The P1 inhibition timing hypothesis
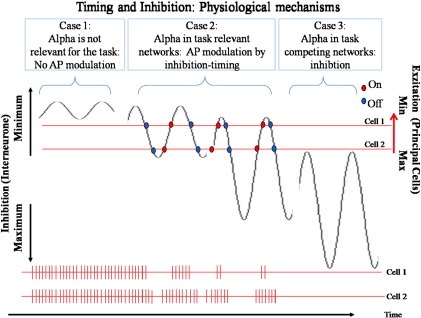
The aim here is to explain the functionality of the P1 on the basis of the inhibition timing hypothesis, which we have applied for the interpretation of alpha oscillations (Klimesch et al. 2007a,b,c). If the amplitudes of an inhibitory oscillation (e.g., an oscillation, generated by inhibitory interneurons) are increased, the time window, in which action potentials (APs) are elicited in target cells, becomes increasingly smaller. Thus, with an increase in amplitudes of an inhibitory oscillation, the timing of excitatory activity becomes more precise. The critical issue here is the assumption that inhibition may increase up to a point where the generation of APs is completely blocked. Such a process cannot be explained by a further increase in amplitudes, because this will only narrow the time window for excitation but will never completely block the generation of APs. Thus, some additional process is necessary to explain blocking of information processing. One possibility lies in the assumption that an increase in amplitudes is accompanied by an increase in firing threshold. Thus, we assume two different types of inhibitory processes. Phasic inhibition modulates the generation of APs in a way that only cells with a very high level of excitation are still able to fire. This may be considered a mechanism that controls the signal to noise ratio (SNR) in task relevant networks. In contrast to phasic inhibition, tonic inhibition leads to a complete blocking of firing. This mechanism is not useful to control information processing in task relevant networks. It is, however, a very efficient mechanism to silence activity in potentially interfering, competing and task irrelevant networks.
The central idea is that the P1 reflects inhibition that is used to control activity in two different neuronal structures, task-relevant and task irrelevant ones. In task relevant structures inhibition is used to increase the SNR during early categorization by enabling precisely timed activity in neurons with a high level of excitation but silencing neurons with a comparatively low level of excitation. As an example, for spatial attention paradigms, the assumption is that inhibition operates to increase the SNR in the contralateral hemisphere only, whereas inhibition is used to block information processing in potentially competing regions of the ipsilateral hemisphere.
Inhibition shapes the P1 component on the basis of three variables, alpha amplitude, phase locking and polarity. A large amplitude with little jitter between trials (reflecting a large extent of phase reorganization or phase locking) and with a polarity that is associated with the inhibitory phase (this most likely is the cycle with positive polarity) is assumed to reflect a high extent of inhibition. The basic assumption, illustrated in Fig. 5A is that the P1 reflects an inhibitory filter (established synchronously in a parallel distributed network) during early categorization that is generated to enhance stimulus processing by increasing the SNR in task relevant networks. For potentially competing networks the P1 reflects the blocking of information processes.
Fig. 5.
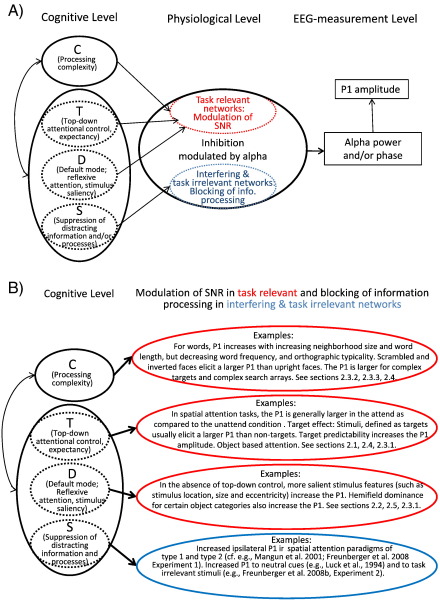
(A) Basic assumptions about the interrelations between cognitive and physiological processes, and the way they affect electrophysiological correlates and measures. Two classes of cognitive processes are considered. One relates to the task specific processing of a stimulus affecting processing complexity (C), the other to different attentional processes, top–down attentional control (T), default mode attentional control (D), and suppression of distracting information and/or processes (S). All of these processes interact in a complex way. In experimentally well controlled tasks, some of these variables can be varied, whereas others can be kept constant. As an example for C, orthographic neighborhood size (N), and word length may be considered variables that directly affect processing complexity. For attentional processes, a pop-out color target may be considered an example affecting D, the focused search for a complex target lacking pop-out features may be considered an example affecting primarily T, whereas the processing of a distractor item may be considered an example for S. With respect to physiology, the central assumption is that inhibition increases the SNR in task relevant networks – by silencing many but the most excitatory cells – and thereby allows for a more selective processing. In interfering/competing networks, however, inhibition is further increased and is used to block information processing. On the measurement level an increase in inhibition is reflected by an increase in alpha amplitudes. The extent of inhibition may very well depend on the extent of excitatory processes in task relevant as well as in potentially interfering networks. The critical prediction for the generation of the P1 component is that it is directly affected by an increase in alpha power and/or phase locking. (B) Overview of findings demonstrating the influence of processing complexity (C) and different attentional processes (T, D, and S).
Inhibition (and the size of the P1) is modulated by different cognitive processes that depend on task demands. Two classes of cognitive processes are considered. One relates to the task specific early categorization of a stimulus affecting processing complexity (C), the other to different attentional processes, such as top–down attentional control (T), default mode attentional control (D), and suppression of distracting information (S). All of these processes interact in a complex way. Nonetheless, in experimentally well controlled tasks, some of these variables can be varied, whereas others can be kept constant. The experimental variation of attentional processes is a typical characteristic of tasks that are used to investigate the P1. Spatial cuing paradigms are a good example. According to our hypotheses, two different processes, T and S are of primary importance in this type of tasks. In type 1 tasks, T is experimentally manipulated by instructing subjects to attend to the left or right hemifield. In type 2 tasks, T is varied by the cue and its validity. T establishes a top–down control process that operates to increase SNR in task relevant networks. In contrast, S is a process that blocks information processing in interfering networks. Thus, attentional benefits – associated with the influence of T – and attentional costs – associated with the influence of S – are both due to an increase in inhibition which leads to an increase in P1 amplitude. The difference between T and S is seen in different inhibitory processes that operate in task relevant vs. interfering networks (cf. Fig. 5A). Attentional processes are not the only class of cognitive processes that affect the P1 component. Processing complexity (C) during early stimulus categorization is another important cognitive process that shapes the P1. As an example, orthographic neighborhood size (N), and word length may be considered variables that directly affect C. A pop-out color target search may be considered an example affecting D, the focused search for a complex target lacking pop-out features may be considered an example affecting primarily T, whereas the processing of a distractor item may be considered an example for S.
3.1. A new interpretation of old findings
In this section we apply the proposed theory particularly to those findings which are difficult to interpret in terms of stimulus evoked activity or on the basis of an enhancement hypothesis. An overview over the findings reviewed in Section 2 and their interpretation on the basis of the P1 inhibition timing hypothesis are presented in Fig. 5B.
