Abstract
The extent to which an animal is affected by the pain or distress of a conspecific will depend on its capacity for empathy. Empathy most probably evolved to facilitate parental care, so the current study assessed whether birds responded to an aversive stimulus directed at their chicks. Domestic hens were exposed to two replicates of the following conditions in a counterbalanced order: control (C; hen and chicks undisturbed), air puff to chicks (APC; air puff directed at chicks at 30 s intervals), air puff to hen (APH; air puff directed at hen at 30 s intervals) and control with noise (CN; noise of air puff at 30 s intervals). During each test, the hens' behaviour and physiology were measured throughout a 10 min pre-treatment and a 10 min treatment period. Hens responded to APH and APC treatments with increased alertness, decreased preening behaviour and a reduction in eye temperature. No such changes occurred during any control period. Increased heart rate and maternal vocalization occurred exclusively during the APC treatment, even though chicks produced few distress vocalizations. The pronounced and specific reaction observed indicates that adult female birds possess at least one of the essential underpinning attributes of empathy.
Keywords: animal welfare, behaviour, bird, chicken, empathy, maternal
1. Introduction
The extent to which animals are affected by the distress of conspecifics is of high relevance to the welfare of farm and laboratory animals. Individuals may be exposed to situations in which they experience visual, olfactory and auditory cues from conspecifics that are distressed or in pain, during routine procedures, transport and slaughter. Their reaction to this exposure will depend on their capacity for empathy.
Empathy can be defined as the ability to be affected by, and to share, the emotional state of another [1]. It can be categorized into different levels [1–3] and has been defined as ‘a multi-level construct extending from simple forms of emotional contagion to complex forms of cognitive perspective taking’ (p. 855 of [4]). The simplest form of empathy, emotional contagion, is an automatic emotional response resulting in a similar emotion being aroused in the observer as a direct result of perceiving the expressed emotion of another [1–3]. This can lead to physiological and behavioural responses, and involves ‘state-matching’—when the emotional state of the observer ‘mirrors’ that of the object. Although birds have not been traditional subjects in this area, recent work suggests a more sophisticated capacity for emotional response to conspecifics than previously realized [5,6].
However, even the apparently simple process of emotional contagion appears to be context-specific. For example, female mice show more freezing behaviour when exposed to the pain of a close relative than when exposed to the pain of a more distant relative [7], suggesting it may serve an adaptive function. If so, empathy may be expected to facilitate parental care in particular, with parents reacting to the distress of their offspring in a manner that improves offspring viability and reproductive success [1]. Studies in this area are few, but changes in maternal behaviour in response to offspring pain have been noted in rats [8].
We used chickens as a model species because, under commercial conditions, chickens will regularly encounter conspecifics showing signs of pain or distress owing to routine husbandry practices or because of the high prevalence of conditions such as bone fractures [9] or leg disorders [10]. In addition, there is considerable background work on their maternal behaviour [11–13]. Hens pay considerable attention to their chicks' behaviour and are sensitive to situations where their chicks make apparent mistakes, altering their maternal feeding display when they perceive their chicks to be eating unpalatable food [14]. This suggests that chickens have a cognitive sensitivity to potential offspring distress, but it is unknown whether they experience an emotional response that could be regarded as empathic.
The current study aimed to provide information on the behaviour and physiological responses of hens while witnessing their chicks being exposed to a mildly aversive stimulus. In particular, as well as general behaviour, heart rate and heart rate variability were used as indicators of sympathetic and parasympathetic nervous system activation. Additionally, surface temperatures of the eye and comb were used to detect possible stress-induced hyperthermia (SIH). If hens can react to their chicks' situation and/or behaviour in response to an aversive stimulus, this would provide an important platform for further investigation into the nature and affective valence of such responses, and ultimately whether chickens show the capacity for empathy.
