Abstract
AIM: To study the ability of human adipose-derived mesenchymal stem cells (AMSCs) to survive over the short and long term, their biodistribution and their biosafety in vivo in tumor-prone environments.
METHODS: We subcutaneously injected human AMSCs from different human donors into immunodeficient SCID mice over both short- (2 and 4 mo) and long- (17 mo) term in young, and aged tumor-prone mice. Presence of human cells was studied by immunohistochemistry and polymerase chain reaction analysis in all organs of injected mice.
RESULTS: Subcutaneously injected AMSCs did not form teratomas at any time point. They did not migrate but remained at the site of injection regardless of animal age, and did not fuse with host cells in any organ examined. AMSCs survived in vivo for at least 17 mo after injection, and differentiated into fibroblasts of the subdermic connective tissue and into mature adipocytes of fat tissue, exclusively at the site of injection.
CONCLUSION: Our results support the assertion that AMSC may be safe candidates for therapy when injected subcutaneously because of their long term inability to form teratomas.
Keywords: Adipose-derived stem cells, Cell transplant, SCID mice, Teratoma, Mesenchymal stem cells, Cell therapy, Biosafety
INTRODUCTION
Adult stem cells, particularly mesenchymal stem cells (MSCs), constitute an essential tool for tissue regeneration in cell therapy studies. Stem cells can induce tissue regeneration either by migrating towards the damaged tissue of the host, and under the influence of local signals, differentiating into cells of suitable phenotype, or by secreting cytokines that can restore tissue homeostasis of the injured tissue[1].
In recent years, different populations of MSCs have been isolated in a variety of adult tissues including bone marrow (BM), skeletal muscle, dental pulp, bone and adipose tissue[2,3]. Adipose tissue is an attractive source of mesenchymal cells as the method of isolation, unlike in other tissues, is easily accessible and not painful for the donor. In addition the cell yield obtained is very high relative to other sources[4]. It has been reported that adipose derived MSCs (AMSCs) are able to differentiate in vitro into several cell types including adipocytes, condrocytes and osteocytes[5]. This ability, together with their strong immunosuppressive effects, makes AMSCs promising candidates for cell therapy. However, further understanding is needed of the mechanisms involved in tissue regeneration by the transplanted MSCs in vivo. Before these cells can be used for patient treatment, there is a need to analyze their ability to migrate and survive over the long term, possible dependence on the route of administration, and aspects of their biosafety including in vivo transformation and tumor aggravation.
Different routes of MSCs transplantation in disease models have been described, including intravenous, intraperitoneal, intra damaged organ, and subcutaneous routes. Systemic intravenous infusion of human BM MSCs in rats showed, after 1 wk, entrapment of the donor cells mostly in the lungs with smaller numbers in the liver, heart, and spleen[6]. In studies using different murine models, grafted MSCs migrated and settled in the lungs, spleen, liver, intestine, BM, and skin, at 48 h[7,8]. However, Aguilar et al[9] reported that after 4 wk less than 0.01% of cells were detectable in the lungs of normal mice. Intraperitoneal xenotransplantation of human AMSC (hAMSC) in mice resulted in engraftment in BM, spleen, lymph node, thymus, liver, kidney, pancreas, lung, heart, brain, and eye at 2 to 4 mo after transplantation[10,11]. Another route of MSC transplantation is to engraft the cells directly onto the damaged host tissue. In a rat model after myocardial infarction, delivery of MSCs by left ventricular cavity infusion enhanced migration and colonization of the cells preferentially to the ischemic myocardium, although MSCs were also identified in the lung, liver, spleen, and BM 1 wk after infusion[6]. Intramuscular implantation of hAMSCs in mice indicated that the liver was the preferred target organ for colonization after 8 mo[12]. Lastly, AMSCs expressing eGFP transgene subcutaneously injected in mice were detected by DNA polymerase chain reaction (PCR) in the spleen, liver, lung, kidney, brain and fat up to 2 mo after transplantation, and in heart, spleen, lung, muscle, and brain up to 2.5 mo after transplantation[11].
Another important issue is the safety of MSC transplantation. Teratoma contribution by subcutaneous injection in immunodeficient mice is a standard technique for studying the teratogenic and oncogenic potential of many different types of stem cells. In fact, it has been described that human MSCs can migrate and integrate into preexisting tumors after intravascular or local delivery, being detected up to 2 mo after transplantation[13-15]. Tumor stroma formation of human BM-MSCs after subcutaneous co-injection with A375SM melanoma cells showed not only passive incorporation of MSCs into the tumor architecture but also MSC proliferation. However, MSCs proliferation was not observed when MSCs were injected alone without malignant cells[13]. Similar results were obtained by Annabi et al[16] after subcutaneous MSC co-injection with malignant glioma cells and by Karnoub et al[17] with human breast cancer cells. Intramuscular injection of hAMSCs in mice showed that the implanted cells tended to maintain a steady state population, did not proliferate rapidly after implantation, and resulted in neither detectable chromosomal abnormalities nor tumors after 8 mo[12].