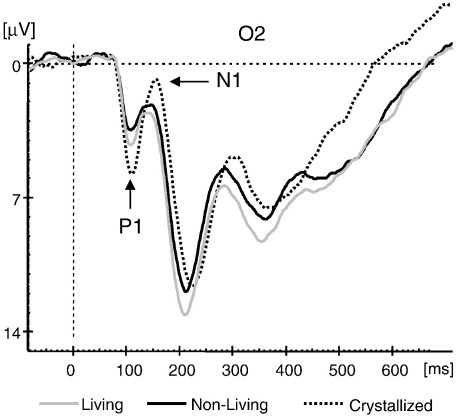
The central prediction of the proposed theory rests on inhibition and on the idea that suppression of task irrelevant and potentially competing information and or neural structures leads to a particularly large increase in the P1 amplitude. Under controlled conditions this suppression related increase will be at least as large or larger than for task relevant processes where inhibition is used to increase the SNR. As a first example let us consider the finding of a large ipsilateral P1 amplitude. We assume that the increased ipsilateral P1 reflects inhibition of task irrelevant and potentially competing processes. Thus, successful suppression of distraction (S) helps to maintain a focused top–down processing mode (T) at contralateral brain regions that enhance information processing. An increased P1, can also be found during recognition of task irrelevant information. As an example let us consider Experiment 2 in the study by Freunberger et al. (2008a). The experiment consisted of a semantic (living/non-living) picture categorization task with meaningful and meaningless pictures. Meaningful pictures represent living, and non-living objects. Meaningless pictures were obtained by distorting pictures of living and non-living objects. We predict that the P1 will be larger for distorted pictures because they can be considered task irrelevant with respect to semantic categorization. Thus, this prediction also focuses on inhibition, but not in the sense of suppressing activity in potentially interfering brain regions, but in the sense of suppressing task irrelevant processes. Distorted pictures (with no semantic meaning) may very early (on the basis of their sensory features) be categorized as semantically meaningless which allows suppression of irrelevant ‘spreading activation processes’ aiming at identifying the stimulus. The findings are in line with this interpretation and show that the P1 for meaningless pictures is delayed and significantly larger than for the ‘task- or processing-relevant’ pictures denoting living and non-living objects (cf. Fig. 6). Most importantly we could also show that the alpha-filtered ERPs exhibit differences in the P1 range that are similar to those of the unfiltered ERPs. Finally, it should be mentioned that in go/no go tasks, where only one type of stimulus must be attended and processed, the P1 will be larger for the go- as compared to the no go-stimulus. (e.g. Rousselet et al., 2007).
Fig. 6.
ERPs for the (living/non-living) semantic categorization task used in Experiment 2 by Freunberger et al. (2008a). Although meaningless, distorted (crystallized) pictures were characterized by a pronounced P1–N1 complex. Reprinted with permission.
Another interesting finding, well in line with our theory is that increasing processing complexity (C) during early categorization is associated with an increase in P1 amplitude. Particularly for faces the inversion of an image has a strong effect on task difficulty. Thus, the increased P1 in response to inverted but also scrambled faces (e.g., Allison et al., 1999; Itier and Taylor, 2004; Linkenkaer-Hansen et al., 1998; Sagiv and Bentin, 2001) can indeed be associated with increased processing demands during early categorization. A very similar interpretation can be applied for the encoding of words or pseudowords. Increased P1 amplitudes were found with increasing orthographic neighborhood size (N) and increasing word-length (for a review, cf. Dien, 2009). According to our hypothesis processing complexity (C) would be high in both cases leading to an increase in SNR that operates to select specific entry points into lexical memory. As a consequence, ERP amplitudes increase in the latency range of the P1. In contrast to neighborhood size and word length, word frequency and orthographic typicality decrease P1 amplitude (Hauk et al. 2006a,b). According to our interpretation, for common and typical words, processing complexity C is low and, thus, P1 amplitude is small.
Findings from target search paradigms are also well in line with the influence of C. The difficult conjunction search elicits a larger P1 than the much easier pop-out search which is associated with D. Both processes, C and D lead to a modulation of SNR in task relevant networks (for a discussion of theoretical considerations see e.g., Navalpakkam and Itti, 2007), but the more difficult of the two processes has a stronger effect on SNR and hence on the size of the P1 amplitude. Another interesting finding is that the P1 is larger for large search arrays which are more difficult to process than small search arrays.
3.2. Evidence for a direct relation between ongoing alpha oscillations and P1 amplitude
Several properties of the P1 show similarities with alpha oscillations. As an example, the latency of the P1 (of about 100 ms) corresponds to the length of the alpha period which is 100 ms for a typical alpha frequency of 10 Hz. More specifically, P1 latency is significantly correlated with individual alpha frequency (Klimesch et al. 2004), and alpha phase locking is largest in the time window of the P1 (Klimesch et al. 2004). Furthermore, alpha power predicts the size of the P1 amplitude (Freunberger et al., 2008a) and significant phase alignment of alpha oscillations predicts P1 latency (Gruber et al. 2005). Finally, under certain task demands, latency differences in the topography of the P1 can be explained by traveling alpha waves (Klimesch et al. 2007c).
It is important to emphasize here that phase reorganization appears as a necessary and logical consequence of an oscillation theory (cf. Klimesch et al. 2007b for an extensive discussion of this issue). If it is assumed that oscillations play an important role for the timing of sensory and cognitive processes this basic function must be evident also during the event-related response and phase reorganization is an obligatory consequence to avoid the potential problem that a stimulus may fall in the unfavorable phase of an oscillatory cycle.
It also should be mentioned that the influence of alpha on the ERP is not limited to early components, such as the P1. There is empirical evidence that baseline shifts of alpha (cf. Nikulin et al., 2007) and asymmetric alpha amplitude modulations (Mazaheri and Jensen, 2008) have a strong influence on slow evoked responses.
In the following, we discuss findings that document a complex relationship between ongoing alpha and the P1 component. We focus on two different aspects. One aspect emphasizes the cognitive-functional relationship between alpha and the P1, and the other focuses on quantitative and physiological aspects.
3.2.1. The functional relationship between ongoing alpha and P1 amplitude
Before we start to consider a quantitative relationship between ongoing alpha and P1 amplitude it is important to emphasize that prestimulus alpha power is predictive for good memory and perceptual performance. For memory performance, we have shown that large resting or prestimulus alpha power is positively associated with performance (Doppelmayr et al., 2002; Klimesch et al., 2000; Vogt et al., 1998) whereas during actual task performance, small power (large event related desynchronization or ERD) is related to good performance (e.g., Doppelmayr et al., 2005; Klimesch et al., 1997). Most interestingly for perceptual performance (in tasks target detection under threshold or near threshold conditions), small prestimulus alpha power (Ergenoglu et al., 2004) and a small ERD or even event related synchronization (ERS) during actual task performance (Hanslmayr et al., 2005) is predictive for good performance. A variety of studies have meanwhile documented that a state of low prestimulus alpha power is associated with improved detection and discriminability of threshold-level stimuli (Hanslmayr et al. 2007a; Mathewson et al. 2009; Romei et al. 2007, 2008; Van Dijk et al. 2008).
There is, thus, good evidence for a double dissociation between pre- and poststimulus alpha power and the type of cognitive performance. Good memory performance is associated with large prestimulus but small poststimulus alpha power, whereas good perception performance is related to small prestimulus power with little or no ERD during perception performance. We have interpreted these findings in terms of cortical inhibition and excitation preceding task performance. Perception performance appears to be enhanced if the cortex already is activated (as indicated by small prestimulus power), whereas memory performance is enhanced if the cortex is not activated (as indicated by large prestimulus power) before a task is performed. This interpretation is quite plausible if we assume that for visual target detection a high level of cortical excitation will be helpful to analyze a visual input. When a specified and well known target must be detected, memory traces are probably ‘preactivated’ and as a consequence inhibition must be reduced. For memory performance, on the other hand, an initial (prestimulus) activation of the cortex may be detrimental because it may interfere with (or even suppress) the high selectivity that is required for accessing a memory trace during actual task performance.
