Abstract
Abdominal wall hernias are common diseases of the abdomen with a global incidence approximately 4%-5%. They are distinguished in external, diaphragmatic and internal hernias on the basis of their localisation. Groin hernias are the most common with a prevalence of 75%, followed by femoral (15%) and umbilical (8%). There is a higher prevalence in males (M:F, 8:1). Diagnosis is usually made on physical examination. However, clinical diagnosis may be difficult, especially in patients with obesity, pain or abdominal wall scarring. In these cases, abdominal imaging may be the first clue to the correct diagnosis and to confirm suspected complications. Different imaging modalities are used: conventional radiographs or barium studies, ultrasonography and Computed Tomography. Imaging modalities can aid in the differential diagnosis of palpable abdominal wall masses and can help to define hernial contents such as fatty tissue, bowel, other organs or fluid. This work focuses on the main radiological findings of abdominal herniations.
Keywords: Abdominal Radiology, Hernia, Intestinal Obstruction, Abdominal Wall, Hiatal Hernia, Internal Hernia, External Hernia, Diagnostic Radiology, Computed tomography, Ultrasonography
INTRODUCTION
Abdominal herniation is a protrusion of part of its content from the abdominal cavity through a normal or abnormal aperture or from wall weakness[1]. Hernias may be congenital or acquired. The first appear prenatally or in infants and are caused by a congenital defect provoking an opening in the abdominal cavity. The second may be caused by conditions that increase the pressure in the abdominal cavity (obesity, coughing, straining), from previous surgical procedure (incisional hernia) or from trauma.
We can distinguish three main types of hernias: external, diaphragmatic and internal. The protrusion in external abdominal herniation occurs through an opening of the abdominal wall[1], while internal herniations happen across mesenteric or peritoneal apertures. Finally, diaphragmatic herniation involves a weakness of the diaphragm.
Abdominal wall hernias are common diseases of the abdomen with a global incidence approximately 4%-5%[2]. They represent one of the most common reasons for emergent surgery performed in patients over 50 years old[3,4]. In fact, they are the second most common indication for surgery after acute appendicitis in Europe and the United States[5].
Groin hernias are the most common with a prevalence of 75% followed by femoral (15%) and umbilical (8%)[2]. Generally there is a higher prevalence in males (M:F, 8:1). However for anatomical reasons, women are more affected by femoral hernias[3]. The most common cause of hernia in the newborn is congenital malformation, in adults, wall stress and in the elderly, weakness of the abdominal wall.
There are many different types of hernias with a wide range of different clinical conditions. Symptoms of abdominal herniations may be absent or non-specific, consisting of mild abdominal discomfort alternating with episodes of intense periumbilical pain and nausea[1]. In some cases, however, they may develop acute complications (incarceration, bowel obstruction, volvulus and strangulation) that necessitate prompt diagnosis and therapy[3].
Diagnosis is usually made at physical examination; however, clinical diagnosis can be difficult, especially in patients with obesity, pain or abdominal wall scarring. In these cases, abdominal imaging may be the first clue to the correct diagnosis[3] and to confirm suspected complications of hernias. Different modalities imaging are used: conventional radiographs or barium studies, ultrasonography (US) and computed tomography (CT). In addition, the cross-sectional imaging modalities, sonography and CT, can aid in the differential diagnosis of palpable abdominal wall masses and help to define hernial contents such as fatty tissue, bowel, other organs or fluid[6].
Conventional radiological techniques provide a useful diagnostic tool to allow detection of the presence and type of hernia and organ involvement. In particular, radiology permits detection of signs of mechanical ileus with bowel loops enlargement, thickening of intestinal folds and air-fluid levels [6]. In emergency cases, a direct exam is usually performed and in non emergencies, a contrast enhancement may be done using a radio opaque contrast agent (barium or water-soluble iodinated in case of obstruction or perforation) that allows gastrointestinal opacification and delineation after oral or rectal administration. These methods are valuable, allowing good diagnostic accuracy, showing any structural abnormality, filling defects and the position and rapports of an opacified organ, recognizing eventual dislocation.
US imaging, like CT, largely finds a mass in the abdominal wall corresponding to the contents of the hernia sac and distinguishes it from other masses such as cysts, hematomas, neoplasms or varicoceles[7]. US may disclose the presence of the hernia signs (Figure 1) and is particularly useful in small midline hernias containing mesenteric fat or to study the pediatric population. US may detect the presence of hernias in the groin with a complete regional evaluation and hiatal hernias. A dynamic study of the gastro-esophageal junction is also possible. In a case of complications, US may provide information on the herniated organs and repercussions in the peritoneal cavity. Signs of mechanical ileus and of decompensation with the presence of peritoneal fluid, the presence or absence of color Doppler signals in the hernial contents and the presence or absence of peristalsis in the herniated bowel loop may be detected[6]. An important sign, with high specificity but limited sensitivity, of incarceration is fluid in the herniated bowel loop with bowel wall thickening and free fluid in the hernial sac. In the evaluation of the groin area, US imaging has an advantage over CT in the ability to evaluate the standing patient, with alternate straining and relaxation[7].
Figure 1.