Given these data, all aspects of biosafety of MSCs including trafficking and differentiation capability, oncogenic transformation, homing to tumor microenvironment and angiogenesis promotion, should be studied. These studies should be performed both over the short and long-term after in vivo transplantation, and in tumor-prone in vivo microenvironments to verify their safe use in host disease models. In this study, we have subcutaneously injected human AMSCs from different human donors into immunodeficient SCID mice at both short- (2 and 4 mo) and long- (17 mo) term, and also both in young and old tumor-prone mice. The presence of human cells was studied by immunohistochemistry and PCR analysis in all organs of injected mice. Subcutaneously-injected AMSCs did not form teratomas at any time point. They did not migrate but remained at the site of injection regardless of animal age, and did not fuse with host cells in any organ examined. AMSCs survived in vivo for at least 17 mo after injection, and differentiated into fibroblasts of the subdermic connective tissue and into mature adipocytes of fat tissue, exclusively at the site of injection.
MATERIALS AND METHODS
AMSCs isolation and cell culture
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. Lipoaspirate was obtained from four donor patients who underwent elective liposuction after giving informed consent. The isolation protocols were approved by the Institutional Review Boards of La Paz Hospital (Madrid, Spain). To obtain AMSCs, the protocol of Zuk et al[4] was followed. Briefly, lipoaspirate was washed extensively with phosphate-buffered saline followed by digestion in 0.075% collagenase Type I for 30 min at 37°C. Digestion was neutralized with Dulbecco’s modified Eagle’s medium (DMEM, Gibco-BRL, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA). The digested adipose tissue was centrifuged at 300 g for 10 min. The cell pellet was cultured in a noninductive medium consisting of DMEM containing 1 mmol/L sodium pyruvate and 2 mmol/L glutamine (Sigma-Aldrich, St. Louis, USA), 10% FBS and 100 U/mL penicillin G and streptomycin (Gibco). The purified cell population is referred to as AMSC cells. Media were removed, and nonadherent cells were flushed away 12-18 h later followed by refeeding with fresh media. Cells were maintained at subconfluence by dissociation with 0.05% trypsin-EDTA (Sigma) and replating under the same culture conditions. Passages were done 1:1 every 72 h. To functionally characterize the isolated AMSC population we tested the cells for their potential for multipotent linage differentiation, by in vitro differentiating the cells into osteo, myo, adipo and chondro lineages following the protocols described in Zuk et al[5] and previously published by our group[18]. Achievement of differentiation was demonstrated by alkaline phosphatase activity for osteogenic differentiation, by anti-sarcomeric-α-actin (Dako, 1:100) for myogenic differentiation, by Oil red staining for adipogenic differentiation and by Alcian blue staining for chondrogenic differentiation. Passages used were P2, which represents 1 population doubling, P3: two population doublings, P6: five population doublings, P7: six population doublings and P11: ten population doublings. Undifferentiated AMSCs were used at 2 and 6 passages in the first group of short-term transplanted NOD/SCID mice (2 mo), and at 7 and 11 passages in the second group of short-term transplanted SCID/NOD mice (4 mo). AMSCs were used at passages 3 and 7 in the long-term transplanted SCID/NOD mice group (17 mo).
Human NTERA-2 cell line,obtained commercially (American Type Culture Collection, Manassas, VA, USA), was used for positive control of teratoma formation, and was cultured in DMEM containing 1 mmol/L sodium pyruvate and 2 mmol/L glutamine, 10% FBS, 0.1mmol/L non-essential amino acids (Gibco) and 100 U/mL penicillin G and streptomycin (Gibco). Cells were kept in a humidified atmosphere with 5% CO2 at 37°C.
Xenotransplantation of human cells
Recipient NOD.CB17-Prkdcscid/J mice were obtained from Harlan Laboratories (Indianapolis, IN, USA) and maintained in a specific pathogen-free environment throughout the experiments. All animal-related procedures were performed in accordance with guidelines set forth by the animal research committees at La Paz Hospital, Spain. To evaluate the biosafety and the possible transformation of human AMSCs over the short- (2 and 4 mo) and long- (17 mo) term experiments were performed using three groups of ten mice each. Group 1 (young mice at short term): Two-month-old mice sacrificed at 2 mo after cell injection. Group 2 (young mice at long term): Three month-old-mice sacrificed at 17 mo after cell injection, and Group 3 (old mice at short term): One-year-old NOD/SCID mice sacrificed at 4 mo after cell injection. Inclusion of a group of old mice at long term was not possible, as average life span of SCID mice homozygotes in an SPF environment is 8.5 mo, allowing insufficient time to complete long term experiments with this mouse model. In each group, 3.5 × 106 AMSCs in 100 μL saline solution was injected subcutaneously into the dorsal side of each mouse (n = 8). One negative control animal per experiment was injected with saline solution alone, and injection of 3.5 × 106 NTERA-2 cells (human embryonal carcinoma cell line, ATCC) was used as a positive control for teratoma formation in one mouse per experiment. The SCID mouse used (NOD.CB17-Prkdcscid/J) is a standardized model for teratoma formation and spontaneous thymic T cell lymphomas are found in more than 10% of homozygotes at 5-9 mo of age, metastasizing to multiple sites and constituting the major cause of death. Therefore, The Committee for Animal Welfare considered that one animal in both the teratoma control group and the saline control group would be enough to ensure that the manipulation of cells, cell injection and animal housing were done correctly, while generating the teratoma or the thymic lymphoma which would certainly cause significant suffering to the animal.