2. Methods
(a). Animals and housing
Thirty-two female chickens aged 50–60 weeks, from two traditional breeds chosen for their increased likelihood of becoming broody (Light Sussex, n = 22; Cotswold Legbar, n = 10), were group-housed in a floor pen (4 × 4 m), bedded with 5 cm of wood shavings, containing nest-boxes and perches. Upon arrival, the temperature in the room was 19°C and the lighting schedule was 12 L : 12 D. Ad libitum layers mash was provided from two suspended feeders and water from two suspended drinkers. The hens were housed with two male Light Sussex chickens to fertilize their eggs. To encourage broodiness, four infertile eggs were kept in each nest-box. Temperature was increased by 1°C and light period by 1 h every two weeks for eight weeks, until a steady state of 23°C and 16 L : 8 D was reached. The hens were then checked three times per day and classified as broody if, during all checks for 2 days, they remained in the nest-box, displayed aggressive behaviour and emitted a characteristic high-pitched vocalization when approached by humans.
(b). Brooding
Broody hens were moved to an adjacent room and housed individually in a pen (100 × 50 × 60 cm) containing a nest-box, a feeder containing layers mash and corn (75% layers mash : 25% corn), and a drinker. The nest-box was bedded with 2 cm of shavings, peat and straw, and contained six infertile eggs. Once the broody hens sat for the majority of their time on the infertile eggs for 24 h, these were swapped with 12 fertile Light Sussex eggs and the hens were allowed to incubate these until hatching. Throughout this period, hens were encouraged to leave the nest-box daily to feed and drink.
Over a period of five months, six Light Sussex and eight Cotswold Legbar hens successfully incubated their eggs. Brood size ranged from four to eight chicks.
(i). Week 1
Each hen and her brood of chicks were transferred to a pen (100 × 100 × 60 cm) bedded with 2 cm of shavings, containing a nest-box, perch, a drinker, a feeder containing layers mash and a chick feeder containing chick crumbs. The hen and chicks were checked three times per day but were not handled further until week 2.
(ii). Week 2: habituation
Each day, for 5 consecutive days, each hen and brood were habituated to the test procedure and apparatus. To prepare hens for non-invasive heart rate monitoring, each hen was fitted with a harness containing material within a pocket, the weight of which was gradually increased until it matched the weight of the heart rate monitor. The harness was made from elastane, fitted around the back and tail and between the legs, and secured behind the neck with hook and loop fastenings, allowing free limb movement. The pocket was positioned over the hen's back. On days 4 and 5, self-adhesive electrode sensors (Ambu Blue sensor M-00-S) were applied before fitting the harness; each hen was gently placed on her back, two small sections of skin overlying the pectoralis muscle either side of the sternum were cleaned using surgical spirit and cotton wool, and the electrode sensors were applied to the cleaned skin. On day 5, a non-invasive telemetric logging system [15] was placed in the pocket and connected to the sensors on the hens' skin using two attached wires.
After the fitting process, the hen and her chicks were placed in the test apparatus and left undisturbed for a period of 20 min. The test apparatus was a 100 × 50 cm wooden structure divided into two sections; the hen box and the chick box, which were separated by a clear Perspex screen. After the 20 min period, the harness was removed.
(iii). Weeks 3–4: testing
Hens were tested on consecutive days for 4 days per week, for two weeks, making up two replicates. Each hen was assigned to one treatment condition (control, C; air puff to chicks, APC; air puff to hen, APH; or control with noise, CN) per day of testing, such that hens experienced all four conditions in a randomized order across each consecutive 4 day testing replicate.
(c). Settling period
Each day, hens were fitted with the heart rate monitor (see above), then placed into the hen box. The hen's brood of chicks was then placed into the chick box and a 5 min settling period commenced. After this, hens were exposed to one of four conditions:
— Control (C). Hen and chicks were left undisturbed for a period of 20 min. For consistency with the other three conditions during analysis, this condition was split into a notional 10 min pre-treatment period and a 10 min treatment period, although no specific treatment was applied.
— Air puff to chicks (APC). After a 10 min pre-treatment period, during which the hen and chicks were left undisturbed, a 10 min treatment period began in which an air puff from a canister of inert compressed air (Sprayduster, AF International, UK) was sprayed into the chick box for 1 s every 30 s.
— Air puff to hen (APH). After the 10 min pre-treatment period, a 10 min treatment period began in which the air puff was sprayed into the hen box for 1 s every 30 s.