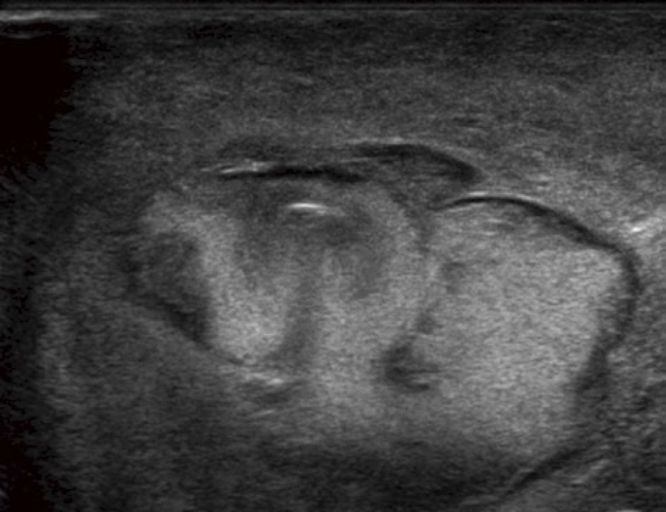
Ultrasonography of Strangulated midline hernia. Hyperechoic haemorrhagic loops surrounded by a transonic (black) thin film of fluid.
For these reasons, US is really helpful in patients with inconclusive or misleading clinical presentations[6]. US is non-invasive, allows for comparison with the asymptomatic side and can be performed in physiological positions with dynamic scanning; for these reasons it plays a fundamental role in evaluating the presence of complications such as strangulation or incarceration and, in some cases, US may detect further pathology in the hernial sac. Operator dependency and the relatively long learning curve are limiting factors. Furthermore, the presence of intestinal gas, often prominent in acute patients, limits the performance of US in emergency conditions.
Among radiological techniques, CT performs better than others, providing an accurate and panoramic view of the abdomen. Advantages of CT include more accurate identification of hernias and their contents and differentiation of hernias from other abdominal masses (tumors, hematomas, abscesses, undescended testes and aneurysms)[8]. Furthermore, because of its superior anatomic detail, multi - detector row CT may help detect subtle signs of complication within the hernia sac, including bowel obstruction, incarceration, strangulation and traumatic wall hernia[8].
CT is also useful in evaluating post-surgical patients, especially those with enlarged masses or exuberant scars. In obese patients, CT helps determine the shape, location and content of abdominal wall hernias[3]. This work focuses on the main radiological findings of abdominal herniations in conventional radiography, US and CT studies.
TECHNIQUE
Conventional radiological techniques provide a useful diagnostic tool, allowing us to detect the presence and type of hernia and organ involvement. In particular, radiology permits detection of signs of mechanical ileus with bowel loops enlargement, thickening of intestinal folds and air-fluid levels[6].
Contrast enhanced radiology allows a better evaluation of the digestive tract and its relationship with surrounding anatomical structures.
According to some authors, fluoroscopy should be performed with the patient in the lateral position because most hernias are not clearly visible in frontal or frontal oblique views, although the presence of an anterior abdominal wall hernia is sometimes indirectly indicated on frontal images by displacement and narrowing or deformity of the herniated bowel loops[8]. The reducibility of bowel-containing hernias also can be assessed during fluoroscopic examination. Therefore, fluoroscopy is useful in barium studies for the detection and characterization of abdominal hernias[9].
US is performed with the 3.5 MHz convex probes usually adopted for abdominal examination and with high frequency 7.5 MHz probes to obtain better resolution of the nearest bowel loops. Color-Doppler analysis increases diagnostic power of the method detecting circulatory alterations. Dynamic study permits checking patency and reducibility of the involved organs[6]. In CT scanning, images are acquired supine, during a single breath-hold, from the diaphragm to the pubic symphysis and eventually before and after bolus injection of intravenous iodinated contrast. Technical parameters are chosen according to the scanner (typically with a 16 slice scanner collimation 1.5 mm, reconstruction slice thickness 2 mm). Multiplanar reformatted (MPR) images, obtained on a workstation, give important information in addition to that provided by axial images as they may better delineate the size and shape of the hernia sac and associated complications[8] (Figure 2A). MDCT can accurately delineate the type of hernia, the location and can identify signs of strangulation, such as mesenteric stranding, poor bowel wall enhancement, wall thickening, free air or fluid in the hernial sac[10].
Figure 2.
