Abstract
Mergenhagen, Stephan E. (National Institutes of Health, Bethesda, Md.). Polysaccharide-lipid complexes from Veillonella parvula. J. Bacteriol. 90:1730–1734. 1965.—A strain of Veillonella parvula (V2) elaborates an extracellular slime when grown in a nutrient medium containing only dialyzable components. Deproteinization with chloroform-butanol of ethyl alcohol-precipitated material from the supernatant culture fluid leads to the isolation of a water-soluble lipopolysaccharide (LPS1). Another component (LPS2), showing similarity in biological and immunological properties to the endotoxic antigen (LPC) isolated from whole cells, was extracted with phenol from the insoluble emulsion remaining after chloroform-butanol extraction of slime. Analysis of polysaccharides by thin-layer chromatography demonstrated the presence of glucose and galactose in LPS1 and glucose, glucosamine, galactosamine, and a methyl pentose in LPC. LPS1 failed to give a positive epinephrine skin test after intravenous injection in rabbits and failed to kill pertussis-sensitized mice, whereas LPS2 and LPC were active in both of these bioassays. Both lipopolysaccharides (LPS1 and LPC) exhibited type-specific haptenic activity in hemagglutination tests with numerous anti-Veillonella rabbit sera. LPS1 was found in these tests to be unrelated to a heterologous strain of Veillonella possessing a related somatic antigen. These experiments reveal the presence of two chemically and immunologically distinguishable polysaccharide-lipid complexes in this strain of V. parvula.
Full text
PDF
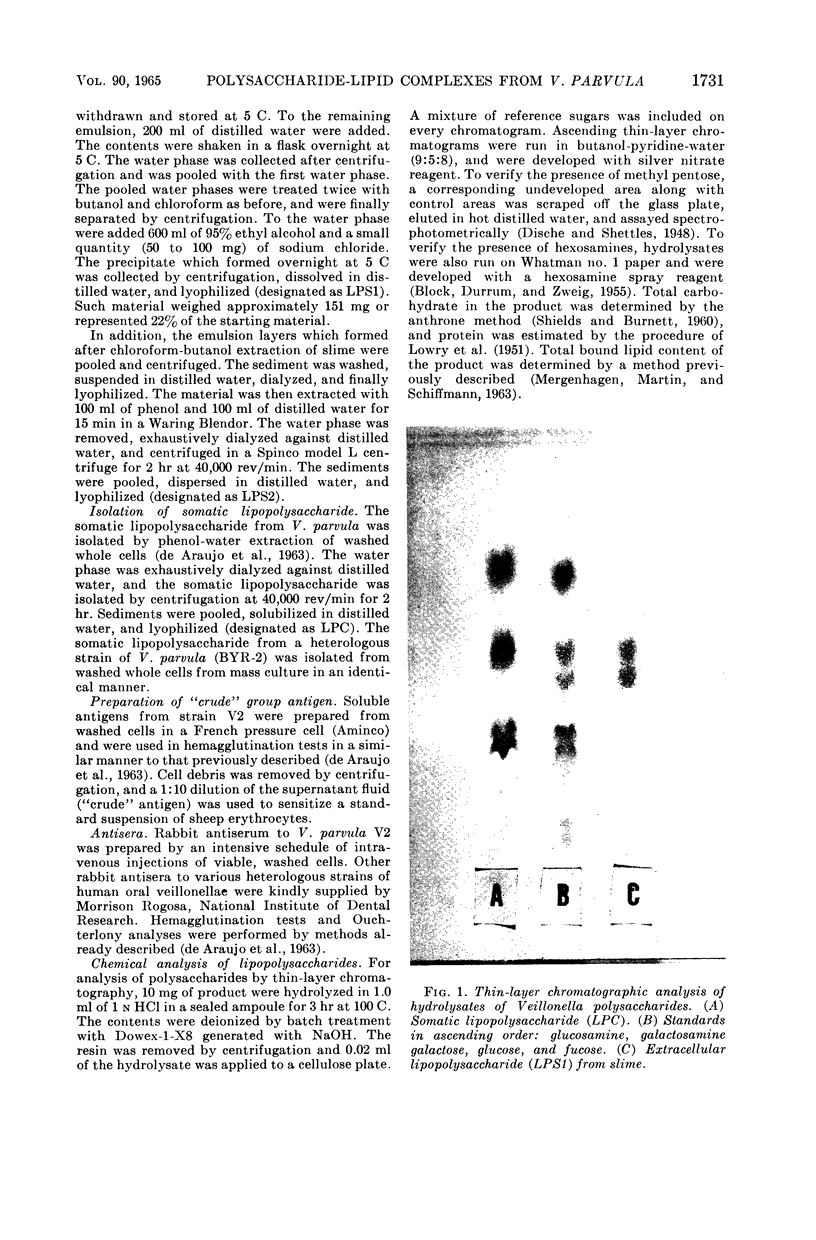
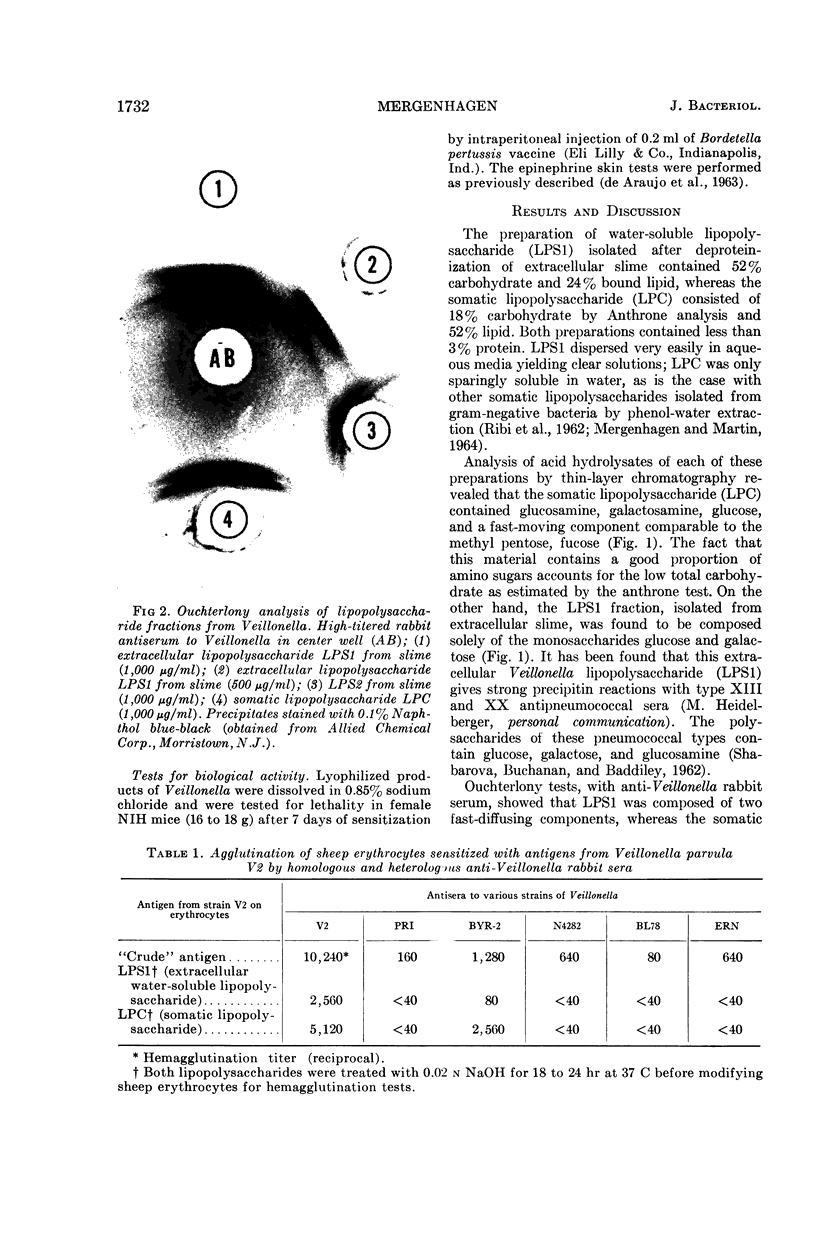


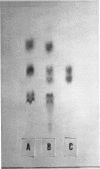
