Pollen tube growth is regulated by female tissue-produced factors that facilitate growth and provide directional guidance. We discuss here signal perception and transduction molecules on the male and the female cell surfaces mediate male-female interactions that underlie successful reproduction.
Abstract
Background
RAC/ROPs are RHO-type GTPases and are known to play diverse signalling roles in plants. Cytoplasmic RAC/ROPs are recruited to the cell membrane and activated in response to extracellular signals perceived and mediated by cell surface-located signalling assemblies, transducing the signals to regulate cellular processes. More than any other cell types in plants, pollen tubes depend on continuous interactions with an extracellular environment produced by their surrounding tissues as they grow within the female organ pistil to deliver sperm to the female gametophyte for fertilization.
Scope
We review studies on pollen tube growth that provide compelling evidence indicating that RAC/ROPs are crucial for regulating the cellular processes that underlie the polarized cell growth process. Efforts to identify cell surface regulators that mediate extracellular signals also point to RAC/ROPs being the molecular switches targeted by growth-regulating female factors for modulation to mediate pollination and fertilization. We discuss a large volume of work spanning more than two decades on a family of pollen-specific receptor kinases and some recent studies on members of the FERONIA family of receptor-like kinases (RLKs).
Significance
The research described shows the crucial roles that two RLK families play in transducing signals from growth regulatory factors to the RAC/ROP switch at the pollen tube apex to mediate and target pollen tube growth to the female gametophyte and signal its disintegration to achieve fertilization once inside the female chamber.
Introduction
G proteins are key components in many eukaryotic signalling pathways. They act as molecular signalling switches by shuttling between an inactive GDP-bound form and an active GTP-bound form (Fig. 1). In the GTP-bound form the GTPase interacts with target proteins to affect cellular changes, until GTP hydrolysis returns the protein to the inactive, GDP-bound state. Of the two main types of G proteins, the RAS-related family of monomeric small GTPases and the heterotrimeric G proteins comprised of an α and a dimeric βγ complex, plants rely considerably more on plant-specific RHO subfamily of RAS-related GTPases, referred to as RAC/ROPs (for RAC-like/RHOs of plants), for transduction of a broad array of signals (see Yalovsky et al. 2010; Wu et al. 2011). For instance, Arabidopsis and a number of other higher plants, including maize, rice and poplar, have close to 10 or more RAC/ROPs, while the moss Physcomitrella patens has 4 (Eklund et al. 2010; Fowler 2010). The most extensively examined functional role for RAC/ROP GTPases is their control of polarized cell growth, exemplified by tip growth in pollen tubes and root hairs (Kost 2008; Yang 2008). The function of the pollen tube is to deliver sperm, transported as cargoes in its cytoplasm, to the female gametophyte for fertilization. Research over the last 25 years and originating from different perspectives related to the pollination process led to the broadly accepted view that RAC/ROPs located at the pollen tube apical membrane (Fig. 1B) represent a major molecular switch regulating the tip growth process. Recent research also implicates potential roles for these small GTPases in regulating pollen tube growth via their actions in female tissues. Several reviews have comprehensively discussed the cellular processes regulated by RAC/ROPs to mediate polarized cell growth (Cheung and Wu 2008; Kost 2008; Yalovsky et al. 2008; Yang 2008). We focus here on the discussion of upstream regulators, in particular receptor-like kinases (RLKs) that may control the ON/OFF state of the RAC/ROP molecular switch to mediate pollen tube growth and pollen–pistil interaction.
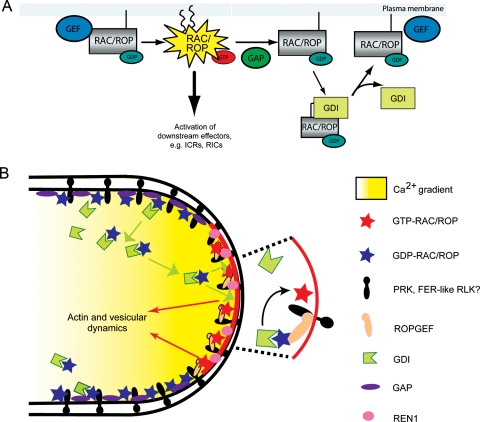
Fig. 1.
RAC/ROP GTPase signalling of polarized pollen tube growth. (A) Regulation of the RAC/ROP molecular switch (modified from Nibau et al. 2006). (B) Apically located RAC/ROP switch in a pollen tube (modified from Cheung and Wu 2008).
Regulated RAC/ROP signalling is crucial for pollen tube growth
Regulation of the RAC/ROP molecular switch
Similar to other RAS-related small G proteins, RAC/ROP activity shuttles between the active GTP-bound form and the inactive GDP-bound form of RAC/ROPs (Fig. 1A). Guanine nucleotide exchange factors (GEFs) catalyse the exchange of GDP for GTP and activate RAC/ROPs (Rossman et al. 2005). In elongating pollen tubes, RAC/ROPs are typically located at the apical membrane domain. Several GEFs, referred to as ROPGEFs, have been located at the apical membrane of elongating pollen tubes, where they serve the functional role of activating apically located RAC/ROPs (Gu et al. 2006; Zhang and McCormick 2007). In their activated state, RAC/ROPs interact with effectors (Wu et al. 2001; Lavy et al. 2007) to mediate cellular target systems. In pollen tubes, two CRIB-domain-containing proteins, referred to as RICs (Wu et al. 2001), have been shown to interact with activated RAC/ROPs and to mediate actin and membrane dynamics (Hwang et al. 2005), processes that are well established to be crucial for maintaining pollen tube growth (Fig. 1B) (Cheung and Wu 2008).
RAC/ROP signalling is terminated by the activity of the GTPase-activating proteins (GAPs) that stimulate the GTPase activity inherent to G proteins, rendering them inactive (Fig. 1A). Studies of a GAP from tobacco, Nt-RhoGAP1, showed that it is located at the pollen tube apical flank cell membrane, just distal to where RAC/ROPs are located (Fig. 1B). The lateral location of Nt-RhoGAP1 to the apically located RAC/ROPs suggests a role in restricting active RAC/ROPs from spreading beyond the apical dome membrane (Klahre and Kost 2006), a model that is quite easy to conceptualize. On the other hand, a novel RhoGAP REN1 from Arabidopsis co-localizes with RAC/ROPs at the apical membrane (Hwang et al. 2008). The overlapping localization domain of RAC/ROPs and a negative regulator to the same membrane domain led to the proposal of a global inhibition model that suggests an elaborate regulatory loop involving ‘alternating and interlinking positive and negative’ feedback interactions between REN1 and RAC/ROPs (Hwang et al. 2008). It is possible that both local and lateral inhibitory mechanisms co-exist to ensure tip growth. It is reasonable to envision that regulated inactivation of RAC/ROPs at the tube apex is crucial for locally modulating their activity while lateral inhibition ensures inactivation of those GTPases that are displaced to the apical flank as the apex migrates forward, ensuring that active signalling is restricted to the tip. Once inactivated, the inactive RAC/ROPs are extracted from the cell membrane by guanine nucleotide dissociation inhibitor (GDI), which also sequesters them in the GDP-bound form in the cytoplasm and recycles them back to the apical dome membrane for activation (Klahre et al. 2006).
Pollen tube growth is dependent on properly regulated RAC/ROP signalling
Directly perturbing the normal level of RAC/ROP signalling activity results in defects in tip growth characteristics. Pollen tubes with elevated RAC/ROP activity, e.g. by the over-expression of constitutively active (CA) RAC/ROPs, are typically highly depolarized, often expanding isotropically resulting in a balloon-tipped or spoon-shaped tube and growth arrest (Kost et al. 1999; Chen et al. 2003; Fu et al. 2001). On the other hand, down-regulating RAC/ROP activity, e.g. by over-expression of dominant-negative (DN) RAC/ROPs, reduces tube elongation rates and pollen tubes are typically broader than rapidly elongating pollen tubes, reflecting that cell expansion has occurred over a broader apical dome region while still capable of maintaining highly asymmetric growth and thus giving rise to a wider tube.