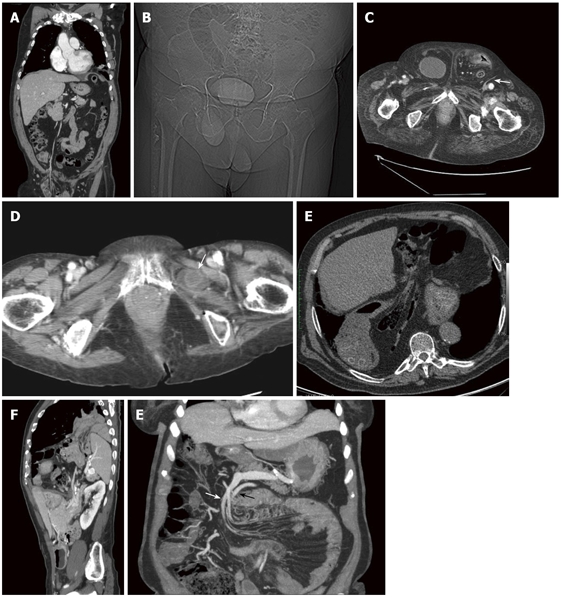
Computed tomography. A: Coronal reconstruction of a right indirect inguinal hernia. Bowel loops are visible in the hernial sac and the vascular axis passing trough the inguinal canal; B: Pilot scan. Bladder underlined by the contrast media in a right direct inguinal hernia; C: Axial scan. Bilateral direct inguinal hernia. On the right contains the bladder, on the left intestinal loops. Note the epigastric vessels lateral to the hernia (arrow); D: Obturator hernias. A thickened bowel loop is located between the external obturator and pectineal muscles (arrow); E: Very large hiatal hernia containing also mesenteric fat and bowel loops; F: Sagittal reconstruction of bockdaleck hernia. The spleen, part of the left kidney and small bowel pass in the thorax through a posterior diaphragmatic defect; G: Thick slab MIP coronal reconstruction of left paraduodenal hernia. Both, the inferior mesenteric vein (white arrow) and the ascending left colic artery (black arrow) can be seen above the herniated loop along the anterior aspect.
To promote the protrusion of the hernia, some authors perform Valsalva and postural maneuvers (prone or lateral decubitus positioning)[3].
Among radiological techniques, CT performs better than others, providing an accurate and panoramic view of the abdomen. Advantages of CT include more accurate identification of hernias and their contents and differentiation of hernias from other abdominal masses (tumors, hematomas, abscesses, undescended testes and aneurysms)[8]. When muscular layers are intact, CT is very useful for diagnosis. When hernias do exist, CT can show which fascial or muscular layers are involved and the content of the hernial sac[11]. Because of its superior anatomical detail, multi - detector row CT may help detect subtle signs of complication within the hernia sac, including bowel obstruction, incarceration, strangulation and traumatic wall hernia[8]. This exam is mandatory in the study of the acute abdomen and is fundamental in deciding what type of treatment is best, surgical or conservative, and is also useful in evaluating post surgical patients, especially those with enlarged masses or exuberant scars.
EXTERNAL HERNIAS
We can divide external hernias in 3 types: groin, ventral and posterior.
Groin
Groin hernias are divided into inguinal (direct and indirect) and femoral[3]. Indirect inguinal hernias are the most common and are caused by the protrusion of peritoneal content through a patent internal inguinal ring, lateral to the inferior epigastric vessels. In men, the hernia can extend along the spermatic cord into the scrotum while in women, the hernia may follow the course of the round ligament into the labia majora[12]. The peritoneal sac containing bowel loops protrudes through the inguinal canal and emerges at the external inguinal ring. Retroperitoneal organs such as the urinary bladder (Figure 2B and C), distal ureters or ascending or descending colon may be incorporated into the hernia[12].
Direct hernias extend through an acquired weakness area in the posterior wall of inguinal canal (the Hesselbach triangle) and pass medially to the inferior epigastric vessels.
Less common than inguinal hernias, femoral hernias occur through a congenital defect in attachment of the transverse fascia to the pubis below the inguinal ligament; the hernia passes through the femoral canal medial to the femoral vein[13], below the inguinal ligament and lateral to the pelvic tubercle and there is a tendency to be right-sided[14]. This type of hernia is more common in women because they have a wider pelvis that enlarges the egress of the femoral canal. They are more likely to incarcerate and strangulate than inguinal hernias.
Ventral hernias
The ventral group includes anterior and lateral abdominal hernias[3]. Anterior defects consist of umbilical, paraumbilical, epigastric and hypogastric hernias[8].
The umbilicus is a scar at the site of attachment of the umbilical cord in the fetus. During the 6th week of development, the abdominal cavity becomes too small to contain the primary intestinal loops so a physiological hernia occurs. In normal conditions, at 12 wk, bowel loops are entirely located in peritoneal cavity. In the fetus, the most common congenital anomaly resulting from failed closure of the umbilical ring is omphalocele. The umbilical cord arises from the hernial sac; intra-abdominal structures herniate into the base of the umbilical cord and the hernia is covered by peritoneum, amnion and Wharton’s jelly.
Umbilical hernias in adults are usually congenital and result from incomplete closure of the abdominal wall after ligation of the umbilical cord. This kind of hernia often remains asymptomatic. Incomplete herniation of the loop may occur and these are called Richter’s hernias.
Acquired umbilical hernias develop more often in obese and multiparous women and strangulation is common.
Epigastric hernias occur on the linea alba between the xiphoid process and umbilicus while hypogastric hernias take place on the midline below the umbilicus. Generally, properitoneal fat, vessels and sometimes solid viscera protrude through the hernial defect[12]. Strangulation (ischemia caused by a compromised blood supply) and incarceration (irreducible sac) are common in all midline hernias[10].
Among lateral hernias, the Spigelian (1.5% of the abdominal hernias) is due to congenital or acquired weakness in the posterior layer of the transverse fascia[12] along the semilunar line in an area located between the rectum sheath and oblique muscles. This defect allows viscera to prolapse between the lateral abdominal wall muscles and form an interstitial hernia. Typically, the omentum or short segmen-
ts of the large or small bowel protrude through the hernial defect[12]. This is uncommon but frequently a cause of incarceration.