Tissue procurement
Animals were sacrificed by CO2 inhalation. All organs of the thirty NOD/SCID mice that were sacrificed at 2, 4 or 17 mo after transplant were extracted. Tissues analyzed were: Brain, trachea, esophagus, thyroid gland, lungs, heart, thymus, stomach, intestine, kidneys, liver, pancreas, spleen, bone, muscle, skin, reproductive glands and testis. The tissues were formalin-fixed, dehydrated and paraffin-embedded or embedded in OTC compound, snap frozen in liquid nitrogen, and stored at -70°C.
Immunohistochemical and histological analysis
Serial sections (4 μm) of the paraffin-embedded tissues were incubated with monoclonal anti-human nuclear ribonucleoprotein antibody (1:200, Millipore, Billerica, MA, USA, MAB1287) and monoclonal anti-human mitochondria antibody (1:50, Millipore, MAB1273). An anti-mouse biotinylated secondary antibody and ABC or ABC-AP methods (Vector Laboratories, Burlingame, CA, USA) were used. Peroxidase or phosphatase activities were revealed by incubation with DAB or Fast Red, respectively. Nuclei were counterstained with Harris hematoxylin and the sections were mounted in Dpex mounting medium (Panreac, Barcelona, Spain) or aqueous mounting medium (ClearMount Solution, Zymed Laboratories, San Francisco, CA, USA). Negative controls for nonspecific staining were treated with the same incubation protocol, omitting the primary antibodies. As positive controls for the immunoreaction, sections of different human tissues were used. As additional negative controls, sections of the same organs of mice injected with saline were used. As positive controls, sections of teratomas formed by the human NTERA-2 cells were used. All fixed and paraffin-embedded tissues were also hematoxylin and eosin (HE) stained to evaluate histological morphology.
Detection of human cells by DNA extraction and PCR analysis
Genomic DNA for PCR analyses was obtained from tissues by phenol chloroform extraction after a Proteinase K digestion, as previously described[19]. The DNA concentration and purity were determined by optical density using a Nanodrop spectrophotometer (NanoDrop Technologies, Thermo Scientific, Wilmington, DE) followed by standard DNA PCR analysis (ProofStart™ DNA Polymerase, Qiagen Sciences, Germantown, MD, USA). To confirm the presence of human AMSCs cells in a murine background we performed PCR using primers for two ubiquitous genes. Primers specific for human β2-microglobulin gene were used to specifically amplify human sequences[20] [huβ2Micro (F): 5'CAGGTTTACTCACGTCATCCAGC3'; huβ2Micro (R): 5'TCACATGGTTCACACGGCAGGC3']. For positive control, PCR amplification of the housekeeping gene β-actin was performed. The β actin gene is highly conserved and we used primers that amplify both human and murine DNA [β-actin (F): 5'GTGACGAGGCCCAGAGCAAGAG3'; β-actin(R): 5'ACGCAGCTCATTGTAGAAGGTGTGG3']. PCR conditions were 95°C × 15 min for one cycle followed by 94°C × 1 min/65°C × 1 min/72°C × 1 min for 31 cycles[20]. For negative controls, no DNA and DNA from non-injected mice was used. For positive controls, DNA from human cornea was used. The 235-base pair (bp) amplified fragments of human β2 microglobulin or 122 bp β-actin were separated on 2% agarose gels.
RESULTS
Morphological characterization and differentiation potential of isolated AMSCs
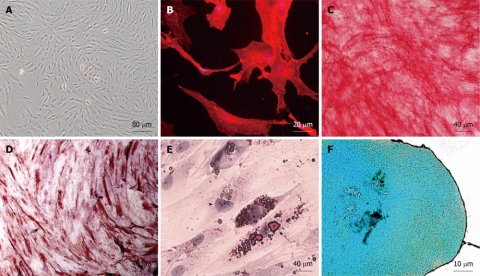
hAMSCs were isolated from lipoaspirates of 4 individual donors. The aspirated cells were indistinguishable from one another in culture, and yielded a primary culture of 1.5 × 107 AMSC adherent cells after 6 ± 1 d (Figure 1A). Cells were phenotypically characterized as CD90 positive (Figure 1B). We obtained a heterogeneous population with most cells (more than 95%) being CD34 negative initially, although the proportion of cells positive for CD34, CD45 and CD105 changed according to the cell passage (not shown). On this basis, our criteria for MSC identification were adherence to plastic and functional characterization by in vitro differentiation potential. For functional characterization, we tested the potential of the isolated AMSC population for multipotent lineage differentiation, by in vitro differentiation into osteo, myo, adipo and chondro lineages (Figure 1C-F).
