Abstract
Easily accessible, primitive chemical structures produced by self-assembly of hydrophobic substances into oil droplets may result in self-moving agents able to sense their environment and move to avoid equilibrium. These structures would constitute very primitive examples of life on the Earth, even more primitive than simple bilayer vesicle structures. A few examples of simple chemical systems are presented that self-organize to produce oil droplets capable of movement, environment remodelling and primitive chemotaxis. These chemical agents are powered by an internal chemical reaction based on the hydrolysis of an oleic anhydride precursor or on the hydrolysis of hydrogen cyanide (HCN) polymer, a plausible prebiotic chemistry. Results are presented on both the behaviour of such droplets and the surface-active properties of HCN polymer products. Such motile agents would be capable of finding resources while escaping equilibrium and sustaining themselves through an internal metabolism, thus providing a working chemical model for a possible origin of life.
Keywords: chemotaxis, hydrogen cyanide, oil droplet, origin of life
1. Introduction
Life on Earth developed from a prebiotic chemistry that provided a complex and rich mixture of organic material. Life somehow self-organized itself out of this organic mess. Comparative evolutionary biology points to a rather sophisticated last universal common ancestor that contains DNA polymers, and full transcription and translation machinery comprising sophisticated biomolecules produced through evolution. There is no record of the composition or functionality of the earlier and simpler forms of life on Earth. We are forced to both speculate on and to create plausible models for the origin of life.
This paper focuses on a model of a primitive form of life that forms a body by self-assembly, contains a simple active metabolism, and it is able to both sense and modify its environment while avoiding equilibrium. This primitive form of life, or protocell, is based on an oil droplet in water. If the avoidance of equilibrium by a structure is the most fundamental prerequisite for life, then this model could be considered as a type of primitive life that could have been possible on the early Earth.
2. Movement in a simple chemical system
We have been studying the fundamental properties of movement in simple non-living systems based on an oil droplet in water [1–4]. When oil is mixed with water containing a surfactant, oil droplets spontaneously form. If a Marangoni instability occurs in the system, through either external manipulation or internal mechanism, the droplet interface will begin to move to resolve the instability. A Marangoni instability describes the flow of surfactant and liquid along an interface to equilibrate an imbalance in interfacial tension and is the underlying physical principle behind the ‘tears of wine’ effect. In a spherical oil droplet when a Marangoni instability occurs, both the interface and the internal volume can move forming a convective cell [5]. If convective flow is coupled with mass leaving the droplet from one pole as organized by the flow structures then the droplet can move through the aqueous environment. Through this purely physical mechanism, the droplet moves in order to preserve itself.
Figure 1 shows a typical moving droplet experiment. Heavy nitrobenzene (NB) oil containing 0.5 M oleic anhydride and Oil Red O as colourant was placed in a glass-bottom disc containing both 10 mM oleate pH 12 micelles and a pH indicator, thymolphthalein. The pH indicator is blue at high pH and colourless below pH 11. As soon as the droplet was introduced to the water phase, it broke symmetry and began to move directionally around the dish (see electronic supplementary material, movie S1). As the droplet moved, it left a trail of low pH solution. This was expected owing to the encapsulated chemistry: the oleic anhydride upon exposure to water at the interface hydrolysed to form two oleic acid molecules. The production of acids at the oil–water interface can result in a local pH change of four units or greater [1]. This local change in pH was enough to change the interfacial tension around the droplet from 5 mN m−1 to nearly 25 mM m–1 [4]. Since the oil–water interface consisted of oleate/oleic acid with a pKa around 8.5, this local change in pH affected the state of the surfactant at the interface causing this shift in interfacial tension. An imbalance in interfacial tension was enough to initiate a Marangoni instability and fluid flow. When a convective cell was established inside the oil droplet, the droplet was able to move through the aqueous phase at speeds up to 1 cm s–1 and for more than 2 h, depending on the starting conditions. This droplet movement was sustained because the convective flow structure that initially forms to eliminate the imbalance in interfacial tension also introduces fresh anhydride from the inner oil phase to the interface where it was hydrolysed. Therefore, the imbalance in tension was maintained as long as there was precursor remaining in the droplet. The coupling of this simple one-step chemical reaction to the spherical oil droplet container formed a feedback loop that sustained a non-equilibrium mobile state over time.
Figure 1.
Droplet moving in an aqueous pool containing surfactant. Droplet (red) was moving from the upper left to the lower right edge. The low pH trail was visualized by using the pH-sensitive dye thymolphthalein. Diameter of the section containing the aqueous pool was 27 mm.
The droplet in this system was moving in response to its own self-generated pH gradient. We have also shown that the droplet was sensitive to externally applied pH gradients, with movement towards high pH. A droplet can sense pH gradients in its local environment and move towards high pH solution using its own particular form of chemotaxis [1]. As shown in figure 2, a droplet moving through the aqueous phase senses a pH gradient (blue), climbs the gradient and stops at the point of highest concentration (electronic supplementary material, movie S2). From such observations, we argued that the droplet has an interface that can sense its local chemical environment and an internal convective flow acting as a motor. Therefore, the system possesses a primitive form of sensory–motor coupling [4].
Figure 2.
Chemotaxis of a droplet in a pH gradient. Each frame represents a 10 s interval. Droplet (red) was moving around aqueous pool until pH gradient was introduced in fourth frame. The dispersing pH gradient was visualized using the pH-sensitive dye, thymolphthalein. Diameter of the section containing the aqueous pool was 27 mm.