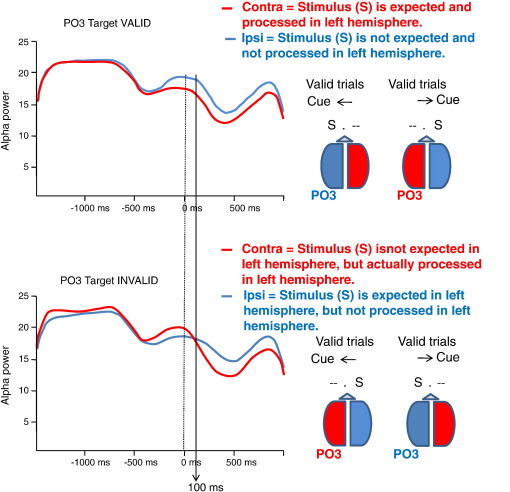
In considering these findings and their interpretation, let us now make predictions for a traditional spatial cuing task in which a target must be detected in the right or left visual field. The prediction for prestimulus alpha power at the contralateral side is a decrease in power, whereas for the ipsilateral side, we expect an increase in power. Because the functional meaning of the P1 amplitude is similar to that of ongoing alpha, we also expect a larger ipsilateral P1. We have tested this prediction in Experiment 1 of the study by Freunberger et al. (2008a). As observed in other studies (e.g., Busch et al. 2004), we also found that the P1 is larger over ipsi- as compared to contralateral recording sites. In our study (using a type 2 paradigm with a jittered ISI between cue and target; cf. Freunberger et al. 2008a) this difference was highly significant. Most importantly, however, we also observed that alpha power is significantly larger over ipsi- as compared to contralateral recordings. In contrast to the P1 which is interpreted as evoked alpha activity (that is short and transient), alpha power can be monitored over the entire time course of a trial. According to our hypothesis that alpha reflects (functional) inhibition, we expected significant differences during the poststimulus period that are due to the side where the target is presented. In general, ipsilateral alpha power should be larger than contralateral alpha power. For the prestimulus period, we expect differences in alpha power that are induced by the cue. In invalid trials subjects will expect the target at the ‘wrong’ location. Thus, for invalid trials, the target is expected in the ipsilateral hemisphere (with respect to actual target presentation) and hence the power in the contralateral hemisphere will be larger. As an example, if in the invalid condition the cue points to the left hemifield, we expect larger prestimulus alpha power over the left hemisphere which is contralateral to the actual target presentation. Thus, for the prestimulus period, we expected larger power over ipsilateral sites in the valid condition but larger power over contralateral sites in the invalid condition. This expected pattern of results could be confirmed statistically and is illustrated in Fig. 7. It should be noted that around 100 ms poststimulus (cf. the black vertical line in Fig. 7) there is the ‘crossing point’ (and, thus, no power difference) between invalid ipsi- and contralateral upper alpha power. Beyond that time, ipsilateral alpha power increases, whereas contralateral power decreases, indicating most likely the (delayed) reorientation of attention to the hemifield where the target appeared.
Fig. 7.
Time course of upper alpha power at Po3 for valid and invalid trials plotted separately for ipsilateral and contralateral target presentations. Note the ‘crossing point‚’ between ipsilateral and contralateral presentations. For a discussion of the findings, see text. Data are from Freunberger et al (2008a). Reprinted with permission.
Indirect evidence for a positive relationship between alpha power and P1 amplitude comes from research about schizophrenia and frontal lobe dysfunction. The prefrontal cortex is considered to play an important role for the inhibition of irrelevant information and the modulation of the P1–N1 complex in attentional cuing paradigms. Studies with schizophrenic patients have shown reliably that resting EEG is characterized by diminished alpha power and increased theta and delta power (e.g., Itil et al., 1972, 1974; Miyauchi et al., 1990; Sponheim et al., 1994, 2000). Sponheim et al. (2000) have demonstrated that even within a group of schizophrenic patients, diminished alpha power is related to increased negative symptomatology and deviant brain morphology. Haenschel et al. (2007) observed that during the encoding of items in a memory scanning task, the P1 is decreased in schizophrenic patients, but increases with load in healthy control subjects. Thus, it appears plausible to assume that the difficulties schizophrenic patients have with inhibiting irrelevant information is reflected by diminished alpha power and a reduced P1.
3.2.2. Quantitative relationships between ongoing alpha and P1 amplitude
When trying to establish that a certain quantitative relationship between ongoing alpha and P1 amplitude exists at least two different aspects must be considered. On the one hand, task type – as described in the previous section – changes the direction of poststimulus reactivity in a complex but predictable way. On the other hand, if early evoked responses are generated/influenced at least in part by ongoing alpha, P1 amplitude will not only depend on alpha power but also by the extent of phase locking of ongoing alpha activity. As a consequence, any simple prediction in the sense that the P1 will be positively or negatively related to prestimulus power must fail if the functionality of alpha (depending on the type of cognitive demand) and the extent of phase locking are ignored.
A good example, demonstrating this problem, is the issue of phase reset. If the influence of task type is not considered, a positive relationship between ongoing oscillatory activity and the amplitude of the evoked response is predicted. The central hypothesis then is that ongoing oscillatory activity simply resets the phase to a certain value (e.g., to the positive peak) in response to the presentation of a stimulus. Thus, if a positive relationship between the amplitude of ongoing oscillatory activity and the amplitude of the evoked response cannot be observed, this is taken as evidence against phase reset (cf. e.g., Becker et al. 2007). Although there is good evidence for phase reset (e.g., Fell et al., 2004; Hanslmayr et al. 2007b; Lakatos et al., 2005), a proof is very difficult because of methodological reasons (for critical reviews see Sauseng et al. 2007; Klimesch et al. 2006). It is important, however, to emphasize that phase reset is only one and a very specific mechanism that can be derived and predicted from an oscillatory ERP model (for a review see Klimesch et al 2007b). Other mechanisms are e.g., evoked oscillations (i.e. an oscillation is elicited by stimulation), prestimulus phase alignment or any type of the influence of (peristimulus) phase on ERPs and performance. In agreement with this notion, several studies have shown that the phase of ongoing alpha oscillations has an influence on ERPs and on task performance (for more recent studies see e.g., Busch et al. 2009; Busch and VanRullen, 2010; Mathewson et al., 2009; Makeig et al. 2002; Lakatos et al. 2008). In addition, it has also been demonstrated that increased alpha phase locking is associated with good performance (e.g., Klimesch et al. 2004; Yamagishi et al. 2008).
The conclusion, thus, is that the investigation of a quantitative relationship must be based on at least the following two requirements, the control of task type and phase. The latter is difficult, but can be based on the following considerations. If a task requires that attention operates in a rhythmic mode (Schroeder and Lakatos, 2009) which is the case in tasks with predictable stimulus occurrence (e.g., in tasks with a fixed – and not jittered – cue to target ISI) anticipation (as reflected by phase locking) may be considered an important factor for task performance. If, however, the processing of a stimulus is not predictable phase locking should be less important and the evoked response should be more dependent on the amplitude of ongoing phase. In proceeding from these considerations, Rajagovindan and Ding (2010) have demonstrated (for a traditional spatial cuing task) that an inverse U-shaped function defines the quantitative relationship between prestimulus alpha power and P1 amplitude. The interesting fact thereby is that the trial to trial fluctuations of prestimulus alpha power are directly related to P1 amplitude in a quantitatively predictive way. The inverse U-shaped function indicates that P1 is largest for a medium level of prestimulus alpha power and smallest either for a very high or low level of alpha.