Figure 1.
In vitro characterization of human adipose-derived mesenchymal stem cell. A: Phase contrast of a primary subconfluent culture showing typical adherent fibroblast-like morphology; B: Anti-CD90 immunofluorescence (in red) of a human adipose-derived mesenchymal stem cell (hAMSC) culture; C: Assessment of in vitro differentiation potential of hAMSC by osteogenic differentiation, demonstrated by alkaline phosphatase activity in red; D: Assessment of in vitro differentiation potential of hAMSC by myogenic differentiation, demonstrated by anti-sarcomeric-α-actin in brown (arrow); E: Assessment of in vitro differentiation potential of hAMSC by adipogenic differentiation, demonstrated by Oil red. Arrow: fat drops in a labeled differentiated cell. Arrowhead: Nucleus of an undifferentiated cell; F: Assessment of in vitro differentiation potential of hAMSC by chondrogenic differentiation, demonstrated by Alcian blue.
Xenotransplantation of human AMSCs: Detection of human cells over the short-term
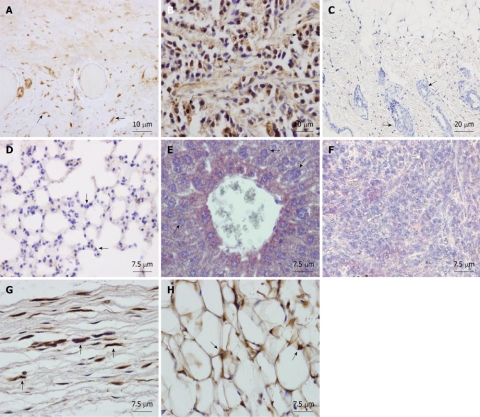
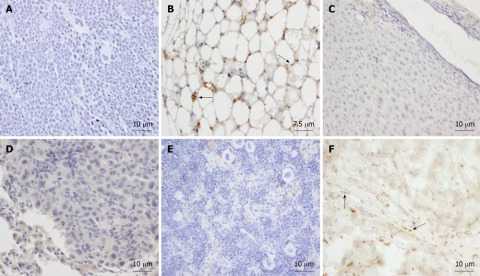
Young animals: Isolated human AMSCs were subcutaneously transplanted into 2 mo-old NOD/SCID mice. To study the biodistribution and transformation potential of human cells in NOD/SCID mice, tissues (brain, trachea, esophagus, thyroid gland, lungs, heart, thymus, stomach, intestine, kidney, liver, pancreas, spleen, bone, muscle, skin, reproductive glands and testis) were harvested after 2 mo and were analyzed by immunohistochemistry, using a monoclonal anti-human nuclear ribonucleoprotein antibody, which specifically labels human cells (Figure 2A). Teratoma formation by NTERA-2 injection in the positive control mouse was achieved at 55 d (Figure 2B). The negative control (saline-injected mouse) did not show a positive reaction in any organ (see, for example, skin at the site of injection, Figure 2C). In the eight mice injected with human AMSC, cells did not migrate (Figure 2D-F) but settled at the site of injection exclusively, differentiating into connective tissue fibroblasts and adipocytes (Figure 2G and H). By using anti-human ribonucleoprotein staining we could exclude cell fusion, as only one nucleus per cell was detected in the human fibroblasts and adipocytes at the site of injection. If cell fusion had occured, one stained and one non-stained nucleus should have been detected in the same cell.
Figure 2.
Tissue sections from NOD/SCID mice transplanted subcutaneously with human adipose-derived mesenchymal stem cells. Mice were sacrificed at two mo and organs were formalin-fixed and paraffin-embedded. Sections were immunostained with monoclonal anti-human nuclear ribonucleoprotein antibody and counterstained with Harris hematoxylin. A: Human Positive control of the antibody, showing intense nuclear immunostaining in the testicular albuginea in brown. Arrows: human labeled fibroblasts; B: Positive control. SCID subepidermal teratoma showing positive nuclear staining for anti-human ribonucleoprotein. Arrows: human labeled teratoma cells; C: Saline injected SCID showing no staining for anti-human ribonucleoprotein in the skin at the site of injection. Arrows: mouse unlabelled cells of the hair follicles; D: Lung of an adipose-derived mesenchymal stem cell (AMSC) injected SCID mouse at 2 mo showing no human AMSC (hAMSC). Arrows: mouse unlabelled cells; E: Liver of an AMSC injected SCID mouse at 2 mo showing no hAMSC. Arrows: mouse unlabelled cells; F: Spleen of an AMSC injected SCID mouse at 2 mo showing no hAMSC. Arrows: mouse unlabelled lymphocytes; G: Subcutaneous connective tissue of an AMSC injected mouse at the site of injection, showing positive nuclear staining in the fibroblasts in brown (arrows); H: Subdermic adipose tissue of an AMSC injected mouse at the site of injection, showing positive nuclear staining in the adipocytes (arrows).