Indirectly altering the endogenous RAC/ROP regulatory capacity in a pollen tube also results in pollen tube growth defects mimicking those induced by over-expressing CA or DN RAC/ROPs. For instance, over-expressing ROPGEFs induced ballooned pollen tubes (Kaothien et al. 2005; Gu et al. 2006; Cheung et al. 2008) and knocking out endogenous RhoGAP in ren1 null mutants resulted in bulbous pollen tubes, mimicking the result of over-expressing wild-type or CA RAC/ROPs, and reduced male transmission (Hwang et al. 2008). On the other hand, over-expression of a GDI, presumably increasing RAC/ROP sequestration in the cytoplasm, also resulted in reduced pollen tube growth (Klahre et al. 2006), similar to the effect of DN RAC/ROP over-expression. In maize and when competing with co-pollinated wild-type pollen, rop2::Mu pollen grains in which the ROP2 mRNA level was significantly reduced as a result of Mu insertion showed compromised transmission capacity (Arthur et al. 2003). These observations provide strong support for properly regulated RAC/ROP signalling being crucial for polarity maintenance at the pollen tube tip and important for optimum reproductive success.
RAC/ROPs as mediators for exogenous pollen tube growth regulators
Pollen tube growth in the pistil is responsive to regulatory factors secreted by female tissues acting e.g. as nutrients and directional guidance molecules. In vitro, pollen tubes are responsive to exogenously applied biological growth regulators originating from female tissues or from pollen themselves. These include native proteins isolated from pistil tissues (Cheung et al. 1995; Wu et al. 1995; Mollet et al. 2000; Kim et al. 2003; Zhang et al. 2008), recombinant proteins expressed in Escherichia coli corresponding to female cell-expressed proteins (Okuda et al. 2009) or synthetic peptides corresponding to pollen-expressed proteins (Covey et al. 2010). Besides the well-known pistil controls that inhibit incompatible pollination in self-incompatible plants (McClure and Franklin-tong 2006; Tantikanjana et al. 2010), negative pollen tube growth regulators from female tissues are also known to play important roles in ensuring successful reproduction. For instance, they are needed to mediate growth arrest and rupture upon pollen tube penetration of the female gametophyte inside the ovule, enabling sperm discharge and fertilization (Huck et al. 2003, Rotman et al. 2003; Escobar-Restrepo et al. 2007; Amien et al. 2010). Late-arriving pollen tubes approaching already penetrated ovules are believed to be repelled by signals emanating from within the ovules (Palanivelu and Preuss 2006), causing them to re-route to receptive, not yet visited ovules, thus preventing polyspermy, ensuring progeny health and maximizing reproductive yield (Shimizu and Okada 2000).
Given the importance of the RAC/ROP GTPases to pollen tube growth, it is easy to envision them as key intracellular signal mediators to convey the signal from some of these growth regulators to the cellular machinery for growth and directionality. Presumably the extracellular to intracellular signalling linkage would be provided by cell surface-located signal perception molecules that will recruit and impact the activity of intracellular ROPGEFs, which in turn regulate the activity of the RAC/ROP switch (Fig. 1B). Thus far, RLKs have been implicated as upstream cell surface regulators for RAC/ROP GTPases and potential mediators for extracellular signals to the RAC/ROP switch. Receptor-like kinases are transmembrane proteins with an extracellular domain (ECD), a single membrane-spanning domain and a cytoplasmic kinase domain. Members of the plant RLK family (Shiu and Bleecker 2003; De Smet et al. 2009; Lehti-Shiu et al. 2009) share conserved catalytic kinase domains and predominantly phosphorylate serine and threonine residues, although some have also been found to phosphorylate tyrosine residues. They perceive signals via specific ligand–ECD interactions and subsequently activate signalling cascades via their cytoplasmic domain (CD). The plant RLK family is enormous; for instance, the Arabidopsis RLK family is comprised of more than 600 members. The ECDs of these RLKs are quite divergent, allowing them to be grouped into 15 subfamilies, one being a leucine-rich repeat (LRR)-containing family (Torii 2004) and the other a Catharanthus roseus RLK1 (CrRLK1)-like family (Hématy and Höfte 2008; Boisson-Dernier et al. 2011; Nibau and Cheung 2011). We focus our discussion here on several members of these two sub-RLK families and the signalling pathways that they regulate as they have been implicated to be cell surface regulators for RAC/ROP signalling for polarized cell growth.
Pollen-specific receptor kinases interact with ROPGEFs and regulate RAC/ROP-signalled pollen tube tip growth
Localization of pollen-specific receptor kinases suggests a cell surface signalling function in pollen tubes
Pollen-specific receptor kinases (PRKs) share features allowing their identification as a family of pollen-specific receptor kinases among a much larger LRR subtype of receptor kinases in plants (Kim et al. 2002). Much of the functional understanding related to PRKs has been derived from two of the earlier identified members of the family, LePRK1 and LePRK2 from tomato, and more recently from At-PRK2a from Arabidopsis. Immunodetection (Muschietti et al. 1998; Kim et al. 2002) and fluorescent protein (FP)-tagged localization of PRKs showed that these receptor kinases are localized along the entire pollen tube cell surface (Fig. 2A) (Cheung et al. 2002; Zhang and McCormick 2007). Immunolocalization analysis using antibodies specific to their respective ECD shows that LePRK1, LePRK2 and a more distantly related homologue LePRK3 occupy overlapping domains along the pollen tube surface but each displaying some noticeable distinction from the others (Kim et al. 2002). Although it is not clear yet whether these localization differences signify functional specialization, biochemical studies to be discussed below suggest that even the closely related LePRK1 and LePRK2 are likely to be differentially involved in their capacity to interact with signals from the female environment. Since dimerization is often associated with receptor kinase activation and LePRK1 and LePRK2 are known to form heterodimers (Wengier et al. 2003), it is conceivable that interactions between the three related LePRKs provide subtle differences expanding their extracellular perception capability and intracellular signalling capacity.
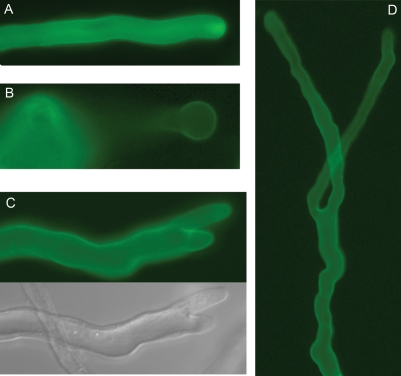
Fig. 2.
Cell membrane-located PRK regulates pollen tube tip growth. LAT52-GFP-Nt-PRK-transformed tobacco pollen tubes are shown. (A) Cell surface localization of GFP-Nt-PRK in elongating pollen tubes. Typical of pollen tube cell membrane-associated proteins, vesicles loaded with FP-PRKs also concentrate in and cycle rapidly in and out of the apical cytoplasm (see also Cheung et al. 2002), which is known to be occupied almost exclusively by transport vesicles. (B–D) Prevalent tip growth defects induced by expression of GFP-Nt-PRK. (B) Balloon tipped and growth arrested. (C) Bifurcated tip; the vacuole-occupied branch (lower, DIC image) was either already growth arrested or likely to cease growth shortly after the image was acquired. (D) A bifurcated GFP-Nt-GFP-expressing pollen tube where growth at both tips had been sustained for some time. Balloon-tipped but not bifurcated tubes were observed previously in LePRK2-transformed pollen tubes (Zhang et al. 2008).
PRKs interact with ROPGEFs
Over-expression of GFP-PRKs in pollen tubes results in growth defects indicative of compromised tip growth (Fig. 2B–D) (Zhang et al. 2008). A long series of molecular genetic studies, which we shall discuss below, implicated the LePRKs as playing important roles in mediating reproductive success. The observation revealing their potential participation in RAC/ROP-regulated pollen tube growth was reported in 2005 when the CDs of LePRK1 and LePRK2 were shown to interact directly with a highly phosphorylated pollen-specific peripheral membrane protein LeKPP (for tomato kinase partner protein) (Kaothien et al. 2005). Over-expression of LeKPP in transformed pollen tubes resulted in ballooned tips and other severely malformed apices, accompanied by disrupted actin organization and vesicle dynamics, reminiscent of phenotypes induced by up-regulating RAC/ROP signalling. Moreover, a family of 14 KPP-like proteins was also identified in Arabidopsis. KPP and the 14 KPP-like Arabidopsis proteins turned out to be the long-sought GEFs for RAC/ROP GTPases (Berken et al. 2005), which had until then eluded identification from direct homology searches. Therefore, the discovery of KPP as a PRK-interacting protein establishes a functional linkage between PRKs and RAC/ROPs in regulating pollen tube tip growth. Similar to RAC/ROPs constituting a plant-specific clade of the RHO GTPase protein family, KPP and ROPGEFs from Arabidopsis and other plant species are also unique to plants. ROPGEFs share a conserved central catalytic domain for GDP to GTP exchange but diverge on their N- and C-terminal regions, presumably providing plasticity in their signalling interactions and thus the range of their signalling capacity.