Posterior hernias
Posterior hernias include the lumbar hernias that occur spontaneously, postsurgically or secondary to trauma, especially after pelvic fracture[3]. Herniation can occur either through defects in the lumbar muscles or the posterior fascia (transversalis or lumbodorsal) in the superior (Grynfeltt-Lesshaft) or inferior (Petit’s) lumbar triangles[3]. Those may be congenital due to defects in the musculoskeletal system (20% of total) or acquired (80%). The Grynfeltt-Lesshaft triangle is defined by the quadratus lumborum muscle medially, twelfth rib superiorly, internal oblique muscle laterally and the erector spinal muscle posteriorly[3]. The floor of the triangle is the transversalis fascia and the aponeurosis of the transversalis muscle of the abdomen. The roof of the triangle is the external oblique and latissimus dorsi muscles. The Petit triangle is bordered by the external oblique muscle anteriorly, the latissimus dorsi muscle posteriorly and the iliac crest inferiorly[3]. Bowel loops, retroperitoneal fat or the kidney or other viscera protrude through the hernial defect. Incarceration or strangulation can occur[12].
Less common hernias
There is another group of less common hernias: sciatic, obturator and perineal hernias.
The obturator hernias, more frequent in elderly women, appear between the external obturator and pectineal muscles[3]. The peritoneal sac and its contents herniate through the obturator canal in the superolateral aspect of the obturator foramen alongside the obturator vessels and nerves and protrude between the external obturator and pectinal muscles or between the layers of the obturator membrane. Occasionally the hernia compresses the obturator nerve, causing pain radiating to the knee. Obturator hernias occur commonly in elderly women. They frequently contain bowel loops; the appendix, omentum, bladder, uterus or adnexal tissue may also protrude through the hernial defect. Incarceration is frequent (Figure 2D). In our experience, this kind of hernia is more frequently observed in association with hip prosthesis or severe coxarthrosis, probably due to the induced atrophy in pectineus and external obturators muscles.
The sciatic hernia takes place through the sciatic foramen, above or below the piriform muscle and under the inferior border of the gluteus maximus muscle, frequently involving the small bowel or the distal ureter[3].
Perineal hernias are uncommon, more frequent in older women through the pelvic floor due to acquired weakness of the pelvic floor and are generally adjacent to the anus, the labia majora or gluteral region[3]. They typically occur at areas of weakness in the urogenital diaphragm, elevator ani muscle or coccygeal muscle.
INCISIONAL HERNIAS
Incisional hernia is one of the most common complications of abdominal surgery at sites of a previous laparotomy, with a reported occurrence rate of up to 20% after laparotomy[15] but may be as high as 41% after aortic surgery[8]. Most incisional hernias develop during the first months after surgery, a critical period for the healing of transected muscular and fibrous layers of the abdominal wall; however, 5%-10% may remain clinically silent for up to 5 years until detection[16].
Generally they occur at the site of a midline or paramedian incision but they may occur at other sites of surgical interruption of soft-tissue layers[12]. A particular type of incisional hernias is parastomal hernias.
Typically, properitoneal fat or the greater omentum protrudes through the hernial defect. If the hernia is left untreated, bowel loops may be incorporated into the hernia and become incarcerated or strangulated[12].
DIAPHRAGMATIC HERNIAS
The diaphragm, the principal muscle of respiration, is made of muscular and membranous structures. It separates the thoracic and abdominal cavities to maintain the pressure differentials in the respective compartments. The muscles of the diaphragm arise from the lower part of the sternum, the lower six ribs and the lumbar vertebrae of the spine and are attached to a central membranous tendon.
The central part is fibrous and consists of the phrenic center; the bulk device is essentially muscle. The phrenic center is shaped like a clover leaf with a front and two side portions. Fleshy bundles branch off from the edge that fit on the inside of the chest wall, splitting into sternal, costal and lumbar bundles. The diaphragm is crossed by the esophagus and by nerve and vascular formations, through the esophageal orifice, the aortic orifice and the orifice for the transition of the inferior cava vein. Two large muscle bundles branch off from the first and second lumbar vertebra, the right and the left medial diaphragmatic pillars. The central tendon of the diaphragm is a thin but strong aponeurosis situated near the center of the vault formed by the muscle but somewhat closer to the front than to the back of the thorax so that the posterior muscular fibers are the longer.
A certain poverty of muscle fibers is found on two small triangular spaces located on either side of the insertion chest and paravertebral level, posteriorly. These spaces are the points of least resistance of the diaphragmatic dome and are called, respectively, Morgagni and Bochdalek foramina. Hernias are the most common diseases of the diaphragm. These can be classified in hiatal hernias, either sliding or paraesophageal hiatal, and lateral. Among lateral hernias, we distinguish anterior (Morgagni hernia) and posterior (Bochdalek hernia) hernias. These hernias are easily detected with conventional radiology, although they are visible on US and especially with CT scans.