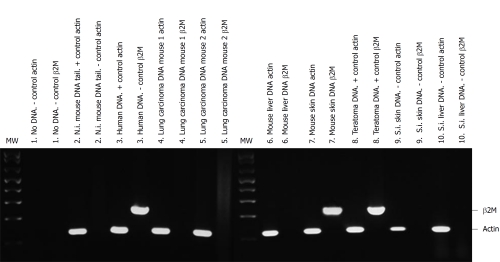
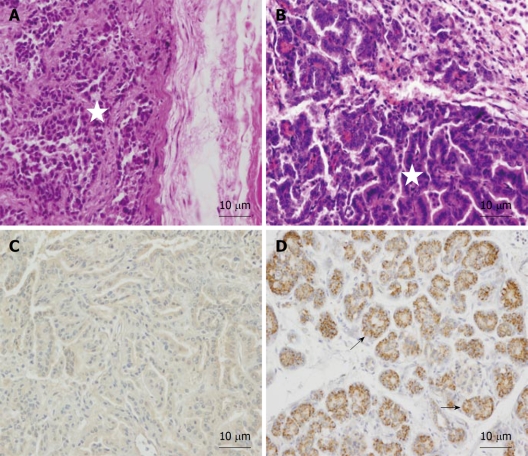
Old animals: In order to study the behavior of transplanted human AMSCs in a tumor-prone microenvironment, isolated human AMSCs were subcutaneously transplanted into 1-year-old NOD/SCID mice, a strain which is prone to develop tumors with aging. In consequence, this strain has a mean age lifespan of only 8.5 mo under pathogen-free conditions, although animals can live more than a year. To study the biodistribution and transformation potential of human cells in NOD/SCID mice, tissues were harvested from NOD/SCID mice at 4 mo after transplantation of human AMSCs, and were frozen for DNA extraction or embedded in paraffin. Presence of human DNA was assayed using PCR for the human-specific gene β2 microglobulin for detection of human donor cells against the murine recipient background. Positive control of teratoma formation by NTERA-2 injection was achieved at 104 d (Figures 3 and 4A). The negative control (saline injected mouse) did not show a positive reaction in any organ (Figure 3) and no tumors were observed. Three of eight AMSC transplanted old animals died before the 4 mo after AMSC injection. One of these had lung and pancreatic tumors, while the other two animals were diagnosed with heart failure after death. At 4 mo the rest of the mice were sacrificed as planned. One additional mouse had a lung tumor, while two more mice showed abnormal lung tissue but no clear signs of tumors in the lung or anywhere else. Pathological microscopic analysis of the two AMSC injected mice showing pulmonary failure associated with a lung mass showed both to be case of lung papillary carcinoma (Figure 4B). However, the tumors were not formed by human transplanted cells, as demonstrated by both immunohistochemistry using a monoclonal anti-human mitochondria antibody (Figure 4C) and by detection of the human-specific β2 microglobulin gene by PCR (Figure 3).
Figure 3.
Detection of human DNA from adipose-derived mesenchymal stem cells in NOD/SCID transplanted mice tissues. Human and mouse DNA were amplified by polymerase chain reaction with specific human β2 microglobulin primers (235 bp, β2M) and with actin primers that recognize mouse and human DNA (122 bp, actin) as mentioned in Materials and Methods. Each pair of lines shows the same DNA amplified with actin and with β2M primers. The following tissues of short-term transplanted mice (4 mo after cell transplantation) are shown: (1) Negative (-) control. No DNA in the polymerase chain reaction with actin or β2M primers; (2) Tail DNA from non-injected (N.i.) mouse tail DNA with actin (+ control of mouse DNA) or β2M primers (- control of human DNA); (3) Positive control. Human corneal tissue (human DNA) with actin or β2M primers; (4) Lung carcinoma DNA of a human adipose-derived mesenchymal stem cell (hAMSC) injected mouse 1 with actin or β2M primers; (5) Lung carcinoma DNA of another hAMSC injected mouse (2) with actin or β2M primers; (6) Liver DNA of a hAMSC injected mouse with actin or β2M primers; (7) Skin DNA of a hAMSC injected mouse with actin or β2M primers; (8) Teratoma DNA of NTERA-2 cells injected mouse with actin or β2M primers (positive (+) control for both sets of primers); (9) Negative control. Skin DNA from the saline injected (S.I.) mouse with actin or β2M primers; and (10) Negative control. Liver DNA from the saline injected mouse with actin or β2M primers.