We are interested in studying the emergence of life-like behaviours (like chemotaxis) in highly reduced and simple physico-chemical systems. The system above contains only five chemical components, including water. The emergent behaviour is due to the self-assembly of the oil molecules into a droplet, the exergonic imbedded hydrolysis reaction, an interface under tension, the flow and redistribution of surfactants along the interface owing to a physical instability initiated and maintained by the chemistry and finally movement primarily because of Newton's third law with the product leaving the interface non-uniformly. All of this is coordinated by the convection flow that breaks the symmetry of the droplet and maintains a feedback loop coupling the chemistry to the physical motion. In principle, similar systems that share the basic characteristics of this system should also be capable of movement and perhaps chemotaxis. Therefore, we explored if such systems could represent a primitive form of life on the early Earth or elsewhere in the universe.
3. The origin of organic material: oil and surfactants
Life, as we understand it, is largely composed of organic material. The sources of organic material on the early Earth could have been: in fall from space, energy-coupled chemistry in the atmosphere and ocean floor vent chemistry. All three sources have been reported to supply from small and simple to large and complex organic molecules. Study of the interstellar medium has produced evidence of short chain alcohols, aldehydes, ketones, acids, aliphatic hydrocarbons, amines, amides, esters, ethers, cyanide derivatives of paraffin, as well as ring structures (e.g. benzene) and large and complex carbon structures (e.g. the cyanopolyynes and polyaromatic hydrocarbons) [5,6]. Simulation of the ultraviolet irradiation of interstellar ices (with water, methanol, CO and ammonia, etc.) produced organic material [7,8] as well as structures that resemble oil droplets and vesicles [9]. Organic crust extracted from carbonaceous chondrites contains both oily substances (including aliphatic and aromatic hydrocarbons [10]) that form oil droplets as well as amphiphilic molecules [10–15] that may form vesicle structures [16–18]. From the ocean floor, the coupled processes of serpentinization and Fisher–Tropsch type synthesis can produce short and medium-chain alkanes [19–24], as well as aromatic hydrocarbons (such as phenol [25]), which could form oil droplets. The chemical environment in such geochemically active spots can be extreme, with pH as high as 12.5 [26]. Simulated early Earth atmosphere studies have produced several different types of molecules depending on conditions, such as amino acids [27,28] and monocarboxylic acids [29,30] along with both complex tar and oil phases [27,31]. Therefore, all three sources could provide molecules capable of forming oil droplets in an aqueous environment as well as simple surfactants such as monocarboxylic acids to form an oil–water interface. Such droplet structures would be much easier to form and more stable to perturbation and aqueous conditions (e.g. salinity and pH) than bilayer vesicles made of simple amphiphiles [16].
One potential source for the generation of complex organic material is hydrogen cyanide (HCN) [32]. It has been shown that there are sources of HCN in the interstellar medium [33]. On Titan in particular, HCN and related compounds are in abundance [34]. Simulated atmosphere experiments help explain the production of HCN and other hydrocarbons on Titan [35]. Self-polymerization reactions of HCN produce extended polymers as an insoluble tar that yields amino acids [36] and nucleobases [37] upon hydrolysis. Also note that many of the simulations of the Earth studies, such as those presented above, produce complex organic oil and tar as the major products of the synthesis. These products are too complex to yield to analysis by chromatography, mass spectrometry, etc., and therefore, the products are often treated offline to produce smaller organic molecules for analysis. For example, in the famous Miller Urey experiment, acid hydrolysis was used before separating and identifying amino acids by chromatography [27].
According to these analyses and simulations, the early Earth could have contained organic material suitable for forming oil phases and simple surfactants such as monocarboxylic acids to form the oil–water interface. In addition, tar-like substances produced from largely uncontrolled geochemical, atmospheric and interstellar processes could have been used as a fuel through hydrolysis in an aqueous medium, aided by acidic or basic conditions. This may have been enough to produce active oil droplets that move, sense the environment, seek out gradients and in doing so would represent a prebiotically plausible dynamic structure that possessed one of the essential hallmarks of life: the ability to avoid equilibrium [38,39].
4. Hydrogen cyanide polymer-powered oil droplets
In order to test the feasibility of moving/chemotactic oil droplets in an early Earth context, the oil droplet system presented above was reworked to include more prebiotically relevant components. Each condition was then tested for its ability to support convection in the oil phase, for the ability of the oil droplet to move through the aqueous phase and in certain cases for chemotaxis of the oil droplet; see results summarized in table 1. The oleic anhydride fuel in the oil phase was substituted with HCN polymer or with activated charcoal (AC) as a control. The NB oil phase was substituted with either diethylphthalate (DEP) or mineral oil (a mixture of medium length chain alkanes). The oleate/oleic acid in the aqueous phase was substituted with other short- and medium-chain saturated monocarboxylic acids.
Table 1.
Oil droplet systems of varying composition.
| polymer | oil phase | aqueous phase | convection | movement | chemotaxis |
|---|---|---|---|---|---|
| HCN | NB | 10 mM oleate (C18) pH 11, 12, 12.5 | yes | yes | yes |
| HCN | NB | 10 mM C4, C6, C7, C8, C10 pH 11 | slow/no | no | n.d. |
| HCN | NB | 100 mM C10 pH 13 | no | no | n.d. |
| HCN | mineral oil | 10 mM oleate (C18) pH 11 | no | no | n.d. |
| HCN | mineral oil | 10 mM oleate pH 12 | no | no | yes |
| HCN | mineral oil | 10 mM oleate pH 12.5 | yes | yes | yes |
| HCN | mineral oil | 10 mM C10 pH 11 | no | no | n.d. |
| HCN | DEP | 10 mM oleate (C18) pH 11, 12, 12.5 | yes | yes | yes |
| HCN | DEP | 10 mM C10 pH 11 | slow | no | n.d. |
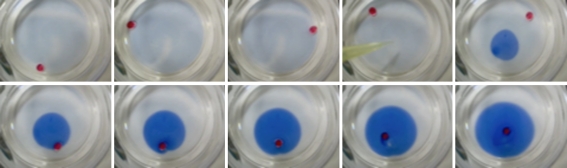
The HCN polymer was mixed with the oil phase. The oil containing the polymer was then placed in the aqueous phase, forming an oil droplet. This formed a messy system with the black polymer inside the oil droplet, at the oil–water interface and also in the aqueous solution. Initially, the system was quiet with the polymer in the droplet slowly coming together to form a collective patch of polymer at the bottom of the droplet. After a lag period of 2–10 min, the droplet became active with clear convection occurring in the oil phase as observed by microscopy. The velocity of the convection increased with time, until finally the droplet began to move around in the aqueous environment. When the droplet was moving, the HCN polymer was concentrated at the trailing end. Figure 3a,b shows two moving droplets composed of HCN polymer in NB or mineral oil, respectively. In figure 3a, one can clearly see the dark HCN particles organized through the centre of the droplet owing to the convective flow pattern. Also see electronic supplementary material, movie S3. Droplets with either AC or no added polymer as controls showed no such activity. Figure 3c shows non-motile droplets of NB with AC. The distribution of AC particles in the droplet was more uniform than in the motile droplets.