For our hypothesis the findings of Rajagovindan and Ding (2010) are of great interest, because they possibly document the operating range of the control of the SNR, as described in Section 3. But the control of the SNR should be effective only for task relevant networks. Indeed, the inverse U-shaped function was found only for attended items in the contralateral hemisphere. For unattended items in the ipsilateral hemisphere the function (between alpha power and the P1) was a flat line. According to our model at ipsilateral sites, alpha and P1-amplitude are increased to a level that enables the blocking of information processing. Thus, there is no modulation of SNR and hence no U-shaped function describing the relationship between alpha power and the P1. Finally, we should mention that in the study by Rajagovindan and Ding (2010) the ipsilateral P1 was not larger than the contralateral P1. This may be due to differences in task demands and the level of excitation in task irrelevant networks. The reason for this consideration is that a certain level of inhibition allowing blocking of information processing may depend on the level of excitation in that network.
3.2.3. Dissociating the influence of alpha power and phase locking on P1 amplitude size
The influence of oscillatory amplitude and phase can be estimated by calculating power and phase locking (e.g., by the phase locking index, PLI cf. Schack and Klimesch, 2002). Increasing power and increasing PLI (decreasing jitter between trials) are capable of increasing the amplitude of an ERP component.
In a recent study we tried to dissociate the influence of these two factors on P1 amplitude size (Freunberger et al. 2009). The basic idea was to use a cue in order to induce a power change that precedes the processing of an item. In a memory scanning task each item of the memory set was preceded by a cue that indicated either to remember or to ignore the next following item. As earlier performed studies (e.g., Klimesch et al. 1999) indicated, a processing mode that is related to the suppression of potentially competing items should lead to an increase in power. On the other hand, a cue indicating that the next item must be remembered should not induce an increase in power, but instead elicit an increase in phase locking possibly reflecting a precise timing in distributed, task-relevant networks. This assumption is based on findings, showing that increased phase locking is associated with an increased probability that an item will later be remembered (Bäuml, et al. 2008, Klimesch et al. 2004). Freunberger et al (2009) could indeed show that the ignore cue elicited an increase in alpha power preceding the presentation of the following item. Most interestingly, despite this increase in alpha power, the P1 was smaller for the ignored items as compared to the to-be-remembered items. On the other hand, phase locking as measured by the PLI was significantly larger for the remembered items. Furthermore, we found that the ratio of the PLI for to-be-remembered vs. not-to-be-remembered items was significantly correlated for alpha but not theta. This finding also suggests that alpha phase locking modulates the P1 component for the to-be-remembered items.
3.3. Predictions
The proposed theory has several consequences for physiological and cognitive processes that can best be described in terms of predictions. One important prediction with respect to physiology is that inhibition leads to the blocking of information processing in task irrelevant and potentially interfering neural structures. It is, however, not clear in which way an oscillation is capable of doing that. One possibility would be to predict a baseline shift as is illustrated in Fig. 8. Another – probably even more interesting – possibility would be to predict that alpha plays a role for phase coding, as was suggested by Nadasdy (2010) for fast frequencies in the gamma range. The central idea is that topographical phase differences in traveling waves code information. A stationary wave, characterized by a lack of topographical phase differences, will not be able to code information but would lead – via spatial summation – to a large amplitude at a scalp electrode.
Fig. 8.
The proposed influence of alpha – as an inhibitory oscillation – on neuronal communication. Three cases are distinguished. Case 1 characterizes a situation in which alpha is not task relevant. The amplitude is small and has no impact on the firing rate which is symbolized by short vertical lines representing action potentials (APs) in the lower panel of the figure. If alpha is task relevant (cf. Case 2), the amplitude increases and starts to inhibit the generation of action potentials (APs) in target cells during the inhibitory phase of the oscillation. Depending on the excitation level of target cells, the impact of the inhibitory oscillation may be small (cf. Cell 2) or large (cf. Cell 1). With an increase in amplitudes, the inhibitory baseline increases, which means that inhibition increases not only during the phases with maximal inhibition (here plotted as troughs) but also during those with minimal inhibition (here plotted as peaks). Case 3 characterizes a situation where inhibition increases further, leading to a complete silencing of target cells. This case is assumed for task irrelevant and potentially competing neuronal structures.
Another important prediction, linking physiological and cognitive processes, is that the P1 amplitude should exhibit topographical phase differences that can be explained by a traveling alpha wave. There are two reasons for this prediction. First, we have assumed that alpha reflects a basic processing mode that controls the flow of information into the brain (Klimesch et al. 2007a,b). Second, this flow of information is associated with early categorization processes in a time window that follows sensory processes and precedes stimulus identification. It is plausible to assume that this process can be described as a spreading activation process from the primary visual cortex to parietal and/or temporal cortices (cf. Klimesch et al. 2007c).
A third prediction can be made stating that the P1 is not a ‘component’ in the traditional sense, but instead is manifested ‘only’ as peak (or trough) in a certain time window and is part of a wave comprising also earlier and later ‘components’. Whether, or not the earlier and later components correspond to the C1 and N1 remains an open question, but there is some empirical evidence for this view (Klimesch et al. 2007c).
Finally, these considerations clearly suggest that neither the P1 nor alpha can be considered a unitary phenomenon. They largely depend on topography, task demands and other factors. This view is well in line with studies showing that pre- and poststimulus alpha depend on each other in a complex way, as e.g., Van Dijk et al. (2008).
4. Discussion
The P1 is responsive to a variety of different task demands, such as e.g., attention to spatial location, target predictability, stimulus saliency, and category specific hemifield dominance. Thus, a simple interpretation of the cognitive functionality of the P1, e.g., in the sense that it reflects ‘early attentional processes’ is hardly possible. A good example is the study by Handy et al. (2003) which found a larger P1 for items belonging to the tool category (as compared to non-tools) in the dominant (as compared to the non-dominant) hemifield even in an incidental encoding paradigm in which subjects were instructed to ignore the meaning of the presented items. Another example is the finding that the P1 may be larger for items that are task irrelevant (e.g., Freunberger et al. 2008b). These findings rule out the possibility to interpret the P1 on the basis of a stimulus enhancement hypothesis reflecting the facilitating influence of early attentional processing.
It is also not possible to explain the functionality of the P1 in terms of a stimulus evoked component. The findings reported by Mangun et al (2001) are particularly impressive because they show the same magnitude of P1 modulation in the contralateral and ipsilateral hemispheres as well. Likewise, in a speeded reaction time task, Fründ et al. (2007) were able to show that – for the same stimulus – the P1 amplitude was significantly larger in trials where subjects gave a fast response. Finally, the ERP lacks a P1 in cases where an expected stimulus cannot be recognized (cf. the ERP to highly distorted pictures in Fig. 3).
Here, we have argued that the P1 reflects inhibition that is needed to filter out relevant stimulus features in task relevant networks and to block information processing in potentially competing and task irrelevant networks. The argument is that this inhibitory filter is used to enable early stimulus categorization by establishing ‘access routes’ to information stored in a complex KS. According to our interpretation, one crucial assumption is that inhibition comprises two different aspects. One aspect relates to the modulation of the SNR in task relevant networks, another to the blocking of information processing in competing and task irrelevant networks or brain regions. The attentional modulation at contra and ipsilateral brain sites can easily be explained on the basis of this assumption, but not e.g., on the basis of a baseline hypothesis that was used to interpret early attentional effects on the P1 (cf. Section 2.1).