Figure 4.
Histopathological findings in paraffin embedded tissue sections of human adipose-derived mesenchymal stem cell transplanted 1-year-old SCID mice after 4 mo. A: NTERA-2 cell derived subepidermal teratoma from positive control transplanted mouse. HE. Star: Teratoma cells; B: Lung papillary carcinoma found in NOD/SCID mice transplanted subcutaneously with human adipose-derived mesenchymal stem cells after 4 mo. HE. Star: Carcinoma cells; C: Immunohistochemical analysis of lung papillary carcinoma showing no staining for anti-human mitochondria; D: Positive control of anti-human mitochondria in human pancreas showing strong immunoreaction in brown (arrows).
Xenotransplantation of human AMSCs: detection of human cells over the long-term
In order to ascertain their biodistribution over the long-term after subcutaneous transplantation, isolated human AMSCs were subcutaneously transplanted into 3 mo old NOD/SCID mice. To study the biodistribution and transformation potential of human cells in NOD/SCID mice over the long term, tissues were harvested at 17 mo and were analyzed by immunohistochemistry, using the monoclonal anti-human mitochondria antibody. The negative control saline-injected mouse did not show a positive reaction in any organ (Figure 5A) and no tumors were observed. In the eight mice injected with human AMSC, only a small number of MSCs survived after 17 mo and had differentiated into subepidermal fibroblasts and adipocytes engrafted at the site of injection (Figure 5B and F). In contrast, we did not observe human MSCs in any other tissues of long-term transplanted NOD/SCID mice (Figure 5C-E). Five of the eight AMSC injected mice developed lung tumors, similarly to the previous experiment.
Figure 5.
Paraffin-embedded tissue sections from NOD/SCID mice transplanted subcutaneously with human adipose-derived mesenchymal stem cells after 17 mo. Sections were immunostained with monoclonal anti-human mitochondria antibody (A-D) or anti-human ribonucleoprotein antibody (E, F) and then were counterstained with hematoxylin. A: Saline injected SCID showing no staining for anti-human mitochondria in spleen. Negative control; B: SCID subcutaneous adipose tissue section at the site of injection at 17 mo showing staining for anti human mitochondria in the adipocytes in brown (arrows); C: Liver of a human adipose-derived mesenchymal stem cell (AMSC) SCID injected mouse at 17 mo showing no human AMSC (hAMSC); D: Lung of a human AMSC SCID injected mouse at 17 mo showing no hAMSCs; E: Spleen of a human AMSC SCID injected mouse at 17 mo showing no hAMSCs; F: SCID subcutaneous dermal tissue section at the site of injection at 17 mo showing staining for anti human ribonucleoprotein in the fibroblasts in brown (arrows).
DISCUSSION
We subcutaneously injected an enriched population of human AMSCs into severe combined immunodeficient mice. The aim of our study was to evaluate the short and long-term behavior of hAMSCs transplanted subcutaneously into NOD/SCID mice of different ages after a short period of cell expansion. Tissue biodistribution of implanted cells was detected through specific anti-human antibodies and specific PCR reaction for human genes. Histological analysis of sections from the implantation sites revealed that the population of human AMSCs transplanted subcutaneously into NOD/SCID mice survived at the injection site for up to 17 mo.
There are no previous studies analyzing the behavior of human AMSCs transplanted subcutaneously in mice over a long period (17 mo) after transplantation, although other authors have studied subcutaneous transplantation of human MSCs into mice over the short term. In a recent study on immunodeficient mice, post-transplanted AMSCs were detected in multiple organs up to 2.5 mo after transplantation and there were no significant differences in tissue distribution according to the administration route (intravenous, subcutaneous and intraperitoneal)[21]. This data disagrees with our findings, as we did not observe a widespread tissue distribution of AMSCs after subcutaneous transplantation. Our results demonstrate that transplanted human cells do not show invasive properties and either maintain fibroblast morphology or differentiate into subepidermal adipocytes engrafting within the site of injection in mice. There was no evidence of migration of AMSCs to different organs regardless of the age of the mouse recipients. Also, in our experiments there was no evidence of spontaneous human-mouse cell fusion in any of the organs analyzed, probably due to the lack of any injuries in the transplanted mice. It is possible that the difference in findings is due to the fact that, in the study described above, cells were transplanted into sublethally irradiated recipients. In fact, in unirradiated animals the level of transplanted MSCs in most tissues was below the limit of detection[21].