Figure 3.
Micrographs of droplets. (a) HCN polymer in NB droplet in 10 mM oleate pH 12. (b) HCN polymer in mineral oil droplet in 10 mM oleate pH 12. (c) AC control in NB droplets in 10 mM oleate pH 11. (d) HCN polymer in NB droplet in 10 mM Triton X-100 control. Scale bar, 500 µm
For the oil phase in general, it was observed that oils with a density value greater than the aqueous medium worked best. These droplets sank into the water phase and confounding effects from the potentially dynamic air–water interface were avoided. Both NB and DEP work well as the supporting oil phase, with DEP droplets exhibiting the liveliest movement (electronic supplementary material, movie S4). Oil droplets consisting of mineral oil showed some convection and directional movement, but the movement with mineral oil was the slowest of all the oils tested (electronic supplementary material, movie S5). The three different oil droplets with HCN polymer were tested for chemotactic movement in an externally generated pH gradient of NaOH. All three oil systems responded to the gradient by convecting and moving towards the source of high pH (electronic supplementary material, movies S6, S7 and S8). It is noted that in the control using AC and DEP in oleate, some internal convection was observed. This was determined to be an artefact owing to the partitioning over time of the DEP into the aqueous phase. First saturating the aqueous phase with DEP and then repeating the experiment eliminated this artefact. In comparison with the original oleic anhydride system, the movement of droplets loaded with HCN polymer was slow, often with a droplet slowly moving around in the local aqueous medium only. But the motion was persistent and was sustained for over an hour in some cases.
Neither NB nor DEP may hold any prebiotic relevance. But if we trust the abiotic synthesis associated with ocean vents, then there is evidence for the production of related phenol [25], benzene, toluene and xylene [40] organic phases. Of course, there is plenty of evidence and mechanism to support the production of alkanes, as presented above. Therefore, the mineral oil sample represents the most prebiotically relevant oil tested.
Oleate acting as a surfactant in the aqueous phase is a rather long single chain monocarboxylic acid with 18 carbons (C18) and a single unsaturated bond. Experiments to substitute out the oleic acid surfactant for simpler, more prebiotically plausible fatty acids resulted in limited success. Single chain saturated fatty acids of length C4, C6, C7, C8 and C10 were tested under the same conditions (10 mM surfactant pH 11–12). The resulting droplets were not able to initiate movement in the system; a slow internal flow of the oil phase was sometimes seen. An increase in decanoate (C10) concentration from 10 to 100 mM resulted in droplets that were more surface active with polymer being continuously redistributed, but no convective flow was supported and no movement was seen.
5. Possible mechanism of hydrogen cyanide polymer oil droplet movement
When an oil droplet containing HCN polymer was placed in an aqueous phase, the aqueous solution slowly became yellow in colour, indicating the presence of organic molecules. Aqueous solutions containing these products were analysed for their surface properties using pendant drop tensiometry. One milligram of HCN polymer was added to 1 ml of aqueous phase: pure water or 10 mm oleate micelles pH 11. Samples were agitated over night at room temperature. An immediate colour change was noted in the aqueous phase of the samples with a strong yellow colour in the oleate sample and very pale yellow colour in the pure water sample. As controls, the same aqueous phases were prepared with AC instead of HCN polymer. No colour change was seen in the AC samples. After the overnight incubation with shaking, the HCN polymer was pelleted and supernatant removed for analysis. The HCN polymer itself was not used in the tensiometry measurements as the presence of the insoluble black polymer tended to confound the results.
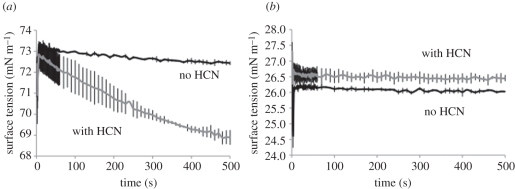
The results of the tensiometry are shown in figure 4. In the pure water sample, the HCN polymer produced a substance in the aqueous phase that was surface active as the surface tension of the sample dropped slowly over time with a total change of about 4 mN m–1 (figure 4a). With the aqueous sample that supported movement in the system (10 mM oleate pH 11), the HCN polymer modified the aqueous phase and reproducibly increased the surface tension by about 0.5 mN m–1 (figure 4b). This is qualitatively similar to values seen previously for the oleic anhydride system [4] where over time the system produced more surfactants, effectively lowering the interfacial tension, but at the same time produced acids that greatly increased the tension around the droplet. The chemical characterization of these surface-active substances produced by the HCN polymer is currently in progress.
Figure 4.
Tensiometry of the aqueous phase. Change in interfacial tension over time with (a) both pure water (black line) and water incubated with HCN polymer (grey line) and (b) 10 mM oleate pH 11 with and without incubation with HCN polymer, grey and black lines, respectively. Experiments were done in triplicate with error bars shown.