The common sliding hiatal hernia and the less common paraesophageal hernia are caused by weakened or torn phrenoesophageal membrane. Sliding hiatal hernias account for 99% of all diaphragmatic hernias, occurring in about 10% of all adults[12]. In this hernia, the gastroesophageal junction is above the esophageal hiatus of the diaphragm. They are often associated with gastroesophageal reflux[12]. In a paraesophageal hernia, all or portions of the stomach herniate into the chest but the gastroesophageal junction is below the diaphragm. There is no correlation with gastro-esophageal reflux and esophagitis unless associated with a sliding hernia.
Weakening of the phrenicoesophageal membrane allows the proximal portion of the stomach to herniate through the esophageal hiatus into the chest. As the hernial defect enlarges, other viscera such as the duodenum, colon, pancreas and mesenteric fat may protrude into the chest (Figure 2E). Gastro-esophageal reflux and esophagitis are frequent and in rare cases, lung fibrosis is possible following severe acid reflux. Complications of sliding hiatal hernias include incarceration and gastric volvulus[12].
With ultrasound, it is possible to see the gastroesophageal junction, especially in children, although direct visualization of a hiatal hernia is elusive.
Bochdalek hernias have a prevalence of 3%-6%[17]. They are usually congenital, resulting from disordered development of the diaphragm but may be acquired as a result of surgery, trauma or infection[12]. The majority of Bochdalek hernias (80%-85%) occur on the left side of the diaphragm (Figure 2F). A large proportion of the remaining cases occur on the right side and a small fraction is bilateral. CT shows discontinuity of the posterolateral part of the diaphragm and a continuous mass above and below the diaphragm (Figure 2F)[18,19].
Morgagni’s hernia is a rare type of diaphragmatic hernia, characterised by herniation through the foramina of Morgagni which is located immediately adjacent to the xiphoid process of the sternum[18]. Protrusion of liver occurs in infants and protrusion of mesenteric fat occurs in adults. Herniation of bowel or stomach can occur in both age groups. The majority of hernias occur on the right side of the body and are generally asymptomatic[12]. In newborns, a diaphragmatic hernia can be detected on obstetric ultrasound examination and in these cases respiratory distress is expected at birth.
Venous congestion and strangulation may occur.
INTERNAL HERNIAS
Internal hernias involve protrusion of the viscera through the peritoneum or mesentery and into a compartment in the abdominal cavity[20]. The orifices can be pre-existing ana-tomical structures, such as foramina, recesses and fossae[20] or congenital defects of the mesentery. In other cases, they
may be caused by surgery, trauma, inflammation or ische-
mic changes[20].
Clinical symptoms, when present, may be intermittent, nonspecific and usually include some degree of nausea, distension, epigastric discomfort and abdominal pain but also chronic digestive problems and recurrent, intermittent intestinal obstruction. Often they may occur with an acute intestinal obstruction of small bowel loops that develops through normal or abnormal apertures[20]. The herniated bowel can return to its normal site or be incarcerated depending on the size of the foramina and the size of the herniated bowel[21]. Internal hernias are better diagnosed by specific sign at CT as well as abnormal location of the small bowel.
The occurrence of abdominal internal hernias is rare and according to the classification of internal abdominal herniations devised by Ghahremani[22], can be separated in to six main groups: paraduodenal hernias (50%-55% of internal abdominal herniations), hernias through the foramen of Winslow (6%-10%), transmesenteric hernias (8%-10%), pericecal hernias (10%-15%), intersigmoid hernias (4%-8%) and paravesical hernias (< 4%)[1].
Paraduodenal hernias account for over half of reported internal hernias. They are basically congenital in origin, representing entrapment of small intestine beneath the mesentery of colon probably occurring due to abnormal embryological rotation of midgut and variation in peritoneal fixation and vascular folds[23]. Two types of paraduodenal hernias must be distinguished: left-sided paraduodenal hernias and right-sided paraduodenal hernias[22]. Most occur on the left side (75%)[22] through the fossa of Landzert and proceed into the descending mesocolon or distal transverse mesocolon (Figure 2G). One-fourth oc-
curs on the right side through the fossa of Waldeyer and proceeds into the ascending mesocolon. Clinical manifestations of paraduodenal hernias can be quite variable from mild abdominal cramps or discomfort to symptoms of bowel obstruction[12]. Postprandial pain with postural variation is a characteristic symptom.
Left-sided paraduodenal hernias are caused by the raising up of a peritoneal fold by the inferior mesenteric vein as it runs along the lateral side of fossa and then above it[1]. The small intestine may herniate through the orifice posteriorly and downward to the left, lateral to the ascending limb of duodenum extending into descending mesocolon and left part of the transverse mesocolon. The free edge of hernia thus contains the inferior mesenteric vein and ascending left colic artery[21].
Radiographical findings of left-sided paraduodenal hernias are well correlated to the anatomic topography. On barium examinations, the typical finding is the presence of a mass of small-bowel loops just lateral to the fourth portion of the duodenum that is separated from the remaining bowel loops. On CT, the location of the herniated small-bowel loops is more clearly visualized, lying behind the ascending left colic artery[12] at the level of or just above and exterior to the ligament of Treitz[1]. The inferior mesenteric vein and the ascending left colic artery can be seen above the herniated loop along the anterior aspect (Figure 2G). The radiologist should search for additional signs of bowel complications: obstruction, vessel engorgement, or even acute small-bowel ischemia, bowel-wall hyperdensity, mesenteric fluid and the presence of parietal air[24].