Human cells could behave differently in a murine model than in humans. However, when allogenic BM MSCs were injected intravenously in baboons, MSCs were found in kidney, lung, thymus, liver, gastrointestinal tissue and skin after 19 mo[22]. This agrees with the results of studies, including our own, in murine models using the same route of administration of human cells, and suggests that hAMSC delivered subcutaneously in humans may not migrate either. In contrast, in a human glioma intracranial xenograft mouse model, hBM-MSCs localized to human gliomas after intraarterial or intracranial delivery[15].
In our experiments hAMSC did not proliferate extensively after transplantation, and did not form teratomas in the standard assay for studying the teratogenic potential of many different types of stem cells. In addition, in our tumor-prone environment studies (long term injection in young animals, or aged NOD/SCID mice that developed lung tumors), hAMSC did not migrate to the tumor niche. When hMSCs were subcutaneously co-injected with melanoma cells of human origin in nude mice, the contribution of MSCs to tumor stroma formation was shown to include both passive incorporation and MSC proliferation. However, in agreement with our results, these authors did not observe MSC proliferation when cells were injected alone[13]. In addition, our injected mice survived for more than a year, which is longer than the average lifespan for untransplanted mice of this strain. However, we cannot exclude the possibility that transplanted hAMSC exacerbate to the tumors observed, as they were observed in the hAMSC transplanted mice but not in the NTERA-2 transplanted mice or in the saline injected mice.
On the other hand, MSCs have been proposed as a selective vehicle to reach cancer cells for specific delivery of anticancer drugs[23]. The inability of our transplanted hAMSC to migrate to the tumor site indicates either that hAMSC are not optimal vehicles for this type of therapy or that the subcutaneous administration route is inadequate for this purpose.
In summary, our results provide further evidence to support the idea that human AMSCs may be safe candidates for cell therapy procedures when injected subcutaneously because of their long term inability to form teratomas. The fact that biodistribution of these cells was restricted to the infusion sites can be of great interest for local therapies with MSC such as the regeneration of epidermis after severe burns or the replacement of connective tissues.
ACKNOWLEDGMENTS
Authors acknowledge Fernando Nuñez, Fatima Dominguez and Carmen Sanchez Palomo for excellent technical assistance and Gareth William Osborne for English language editing of the manuscript.
COMMENTS
Background
Adipose tissue derived stem cells constitute an easy and highly efficient source of adipose-derived mesenchymal stem cells (AMSCs). However, further understanding of all aspects of the biosafety of AMSCs, including trafficking and differentiation capability, oncogenic transformation, homing to tumor microenvironment and angiogenesis promotion should be studied before these cells can be used for patient treatment. Biodistribution of transplanted cells in animal models has been described mainly over the short term, and cells have been detected in many different organs. This article studies the ability of human AMSC to survive over both short and long term, their biodistribution, and their biosafety in vivo in tumor-prone environments when the cells are injected subcutaneously into immunodeficient SCID mice.
Research frontiers
The application of regenerative therapies using stem cells is a great promise for the cure of many different degenerative diseases. Before any application of such therapies, there is a need to ensure that such stem cells are safe for transplantation in humans, especially over the long term and in environments where there is a pre-existing tumor or in tumor-prone environments. Another fact to take into account is the possibility of wide biodistribution of the cells depending on the route of administration, outside of the target organ.
Innovations and breakthroughs
In the previous application of stem cells in mouse disease models, different routes of mesenchymal stem cells (MSCs) transplantation have been described, including intravenous, intraperitoneal, intra damaged organ, and subcutaneous routes. Biodistribution of transplanted cells has been described mainly over the short term, and cells have been detected in many different organs. We have subcutaneously injected human AMSCs from different human donors into immunodeficient SCID mice over both short- (2 and 4 mo) and long- (17 mo) term in young, and aged tumor-prone mice. Presence of human cells was studied by immunohistochemistry and PCR analysis in all organs of injected mice. AMSCs did not form teratomas at any time point when injected subcutaneously, they did not migrate but remained at the site of injection independently of animal age, and did not fuse with host cells in any organ examined. AMSCs survived in vivo for at least 17 mo after injection, and differentiated into fibroblasts of the subdermic connective tissue and into mature adipocytes of fat tissue, exclusively at the site of injection.
Applications
Evidence is presented supporting the assertion that AMSC may be safe candidates for therapy when injected subcutaneously because of their long term inability to form teratomas. The fact that biodistribution of these cells was restricted to the infusion sites can be of great interest for local therapies with MSC such as the regeneration of epidermis after severe burns or the replacement of connective tissues.
Peer review
This article is very interesting, well organized and has originality in terms of investigating long-term effect on ASCs transplantation in vivo. According to the results, subcutaneously transplanted ASCs would be able to survive for 17 wk without producing tetratoma. Thus, this article would provide a high impact on stem cell researchers especially studying stem cell therapy.