Several experiments and controls were conducted based on the following reference system that showed convection, movement and chemotaxis: 10 mg ml–1 HCN polymer in NB as oil phase and 10 mM oleate micelles pH 11 as aqueous phase. The HCN polymer was substituted with AC as a control, and the pH-sensitive oleate surfactant was substituted with the pH-insensitive synthetic non-ionic surfactant, Triton X-100. The results are shown in table 2. By removing the HCN polymer, all movement and convection in the system was stopped (also see figure 3c). But the system was still able to respond to external pH gradients with brief chemotaxis. Therefore, the interface was able to act as a sensor for the environmental pH but the droplet was not able to move itself without the HCN polymer fuel. When both the HCN polymer fuel and the oleate pH-sensitive surfactant were replaced with unreactive AC and insensitive Triton X-100, all convection, movement and chemotaxis activity stopped. It was also noted in cases where there was no movement, pieces of HCN polymer and yellow solution slowly distributed all around the sessile droplet (figure 3d). This more or less homogeneous dissipation of HCN polymer and products was not seen in the moving droplet system. This suggests that both the HCN polymer source and the pH-sensitive oleate were necessary for movement. Furthermore, when the HCN polymer was present and the pH-sensitive oleate was replaced with Triton X-100, a small amount of chemotaxis capability was retained. This suggests that the surface-active substances produced by the HCN polymer (as seen in figure 4) were produced and could sense and react to pH gradients in the environment.
Table 2.
Motile oil droplet system and controls.
| polymer | oil phase | aqueous phase | convection | movement | chemotaxis |
|---|---|---|---|---|---|
| HCN | NB | 10 mM oleate pH 11 | ++ | + | +++ |
| AC | NB | 10 mM oleate pH 11 | − | − | +++ |
| HCN | NB | 10 mM Triton X-100 pH 11 | − | − | + |
| AC | NB | 10 mM Triton X-100 pH 11 | − | − | − |
Therefore, the movement in the HCN polymer oil droplet system was probably due to two main factors: the breaking of symmetry and the expulsion of material from the droplet. The droplet was able to break symmetry when oleate surfactant was included in the system, as was seen in the oleic anhydride system [1]. This would allow the directional expulsion of HCN polymer products over time with consequent movement according to Newton's third law. The droplet was not able to break symmetry or maintain asymmetry with other surfactants, and therefore products of the HCN polymer dispersed omnidirectionally around the droplet with no motion supported. The HCN polymer itself produced substances that affect the tension at the oil–water interface, and therefore may influence the breaking of symmetry, the maintenance of asymmetry and the direction of movement of the motile droplet. Further characterization of the chemical composition of the HCN polymer products is currently under investigation.
6. Discussion and conclusion
Making protocell models out of precise ratios of highly purified and activated compounds is a product of wishful thinking that bears little relevance to the origin of life. If we follow both the development of geochemistry and simulated geochemical, atmospheric and interstellar processes in the production of organic compounds from simple precursors, then it is obvious that life needed to organize itself out of a vast mess of organic and inorganic complexity. There were no pure compounds or highly constrained metabolic pathways. As we are all taught in beginner's chemistry, largely uncontrolled organic chemistry leads to tar. This is also proven by all simulated early Earth chemical experiments where the majority of the products are not pure amino acids and the like but highly complex polymeric tar. It is from this tar, subsequently treated with strong acid to break it apart, that we see the minor monomeric products, some of which have biological relevance.
Therefore, some intermediate processes between the organic soup and evolving protocells must have been present during the origin of life. The oil droplet model for the origin of life is proposed because it is an easy system to build from scratch using the organic mixtures that would have been present on the early Earth. The oil droplet would be much easier to form from a complex mix of organic molecules and would have a higher degree of robustness when compared with the typical container thought to be relevant to the origin of life, the bilayer vesicle [9,16,41]. Dynamics emerge from the oil droplet system, such as movement coupled with metabolism and the ability to perform chemotaxis.
Simple physico-chemical systems capable of self-movement have been demonstrated consisting of purified components [13,42–45]. Here, a more primitive and dirty system of self-movement is presented with an oil phase consisting of a mixture of medium length chain alkanes, a simple single chain monocarboxylic acid as a surfactant, and insoluble HCN polymer in the form of tar as the source of fuel for the system. The droplet is under the stress produced by its internal chemistry. The droplet seeks to relieve the stress by movement and therefore the droplet preserves itself [4]. This system is able to break symmetry, support convective flow in the oil droplet, move through the aqueous environment and sense local chemical gradients. With such motion the droplets were able to escape from their slowly diffusing waste products and effectively avoid equilibrium, which is the most fundamental aspect of living systems [38,39].
Current and future studies will determine the chemical and molecular composition of the products of HCN polymer hydrolysis, here seen as a yellow aqueous liquid that is surface active. The hydrolysis of the HCN polymer should produce a variety of acids [46] that could support the changes in interfacial tension seen here. Also other surfactants and mixtures of surfactants able to initiate and support droplet movement will be tested in order to further define more primitive initial compositions for the emergence of self-moving containers at the origin of life.
The well-studied oleic anhydride-fuelled oil droplets contain a simple one-step hydrolysis reaction that is exergonic. There is no reason why an oil droplet could not host a more complex pathway or network of chemical reactions. One could build up the complexity of the internal chemical metabolism by coupling together both endergonic and exergonic reactions as seen in nature with the final step being exergonic [47]. It is not known how simple or complex the chemistry of the HCN polymer is, and steps will be taken to analyse this further.