— Control with noise (CN). After the 10 min pre-treatment period, a 10 min treatment period began in which the air puff was sprayed from the same location as APC but was directed away from both the hen and chick boxes, so that the hen was subject only to the noise of the air puff. This occurred for 1 s every 30 s.
Immediately after each test the harness and heart rate monitor was removed.
3. Physiological and behavioural responses
All physiological and behavioural parameters were monitored throughout the pre-treatment and treatment periods for all four conditions.
(a). Physiology
A thermal imaging camera (ThermaCam E4, FLIR) was used to capture a thermal image of the hen's head every 1 min, and maximum eye and comb temperature were obtained using ThermaCam Reporter 2000 Professional. A time window of 5 s at either side of the 1 min mark was allowed to ensure a clear image of the side of each hen's head was obtained. Distance from the hen was maintained at 1 m and the thermal camera was set to an emissivity of 0.96. The ambient temperature of the testing room was maintained at 23°C.
Heart rate data were obtained and analysed using a non-invasive telemetric logging system and software [15]. Heart rate variability was calculated using Spike 2 (Cambridge Electronic Design, UK). Owing to technical difficulties, it was not possible to obtain a complete set of heart rate data for two hens, so these were excluded from heart rate analysis.
(b). Behaviour
Hen behaviour, hen vocalizations and chick distress vocalizations were recorded continuously using a video camera positioned over the test apparatus. Videos were analysed using Observer v. 5.0 (Noldus). All the behaviours and vocalizations listed by previous authors ([16] and [17], respectively) were included, using the same descriptors.
(c). Statistical analyses
Heart rate measured in beats per minute (b.p.m.) was calculated every minute. Heart rate variability as the square root of the mean of the sum of the squares of differences between successive inter-beat intervals (RMSSD) and standard deviation of the inter-beat intervals (SDNN) was calculated every 2 min. Heart rate, heart rate variability and temperature data were averaged over each 10 min period to produce one data point per 10 min period. The durations (percentage of time) of all performed behaviours and vocalizations were calculated for each 10 min period.
Data were analysed using PASW Statistics v. 17. All data were checked for normality using a Kolmogorov–Smirnov test and all non-normal data were transformed using the formula x = √(x + 0.5). A repeated-measures ANOVA was conducted with condition and period as within-subjects factors. Post hoc tests (least significant difference) were conducted in the event of a significant interaction effect from an ANOVA, during which pre-treatment and treatment periods were compared for each condition. A mixed between-within-subjects model was able to consider the influence of two additional factors: (1) the order of testing and hence the prior experience of the hens (namely whether hens received (i) APC before APH (n = 7) or (ii) APH before APC (n = 7)); and (2) the breed of the hens ((i) Light Sussex (n = 6) or (ii) Cotswold Legbar (n = 8)). Spearman's rank order correlations were used to test for associations between hen vocalizations and chick distress vocalizations.
4. Results
Table 1 shows the physiological and behavioural responses of hens to each condition.
Table 1.
Physiological and behavioural responses (mean and standard error (s.e.)) of the hens during the pre-treatment (Pre) and treatment (Treat) periods of the four conditions (C, control; APC, air puff to chicks; APH, air puff to hen; CN, control with noise).