Another important aspect of the presented hypothesis is that the functionality of alpha is very similar to those of a ‘default mode system’ (as suggested by Raichle et al. 2001) which plays an important role for consciousness. This system provides us with the very basic function to monitor the world around us and to continuously update semantic information. It enables us to be ‘semantically’ oriented in a constantly changing environment. It represents the meaning of objects surrounding us and events we encounter. Or put in other words, it allows us to be oriented in time and space. In a similar way as alpha, the default mode system is not associated with working memory demands. However, activation of the working memory system would require resources of the default mode system (see e.g., Scheeringa et al. 2008), but not vice versa. Based on these considerations, the P1 is the event-related manifestation of ongoing alpha oscillations. It is interesting to note that in the visual domain, eye fixations play an important role for monitoring the world around us and that in response to the onset of a saccade a large P1 can be observed (by using the method of fixation related potentials) that apparently is modulated at least in part by alpha oscillations (Ossandon et al., 2010).
One critical aspect of our hypothesis is that the P1 is generated at least in part by alpha oscillations. It is important, however, to emphasize that this assumption does not necessarily depend on a phase reset. The controversy between the evoked and phase reset model for the generation of early ERP components has unnecessarily narrowed and focused the potential influence of oscillations on ERPs by considering only one and highly specific mechanism, namely phase reset. There are different mechanisms other than phase reset that may have an important influence on the generation of ERPs (for a discussion see Klimesch et al., 2007b). One such mechanism is prestimulus phase alignment in cases where the appearance of a stimulus or event can easily be predicted. It also should be emphasized that even in a case where alpha would be the only driving force for the generation of the P1, its amplitude may very well be influenced by stimulus evoked processes. On the other hand, however, as we have argued, the P1 cannot be considered to be solely generated by an evoked response to a stimulus.
The ultimate aim of any theory is the formulation of a quantitative relationship between the postulated processes to enable a precise prediction of the neural and behavioral response. Future research may reveal, whether a specific quantitative function regulates the extent of an event-related change in inhibition as well as in excitation in response to a stimulus and/or task demands. Several studies proceed from a sigmoid function that characterizes the ‘local’ neuronal gain related to a sensory input (cf. Destexhe et al. 2001; Freeman, 1979; Rajagovindan and Ding, 2010). The basic idea is that an increase in excitation in a task relevant network depends on background/spontaneous activity. The larger this activity is, the larger the gain. This relationship is not linear but obeys a sigmoidal function. The important point for our theory is that we have to consider two functions, one for excitatory and another for inhibitory activity. The latter regulates the local inhibitory gain in the task relevant network in order to optimize SNR. This means that the inhibitory background activity and the event-related inhibitory gain depend on the excitatory background activity and the excitatory event-related gain. As a consequence, in order to increase the SNR in task relevant networks inhibition will increase as excitation increases. These considerations suggest that the P1 reflects the event related change in background inhibitory activity and allows the following predictions. (i) For task relevant networks, an inverted U-shaped function may be predicted between prestimulus (ongoing) alpha power (reflecting inhibitory background activity) and P1 amplitude (reflecting the event related change in inhibition), provided phase locking does not play a specific or interfering role. The inverted U-shaped function simply means that beyond a certain level of background activity, the level of event-related inhibition is reduced in order to avoid blocking of information processing in task relevant networks. This prediction is very similar to that of Rajagovindan and Ding (2010) with the only but important difference that (according to their view) the inverted U-shaped function (between ongoing alpha and P1 amplitude) is thought to reflect excitatory processes. (ii) For task competing networks, there is no need to control/modify the SNR. Thus, inhibition may be set to a certain level (depending again on excitation), which does not reflect the local inhibitory gain (and the modulation of SNR) but the blocking of information processing.
Acknowledgments
I am grateful for insightful and critical discussions with my colleagues Robert Fellinger and Roman Freunberger. I am also very grateful for critical comments of 3 Reviewers who helped to improve earlier drafts of this article.
Footnotes
This research was supported by the Austrian Science Foundation (FWF Project P21503-B18).
References
- Allison T., Puce A., McCarthy G. Category-sensitive excitatory and inhibitory processes in human extrastriate cortex. J. Neurophysiol. 2002;88:2864–2868. doi: 10.1152/jn.00202.2002. [DOI] [PubMed] [Google Scholar]
- Allison T., Puce A., Spencer D.D., McCarthy G. Electrophysiological studies of human face perception. I: potentials generated in occipitotemporal cortex by face and non-face stimuli. Cereb. Cort. 1999;9(5):415–430. doi: 10.1093/cercor/9.5.415. [DOI] [PubMed] [Google Scholar]
- Anderson J.R. Effects of prior knowledge on memory for new information. Mem. Cogn. 1981;9:237–246. [Google Scholar]
- Anderson J.R. The Harvard University Press; Cambridge, MA: 1983. The Architecture of Cognition. [Google Scholar]
- Anderson J.R., Pirolli P.L. The spread of activation. J. Exp. Psychol. Learn. Mem. Cogn. 1984;10:791–798. [Google Scholar]
- Becker R., Ritter P., Villringer A. Influence of ongoing alpha rhythm on the visual evoked potential. Neuroimage. 2007;39(2):707–716. doi: 10.1016/j.neuroimage.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Bäuml K.H., Hanslmayr S., Pastötter B., Klimesch W. Oscillatory correlates of intentional updating in episodic memory. Neuroimage. 2008;41:596–604. doi: 10.1016/j.neuroimage.2008.02.053. [DOI] [PubMed] [Google Scholar]
- Batty M., Taylor M.J. Visual categorisation during childhood: an ERP study. Psychophysiology. 2002;39:482–490. doi: 10.1017.S0048577202010764. [DOI] [PubMed] [Google Scholar]
- Busch N.A., Debener S., Kranczioch C., Engel A.K., Herrmann C.S. Size matters: effects of stimulus size, duration and eccentricity on the visual gamma-band response. Clin. Neurophysiol. 2004;115(8):1810–1820. doi: 10.1016/j.clinph.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Busch N.A., Herrmann C.S., Müller M.M., Lenz D., Gruber T. A cross-laboratory study of event-related gamma activity in a standard object recognition paradigm. Neuroimage. 2006;33(4):1169–1177. doi: 10.1016/j.neuroimage.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Busch N.A., Schadow J., Fründ I., Herrmann C.S. Time-frequency analysis of target detection reveals an early interface between bottom-up and top–down processes in the gamma-band. Neuroimage. 2006;29:1106–1116. doi: 10.1016/j.neuroimage.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Busch N.A., Dubois J., VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. J. Neurosci. 2009;29(24):7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch N.A., VanRullen R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proc. Natl. Acad. Sci. USA. 2010;107(37):16048–16053. doi: 10.1073/pnas.1004801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltheart M., Davelaar E., Jonasson J.T., Besner D. Access to the internal lexicon. In: Dornic S., editor. vol. 6. Academic Press; New York: 1977. pp. 535–555. (Attention and Performance). [Google Scholar]