PRK regulates RAC/ROP-mediated pollen tube tip growth
Over-expression of the Arabidopsis ROPGEF1 in tobacco pollen tubes induces a highly ballooned apical morphology and disorganized actin organization (Gu et al. 2006; Cheung et al. 2008), similar to the effect of over-expressing CA RAC/ROPs. GFP-ROPGEF1 is localized throughout the ballooned apical region (Gu et al. 2006), consistent with the induced isotropic growth having resulted from the spread of the RAC/ROP activation to beyond the apical membrane domain. ROPGEF1 is broadly expressed in Arabidopsis (Zimmermann et al. 2004), but its transcripts are much less abundant than those of other ROPGEFs identified as pollen-specific or pollen-enriched (Gu et al. 2006; Zhang and McCormick 2007). Unlike ROPGEF1, the GFP-labelled pollen-specific or -enriched ROPGEFs were located in a limited membrane region around the apical dome when they were over-expressed in pollen tubes and induced only slightly depolarized growth resulting in broader pollen tubes. This was consistent with increased RAC/ROP activation over a broader apical membrane area but nonetheless still confined to the extending tip region, therefore sustaining asymmetric growth albeit over a broader apical membrane domain, yielding the wider tubes. The more restricted apical localization for these pollen-specific or -enriched ROPGEFs suggests the presence of endogenous mechanisms to preclude their activating RAC/ROP to beyond the apical flank region. Such mechanisms apparently do not apply to the constitutively expressed ROPGEF1, which is not highly pollen prevalent.
To directly establish a signalling linkage between PRKs and RAC/ROPs in pollen tube growth, Zhang and McCormick (2007) used bimolecular fluorescence complementation to establish that At-PRK2a and ROPGEF12 directly interact along the pollen tube cell membrane. When At-PRK2a and full-length ROPGEF12, which is auto-inhibited by its C-terminal domain, were over-expressed individually in transformed tobacco pollen tubes, they induced the relatively weak phenotype of wider tubes. Their co-overexpression, however, resulted in the severe phenotype of isotropic growth. Similarly, co-expression of LePRK2 and LeKPP in transiently transformed tobacco pollen tubes also resulted in a similar synergistic effect (Zhang et al. 2008). Furthermore, biochemical studies showed that LePRK1 and LePRK2 associate with a high-molecular-weight (∼400 kDa) protein complex in germinating tomato pollen (Wengier et al. 2003). RAC/ROPs and LeKPP were reportedly also detected in the same column fractions (see Wengier et al. 2003), suggesting that the 400-kDa complex might represent a PRK-signalling complex that initiates RAC/ROP signalling in pollen tubes. Taken together, these observations show that PRKs function as cell surface regulators interacting with ROPGEFs to mediate a level of spatially restricted RAC/ROP signalling activities at the pollen tube apex to ensure tip-focused growth.
PRKs interact with pollen tube growth regulators to mediate reproductive success
Biochemical and molecular genetic studies show that the LePRKs interact with molecules secreted either from female tissues or from pollen (Tang et al. 2002, 2004). Results from studies based on antisense suppression of LePRK2 (Zhang et al. 2008) and of two LePRK2-interacting pollen proteins, LAT52 (Tang et al. 2002) and a homologue of SHY (Tang et al. 2004) from Petunia (Guyon et al. 2004), and analysis of pollen tube growth regulatory activities of two LePRK2-interacting female molecules, STIL (Zhang et al. 2008; Wengier et al. 2010) and STIG1 (Tang et al. 2004), are consistent with the PRK-mediated signalling pathway being important for pollen germination and tube growth.
LePRK2 interacts with a stylar-secreted pollen germination and tube growth promoting molecule, STIL
LePRK1 and 2 are closely related and their E. coli-produced kinase domains are active kinases and autophosphorylate (Muschietti et al. 1998). Immunoprecipitation studies showed that they form heterodimers in the cell membrane of pollen and of yeast when co-expressed in the heterologous system (Wengier et al. 2003). However, LePRK1 and 2 are not functionally equivalent. At the gene expression level, LePRK1 and 2 are expressed late in pollen development but only the level of LePRK2 increases further when pollen germinates. Phosphorylation assays using pollen membrane fractions showed that LePRK2, but not LePRK1, is phosphorylated. Furthermore, heterodimerization apparently relies on LePRK2 being an active kinase as dimer formation in yeast between the wild-type LePRK1 and a kinase-inactive form of LePRK2 was prohibited, whereas that between the wild-type LePRK2 and kinase-inactive LePRK1 was not affected (Wengier et al. 2003).
Phosphorylation of LePRK2 is apparently regulated in vivo as pollen germinates and during tube growth. A low-molecular-weight species from the tomato stylar exudates, STIL (stylar interactor for LePRKs), dephosphorylates LePRK2 when added to in vitro phosphorylation assays by pollen membrane fractions (Muschietti et al. 1998; Wengier et al. 2010). Furthermore, stylar fractions, when added to germinating pollen grains, disrupted the LePRK-containing 400-kDa protein complexes and rendered the majority of these PRKs to molecular weight fractions closer to their monomeric molecular mass (Wengier et al. 2003). Moreover, the LePRK1 and LePRK2 heterodimers formed in yeast were disrupted when stylar fractions enriched in STIL were added to membrane fractions from the LePRK1 and LePRK2 co-expressing yeast, presumably as a result of STIL dephosphorylation of LePRK2.
STIL, described as a ‘peculiar’ molecule from the style, is a low-molecular-weight species of 3350 Da (Wengier et al. 2010). Its activity to dephosphorylate LePRK2 is resistant to heat, base, dithiothreitol and protease treatment. STIL is also resistant to traditional acid treatment (1 N HCl at 100 °C for 20 h) but sensitive to microwave-assisted acid treatment (1.5 N HCl for 10 min at 900 W), which apparently mediated more extensive hydrolysis of STIL than acid treatment under normal conditions. The exact chemical nature of STIL remains to be determined, although it is likely to be at least partially peptidic as it absorbs at 280 nm and has amino acid content. When added to wild-type pollen, STIL promoted germination and enhanced tube growth rates (Zhang et al. 2008). Transformed tomato pollen tubes with antisense-suppressed LePRK2 levels failed to respond to STIL-stimulated growth, providing evidence for a LePRK-regulated pathway underlying the STIL-stimulated growth responses (Zhang et al. 2008).
Taking into account the molecular genetics, biochemical and cellular observations surrounding the LePRKs, a picture of a LePRK2-mediated signalling pathway emerges whereby the stylar factor STIL promotes pollen germination and tube growth by dephosphorylating LePRK2. This in turn disrupts the LePRK1 and LePRK2 heterodimer and its association with a high-molecular-weight protein complex, potentially impacting interaction with KPP, and modulates downstream RAC/ROP signalling activity to promote tip growth.
LePRK2 interacts with pollen tube growth regulatory molecules from pollen or pistil
The signalling capacity of LePRK1 and LePRK2 can apparently be modulated by proteins from both pollen and pistil through their interactions with their ECDs. Yeast two-hybrid screens and biochemical protein–protein interaction assays showed that the ECD of LePRK2 interacts with at least two pollen glycoproteins, the secreted cysteine-rich LAT52 (Tang et al. 2002) and the cell wall-located SHY (Guyon et al. 2004; Tang et al. 2004). Moreover, LePRK2 interacts with LAT52 before but not after pollen germination, hinting that the pistillate environment encountered by the germinating pollen must have resulted in conditions that disrupt their interaction. Both LAT52 and SHY apparently contribute to ensure productive pollination resulting in fertilization. Antisense suppression of LAT52 resulted in the inability of pollen grains to hydrate properly in vitro, abortive tube growth in the style and reduced male transmission (Muschietti et al. 1994). Antisense-mediated reduction of the SHY homologue in Petunia severely inhibited pollen germination and for those SHY-suppressed pollen grains that managed to germinate, their tubes were arrested just above the ovary, so overall seed yields in these plants were poor (Guyon et al. 2004).