What would oil-based life look like? The self-moving oil droplet as a model of primitive life already possesses some relevant characteristics of life, such as a perpetuated body or identity, an embedded metabolism and the ability to avoid equilibrium. The droplet can also change in time owing to its past experiences. We have analysed the stop–go movement behaviour of many droplets and discovered that the behaviour at time x + 1 is not independent of behaviour at time x, indicating that the droplet system may possess a kind of memory [48]. The droplet system would therefore be capable of evolution, presumably Lamarckian evolution, over time. The system could evolve into a smart system capable of memory through self-modification and modification of its environment [4]. A chemical language shared by a population of droplets could result in group dynamics and higher order behaviours [49].
It is not claimed that a moving oil droplet that avoids equilibrium is alive, but it may be a necessary step towards life. By its action of feeding on organics, breaking them down to smaller products and making chemical gradients in its environment, the motile oil droplet may provide some key structural elements needed to assemble the first living cells. It may in effect provide molecular building blocks. It may provide active molecular precursors through its oil-based metabolism that would be impossible to form in the water phase. It is noted that all contemporary protocell models that are water-based use offline synthesis in anhydrous solvents to make molecules. Even in such systems that the scientific community accepts are more plausible, an anhydrous molecular synthesis apparatus is needed for support. The oil droplet could, in principle, supply this support in a prebiotic context.
The oil environment would also be a good place to host chemical reactions, especially as so many organic reactions and molecules are sensitive to water [38]. Enzymatic function would be possible as well as very strong hydrogen bonding in the oil phase [50,51]. The oil droplet could protect water-labile fuel sources encapsulated within the oil phase and could regulate, in principle, the processing of the fuel in a controlled way by regulating the convective flow and movement. Further specialization by the inclusion of compartments within the oil phase could help increase the sophistication of function. It has been noted in oil and water systems that reverse micelles spontaneously form [1,52,53]. In addition, mono-layers of simple surfactants at an oil–water interface can serve to regulate the exchange of material as well as heat [54], thus forming internal compartments capable of creating gradients. Mechanisms for replication can also be envisioned where instabilities in the oil droplet do not result in organized convective flow but in more turbulent flow that effectively causes the droplet to self-divide (F. Caschera, S. Rasmussen, M. Hanczyc 2011, unpublished data). Self-division and subsequent feeding or fusion would result in a primitive replication cycle. Replication may be necessary for life, as it would provide a means for evolution. Currently, these primitive dynamic systems do not contain genetic information. The information would be present in the form of compositional information, both in the external modified environment and in the internal state of the droplet.
It is not clear that extant water-centric life evolved from the oil-centric model. The self-moving oil droplet is a good model of ‘weird life’ as detailed by the Committee on the Limits of Organic Life in Planetary Systems [39,55]. The droplet is very much unlike life that currently exists on Earth, but could be considered as a model for the origin of life on Earth or of life on other planets since it represents a structure that could arise spontaneously from an organic soup, supports chemical bonding and actively avoids equilibrium. Even if the motile oil droplets existed on the early Earth, they might have been evolutionary dead ends. But this argument can be used for any of the protocell models that currently exist. It is proposed that the oil droplet origin of life model may have been a very early step in life emerging from the organic soup and that step remodelled the chemical landscape on the early Earth in such a way that water-centric life could have emerged. Since so much rich and ‘vital’ chemistry can be produced in the oil phase and since such oil droplet structures form easily, extant oil-centric life, orthogonal to water-centric life, can be searched for both on this planet and elsewhere in the Universe.
7. Material and methods
Oleic acid (C18), oleic anhydride, sodium hydroxide, DEP, NB, mineral oil, AC, decanoic acid (C10), butyric acid (C4), hexanoic acid (C6), oenanthic acid (C7), caprylic acid (C8), Triton X-100, Oil Red O and thymolphthalein were all purchased from Sigma Aldrich.
Surfactants in the aqueous phase were prepared at the desired concentration (typically 10 mM) in water using 5 M NaOH to raise the pH of the resulting solution, typically 10–13.
pH indicator, thymolphthalein, was prepared at 10× working concentration: 6 mg thymolphthalein, 5 ml water, 20 µl 3 M NaOH, 900 µl ethanol.
HCN polymer was kindly supplied by Robert D. Minard. The polymer was formed in toluene with 1 mol per cent triethylamine as catalyst [46]. The dried black polymer was mixed with oil at 10 mg ml–1 and then heated to 60°C for 20 min before use. Over time, the black polymer would settle to the bottom of the tube, so gentle mixing by hand to disperse the polymer was performed before the oil was used in an experiment. The dispersed polymer in the oil phase appeared like a suspension of dark particles. Samples were stored at room temperature (20°C–22°C).
(a). Microscopy
All conditions tested were observed with an inverted light microscope Nikon Eclipse TE2000-S and images and movies captured with a Photometrics Cascade II 512 camera and in-house software. Aqueous phase (100 µl) was added to a glass slide (VWR, Italy) with a concave depression of 1.5 cm in diameter. One or more oil droplets (1 µl total) were then added and monitored over time. When a pH gradient was applied, a pipette was loaded with 1 µl 5 M NaOH and positioned near the oil droplet on the slide. The contents of the pipette were not released manually but were allowed to diffuse out slowly. The result of the added gradient on the droplets was observed by microscopy.
(b). Macroscopic observation of droplet movement
Eight hundred microlitres of aqueous phase was added to a glass-bottom dish of 35 mm with a quartz base of 27 mm in diameter. The aqueous phase consisted of 10 mM oleate pH 11–12.5 and when appropriate 0.1 mg ml–1 thymolphthalein pH indicator. A 5 µl droplet containing 0.5 M oleic anhydride and 1 mg ml–1 Oil Red O as colourant was then added to the system. For the HCN system, an oil droplet consisting of 10 mg ml–1 HCN polymer was added to a glass dish or a glass microscope slide containing surfactant as indicated in tables 1 and 2. The same amount of AC instead of HCN polymer was added to the oil phase as a control. Movement was recorded using an iSight digital video camera and iMovie or BTVPro software. To make a pH gradient, 1 µl of 3 M NaOH was added manually to the system after the droplet had started to move.