- Cristescu T.C., Nobre A.C. Differential modulation of word recognition by semantic and spatial orienting of attention. J. Cogn. Neurosci. 2008;20(5):787–801. doi: 10.1162/jocn.2008.20503. [DOI] [PubMed] [Google Scholar]
- Danckert J., Goodale M.A. Superior performance for visually guided pointing in the lower visual field. Exp. Brain Res. 2001;137(3–4):303–308. doi: 10.1007/s002210000653. [DOI] [PubMed] [Google Scholar]
- Debruille J.B., Guillem F., Renault B. ERPs and chronometry of face recognition: following-up Seeck et al. and George et al. Neuroreport. 1998;9(15):3349–3353. doi: 10.1097/00001756-199810260-00002. [DOI] [PubMed] [Google Scholar]
- Destexhe A., Rudolph M., Fellous J.M., Sejnowski T.J. Fluctuating synaptic conductances recreate in vivo-like activity in neocortical neurons. Neuroscience. 2001;107:13–24. doi: 10.1016/s0306-4522(01)00344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J. The neurocognitive basis of reading single words as seen through early latency ERPs: a model of converging pathways. Biol. Psychol. 2009;80(1):10–22. doi: 10.1016/j.biopsycho.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Di Russo F., Martínez A., Sereno M.I., Pitzalis S., Hillyard S.A. Cortical sources of the early components of the visual evoked potential. Hum. Brain Mapp. 2002;15:95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniger G.M., Foxe J.J., Murray M.M., Higgins B.A., Snodgrass J.G., Schroeder C.E. Activation time course of ventral visual stream object-recognition areas: high density electrical mapping of perceptual closure processes. J. Cogn. Neurosci. 2000;12(4):615–621. doi: 10.1162/089892900562372. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M., Klimesch W., Stadler W., Pöllhuber D., Heine C. EEG alpha power and intelligence. Intelligence. 2002;30:289–302. [Google Scholar]
- Doppelmayr M., Klimesch W., Hödlmoser K., Sauseng P., Gruber W. Intelligence related upper alpha desynchronization in a semantic memory task. Brain Res. Bull. 2005;66(2):171–177. doi: 10.1016/j.brainresbull.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Ergenoglu T., Demiralp T., Bayraktaroglu Z., Ergen M., Beydagi H., Uresin Y. Alpha rhythm of the EEG modulates visual detection performance in humans. Cognitive Brain Res. 2004;20:376–383. doi: 10.1016/j.cogbrainres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Fell J., Dietl T., Grunwald T., Kurthen M., Klaver P., Trautner P., Schaler C., Elger C.E., Fernández G. Neural bases of cognitive ERPs: more than phase reset. J. Cogn. Neurosci. 2004;16:1595–1604. doi: 10.1162/0898929042568514. [DOI] [PubMed] [Google Scholar]
- Foxe J.J., Simpson G.V. Flow of activation from V1 to frontal cortex in humans. A framework for defining “early” visual processing. Exp. Brain Res. 2002;142(1):139–150. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- Freeman W. Non-linear gain mediating cortical stimulus-response relations. Biol. Cybern. 1979;33:237–247. doi: 10.1007/BF00337412. [DOI] [PubMed] [Google Scholar]
- Freunberger R., Höller Y., Griesmayr B., Gruber W., Sauseng P., Klimesch W. Functional similarities between the P1 component and alpha oscillations. Europ. J. Neurosci. 2008;27:2330–2340. doi: 10.1111/j.1460-9568.2008.06190.x. [DOI] [PubMed] [Google Scholar]
- Freunberger R., Klimesch W., Griesmayr B., Sauseng P., Gruber W. Alpha phase coupling reflects object recognition. Neuroimage. 2008;42:928–935. doi: 10.1016/j.neuroimage.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Freunberger R., Fellinger R., Sauseng P., Gruber W., Klimesch W. Dissociation between phase-locked and nonphase-locked alpha oscillations in a working memory task. Hum. Brain Mapp. 2009;30(10):3417–3425. doi: 10.1002/hbm.20766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fründ I., Busch N.A., Schadow J., Koerner U., Herrmann C.S. From perception to action: phase-locked gamma oscillations correlate with reaction times in a speeded response task. BMC Neurosci. 2007;8:1–11. doi: 10.1186/1471-2202-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A., Clapp W., Kelley J., McEvoy K., Knight R.T., D'Esposito M. Age-related top–down suppression deficit in the early stages of cortical visual memory processing. Proc. Natl. Acad. Sci. USA. 2008;105(35):13122–13126. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber W.R., Klimesch W., Sauseng P., Doppelmayr M. Alpha phase synchronization predicts P1 and N1 latency and amplitude size. Cereb. Cort. 2005;15:371–377. doi: 10.1093/cercor/bhh139. [DOI] [PubMed] [Google Scholar]
- Gunter T.V., Wijers A.A., Jackson J.L., Mulder G. Visual spatial attention to stimuli presented on the vertical and horizontal meridian: an ERP study. Psychophysiology. 1994;31:140–153. doi: 10.1111/j.1469-8986.1994.tb01034.x. [DOI] [PubMed] [Google Scholar]
- Haenschel C., Bittner R.A., Haertling F., Rotarska-Jagiela A., Maurer K., Singer W. Contribution of impaired early-stage visual processing to working memory dysfunction in adolescents with schizophrenia — a study with event-related potentials and functional magnetic resonance imaging. Arch. Gen. Psychiat. 2007;64:1229–1240. doi: 10.1001/archpsyc.64.11.1229. [DOI] [PubMed] [Google Scholar]
- Handy T.C., Grafton S.T., Shroff N.M., Ketay S., Gazzaniga M.S. Graspable objects grab attention when the potential for action is recognized. Nat. Neurosci. 2003;6(4):421–427. doi: 10.1038/nn1031. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S., Klimesch W., Sauseng P., Gruber W., Doppelmayr M., Freunberger R., Pecherstorfer T. Visual discrimination performance is related to decreased alpha amplitude but increased phase locking. Neurosci. Lett. 2005;375:64–68. doi: 10.1016/j.neulet.2004.10.092. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S., Aslan A., Staudigl T., Klimesch W., Herrmann C., Bäuml K.H. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;37:1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S., Klimesch W., Sauseng P., Gruber W., Doppelmayr M., Freunberger R. Alpha phase reset contributes to the generation of ERPs. Cereb. Cort. 2007;17:1–8. doi: 10.1093/cercor/bhj129. [DOI] [PubMed] [Google Scholar]
- Hauk O., Pulvermüller F. Effects of word length and frequency on the human event-related potential. Clin. Neurophysiol. 2004;115(5):1090–1103. doi: 10.1016/j.clinph.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Hauk O., Davis M.H., Ford M., Pulvermüller F., Marslen-Wilson W.D. The time course of visual word recognition as revealed by linear regression analysis of ERP data. Neuroimage. 2006;30(4):1383–1400. doi: 10.1016/j.neuroimage.2005.11.048. [DOI] [PubMed] [Google Scholar]