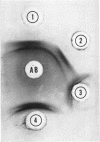
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DEARAUJO W. C., VARAH E., MERGENHAGEN S. E. IMMUNOCHEMICAL ANALYSIS OF HUMAN ORAL STRAINS OF FUSOBACTERIUM AND LEPTOTRICHIA. J Bacteriol. 1963 Oct;86:837–844. doi: 10.1128/jb.86.4.837-844.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MERGENHAGEN S. E., MARTIN G. R. PROPERTIES OF A LYSOZYME-DISSOCIATED ENDOTOXIC FRACTION FROM ESCHERICHIA COLI. J Bacteriol. 1964 Oct;88:1169–1174. doi: 10.1128/jb.88.4.1169-1174.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERGENHAGEN S. E., MARTIN G. R., SCHIFFMANN E. Studies on an endotoxin of a group C Neisseria meningitidis. J Immunol. 1963 Feb;90:312–317. [PubMed] [Google Scholar]
- MERGENHAGEN S. E., VARAH E. Serologically specific lipopolysaccharides from oral veillonella. Arch Oral Biol. 1963 Jan-Feb;8:31–36. doi: 10.1016/0003-9969(63)90089-x. [DOI] [PubMed] [Google Scholar]
- RIBI E., HASKINS W. T., MILNER K. C., ANACKER R. L., RITTER D. B., GOODE G., TRAPANI R. J., LANDY M. Physicochemical changes in endotoxin associated with loss of biological potency. J Bacteriol. 1962 Oct;84:803–814. doi: 10.1128/jb.84.4.803-814.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHABAROVA Z. A., BUCHANAN J. G., BADDILEY J. The composition of pneumococcus type-specific substances containing phosphorus. Biochim Biophys Acta. 1962 Feb 12;57:146–148. doi: 10.1016/0006-3002(62)91091-0. [DOI] [PubMed] [Google Scholar]
- SRIVASTAVA H. C., BREUNINGER E., CREECH H. J., ADAMS G. A. Preparation and properties of polysaccharide-lipid complexes from Serratia marcescens. Can J Biochem Physiol. 1962 Jul;40:905–918. [PubMed] [Google Scholar]