Right-sided paraduodenal hernias are congenital diseases that may be related to the incomplete or absent 180° rotation of the embryological intestine[1]. Right paraduodenal hernias represent an entrapment of small bowel behind the ascending mesocolon and right half of transverse mesocolon. The superior mesenteric artery and the right colic vein are in the free edge of the hernia[23]. The typical clinical presentations of right and left-sided paraduodenal hernias are similar; however, both conventional barium studies and CT can be used to distinguish between the two[1].
Internal abdominal hernias through the foramen of Winslow account for 6%-10% of all internal hernias. The small bowel is the herniated viscera in 60%-70% of cases. The terminal ileum, cecum and ascending colon are involved in about 25%-30%[20]. Other viscera such as the transverse colon, gallbladder and omentum have also been reported[22,25]. The formation mechanism of these hernias is distinct from that of paraduodenal because the foramen of Winslow is a normal peritoneal opening allowing a communication between the lesser sac and the remainder of the peritoneal cavity[1]. The foramen is situated in the portacaval space lying between the portal vein anteriorly and the inferior vena cava posteriorly including the portal vein, common bile duct and hepatic artery[1].
Predisposing factors include an enlarged foramen of Winslow and excessively mobile intestinal loops because of a long mesentery or persistence of the ascending mesocolon[22,26]. Patients present with acute onset of progressive upper abdominal pain and small bowel obstruction. Physical examination usually reveals localized tenderness and distension in the epigastric regions. Radiographical features of internal abdominal herniations through the foramen of Winslow can vary depending on which of the organs are entrapped.
Transmesenteric hernias are 5%-10% of internal abdominal herniations overall and are the most common internal hernia in children (Figure 3)[1]. In fact, almost 35% of those hernias occur during the pediatric period due to a congenital defect in the small-bowel mesentery in the ileocecal region, while in adults, surgical procedures raise the opening of a foramen, through which the bowel crosses[20]. Most occur on the right side of the greater omentum[27]. Furthermore, a high incidence of transmesenteric hernias after abdominal surgery has been described, especially after the creation of a Roux-en-Y anastomosis[28].
Figure 3.
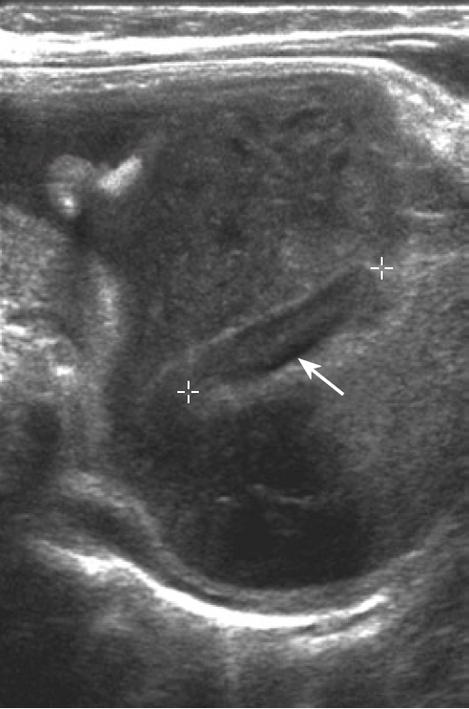
Ultrasonography of transmesenteric hernia in an infant (calipers).
Clinical symptoms frequently include signs of acute small-bowel obstruction[1]. Because of the absence of a limiting hernial sac, it can often be difficult to distinguish between a transmesenteric hernia and a small-bowel volvulus. Because of the difficulty of identification, detection of a group of small bowel loops and abnormalities of the mesenteric vessels plays an important role in diagnosis of transmesenteric hernia. CT shows that converging mesenteric vessels are located at the entrance of the hernial sac and there is displacement of the main mesenteric trunk[29].
Pericecal hernias account for only 6%-13% of internal abdominal herniations[1]. The pericecal fossa is located behind the cecum and ascending colon and is limited by the parietocecal fold outwards and the mesentericocecal fold inwards[1]. Pericecal hernias usually involve an ileal loop that goes through a defect in the cecal mesentery and occupies the pericecal fossa, especially the right paracolic gutter[1]. Clinical symptoms are often characterized by episodes of intense lower abdominal pain, like a colicky right lower quadrant pain very similar to the appendiceal pain, often causing confusion[30]. Through barium or CT, pericecal hernias occur as dilated and fixed small-bowel loops located posteriorly and laterally in relationship to the cecum, often into the right paracolic gutter.
The sigmoid mesocolon is a peritoneal fold that anchors the sigmoid colon to the pelvic wall and near the left common iliac artery there is a potential site for an internal hernia[20]. Herniation of small-bowel segments through the mesosigmoid occurs because of an incomplete defect of the mesentery. These types of internal hernias account for 6% of all internal hernias and are divided into three groups: intersigmoid hernia, transmesosigmoid hernia and intermesosigmoid hernia[20].