- Hauk O., Patterson K., Woollams A., Watling L., Pulvermüller F., Rogers T.T. When would you prefer a SOSSAGE to a SAUSAGE? ERP correlates of orthographic typicality and lexicality in written word recognition. J. Cogn. Neurosci. 2006;18(5):818–832. doi: 10.1162/jocn.2006.18.5.818. [DOI] [PubMed] [Google Scholar]
- Hauk O., Pulvermüller F., Ford M., Marslen-Wilson W.D., Davis M.H. Can I have a quick word? Early electrophysiological manifestations of psycholinguistic processes revealed by event-related regression analysis of the EEG. Biol. Psychol. 2009;80(1):64–74. doi: 10.1016/j.biopsycho.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Heinze H.J., Mangun G.R. Electrophysiological signs of sustained and transient attention to spatial locations. Neuropsychologia. 1995;33(7):889–908. doi: 10.1016/0028-3932(95)00023-v. [DOI] [PubMed] [Google Scholar]
- Heinze H.J., Mangun G.R., Burchert W., Hinrichs H., Scholz M., Munte T.F. Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature. 1994;372(6506):543–546. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- Hillyard S.A., Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc. Natl. Acad. Sci. USA. 1998;95(3):781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard S.A., Vogel E.K., Luck S.J. Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philos. T. Roy. Soc. B. 1998;353(1373):1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf J.M., Mangun G.R. Shifting visual attention in space: an electrophysiological analysis using high spatial resolution mapping. Clin. Neurophysiol. 2000;111(7):1241–1257. doi: 10.1016/s1388-2457(00)00313-8. [DOI] [PubMed] [Google Scholar]
- Hopfinger J.B., Mangun G.R. Reflexive attention modulates processing of visual stimuli in human extrastriate cortex. Psychol. Sci. 1998;9(6):441–447. doi: 10.1111/1467-9280.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier R.J., Taylor M.J. N170 or N1? Spatiotemporal differences between object and face processing using ERPs. Cereb. Cort. 2004;14:132–142. doi: 10.1093/cercor/bhg111. [DOI] [PubMed] [Google Scholar]
- Itil T.M., Saletu B., Davis S. EEG findings in chronic schizophrenics based on digital computer period analysis and analog power spectra. Biol. Psychiatry. 1972;5:1–13. [PubMed] [Google Scholar]
- Itil T.M., Saletu B., Davis S., Allen M. Stability studies in schizophrenics and normals using computer-analyzed EEG. Biol. Psychiatry. 1974;8:321–335. [PubMed] [Google Scholar]
- Kenemans J.L., Baas J.M.P., Mangun G.R., Lijffijt M., Verbaten M.N. On the processing of spatial frequencies as revealed by evoked-potential source modeling. Clin. Neurophysiol. 2000;111(6):1113–1123. doi: 10.1016/s1388-2457(00)00270-4. [DOI] [PubMed] [Google Scholar]
- Khateb A., Pegna A.J., Michel C.M., Landis T., Annoni J.M. Dynamics of brain activation during an explicit word and image recognition task: an electrophysiological study. Brain Topogr. 2002;14(3):197–213. doi: 10.1023/a:1014502925003. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Lawrence Erlbaum; Hillsdale, NJ: 1994. The Structure of Long-term Memory: A Connectivity Model of Semantic Processing. [Google Scholar]
- Klimesch W., Doppelmayr M., Pachinger T., Ripper B. Brain oscillations and human memory performance: EEG correlates in the upper alpha and theta bands. Neurosci. Lett. 1997;238:9–12. doi: 10.1016/s0304-3940(97)00771-4. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Doppelmayr M., Schwaiger J., Auinger P., Winkler T. ‘Paradoxical’ alpha synchronization in a memory task. Cognitive Brain Res. 1999;7:493–501. doi: 10.1016/s0926-6410(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Vogt F., Doppelmayr M. Interindividual differences in alpha and theta power reflect memory performance. Intelligence. 2000;27(4):347–362. [Google Scholar]
- Klimesch W., Schack B., Schabus M., Doppelmayr M., Gruber W., Sauseng P. Phase locked alpha and theta oscillations generate the P1–N1 complex and are related to memory performance. Cognitive Brain Res. 2004;19:302–316. doi: 10.1016/j.cogbrainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Hanslmayr S., Sauseng P., Gruber W. Distinguishing the evoked response from phase reset: a comment to Mäkinen et al. Neuroimage. 2006;29:808–811. doi: 10.1016/j.neuroimage.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res. Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Hanslmayr S., Gruber W., Freunberger R. Event-related phase reorganization may explain evoked neural dynamics. Neurosci. Biobehav. Rev. 2007;31:1003–1016. doi: 10.1016/j.neubiorev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Hanslmayr S., Sauseng P., Gruber W., Doppelmayr M. The P1 and traveling alpha waves: evidence for evoked oscillations. J. Neurophysiol. 2007;97:1311–1318. doi: 10.1152/jn.00876.2006. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Freunberger R., Sauseng P. Oscillatory mechanisms of process binding in memory. Neurosci. Biobehav. Rev. 2010;34:1002–1014. doi: 10.1016/j.neubiorev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Lakatos P., Karmos G., Mehta A.D., Ulbert I., Schroeder C.E. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- Lakatos P., Shah A.S., Knuth K.H., Ulbert I., Karmos G., Schroeder C.E. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J. Neurophysiol. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K., Palva J.M., Sams M., Hietanen J.K., Aronen H.J., Ilmoniemi R.J. Face-selective processing in human extrastriate cortex around 120 ms after stimulus onset revealed by magneto- and electroencephalography. Neurosci. Lett. 1998;253(3):147–150. doi: 10.1016/s0304-3940(98)00586-2. [DOI] [PubMed] [Google Scholar]
- Luck S.J., Hillyard S.A. The role of attention in feature detection and conjunction discrimination: an electrophysiological analysis. Int. J. Neurosci. 1995;80(1–4):281–297. doi: 10.3109/00207459508986105. [DOI] [PubMed] [Google Scholar]
- Luck S.J., Hillyard S.A., Mouloua M., Woldorff M.G., Clark V.P., Hawkins H.L. Effects of spatial cuing on luminance detectability: psychophysical and electrophysiological evidence for early selection. J. Exp. Psychol. Human. 1994;20(4):887–904. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Makeig S., Westerfield M., Jung T.P., Enghoff S., Townsend J., Courchesne E. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Mangun G.R., Buck L.A. Sustained visual–spatial attention produces costs and benefits in response time and evoked neural activity. Neuropsychologia. 1998;36(3):189–200. doi: 10.1016/s0028-3932(97)00123-1. [DOI] [PubMed] [Google Scholar]
- Mangun G.R., Hinrichs H., Scholz M., Mueller-Gaertner H.W., Herzog H., Krause B.J. Integrating electrophysiology and neuroimaging of spatial selective attention to simple isolated visual stimuli. Vision Res. 2001;41(10–11):1423–1435. doi: 10.1016/s0042-6989(01)00046-3. [DOI] [PubMed] [Google Scholar]
- Mangun G.R., Hopfinger J.B., Kussmaul C.L., Fletcher E.M., Heinze H.J. Covariations in ERP and PET measures of spatial selective attention in human extrastriate visual cortex. Hum. Brain Mapp. 1997;5(4):273–279. doi: 10.1002/(SICI)1097-0193(1997)5:4<273::AID-HBM12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Mangun G.R. Neural mechanisms of attention. In: Zani A., Proverbio A.M., editors. The Cognitive Electrophysiology of Mind and Brain. Academic Press; New York: 2003. pp. 247–258. [Google Scholar]