The ECDs of LePRK1 and LePRK2 both interact physically with a female partner LeSTIG1, a cysteine-rich protein secreted by the stigma (Tang et al. 2004) where pollen grains are deposited and germinate. Escherichia coli-produced recombinant STIG1 stimulated in vitro pollen tube growth when added to pollen germination cultures. In vitro, STIG1 bound to the ECD of LePRK2 and displaced binding by the pollen-produced LAT52. Displacement of LAT52 from complexing with LePRK2 by STIG1 observed in vitro provided a plausible explanation for the observation that LAT52 binds to LePRK2 before but not after pollen germination, and led to the proposition that LePRK2 changes interacting partners when on the surface of pollen grains and upon tube germination (Tang et al. 2004). How the pollen partners LAT52 and SHY and the pistil partner LeSTIG1 impact the signalling capacity of LePRKs remains to be established. Observations that each of these pollen- and pistil-secreted proteins has a biological impact on pollen germination and tube growth in vitro or for the reproductive process in vivo provide strong implications for the PRK-regulated signalling pathway being crucial for reproduction.
Finely tuned phosphoregulation of LePRK2 underlies its activity in regulating pollen tube growth
Studies from STIL and the other LePRK-interacting pollen and pistil proteins show that PRK-mediated signalling capacity could be modulated in several ways and during different stages of pollination. That dephosphorylation of LePRK2 by STIL disrupts its complex formation with other potential signalling partners implies important functional significance for LePRK2 phosphorylation. It was shown recently that LePRK2 exists as multiple isoforms in the pollen cell membrane, apparently phosphorylated on two phosphorylatable motifs located in the cytoplasmic juxtamembrane region (Salem et al. 2011). Over-expression of wild-type and site-directed mutant forms of LePRK2 showed that these two motifs act antagonistically in affecting how LePRK2 impacts pollen tube growth. When transiently expressed in tobacco pollen tubes, mutations rendering the more N-terminally located motif non-phosphorylatable (serines to alanines) resulted in longer pollen tubes, suggesting that phosphorylation negatively impacts growth. Conversely, similar mutations in the more distally located motif resulted in shorter pollen tubes, suggesting that phosphorylation stimulates growth. Furthermore, the results obtained by using phospho-mimicking mutations in these motifs led to inverse observations and therefore similar conclusions on the antagonistic effects of these LePRK2 phosphorylatable motifs. Thus it is possible to envision that the output signalling activity from the PRK signalling module is maintained by varying the level of phosphorylation on these two motifs. The multiple interacting male and female partners and the two antagonistic phosphorylated motifs argue for the presence of mechanisms capable of fine-tuning the PRK signalling capacity to facilitate interactions with different partners and modulate RAC/ROP GTPase activity to mediate polarized growth throughout the long pollen tube growth journey in the pistil.
The FERONIA family of RLKs as cell surface regulators for pollen tube growth and pollen–pistil interaction
The FERONIA (FER) family of RLKs (more frequently referred to as the CrRLK family after its founding member found in C. roseus) is emerging as an important subfamily of plant RLKs regulating plant growth and development. CrRLKs are conserved among plants; in Arabidopsis they comprise a 17-membered family (Shiu and Bleecker 2003; Lehti-Shiu et al. 2009). Studies on five of these, including FER (Huck et al. 2003; Rotman et al. 2003; Escobar-Restrepo et al. 2007; Guo et al. 2009; Deslauriers and Larsen 2010; Duan et al. 2010), THESEUS1 (THE1) (Hématy et al. 2007), HERCULES1 (Guo et al. 2009), ANXUR1 and ANXUR2 (ANX1 and ANX2) (Boisson-Dernier et al. 2009; Miyazaki et al. 2009), have already revealed important roles for these RLKs in regulating cell growth processes in different tissues and under different development conditions. Recent reviews have discussed the structure and emerging knowledge about the biological roles of these FER family RLKs (Hématy and Höfte 2008; Boisson-Dernier et al. 2011; Nibau and Cheung 2011). The ECDs of these FER RLKs are distinguishable from other RLKs in having two domains with recognizable homology with malectin, a conserved animal endoplasmic reticulum-anchored membrane protein that binds di-glucose (Schallus et al. 2008). Although the functional significance of these potential sugar-binding domains is unclear, their presence is intriguing considering that FER, ANX1 and ANX2 control pollen tube integrity, and that THE1 regulates cell growth in response to the cell wall environment and suppresses plant growth under cellulose deficiency. Here, we focus on the FER-related RLKs known to impact the reproductive process, FER, ANX1 and ANX2 and their potential roles in RAC/ROP-regulated processes in reproduction.
FER regulates pollen tube–ovule interaction and induces pollen tube disintegration
The Arabidopsis Feronia (Fer)/Sirène (Srn) (for simplicity, Fer will be used from here on unless the srn mutant is referred to specifically) was initially identified through genetic screens as an important regulator for female gametophytic function (Huck et al. 2003; Rotman et al. 2003). Molecular analysis of wild-type and mutant alleles of fer showed that it encodes an RLK belonging to the broader CrRLK family (Escobar-Restrepo et al. 2007). In wild-type plants, after being guided by attractants that emanate from the female gametophyte to enter the ovule, the pollen tube and ovule engage in a series of pollen tube–ovule interactive events referred to as pollen tube reception whereby the first-arriving pollen tube enters one of the paired synergid cells at the entrance to the female gametophyte and ruptures, resulting in sperm discharge and fertilization (see Dresselhaus and Marton 2009). Late-arriving pollen tubes are prevented from entering an already penetrated ovule (Palanivelu and Preuss 2006; Sandaklie-Nikolova et al. 2007). In the ovules, FER is expressed most predominantly in the synergid cells, and GFP-labelled FER localizes to synergid cell membrane and accumulates prominently in the filiform apparatus (Escobar-Restrepo et al. 2007), a thickened cell wall region at the entrance to the female gametophyte secreted by the synergid cells and with a high density of criss-crossing synergid cell membrane (Punwani et al. 2007). fer female gametophytes attract pollen tubes normally but fail to induce rupture of the penetrated pollen tube and prevent multiple pollen tube penetration. This results in supernumerary pollen tube entrance yet female sterility as sperm release and therefore fertilization fail. Because of multiple pollen tube entry and their overgrowth inside the female gametophyte, fer ovules show the dramatic phenotype of pollen tube pile-up inside the female gametophyte and sometimes bundles of pollen tubes gather at the entrance to the ovule (Fig. 3A) (Huck et al. 2003; Rotman et al. 2003; Escobar-Restrepo et al. 2007).
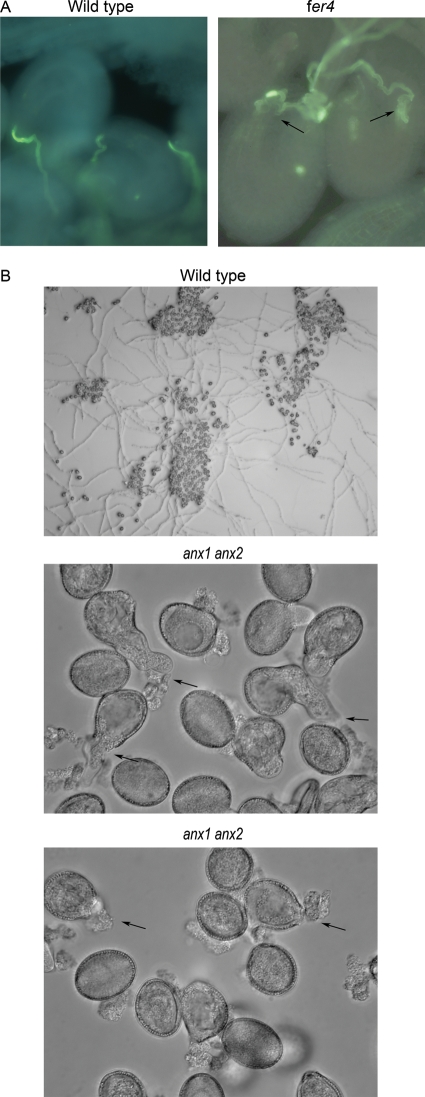
Fig. 3.