Footnotes
Supported by Project grants SAF2008-03837 and SAF2010-19230 from Ministry of Science and Innovation, and Agencia Laín Entralgo, Madrid, Spain, and from BioMedical Foundation Mutua Madrileña, Spain
Peer reviewers: Niels Olsen Saraiva Camara, MD, Associate Professor, Laboratory of Transplantation Immunobiology, Department of Immunology, Institute of Biomedical Sciences IV, University of São Paulo, Rua Prof. Dr. Lineu Prestes 1730, Cidade Universitária, 05508-900, São Paulo, Brazil; Soo-Hong Lee, PhD, Assistant Professor, College of Life Science, CHA Stem Cell Institute, CHA University, 606-16 Yeoksam 1-dong, Gangnam-gu, Seoul 135-081, South Korea; Wolfgang Wagner, MD, PhD, Professor, Helmholtz-Institute for Biomedical Engineering, Stem Cell Biology and Cellular Engineering, University of Aachen Medical School (RWTH Aachen), 52074 Aachen, Germany
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
References
- 1.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 3.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 6.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Guo ZK, Li XS, Hou CM, Tang PH, Mao N. Functional and phenotypic alteration of intrasplenic lymphocytes affected by mesenchymal stem cells in a murine allosplenocyte transfusion model. Cell Transplant. 2007;16:85–95. [PubMed] [Google Scholar]
- 8.Li H, Guo Z, Jiang X, Zhu H, Li X, Mao N. Mesenchymal stem cells alter migratory property of T and dendritic cells to delay the development of murine lethal acute graft-versus-host disease. Stem Cells. 2008;26:2531–2541. doi: 10.1634/stemcells.2008-0146. [DOI] [PubMed] [Google Scholar]
- 9.Aguilar S, Nye E, Chan J, Loebinger M, Spencer-Dene B, Fisk N, Stamp G, Bonnet D, Janes SM. Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung. Stem Cells. 2007;25:1586–1594. doi: 10.1634/stemcells.2006-0762. [DOI] [PubMed] [Google Scholar]
- 10.Hofling AA, Vogler C, Creer MH, Sands MS. Engraftment of human CD34+ cells leads to widespread distribution of donor-derived cells and correction of tissue pathology in a novel murine xenotransplantation model of lysosomal storage disease. Blood. 2003;101:2054–2063. doi: 10.1182/blood-2002-08-2597. [DOI] [PubMed] [Google Scholar]
- 11.Meyerrose TE, Roberts M, Ohlemiller KK, Vogler CA, Wirthlin L, Nolta JA, Sands MS. Lentiviral-transduced human mesenchymal stem cells persistently express therapeutic levels of enzyme in a xenotransplantation model of human disease. Stem Cells. 2008;26:1713–1722. doi: 10.1634/stemcells.2008-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vilalta M, Dégano IR, Bagó J, Gould D, Santos M, García-Arranz M, Ayats R, Fuster C, Chernajovsky Y, García-Olmo D, et al. Biodistribution, long-term survival, and safety of human adipose tissue-derived mesenchymal stem cells transplanted in nude mice by high sensitivity non-invasive bioluminescence imaging. Stem Cells Dev. 2008;17:993–1003. doi: 10.1089/scd.2007.0201. [DOI] [PubMed] [Google Scholar]
- 13.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 14.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 15.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 16.Annabi B, Naud E, Lee YT, Eliopoulos N, Galipeau J. Vascular progenitors derived from murine bone marrow stromal cells are regulated by fibroblast growth factor and are avidly recruited by vascularizing tumors. J Cell Biochem. 2004;91:1146–1158. doi: 10.1002/jcb.10763. [DOI] [PubMed] [Google Scholar]
- 17.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 18.Arnalich-Montiel F, Pastor S, Blazquez-Martinez A, Fernandez-Delgado J, Nistal M, Alio JL, De Miguel MP. Adipose-derived stem cells are a source for cell therapy of the corneal stroma. Stem Cells. 2008;26:570–579. doi: 10.1634/stemcells.2007-0653. [DOI] [PubMed] [Google Scholar]
- 19.Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice AM, Wood JA, Milross CG, Collins CJ, McCarthy NF, Vowels MR. Conditions that enable human hematopoietic stem cell engraftment in all NOD-SCID mice. Transplantation. 2000;69:927–935. doi: 10.1097/00007890-200003150-00044. [DOI] [PubMed] [Google Scholar]
- 21.Meyerrose TE, De Ugarte DA, Hofling AA, Herrbrich PE, Cordonnier TD, Shultz LD, Eagon JC, Wirthlin L, Sands MS, Hedrick MA, et al. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells. 2007;25:220–227. doi: 10.1634/stemcells.2006-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 23.Galderisi U, Giordano A, Paggi MG. The bad and the good of mesenchymal stem cells in cancer: Boosters of tumor growth and vehicles for targeted delivery of anticancer agents. World J Stem Cells. 2010;2:5–12. doi: 10.4252/wjsc.v2.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]