(c). Tensiometry
Interfacial tension of the oil–water interface was determined using a PAT1D tensiometer (Sinterface, Germany) by the pendent drop method using the Sinterface software. To prepare the samples for tensiometry, 1 mg of HCN polymer was added to 1 ml of aqueous phase: water or 10 mm oleate micelles pH 11 (adjusted with NaOH). Samples were shaken in an IKA Vibrax basic shaker at 500 r.p.m. overnight at room temperature (20°C). As controls, the same aqueous phases were prepared with AC instead of HCN polymer. After the overnight incubation with shaking, all samples were spun down at 13 000 r.p.m. for 30 min in Heraeus Biofuge Pico and supernatant removed for analysis. Tensiometry was performed with the aqueous phase as the internal phase hanging in air. Each condition was tested in triplicate.
Acknowledgements
I would like to thank Bob Minard for the HCN polymer samples, as well as Mark Doerr and Hans Toftlund for helpful discussions. This work was done both at ProtoLife Srl, Italy and the University of Southern Denmark under the European Commission grants on Programmable Artificial Cell Evolution (PACE) and MAtrix for CHemical IT (MATCHIT). M.M.H. is supported by the Danish National Research Foundation under FLinT (the Centre for Fundamental Living Technology).
References
- 1.Hanczyc M. M., Toyota T., Ikegami T., Packard N., Sugawara T. 2007. Fatty acid chemistry at the oil–water interface: self-propelled oil droplets. J. Am. Chem. Soc. 129, 9386–9391 10.1021/ja0706955 (doi:10.1021/ja0706955) [DOI] [PubMed] [Google Scholar]
- 2.Toyota T., Maru N., Hanczyc M. M., Ikegami T., Sugawara T. 2009. Self-propelled oil droplets consuming ‘fuel’ surfactant. J. Am. Chem. Soc 131, 5012–5013 10.1021/ja806689p (doi:10.1021/ja806689p) [DOI] [PubMed] [Google Scholar]
- 3.Matsuno H., Hanczyc M. M., Ikegami T. 2007. Self-maintained movements of droplets with convection flow. In Proc. 3rd Australian Conf. on Progress in Artificial Life, 2007, pp. 179–188 Berlin, Heidelberg: Springer-Verlag [Google Scholar]
- 4.Hanczyc M. M., Ikegami T. 2010. Chemical basis for minimal cognition. Artif. Life 16, 233–243 10.1162/artl_a_00002 (doi:10.1162/artl_a_00002) [DOI] [PubMed] [Google Scholar]
- 5.Kwok S. 2007. Physics and chemistry of the interstellar medium, pp. 517–519 Mill Valley, CA: University Science Books [Google Scholar]
- 6.Henning T. h., Salama F. 1998. Carbon in the universe. Science 282, 2204–2210 10.1126/science.282.5397.2204 (doi:10.1126/science.282.5397.2204) [DOI] [PubMed] [Google Scholar]
- 7.Bernstein M. P., Sandford S. A., Allamandola L. J., Chang S., Scharberg M. A. 1995. Organic compounds produced by photolysis of realistic interstellar and cometary ice analogs containing methanol. Astrophys. J. 454, 327–344 10.1086/176485 (doi:10.1086/176485) [DOI] [Google Scholar]
- 8.Gerakines P. A., Moore M. H., Hudson R. L. 2000. Energetic processing of laboratory ice analogs: UV photolysis versus ion bombardment. J. Geophys. Res. 106, 33381–33385 10.1029/2000JE001320 (doi:10.1029/2000JE001320) [DOI] [Google Scholar]
- 9.Dworkin J. P., Deamer D. W., Sandford S. A., Allamandola L. J. 2001. Self-assembling amphiphilic molecules: synthesis in simulated interstellar/precometary ices. Proc. Natl Acad. Sci. USA 98, 815–819 10.1073/pnas.98.3.815 (doi:10.1073/pnas.98.3.815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnamurthy R. V., Epstein S., Cronin J. R., Pizzarello S., Yuen G. U. 1992. Isotopic and molecular analyses of hydrocarbons and monocarboxylic acids of the Murchison meteorite. Geochim. Cosmochim. Acta 56, 4045–4058 10.1016/0016-7037(92)90015-B (doi:10.1016/0016-7037(92)90015-B) [DOI] [PubMed] [Google Scholar]
- 11.Yuen G. U., Kvenvolden K. A. 1973. Monocarboxylic acids in Murray and Murchison carbonaceous meterorites. Nature 246, 301–303 10.1038/246301a0 (doi:10.1038/246301a0) [DOI] [Google Scholar]
- 12.Lawless J. G., Yuen G. U. 1979. Quantitation of monocarboxylic acids in the Murchison carbonaceous meteorite. Nature 282, 431–454 10.1038/282396a0 (doi:10.1038/282396a0) [DOI] [Google Scholar]
- 13.Yuen G., Blair N., Des Marais D. J., Chang S. 1984. Carbon isotope composition of low molecular weight hydrocarbons and monocarboxylic acids from Murchison meteorite. Nature 307, 252–254 10.1038/307252a0 (doi:10.1038/307252a0) [DOI] [PubMed] [Google Scholar]
- 14.Epstein S., Krishnamurthy R. V., Cronin J. R., Pizzarello S., Yuen G. U. 1987. Unusual stable isotope ratios in amino acid and carboxylic acid extracts from the Murchison meteorite. Nature 26, 477–479 10.1038/326477a0 (doi:10.1038/326477a0) [DOI] [PubMed] [Google Scholar]