Intersigmoid hernia, the most common type, is herniation into a peritoneal pocket formed between two adjacent sigmoid segments and their mesentery, the intersigmoid fossa[1], situated in the attachment of the lateral aspect of the sigmoid mesocolon[20].
In the transmesosigmoid hernia, the small bowel loops goes through a foramina in the sigmoid mesocolon, especially the left lower of the abdomen, posteriorly-laterally to the sigmoid colon. Intramesosigmoid hernia is incarceration with a hernial sac through a congenital defect, present in only one of the constituent leaves of the sigmoid mesentery[20].
Supravesical hernias, although rare, are the cause of most pelvic hernias[1]. Herniation occurs in the supravesical fossa, between the remnants of the median and the left or right umbilical ligaments[1,20]. Herniated bowel loops can either remain within or extend above the pelvis. Internal supravesical hernias are divided into three categories: anterior, lateral, and posterior, which are based on whether the course is in front of, beside or behind the bladder[20].
CONCLUSION
There are several situations where an accurate clinical exam may be difficult or impossible. This may be due to pain, obesity or excessive scar formation over a small, deep peritoneal defect. Moreover, the herniated segments occasionally dissect and hide between muscular, aponeurotic and fascial layers of the abdominal wall. These interparietal or interstitial hernias often present with localized swelling and tenderness adjacent to the surgical scar but their actual content and internal orifice are seldom palpable. In all those cases, the presence of a difficult clinical examination of the hernia can be documented by gastrointestinal barium studies, sonography or CT to give the correct diagnosis[16].
Imaging studies become fundamental, especially in those patients with important pain or in obese where the abundant subcutaneous fat can prevent the palpation of a deeply seated peritoneal defect and the protruding intestinal loop or greater omentum[12]. Radiological studies may then be used to visualize the herniated segments and to evaluate associated complications such as intestinal obstruction. CT sections also give the size of the defect and content of the hernia[16].
Footnotes
Peer reviewers: Kenneth Kak Yuen Wong, MD, PhD, Assistant Professor, Department of Surgery, The University of Hong Kong, Queen Mary Hospital, Pokfulam Road, Hong Kong, China
S- Editor Zhang HN L- Editor Roemmele A E- Editor Zhang L
References
- 1.Mathieu D, Luciani A. Internal abdominal herniations. AJR Am J Roentgenol. 2004;183:397–404. doi: 10.2214/ajr.183.2.1830397. [DOI] [PubMed] [Google Scholar]
- 2.Digestive diseases in the United States: Epidemiology and Impact. NIH Publication No. 94-1447. Bethesda: NIDDK; 1994. [Google Scholar]
- 3.Aguirre DA, Casola G, Sirlin C. Abdominal wall hernias: MDCT findings. AJR Am J Roentgenol. 2004;183:681–690. doi: 10.2214/ajr.183.3.1830681. [DOI] [PubMed] [Google Scholar]
- 4.Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003;83:1045–151, 1045-151. doi: 10.1016/S0039-6109(03)00132-4. [DOI] [PubMed] [Google Scholar]
- 5.Madoz A, Franpas E, D’Alincourt A, Perret C, Laute F, Liebault B, Dupas B. Imagerie des hernies pariétale abdominales. Elsevier Masson SAS. 2007;33:A39. [Google Scholar]
- 6.Rettenbacher T, Hollerweger A, Macheiner P, Gritzmann N, Gotwald T, Frass R, Schneider B. Abdominal wall hernias: cross-sectional imaging signs of incarceration determined with sonography. AJR Am J Roentgenol. 2001;177:1061–1066. doi: 10.2214/ajr.177.5.1771061. [DOI] [PubMed] [Google Scholar]
- 7.Bendavid R, Abrahamson J, Arregui ME, Flament JB, Phillips EH (Ed. ). Abdominal Wall Hernias: Principles and Management. New York: Springer-Verlag; 2001. [Google Scholar]
- 8.Aguirre DA, Santosa AC, Casola G, Sirlin CB. Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at multi-detector row CT. Radiographics. 2005;25:1501–1520. doi: 10.1148/rg.256055018. [DOI] [PubMed] [Google Scholar]
- 9.Zafar HM, Levine MS, Rubesin SE, Laufer I. Anterior abdominal wall hernias: findings in barium studies. Radiographics. 2006;26:691–699. doi: 10.1148/rg.263055714. [DOI] [PubMed] [Google Scholar]
- 10.Ianora AA, Midiri M, Vinci R, Rotondo A, Angelelli G. Abdominal wall hernias: imaging with spiral CT. Eur Radiol. 2000;10:914–919. doi: 10.1007/s003300051036. [DOI] [PubMed] [Google Scholar]