FER-family RLKs regulating pollen and pistil functions. (A) Single pollen tube entrance in wild-type (left) and supernumerary pollen tube entrance and overgrowth (arrows) in fer ovules (right). (B) As reported in Miyazaki et al. (2009) and Boisson-Dernier et al. (2009), pollen from double anx1 anx2 mutant rupture (arrows, middle panel) immediately or shortly after tube emergence (arrows, lower panel), while wild-type pollen tubes (top panel) elongate efficiently under similar culture conditions.
ANX1 and ANX2 maintain pollen tube integrity
ANX1 and ANX2 are two of four pollen-specific FER-like RLKs in Arabidopsis and their transcripts accumulate to very high levels in mature pollen (Zimmermann et al. 2004). GFP-labelled ANXs are localized to the apical and subapical membrane (Boisson-Dernier et al. 2009; Miyazaki et al. 2009), a region where spatially and temporally regulated cell expansion is crucial for maintaining tip-focused growth. Mutant analyses showed that ANX1 and ANX2 act redundantly to maintain pollen tube integrity. Plants with homozygous mutant alleles in one of the ANXs and heterozygous ones in the other are normal, whereas double anx1 anx2 mutants are male sterile and their pollen tubes rupture in vitro (Fig. 3B) and burst precociously while growing in the pistil, never reaching the ovules (Boisson-Dernier et al. 2009; Miyazaki et al. 2009). Therefore, the activity from ANXs is apparently needed to maintain cell integrity while pollen tubes are growing within the maternal tissue but must somehow be suppressed upon arrival at the female gametophyte to permit tube disintegration, enabling sperm discharge and therefore fertilization.
Taken together with the function of FER as a facilitator of pollen tube disintegration in the female gametophyte, we cannot avoid speculating that somehow male–female interaction at the point of pollen tube contact with and entrance into the female gametophyte underlies the activation of FER-mediated activity and inactivation of the ANX-regulated pathway. Models have been proposed (Escobar-Restrepo et al. 2007; Boisson-Dernier et al. 2009; Miyazaki et al. 2009; Kanaoka and Torii 2010). For instance, male arrival may trigger activation of FER in the female gametophyte, which in turn inhibits pollen tube growth followed by tube rupture. Alternatively, FER and ANXs may share the same ligand and that in the female gametophyte FER sequesters the ligand, thus preventing ANX-mediated signalling in the pollen tube, resulting in its growth arrest and rupture. Furthermore, it is also possible that as the pollen tube penetrates the female gametophyte, direct interaction between the ANX ECDs on the pollen tip and the ECD of FER along the synergid cell surface may trigger downstream events in both cells. Determining if and how the FER- and ANX-regulated pathways interact allowing the female-controlled pathway to induce male disintegration and the male-operating pathway to permit its own destruction enabling sperm release and fertilization should provide important insight into pollen tube–ovule interaction.
A multifunctional FER is a cell surface regulator for RAC/ROP signalling
FER is broadly expressed except in pollen (Zimmermann et al. 2004). Besides its female gametophytic function in regulating pollen tube growth, FER is important for a broad range of growth and development-related processes, including polarized root hair growth, maintenance of normal trichome morphology and mediation of hormone responses including those of brassinolides, ethylene and auxin (Guo et al. 2009; Deslauriers and Larsen 2010; Duan et al. 2010). Homozygous fer and RNAi-suppressed fer mutants are noticeably growth inhibited throughout development. We discuss here studies that reveal FER as an upstream regulator for RAC/ROP-signalled NADPH oxidase-dependent polarized cell growth (Fig. 4) (Duan et al. 2010).
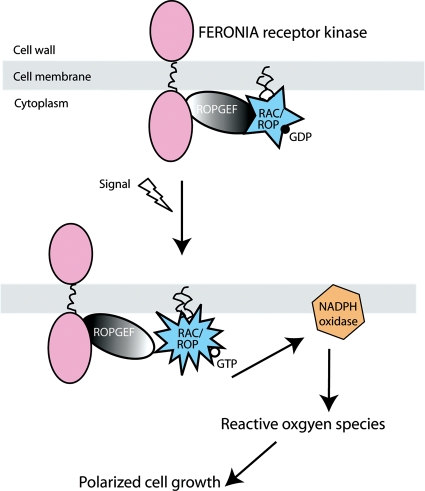
Fig. 4.
A model for FER-mediated RAC/ROP-signalled NADPH oxidase-dependent ROS-regulated polarized cell growth (modified from Duan et al. 2010).
A FER RLK linkage to RAC/ROP signalling
FER was identified as an interacting protein with several Arabidopsis ROPGEFs in a direct effort to identify potential upstream regulators for RAC/ROP signalling by yeast two-hybrid analysis, followed by bimolecular fluorescence complementation assays in plant cells (Duan et al. 2010). Biochemically, an epitope-tagged FER expressed in transformed seedlings and protoplasts was pulled down by RAC/ROPs, which also interact with activator ROPGEFs (Berken et al. 2005; Gu et al. 2006), suggesting at least a tripartite complex with RAC/ROP, FER and ROPGEF providing the linkage between its upstream regulator and downstream effector. Furthermore, RAC/ROP pull-down of FER was considerably more favoured by GDP-saturated RAC/ROPs, consistent with the bridging ROPGEFs interacting preferentially with the inactive form of the GTPases to stimulate GDP/GTP exchange, as has been shown previously in the biochemical characterization of ROPGEFs (Berken et al. 2005). Presumably, upon activation the GTP-bound RAC/ROPs would interact with effectors to activate downstream target pathways, weakening RAC/ROP association with the upstream regulators (Fig. 4).
FER as a cell surface regulator for RAC/ROP-mediated polarized cell growth
Analysis of a T-DNA-induced knockout mutant fer4 and one of the originally described fer mutants, srn (Rotman et al. 2003), showed that they are severely defective in root hair development, along with other growth-suppressed phenotypes (Duan et al. 2010). Like pollen tubes, root hairs grow exclusively at the tip and it is also well established that RAC/ROP signalling at the root hair tip is crucial for proper hair cell formation, starting from the emergence from hair-forming cells, trichoblasts, on the root epidermis to maintaining tip growth. Up-regulating RAC/ROP signalling most prevalently led to highly depolarized growth resulting in ballooned hairs, whereas down-regulating these small GTPases resulted in shorter and wavier root hairs (Molendijk et al. 2001; Jones et al. 2002; Tao et al. 2002). GFP-labelled RAC/ROPs locate to the bulges on trichoblasts that mark the sites of root hair emergence and to the root hair apical membrane throughout its development (Molendijk et al. 2001; Jones et al. 2002). Up-regulating RAC/ROP signalling by over-expressing At-ROP2 also induced more and ectopic emergence of root hairs, some of them developing split tips (Jones et al. 2002), consistent with ectopic activation of RAC/ROP signalling as underlying ectopic growth fronts.
In a series of molecular genetics and physiological studies involving Arabidopsis mutants defective in Root hair defective2 [Rhd2/RbohC encoding an NADPH oxidase that produces reactive oxygen species (ROS)] and Supercentipede1 (Scn1/AtRhoGdi encoding a GDI for RAC/ROPs, thus resulting in up-regulated RAC/ROP signalling), Dolan and colleagues demonstrated a RAC/ROP-dependent pathway whereby active RAC/ROPs are crucial for focusing NADPH oxidase-dependent ROS production at the root hair apical region mediating tip growth (Foreman et al. 2003; Carol et al. 2005; Carol and Dolan 2006). fer root hairs morphologically mimic those observed in rhd2 (Duan et al. 2010) and lack NADPH oxidase-dependent ROS. Although the manner in which ROS regulate cell growth is complex (Knight 2007; Monshausen et al. 2007; Swanson and Gilroy 2010), the fer phenotypes nonetheless implicate FER as functioning in the RAC/ROP-regulated pathway to mediate polarized root hair growth. Moreover, relative to wild-type plants, fer seedlings accumulate significantly lower levels of activated RAC/ROPs while over-expressing RAC/ROPs in fer restores root hair morphology, ROS level and auxin-regulated ROS production and root hair development to normal (Duan et al. 2010). Taken together with results showing physical interactions between FER and ROPGEFs, these functional studies provide strong evidence supporting FER as an upstream regulator for RAC/ROP signalling, specifically that of NADPH oxidase-dependent ROS-mediated polarized root hair growth during seedling development (Fig. 4).