- 15.Naraoka H., Shimoyama A., Harada K. 1999. Molecular distribution of monocarboxylic acids in Asuka carbonaceous chondrites from Antarctica. Orig. Life Evol. Biosph. 29, 187–201 10.1023/A:1006547127028 (doi:10.1023/A:1006547127028) [DOI] [PubMed] [Google Scholar]
- 16.Monnard P.-E., Deamer D. W. 2002. Membrane self-assembly processes: steps toward the first cellular life. Anat. Rec. 268, 196–207 10.1002/ar.10154 (doi:10.1002/ar.10154) [DOI] [PubMed] [Google Scholar]
- 17.Deamer D. W. 1985. Boundary structures are formed by organic components of the Murchison carbonaceous chondrite. Nature 317, 792–794 10.1038/317792a0 (doi:10.1038/317792a0) [DOI] [Google Scholar]
- 18.Deamer D. W., Pashley R. M. 1989. Amphiphilic components of the murchison carbonaceous chondrite: surface properties and membrane formation. Orig. Life Evol. Biosph. 19, 21–38 10.1007/BF01808285 (doi:10.1007/BF01808285) [DOI] [PubMed] [Google Scholar]
- 19.Macleod G., McKeown C., Hall A. J., Russell M. J. 1994. Hydrothermal and oceanic pH conditions of possible relevance to the origin of life. Orig. Life Evol. Biosph. 24, 19–41 10.1007/BF01582037 (doi:10.1007/BF01582037) [DOI] [PubMed] [Google Scholar]
- 20.Simoneit B. R. T. 1995. Evidence for organic synthesis in high temperature aqueous media—facts and prognosis. Orig. Life Evol. Biosph. 25, 119–140 10.1007/BF01581578 (doi:10.1007/BF01581578) [DOI] [PubMed] [Google Scholar]
- 21.Holm N. G., Charlou J. L. 2001. Initial indications of abiotic formation of hydrocarbons in the Rainbow ultramafic hydrothermal system, Mid-Atlantic Ridge. Earth Planet. Sci. Lett. 191, 1–8 10.1016/S0012-821X(01)00397-1 (doi:10.1016/S0012-821X(01)00397-1) [DOI] [Google Scholar]
- 22.Sherwood-Lollar B., Westgate T. D., Ward J. A., Slater G. F., Lacrampe-Couloume G. 2002. Abiotic formation of alkanes in the Earth's crust as a minor source for global hydrocarbon reservoirs. Nature 416, 522–524 10.1038/416522a (doi:10.1038/416522a) [DOI] [PubMed] [Google Scholar]
- 23.Sleep N. H., Meibom A., Fridriksson T. h., Coleman R. G., Bird D. K. 2004. H2-rich fluids from serpentinization: geochemical and biotic implications. Proc. Natl Acad. Sci. USA 101, 12 818–12 823 10.1073/pnas.0405289101 (doi:10.1073/pnas.0405289101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulte M., Blake D., Hoehler T., McCollom T. 2006. Serpentinization and its implications for life on the early Earth and Mars. Astrobiology 6, 364–376 10.1089/ast.2006.6.364 (doi:10.1089/ast.2006.6.364) [DOI] [PubMed] [Google Scholar]
- 25.Tian G., Yuan H., Mu Y., He C., Feng S. 2007. Hydrothermal reactions from sodium hydrogen carbonate to phenol. Org. Lett. 9, 2019–2021 10.1021/ol070597o (doi:10.1021/ol070597o) [DOI] [PubMed] [Google Scholar]
- 26.Mottl M. J., Komor S. C., Fryer P., Moyer C. L. 2003. Deep-slab fluids fuel extremophilic Archaea on a Mariana forearc serpentinite mud volcano: Ocean Drilling Program Leg 195. Geochem. Geophys. Geosyst. 4, 1–14 10.1029/2003GC000588 (doi:10.1029/2003GC000588) [DOI] [Google Scholar]
- 27.Miller S. L. 1953. Production of amino acids under possible primitive Earth conditions. Science 117, 528–529 10.1126/science.117.3046.528 (doi:10.1126/science.117.3046.528) [DOI] [PubMed] [Google Scholar]
- 28.Rode B. M. 1999. Peptides and the origin of life. Peptides 20, 773–786 10.1016/S0196-9781(99)00062-5 (doi:10.1016/S0196-9781(99)00062-5) [DOI] [PubMed] [Google Scholar]
- 29.Allen W. V., Ponnamperuma C. 1967. A possible prebiotic synthesis of monocarboxylic acids. Curr. Mod. Biol. 1, 24–28 [DOI] [PubMed] [Google Scholar]
- 30.Yuen G. U., Lawless J. G., Edelson E. H. 1981. Quantification of monocarboxylic acids from a spark discharge synthesis. J. Mol. Evol. 17, 43–47 10.1007/BF01792423 (doi:10.1007/BF01792423) [DOI] [Google Scholar]
- 31.Martin W., Baross J., Kelley D., Russell M. L. 2008. Hydrothermal vents and the origin of life. Nat. Rev. 6, 805–814 10.1038/nrmicro1991 (doi:10.1038/nrmicro1991) [DOI] [PubMed] [Google Scholar]
- 32.Matthews C. N. 2005. The HCN world, establishing protein–nucleic acid life via hydrogen cyanide polymers. Earth Environ. Sci. 6, 121–135 [Google Scholar]
- 33.Snyder L. E., Buhl D. 1971. Observations of radio emission from interstellar hydrogen cyanide. Astrophys. J. 163, L47–L52 10.1086/180664 (doi:10.1086/180664) [DOI] [Google Scholar]
- 34.Marten A., Hidayat T., Biraud Y., Moreno R. 2002. New millimeter heterodyne observations of Titan: vertical distributions of nitriles HCN, HC3N, CH3CN and the isotopic ratio 15N/14N in its atmosphere. Icarus 158, 532–544 10.1006/icar.2002.6897 (doi:10.1006/icar.2002.6897) [DOI] [Google Scholar]