- Mazaheri A., Jensen O. Asymmetric amplitude modulations of brain oscillations generate slow evoked responses. J. Neurosci. 2008;28(31):7781–7787. doi: 10.1523/JNEUROSCI.1631-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson K.E., Gratton G., Fabiani M., Beck D.M., Ro T. To see or not to see: prestimulus phase predicts visual awareness. J. Neurosci. 2009;29:2725–2732. doi: 10.1523/JNEUROSCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi T., Tanaka K., Hagimoto H., Miura T., Kishimoto H., Matsushita M. Computerized EEG in schizophrenia patients. Biol. Psychiatry. 1990;28:488–494. doi: 10.1016/0006-3223(90)90482-h. [DOI] [PubMed] [Google Scholar]
- Nobre A.C., Rao A., Chelazzi L. Selective attention to specific features within objects: behavioral and electrophysiological evidence. J. Cogn. Neurosci. 2006;18(4):539–561. doi: 10.1162/jocn.2006.18.4.539. [DOI] [PubMed] [Google Scholar]
- Nadasdy Z. Binding by asynchrony: the neuronal phase code. Front. Neurosci. 2010;4:1–11. doi: 10.3389/fnins.2010.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navalpakkam V., Itti L. Search goal tunes visual features optimally. Neuron. 2007;53(15):605–617. doi: 10.1016/j.neuron.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Nikulin V., Linkenkaer-Hansen K., Nolte G., Lemm S., Müller K., Ilmoniemi R., Curio G. A novel mechanism for evoked responses in the human brain. Europ. J. Neurosci. 2007;25:3146–3154. doi: 10.1111/j.1460-9568.2007.05553.x. [DOI] [PubMed] [Google Scholar]
- Ossandon J.P., Helo A.V., Montefusco-Siegmund R., Maldonado P.E. Superposition model predicts EEG occipital activity during free viewing of natural scenes. J. Neurosci. 2010;30(13):4787–4795. doi: 10.1523/JNEUROSCI.5769-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M.I. Orienting of attention. Q. J. Exp. Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagovindan R., Ding M. From prestimulus alpha oscillations to visual-evoked response: an inverted-U function and its attentional modulation. J. Cogn. Neurosci. 2010;23(6):1379–1394. doi: 10.1162/jocn.2010.21478. [DOI] [PubMed] [Google Scholar]
- Roland P. Six principles of visual cortical dynamics. Front. Neurosci. 2010;4:1–21. doi: 10.3389/fnsys.2010.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V., Brodbeck V., Michel C., Amedi A., Pascual-Leone A., Thut G. Spontaneous fluctuations in posterior {alpha}-band EEG activity reflect variability in excitability of human visual areas. Cereb. Cort. 2007;18:2010–2018. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V., Rihs T., Brodbeck V., Thut G. Resting electroencephalogram alpha-power over posterior sites indexes baseline visual cortex excitability. Neuroreport. 2008;19:203–208. doi: 10.1097/WNR.0b013e3282f454c4. [DOI] [PubMed] [Google Scholar]
- Rousselet G.A., Mace M., Thorpe S., Fabre-Thorpe M. Limits of event-related potential differences in tracking object processing speed. J. Cogn. Neurosci. 2007;19:1241–1258. doi: 10.1162/jocn.2007.19.8.1241. [DOI] [PubMed] [Google Scholar]
- Ruz M., Nobre A.C. Attention modulates initial stages of visual word processing. J. Cogn. Neurosci. 2008;20(9):1727–1736. doi: 10.1162/jocn.2008.20119. [DOI] [PubMed] [Google Scholar]
- Sagiv N., Bentin S. Structural encoding of human and schematic faces: holistic and part-based processes. J. Cogn. Neurosci. 2001;13(7):937–951. doi: 10.1162/089892901753165854. [DOI] [PubMed] [Google Scholar]
- Schack B., Klimesch W. Frequency characteristic of evoked and oscillatory electroencephalographic activity in a human memory scanning task. Neurosci. Lett. 2002;331:107–110. doi: 10.1016/s0304-3940(02)00846-7. [DOI] [PubMed] [Google Scholar]
- Sauseng P., Klimesch W., Gruber W.R., Hanslmayr S., Freunberger R., Doppelmayr M. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience. 2007;146:1435–1444. doi: 10.1016/j.neuroscience.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Scheeringa R., Bastiaansen M., Petersson K., Oostenveld R., Norris D., Hagoort P. Frontal theta EEG activity correlates negatively with the default mode network in resting state. Int. J. Psychophysiol. 2008;67:242–251. doi: 10.1016/j.ijpsycho.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Schroeder C.E., Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:918. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalowitz S., Zheng X. An ERP study of category priming: evidence of early lexical semantic access. Biol. Psychol. 2009;80:122–129. doi: 10.1016/j.biopsycho.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Sponheim S.R., Clementz B.A., Iacono W.G., Beiser M. Resting EEG in first-episode and chronic schizophrenia. Psychophysiology. 1994;31:37–43. doi: 10.1111/j.1469-8986.1994.tb01023.x. [DOI] [PubMed] [Google Scholar]
- Sponheim S.R., Clementz B.A., Iacono W.G., Beiser M. Clinical and biological concomitants of resting state EEG power abnormalities in schizophrenia. Biol. Psychiatry. 2000;48:1088–1097. doi: 10.1016/s0006-3223(00)00907-0. [DOI] [PubMed] [Google Scholar]
- Taylor M.J. Non-spatial attentional effects on P1. Clin. Neurophysiol. 2002;113:1903–1908. doi: 10.1016/s1388-2457(02)00309-7. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Khan S.C. Top–down modulation of early selective attention processes in children. Int. J. Psychophysiol. 2000;37:135–147. doi: 10.1016/s0167-8760(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Chevalier H.A., Lobaugh N.J. ERP measures of feature and conjunction processing. Psychophysiology. 1999;36(Suppl 1):58. [Google Scholar]
- Taylor M.J., Chevalier H., Lobaugh N.J. Discrimination of single features and conjunctions by children. Neuroimage. 2001;13:S949. doi: 10.1016/s0167-8760(03)00155-7. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Chevalier H., Lobaugh N.J. ERP measures of early processing in feature and conjunction search: increased speed with increased array size. Psychophysiology. 2001;38(Suppl 1):S94. [Google Scholar]
- Taylor M.J., Edmonds G.E., McCarthy G., Allison T. Eyes first! Eye processing develops before face processing in children. Neuroreport. 2001;12:1671–1676. doi: 10.1097/00001756-200106130-00031. [DOI] [PubMed] [Google Scholar]
- Thorpe S., Fize D., Marlot C. Speed of processing in the human visual system. Nature. 1996;381:520–522. doi: 10.1038/381520a0. [DOI] [PubMed] [Google Scholar]
- Valdes-Sosa M., Bobes M.A., Rodriguez V., Pinilla T. Switching attention without shifting the spotlight: object-based attentional modulation of brain potentials. J. Cogn. Neurosci. 1998;10(1):137–151. doi: 10.1162/089892998563743. [DOI] [PubMed] [Google Scholar]
- Van Dijk H., Schoffelen J.M., Oostenveld R., Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J. Neurosci. 2008;28:1816–1823. doi: 10.1523/JNEUROSCI.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRullen R., Thorpe S.J. Is it a bird? Is it a plane? Ultra-rapid visual categorization of natural and artifactual objects. Perception. 2001;30:655–668. doi: 10.1068/p3029. [DOI] [PubMed] [Google Scholar]
- VanRullen R., Thorpe S.J. The time course of visual processing: from early perception to decision making. J. Cogn. Neurosci. 2001;13:454–461. doi: 10.1162/08989290152001880. [DOI] [PubMed] [Google Scholar]
- Vogt F., Klimesch W., Doppelmayr M. High frequency components in the alpha band and memory performance. J. Clin. Neurophysiol. 1998;15(2):167–172. doi: 10.1097/00004691-199803000-00011. [DOI] [PubMed] [Google Scholar]
- Yamagishi N., Callan D.E., Anderson S.J., Kawato M. Attentional changes in pre-stimulus oscillatory activity within early visual cortex are predictive of human visual performance. Brain Res. 2008;1197:115–122. doi: 10.1016/j.brainres.2007.12.063. [DOI] [PubMed] [Google Scholar]