Conclusions and forward look
The discovery of FER acting as an upstream regulator of a well-established RAC/ROP-regulated pathway and the fact that FER, ANX1 and ANX2 play key roles in regulating pollen tube integrity during growth in the pistil suggest potentially that a subset of FER family members could act as surface regulators for RAC/ROP-regulated processes in pollen tube growth. Tip-located ROS and extracellular superoxide to the pollen tubes apex have been detected in growing pollen tubes (Potocký et al. 2007). Reducing ROS by antisense oligonucleotide-mediated suppression of NADPH oxidase expression, application of inhibitors for ROS production or ROS scavengers all inhibited tube growth whereas growth in these NADPH oxidase-suppressed pollen tube could be restored by H2O2 application. Together these observations provide support for ROS playing an important role in regulating pollen tube growth. Given the linkage between FER and RAC/ROP-regulated NADPH oxidase-dependent ROS production in root hairs, it is reasonable to envisage that the role of ANX1 and ANX2 is to regulate a similar pathway in pollen tubes to sustain tip growth. Furthermore, Ca2+ augments apical ROS accumulation in pollen tubes (Potocký et al. 2007). The Ca2+-augmented apical superoxide production apparently requires proper PRK signalling as it could not be induced in LePRK2-suppressed tomato pollen tubes (Zhang et al. 2008). Therefore, it is plausible to suggest convergence of PRK-mediated and the potentially ANX1- and ANX2-regulated pathways in yet unknown crosstalk interactions between these two RLKs.
FER, ANX1 and ANX2 are not the only FER family RLKs expressed in reproductive tissues in Arabidopsis. There are two other closely related pollen-specific family members (At4g39110 and At2g21480) that clearly are functionally non-redundant with ANX1 and ANX2. Furthermore, FER is also prominently expressed in the stigma (Zimmermann et al. 2004; Duan et al. 2010), along with another family member (At5g59700), which is almost exclusively expressed in the stigma (Zimmermann et al. 2004). Determining the biological roles for these yet to be characterized FER family RLKs in the pollination and fertilization processes, and mechanistically dissecting their signalling pathways, will reveal whether they represent additional cell surface regulators for the reproductive process and whether their signalling activity is also mediated by the RAC/ROP switch.
Sources of funding
Work carried out in our laboratory was supported by grants from the United States Department of Agriculture (CSREES 2004-353-4-14873) and the US National Science Foundation (IOB0544222).
Contributions by the authors
All authors contributed to the preparation of the manuscript. The first author contributed to images shown in Figs 2 and 3. The second author contributed to some background studies related to images shown in Fig. 3 and contents in the introductory sections of the manuscripts. The third author contributed to plant materials used for Fig. 3. The fourth and fifth authors contributed to all aspects of the presented work.
Conflicts of interest statement
None declared.
Acknowledgements
We thank Jorge Muschietti, Instituto de Ingeniería Genética y Biología Molecular, Argentina, for helpful suggestions on the manuscript.
References
- Amien S, Kliwer I, Marton ML, Debener T, Geiger D, Becker D, Dresselhaus T. Defensin-like ZmES4 mediates pollen tube burst in maize via opening of the potassium channel KZM1. PLoS Biology. 2010;8:e1000388. doi: 10.1371/journal.pbio.1000388. doi:10.1371/journal.pbio.1000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur KM, Vejlupkova Z, Meeley RB, Fowler JE. Maize ROP2 GTPase provides a competitive advantage to the male gametophyte. Genetics. 2003;165:2137–2151. doi: 10.1093/genetics/165.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berken A, Thomas C, Wittinghofer A. A new family of RhoGEFs activates the Rop molecular switch in plants. Nature. 2005;436:1176–1180. doi: 10.1038/nature03883. doi:10.1038/nature03883. [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier A, Roy S, Kritsas K, Brobei MA, Jaciubek M, Schroeder JI, Brossniklaus U. Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development. 2009;136:3279–3288. doi: 10.1242/dev.040071. doi:10.1242/dev.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A, Kessler SA, Grossniklaus U. The walls have ears: the role of plnt CrRLK1s in sensing and transducing extracellular signals. Journal of Experimental Botany. 2011;62:1581–1591. doi: 10.1093/jxb/erq445. doi:10.1093/jxb/erq445. [DOI] [PubMed] [Google Scholar]
- Carol RJ, Dolan L. The role of reactive oxygen species in cell growth: lessons from root hairs. Journal of Experimental Botany. 2006;57:1829–1834. doi: 10.1093/jxb/erj201. [DOI] [PubMed] [Google Scholar]
- Carol RJ, Takeda S, Linstead P, Currant MC, Kakesova H, Derbyshire P, Drea S, Zarsky V, Dolan L. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature. 2005;438:1013–1016. doi: 10.1038/nature04198. doi:10.1038/nature04198. [DOI] [PubMed] [Google Scholar]
- Chen CY, Cheung AY, Wu HM. Actin depolymerizing factor mediates Rac/Rop GTPase regulated pollen tube growth. Plant Cell. 2003;15:237–249. doi: 10.1105/tpc.007153. doi:10.1105/tpc.007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Wu HM. Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annual Review of Plant Biology. 2008;59:547–572. doi: 10.1146/annurev.arplant.59.032607.092921. doi:10.1146/annurev.arplant.59.032607.092921. [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wang H, Wu HM. A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell. 1995;82:383–393. doi: 10.1016/0092-8674(95)90427-1. doi:10.1016/0092-8674(95)90427-1. [DOI] [PubMed] [Google Scholar]
- Cheung AY, Chen C, Glaven R, Vidali L, Hepler PK, Wu H-M. Rab2 regulate vesicular transport between endoplasmic reticulum and Golgi bodies and is important for pollen tube elongation. Plant Cell. 2002;14:945–962. doi: 10.1105/tpc.000836. doi:10.1105/tpc.000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Duan Q, de Costa S, de Graaf B, di Stilio V, Wu HM. The dynamic pollen tube cytoskeleton: live cell studies using actin-binding and microtubule-binding reporter proteins. Molecular Plant. 2008;1:686–702. doi: 10.1093/mp/ssn026. doi:10.1093/mp/ssn026. [DOI] [PubMed] [Google Scholar]
- Covey PA, Subbaiah CC, Parsons RL, Pearce G, Lay FT, Anderson MA, Ryan CA, Bedinger PA. A pollen-specific RALF from tomato that regulates pollen tube elongation. Plant Physiology. 2010;153:703–715. doi: 10.1104/pp.110.155457. doi:10.1104/pp.110.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I, Vos U, Jurgen G, Beeckman T. Receptor-like kinases shape the plant. Nature Cell Biology. 2009;11:1166–1172. doi: 10.1038/ncb1009-1166. doi:10.1038/ncb1009-1166. [DOI] [PubMed] [Google Scholar]
- Deslauriers SD, Larsen PB. FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Molecular Plant. 2010;3:626–640. doi: 10.1093/mp/ssq015. doi:10.1093/mp/ssq015. [DOI] [PubMed] [Google Scholar]
- Dresselhaus T, Marton ML. Micropylar pollen tube guidance and burst: adapted from defense mechanism? Current Opinion in Plant Biology. 2009;12:773–780. doi: 10.1016/j.pbi.2009.09.015. doi:10.1016/j.pbi.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Duan Q, Kita D, Li C, Cheung AY, Wu HM. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proceedings of the National Academy of Sciences of the USA. 2010;107:17821–17826. doi: 10.1073/pnas.1005366107. doi:10.1073/pnas.1005366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund DM, Svensson EM, Kost B. Physcomitrella patens: a model to investigate the role of RAC/ROP GTPase signalling in tip growth. Journal of Experimental Botany. 2010;61:1917–1937. doi: 10.1093/jxb/erq080. doi:10.1093/jxb/erq080. [DOI] [PubMed] [Google Scholar]