- 35.Borucki W. J., Giver L. P., McKay C. P., Scattergood T., Parris J. E. 1988. Lightening production of hydrocarbons and HCN on Titan: laboratory measurements. Icarus 76, 125–134 10.1016/0019-1035(88)90145-5 (doi:10.1016/0019-1035(88)90145-5) [DOI] [PubMed] [Google Scholar]
- 36.Oró J., Kamat S. S. 1961. Amino-acid synthesis from hydrogen cyanide under possible primitive earth conditions. Nature 190, 442–443 10.1038/190442a0 (doi:10.1038/190442a0) [DOI] [PubMed] [Google Scholar]
- 37.Oró J., Kimball A. P. 1961. Synthesis of purines under possible primitive earth conditions. I. Adenine from hydrogen cyanide. Arch. Biochem. Biophys. 94, 217–227 10.1016/0003-9861(61)90033-9 (doi:10.1016/0003-9861(61)90033-9) [DOI] [PubMed] [Google Scholar]
- 38.Benner S. A., Ricardo A., Carrigan M. A. 2004. Is there a common chemical model for life in the universe? Curr. Opin. Chem. Biol. 8, 672–689 10.1016/j.cbpa.2004.10.003 (doi:10.1016/j.cbpa.2004.10.003) [DOI] [PubMed] [Google Scholar]
- 39.Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council 2007. The limits of organic life in planetary systems. Washington, DC: The National Academies Press [Google Scholar]
- 40.Haggerty J. A. 1991. Evidence from fluid seeps atop serpentine seamounts in the Mariana Forearc: clues for emplacement of the seamounts and their relationship to forearc tectonics. Mar. Geol. 102, 293–309 10.1016/0025-3227(91)90013-T (doi:10.1016/0025-3227(91)90013-T) [DOI] [Google Scholar]
- 41.Hanczyc M. M., Fujikawa S. M., Szostak J. W. 2003. Experimental models of primitive cellular compartments: encapsulation, growth, and division. Science 302, 618–622 10.1126/science.1089904 (doi:10.1126/science.1089904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaudhury M. K., Whitesides G. M. 1992. How to make water run uphill. Science 256, 1539–1541 10.1126/science.256.5063.1539 (doi:10.1126/science.256.5063.1539) [DOI] [PubMed] [Google Scholar]
- 43.Tao Y.-G., Kapral R. 2008. Design of chemically propelled nanodimer motors. J. Chem. Phys. 128, 164518. 10.1063/1.2908078 (doi:10.1063/1.2908078) [DOI] [PubMed] [Google Scholar]
- 44.Mangome N., Yoshikawa K. 1996. Nonlinear oscillation and ameba-like motion in an oil/water system. J. Phys. Chem. 100, 19 102–19 105 10.1021/jp9616876 (doi:10.1021/jp9616876) [DOI] [Google Scholar]
- 45.Sumino Y., Kitahata H., Yoshikawa K., Nagayama M., Nomura S.-i. M., Magome N., Mori Y. 2005. Chemosensitive running droplet. Phys. Rev. E 72, 041603. 10.1103/PhysRevE.72.041603 (doi:10.1103/PhysRevE.72.041603) [DOI] [PubMed] [Google Scholar]
- 46.Matthews C. N., Moser R. E. 1966. Prebiological protein synthesis. Proc. Natl Acad. Sci. USA 56, 1087–1094 10.1073/pnas.56.4.1087 (doi:10.1073/pnas.56.4.1087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voet D., Voet J. 2004. Biochemistry. Hoboken, NJ: John Wiley & Sons; [DOI] [PubMed] [Google Scholar]
- 48.Horibe N., Hanczyc M. M., Ikegami T. Submitted Shape and motion dynamics in self-moving oil droplets. Robot. Auton. Syst. [Google Scholar]
- 49.Horibe N., Hanczyc M. M., Ikegami T. 2011. Mode switching and collective behavior in chemical oil droplets. Entropy 13, 709–719 10.3390/e13030709 (doi:10.3390/e13030709) [DOI] [Google Scholar]
- 50.Zaks A., Klibanov A. M. 1985. Enzyme-catalyzed processes in organic solvents. Proc. Natl Acad. Sci. USA 82, 3192–3196 10.1073/pnas.82.10.3192 (doi:10.1073/pnas.82.10.3192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klibanov A. M. 2001. Improving enzymes by using them in organic solvents. Nature 409, 241–246 10.1038/35051719 (doi:10.1038/35051719) [DOI] [PubMed] [Google Scholar]
- 52.Ikezoe Y., Ishizaki S., Yui H., Fujinami M., Sawada T. 2004. Direct observation of chemical oscillation at a water/nitrobenzene interface with a sodium-alkyl-sulfate system. Anal. Sci. 20, 1509–1514 10.2116/analsci.20.1509 (doi:10.2116/analsci.20.1509) [DOI] [PubMed] [Google Scholar]
- 53.Ikezoe Y., Ishizaki S., Yui H., Fujinami M., Sawada T. 2004. Chemical oscillation with periodic adsorption and desorption of surfactant ions at a water/nitrobenzene interface. Anal. Sci. 20, 435–440 10.2116/analsci.20.435 (doi:10.2116/analsci.20.435) [DOI] [PubMed] [Google Scholar]
- 54.Feng H., Shah D. O. 1998. The effect of surfactant monolayers on the heat transfer through air/water and oil/water interfaces using IR imaging technique. J. Coll. Inter. Sci. 205, 531–534 10.1006/jcis.1998.5607 (doi:10.1006/jcis.1998.5607) [DOI] [PubMed] [Google Scholar]
- 55.Zimmer C. 2007. Scientists urge a search for life not as we know it. The New York Times, 7 July [Google Scholar]