- 11.Baker ME, Weinerth JL, Andriani RT, Cohan RH, Dunnick NR. Lumbar hernia: diagnosis by CT. AJR Am J Roentgenol. 1987;148:565–567. doi: 10.2214/ajr.148.3.565. [DOI] [PubMed] [Google Scholar]
- 12.Miller PA, Mezwa DG, Feczko PJ, Jafri ZH, Madrazo BL. Imaging of abdominal hernias. Radiographics. 1995;15:333–347. doi: 10.1148/radiographics.15.2.7761639. [DOI] [PubMed] [Google Scholar]
- 13.Shadbolt CL, Heinze SB, Dietrich RB. Imaging of groin masses: inguinal anatomy and pathologic conditions revisited. Radiographics. 2001;21 Spec No:S261–S271. doi: 10.1148/radiographics.21.suppl_1.g01oc17s261. [DOI] [PubMed] [Google Scholar]
- 14.Zarvan NP, Lee FT, Yandow DR, Unger JS. Abdominal hernias: CT findings. AJR Am J Roentgenol. 1995;164:1391–1395. doi: 10.2214/ajr.164.6.7754880. [DOI] [PubMed] [Google Scholar]
- 15.Craft RO, Harold KL. Laparoscopic repair of incisional and other complex abdominal wall hernias. Perm J. 2009;13:38–42. doi: 10.7812/tpp/09-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghahremani GG, Jimenez MA, Rosenfeld M, Rochester D. CT diagnosis of occult incisional hernias. AJR Am J Roentgenol. 1987;148:139–142. doi: 10.2214/ajr.148.1.139. [DOI] [PubMed] [Google Scholar]
- 17.Meyers MA. Dynamic radiology of the abdomen: normal and pathologic anatomy. 3rd ed. New York, NY: Springer-Verlag; 1988. [Google Scholar]
- 18.Torfs CP, Curry CJ, Bateson TF, Honoré LH. A population-based study of congenital diaphragmatic hernia. Teratology. 1992;46:555–565. doi: 10.1002/tera.1420460605. [DOI] [PubMed] [Google Scholar]
- 19.Yang W, Carmichael SL, Harris JA, Shaw GM. Epidemiologic characteristics of congenital diaphragmatic hernia among 2.5 million California births, 1989-1997. Birth Defects Res A Clin Mol Teratol. 2006;76:170–174. doi: 10.1002/bdra.20230. [DOI] [PubMed] [Google Scholar]
- 20.Takeyama N, Gokan T, Ohgiya Y, Satoh S, Hashizume T, Hataya K, Kushiro H, Nakanishi M, Kusano M, Munechika H. CT of internal hernias. Radiographics. 2005;25:997–1015. doi: 10.1148/rg.254045035. [DOI] [PubMed] [Google Scholar]
- 21.Chou CK, Mak CW, Wu RH, Chang JM. Combined transmesocolic-transomental internal hernia. AJR Am J Roentgenol. 2005;184:1532–1534. doi: 10.2214/ajr.184.5.01841532. [DOI] [PubMed] [Google Scholar]
- 22.Ghahremani GG. Abdominal and Pelvic Hernias. In: Gore RM, Levine MS, eds , editors. Textbook of Gastrointestinal Radiology , 2nd ed. Philadelphia, PA: Saunders; 1994. [Google Scholar]
- 23.Dayananda L, Sreekumar KP, Moorthy S, Pabhu NK. Para duodenal hernias- a pictorial essay. Ind J Radiol Imag. 2006;16:469–471. [Google Scholar]
- 24.Frager D, Baer JW, Medwid SW, Rothpearl A, Bossart P. Detection of intestinal ischemia in patients with acute small-bowel obstruction due to adhesions or hernia: efficacy of CT. AJR Am J Roentgenol. 1996;166:67–71. doi: 10.2214/ajr.166.1.8571907. [DOI] [PubMed] [Google Scholar]
- 25.Ghahremani GG. Internal abdominal hernias. Surg Clin North Am. 1984;64:393–406. doi: 10.1016/s0039-6109(16)43293-7. [DOI] [PubMed] [Google Scholar]
- 26.ZIMMERMAN LM, LAUFMAN H. Intraabdominal hernias due to developmental and rotational anomalies. Ann Surg. 1953;138:82–91. doi: 10.1097/00000658-195307000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kulacoglu H, Tumer H, Aktimur R, Kusdemir A. Internal herniation with fatal outcome: herniation through an unusual apertura between epiploic appendices and greater omentum. Acta Chir Belg. 2006;106:109–111. doi: 10.1080/00015458.2006.11679849. [DOI] [PubMed] [Google Scholar]
- 28.Blachar A, Federle MP, Dodson SF. Internal hernia: clinical and imaging findings in 17 patients with emphasis on CT criteria. Radiology. 2001;218:68–74. doi: 10.1148/radiology.218.1.r01ja5368. [DOI] [PubMed] [Google Scholar]
- 29.Delabrousse E, Couvreur M, Saguet O, Heyd B, Brunelle S, Kastler B. Strangulated transomental hernia: CT findings. Abdom Imaging. 2001;26:86–88. doi: 10.1007/s002610000135. [DOI] [PubMed] [Google Scholar]
- 30.Pessaux P, Tuech JJ, Derouet N, Du Plessis R, Ronceray J, Arnaud JP. [Internal hernia: a rare cause of intestinal obstruction. Apropos of 14 cases] Ann Chir. 1999;53:870–873. [PubMed] [Google Scholar]
- 31.Lee GH, Cohen AJ. CT imaging of abdominal hernias. AJR Am J Roentgenol. 1993;161:1209–1213. doi: 10.2214/ajr.161.6.8249727. [DOI] [PubMed] [Google Scholar]