- Escobar-Restrepo J-M, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang W-C, Grossniklaus U. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;317:656–660. doi: 10.1126/science.1143562. doi:10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, Davies JM, Dolan L. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. doi:10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- Fowler JE. Evolution of the ROP GTPase signaling module. In: Yalovsky S, Baluska F, Jones A, editors. Integrated G proteins signaling in plants. Berlin: Springer; 2010. pp. 305–327. [Google Scholar]
- Fu Y, Wu G, Yang Z. Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. Journal of Cell Biology. 2001;152:1019–1032. doi: 10.1083/jcb.152.5.1019. doi:10.1083/jcb.152.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Li S, Lord EM, Yang Z. Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. Plant Cell. 2006;18:366–381. doi: 10.1105/tpc.105.036434. doi:10.1105/tpc.105.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo HQ, Li L, Ye HX, Yu XF, Algreen A, Yin YH. Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the USA. 2009;106:7648–7653. doi: 10.1073/pnas.0812346106. doi:10.1073/pnas.0812346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon V, Tang WH, Monti MM, Raiola A, Lorenzo GD, McCormick S, Taylor LP. Antisense phenotypes reveal a role for SHY, a pollen-specific leucine-rich repeat protein, in pollen tube growth. Plant Journal. 2004;39:643–654. doi: 10.1111/j.1365-313X.2004.02162.x. doi:10.1111/j.1365-313X.2004.02162.x. [DOI] [PubMed] [Google Scholar]
- Hématy K, Höfte H. Novel receptor kinases involved in growth regulation. Current Opinion in Plant Biology. 2008;11:321–328. doi: 10.1016/j.pbi.2008.02.008. doi:10.1016/j.pbi.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Hématy K, Sado PE, Van Tuinen A, Rochange S, Desnos T, Balzegue S, Pelletier S, Renou JP, Hofte H. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Current Biology. 2007;17:922–931. doi: 10.1016/j.cub.2007.05.018. doi:10.1016/j.cub.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Huck N, Moore JM, Federer M, Grossniklaus U. The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development. 2003;130:2149–2159. doi: 10.1242/dev.00458. doi:10.1242/dev.00458. [DOI] [PubMed] [Google Scholar]
- Hwang JU, Gu Y, Lee YJ, Yang Z. Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Molecular Biology of the Cell. 2005;16:5385–5399. doi: 10.1091/mbc.E05-05-0409. doi:10.1091/mbc.E05-05-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JU, Vernoud V, Szumlanski A, Nielsen E, Yang Z. A tip-localized RhoGAP controls cell polarity by globally inhibiting Rho GTPase at the cell apex. Current Biology. 2008;18:1907–1916. doi: 10.1016/j.cub.2008.11.057. doi:10.1016/j.cub.2008.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MA, Shen JJ, Fu Y, Li H, Yang Z, Grierson CS. The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell. 2002;14:763–776. doi: 10.1105/tpc.010359. doi:10.1105/tpc.010359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaoka MM, Torii KU. FERONIA as an upstream receptor kinase for polar cell growth in plants. Proceedings of the National Academy of Sciences of the USA. 2010;107:17461–17462. doi: 10.1073/pnas.1013090107. doi:10.1073/pnas.1013090107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaothien P, Ok SH, Shuai B, Wengier D, Cotter R, Kelley D, Kiriakopolos S, Muschietti J, McCormick S. Kinase partner protein interacts with the LePRK1 and LePRK2 receptor kinases and plays a role in polarized pollen tube growth. Plant Journal. 2005;42:492–503. doi: 10.1111/j.1365-313X.2005.02388.x. doi:10.1111/j.1365-313X.2005.02388.x. [DOI] [PubMed] [Google Scholar]
- Kim HU, Cotter R, Johnson S, Senda M, Dodds P, Kulikauskas R, Tang W, Ezcurra I, Herzmark P, McCormick S. New pollen-specific receptor kinases identified in tomato, maize and Arabidopsis: the tomato kinases show overlapping but distinct localization patterns on pollen tubes. Plant Molecular Biology. 2002;50:1–16. doi: 10.1023/a:1016077014583. doi:10.1023/A:1016077014583. [DOI] [PubMed] [Google Scholar]
- Kim S, Mollet JC, Dong J, Zhang K, Park SY, Lord EM. Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proceedings of the National Academy of Sciences of the USA. 2003;100:16125–16130. doi: 10.1073/pnas.2533800100. doi:10.1073/pnas.2533800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahre U, Kost B. Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. Plant Cell. 2006;18:3033–3046. doi: 10.1105/tpc.106.045336. doi:10.1105/tpc.106.045336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahre U, Becker C, Schmitt AC, Kost B. Nt-RhoGDI2 regulates Rac/Rop signaling and polar cell growth in tobacco pollen tubes. Plant Journal. 2006;46:1018–1031. doi: 10.1111/j.1365-313X.2006.02757.x. doi:10.1111/j.1365-313X.2006.02757.x. [DOI] [PubMed] [Google Scholar]
- Knight MR. New ideas on root hair growth appear from the flanks. Proceedings of the National Academy of Sciences of the USA. 2007;104:20649–20650. doi: 10.1073/pnas.0710632105. doi:10.1073/pnas.0710632105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B. Spatial control of Rho (Rac-Rop) signaling in tip-growing plant cells. Trends in Cell Biology. 2008;18:119–127. doi: 10.1016/j.tcb.2008.01.003. doi:10.1016/j.tcb.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Kost B, Lemichez E, Spielhofer P, Hong Y, Tolias K, Carpenter C, Chua NH. Rac homologurs and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. Journal of Cell Biology. 1999;145:317–330. doi: 10.1083/jcb.145.2.317. doi:10.1083/jcb.145.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavy M, Bloch D, Hazak O, Gutman I, Poraty L, Sorek N, Sternberg H, Yalovsky S. A novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Current Biology. 2007;17:947–952. doi: 10.1016/j.cub.2007.04.038. doi:10.1016/j.cub.2007.04.038. [DOI] [PubMed] [Google Scholar]
- Lehti-Shiu MD, Zou C, Hanada K, Shiu S-H. Evolutionary history and stress regulation of plant receptor-like Kinase/Pelle genes. Plant Physiology. 2009;150:12–26. doi: 10.1104/pp.108.134353. doi:10.1104/pp.108.134353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure BA, Franklin-Tong V. Gametophytic self-incompatibility: understanding the cellular mechanisms involved in ‘self’ pollen tube inhibition. Planta. 2006;224:233–245. doi: 10.1007/s00425-006-0284-2. doi:10.1007/s00425-006-0284-2. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Murata T, Sakurai-Ozato N, Kubo M, Demura T, Fukudo H. ANXUR1 and 2, Sister genes to FERONIA/SIRENE, are male factors for coordinated fertilization. Current Biology. 2009;19:1327–1331. doi: 10.1016/j.cub.2009.06.064. doi:10.1016/j.cub.2009.06.064. [DOI] [PubMed] [Google Scholar]
- Molendijk AJ, Bischoff F, Rajendrakumar CS, Friml J, Braun M, Gilroy S, Palme K. Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO Journal. 2001;20:2779–2788. doi: 10.1093/emboj/20.11.2779. doi:10.1093/emboj/20.11.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollet JC, Park SY, Nothnagel EA, Lord EM. A lily stylar pectin is necessary for pollen tube adhesion to an in vitro stylar matrix. Plant Cell. 2000;12:1737–1750. doi: 10.1105/tpc.12.9.1737. doi:10.1105/tpc.12.9.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proceedings of the National Academy of Sciences of the USA. 2007;104:20996–21001. doi: 10.1073/pnas.0708586104. doi:10.1073/pnas.0708586104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschietti J, Dircks L, Vancanneyt G, McCormick S. LAT52 protein is essential for tomato pollen development: pollen expressing antisense LAT52 RNA hydrates and germinates abnormally and cannot achieve fertilization. Plant Journal. 1994;6:321–338. doi: 10.1046/j.1365-313x.1994.06030321.x. doi:10.1046/j.1365-313X.1994.06030321.x. [DOI] [PubMed] [Google Scholar]
- Muschietti J, Eyal Y, McCormick S. Pollen tube localization implies a role in pollen-pistil interactions for the tomato receptor-like protein kinases LePRK1 and LePRK2. Plant Cell. 1998;10:319–330. doi: 10.1105/tpc.10.3.319. doi:10.1105/tpc.10.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibau C, Cheung A. New insights into the functional roles of CrRLKs in the control of plant cell growth and development. Plant Signaling Behaviour. 2011;6:1–5. doi: 10.4161/psb.6.5.14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibau C, Wu H-M, Cheung AY. RAC/ROP GTPases: ‘hubs’ for signal integration and diversification in plants. Trends in Plant Science. 2006;11:309–315. doi: 10.1016/j.tplants.2006.04.003. doi:10.1016/j.tplants.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Okuda S, et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature. 2009;458:357–362. doi: 10.1038/nature07882. 22 other coauthors doi:10.1038/nature07882. [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Preuss D. Distinct short-range ovule signals attract or repel Arabidopsis thaliana pollen tubes in vitro. BMC Plant Biology. 2006;6 doi: 10.1186/1471-2229-6-7. 7. doi:10.1186/1471-2229-6-7 doi: 10.1186/1471-2229-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocký M, Jones MA, Bezvoda R, Smirnoff N, Žárský V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytologist. 2007;174:742–751. doi: 10.1111/j.1469-8137.2007.02042.x. doi:10.1111/j.1469-8137.2007.02042.x. [DOI] [PubMed] [Google Scholar]
- Punwani JA, Rabiger DS, Drews GN. MYB98 positively regulates a battery of synergid-expressed genes encoding filiform apparatus-localized proteins. Plant Cell. 2007;19:2557–2568. doi: 10.1105/tpc.107.052076. doi:10.1105/tpc.107.052076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Soondek J. GEF means GO: turning on RHO GTPases with guanine nucleotide-exchange factors. Nature Molecular Cell Biology. 2005;6:167–179. doi: 10.1038/nrm1587. doi:10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Rotman N, Rozier F, Boavida L, Dumas C, Berger F, Faure JE. Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Current Biology. 2003;13:432–436. doi: 10.1016/s0960-9822(03)00093-9. doi:10.1016/S0960-9822(03)00093-9. [DOI] [PubMed] [Google Scholar]
- Salem T, Mazzella A, Barberini ML, Wengier D, Motillo V, Parisi G, Muschietti J. Mutations in two putative phosphorylation motifs in the tomato pollen receptor kinase LePRK2 show antagonistic effects on pollen tube length. Journal of Biological Chemistry. 2011;286:4882–4891. doi: 10.1074/jbc.M110.147512. doi:10.1074/jbc.M110.147512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandaklie-Nikolova L, Palanivelu R, King EJ, Copenhaver GP, Drews GN. Synergid cell death in Arabidopsis is triggered following direct interaction with the pollen tube. Plant Physiology. 2007;144:1753–1762. doi: 10.1104/pp.107.098236. doi:10.1104/pp.107.098236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallus T, Jaeckh C, Feher K, Palma AS, Liu Y, Simpson JC, Mackeen M, Stier G, Gibson TJ, Feizi T, Pieler T, Muhle-Goll C. Melectin: a novel carbohydrate-binding protein of the endoplasmic reticulum and a candidate player in the early steps of protein N-glycosylation. Molecular Biology of the Cell. 2008;19:3404–3414. doi: 10.1091/mbc.E08-04-0354. doi:10.1091/mbc.E08-04-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu KK, Okada K. Attractive and repulsive interactions between female and male gametophytes in Arabidopsis pollen tube guidance. Development. 2000;127:4511–4518. doi: 10.1242/dev.127.20.4511. [DOI] [PubMed] [Google Scholar]
- Shiu S-H, Bleecker AB. Expansion of the receptor-like kinase/pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiology. 2003;132:530–543. doi: 10.1104/pp.103.021964. doi:10.1104/pp.103.021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson S, Gilroy S. ROS in plant development. Physiologia Plantarum. 2010;138:384–392. doi: 10.1111/j.1399-3054.2009.01313.x. doi:10.1111/j.1399-3054.2009.01313.x. [DOI] [PubMed] [Google Scholar]
- Tang W, Ezcurra I, Muschietti J, McCormick S. A cysteine-rich extracellular protein, LAT52, interacts with the extracellular domain of the pollen receptor kinase LePRK2. Plant Cell. 2002;14:2277–2287. doi: 10.1105/tpc.003103. doi:10.1105/tpc.003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Kelley D, Ezcurra I, Cotter R, McCormick S. LeSTIG1, an extracellular binding partner for the pollen receptor kinases LePRK1 and LePRK2, promotes pollen tube growth in vitro. Plant Journal. 2004;39:343–353. doi: 10.1111/j.1365-313X.2004.02139.x. doi:10.1111/j.1365-313X.2004.02139.x. [DOI] [PubMed] [Google Scholar]
- Tantikanjana T, Nasrallah ME, Nasrallah JB. Complex networks of self-incompatiblity signaling in the Brassicaceae. Current Opinion in Plant Biology. 2010;13:520–526. doi: 10.1016/j.pbi.2010.06.004. doi:10.1016/j.pbi.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Tao LZ, Cheung AY, Wu H-M. Plant Rac-like GTPases are activated by auxin and mediate auxin-responsive gene expression. Plant Cell. 2002;14:2745–2760. doi: 10.1105/tpc.006320. doi:10.1105/tpc.006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii KU. Leucine-rich repeat receptor kinases in plants: structure, function, and signal transduction pathways. International Review of Cytology. 2004;234:1–46. doi: 10.1016/S0074-7696(04)34001-5. doi:10.1016/S0074-7696(04)34001-5. [DOI] [PubMed] [Google Scholar]
- Wengier D, Valsecchi I, Cabanas ML, Tang WH, McCormick S, Muschietti J. The receptor kinases LePRK1 and LePRK2 associate in pollen and when expressed in yeast, but dissociate in the presence of style extract. Proceedings of the National Academy of Sciences of the USA. 2003;100:6860–6865. doi: 10.1073/pnas.0631728100. doi:10.1073/pnas.0631728100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengier D, Massella MA, Salem TM, McCormick S, Muschietti JP. STIL, a peculiar molecule from styles, specifically dephosphorylates the pollen receptor kinase LePRK2 and stimulates pollen tube growth in vitro. BMC Plant Biology. 2010;10:33. doi: 10.1186/1471-2229-10-33. doi:10.1186/1471-2229-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Gu Y, Li S, Yang Z. A genome-wide analysis of Arabidopsis Rop-interactive CRIB motif-containing proteins that act as Rop GTPase targets. Plant Cell. 2001;13:2841–2856. doi: 10.1105/tpc.010218. doi:10.1105/tpc.13.12.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HM, Wang H, Cheung AY. A pollen tube growth stimulatory glycoprotein is deglycosylated by pollen tubes and displays a glycosylation gradient in the flower. Cell. 1995;82:395–403. doi: 10.1016/0092-8674(95)90428-x. doi:10.1016/0092-8674(95)90428-X. [DOI] [PubMed] [Google Scholar]
- Wu H-M, Hazak O, Cheung AY, Yalvolsky S. 2011. Rac/Rop GTPases in auxin signaling: a perspective. Plant Cell, in press, April.
- Yalovsky S, Bloch D, Sorek N, Kost B. Regulation of membrane trafficking, cytoskeleton dynamics, and cell polarity by ROP/RAC GTPases. Plant Physiology. 2008;147:1527–1543. doi: 10.1104/pp.108.122150. doi:10.1104/pp.108.122150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky S, Baluska F, Jones A. Integrated G proteins signaling in plants. Berlin: Springer; 2010. [Google Scholar]
- Yang Z. Cell polarity signaling in Arabidopsis. Annual Review of Cell and Developmental Biology. 2008;24:551–575. doi: 10.1146/annurev.cellbio.23.090506.123233. doi:10.1146/annurev.cellbio.23.090506.123233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Diego W, Shuai B, Gui C, Muschietti J, McCormick S, Tang W-H. The pollen receptor kinase LePRK2 mediates growth promoting signals and positively regulates pollen germination and tube growth. Plant Physiology. 2008;148:1368–1379. doi: 10.1104/pp.108.124420. doi:10.1104/pp.108.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, McCormick S. A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the USA. 2007;104:18830–18835. doi: 10.1073/pnas.0705874104. doi:10.1073/pnas.0705874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiology. 2004;136:2621–2632. doi: 10.1104/pp.104.046367. doi:10.1104/pp.104.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]