| C Pre | C Treat | APC Pre | APC Treat | APH Pre | APH Treat | CN Pre | CN Treat | ||
|---|---|---|---|---|---|---|---|---|---|
| physiology | heart rate (b.p.m.) | 288.98 | 282.28 | 287.30 | 304.83 | 288.22 | 293.21 | 289.83 | 280.26 |
| s.e. | 7.93 | 9.14 | 6.98 | 6.98 | 7.39 | 22.22 | 6.81 | 6.66 | |
| eye temperature (°C) | 33.83 | 34.18 | 33.67 | 33.12 | 33.65 | 32.60 | 33.81 | 34.01 | |
| s.e. | 0.20 | 0.30 | 0.30 | 0.32 | 0.37 | 0.22 | 0.28 | 0.29 | |
| comb temperature (°C) | 36.18 | 36.90 | 35.39 | 35.39 | 36.25 | 35.33 | 36.73 | 37.17 | |
| s.e. | 0.72 | 0.69 | 0.71 | 0.69 | 0.76 | 0.73 | 0.55 | 0.60 | |
| behaviour | stand alert (% time) | 29.16 | 34.06 | 40.14 | 57.54 | 37.18 | 66.32 | 41.75 | 35.22 |
| s.e. | 5.12 | 6.35 | 7.38 | 8.58 | 6.34 | 6.42 | 7.55 | 5.80 | |
| preen (% time) | 41.56 | 36.05 | 35.70 | 18.67 | 37.91 | 7.53 | 35.25 | 36.57 | |
| s.e. | 6.23 | 6.15 | 4.94 | 5.60 | 7.07 | 2.69 | 7.19 | 4.61 | |
| total vocalizations (% time) | 0.04 | 0.06 | 2.47 | 11.23 | 0.01 | 3.24 | 0.95 | 0.26 | |
| s.e. | 0.04 | 0.06 | 1.75 | 6.41 | 0.01 | 1.44 | 0.95 | 0.17 | |
| maternal cluck (% time) | 0.04 | 0.06 | 1.69 | 8.61 | 0.01 | 0.54 | 0.47 | 0.19 | |
| s.e. | 0.04 | 0.06 | 1.14 | 4.56 | 0.01 | 0.41 | 0.47 | 0.17 |
(a). Physiological recordings
During C and CN, there were no significant differences in heart rate, heart rate variability and eye temperature between the pre-treatment and treatment periods.
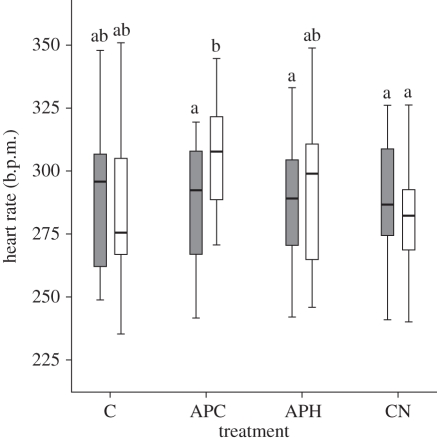
For heart rate (figure 1), there was a significant interaction effect between condition and period (Wilks's Lambda = 0.409, F3,9 = 4.898, p = 0.038, partial eta-squared = 0.591) with a significant increase between the pre-treatment and treatment period for APC. There was no significant increase in the heart rate for APH.
Figure 1.
Heart rate (b.p.m.) of hens during the four conditions. Different letters above bars indicate a significant difference (p < 0.05). C, control; APC, air puff to chicks; APH, air puff to hen; CN, control with noise. Grey bars, pre-treatment; white bars, treatment.
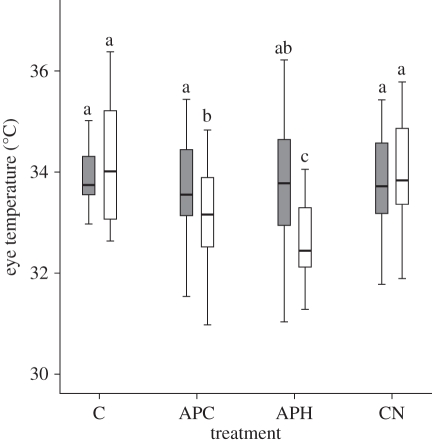
A significant interaction effect between condition and period was also noted for eye temperature (figure 2; Wilks's Lambda = 0.372, F3,11 = 6.188, p = 0.010, partial eta-squared = 0.628) and comb temperature (Wilks's Lambda = 0.303, F3,11 = 8.448, p = 0.003, partial eta-squared = 0.697). Specifically, eye temperature decreased between the pre-treatment and treatment period for APC and APH, and comb temperature decreased between the pre-treatment and treatment period for APH. Conversely, there was an increase in comb temperature between the pre-treatment and treatment periods for C.
Figure 2.
Eye temperature (°C) of the hens during the four conditions. Different letters above bars indicate a significant difference (p < 0.05). C, control; APC, air puff to chicks; APH, air puff to hen; CN, control with noise. Grey bars, pre-treatment; white bars, treatment.
There was no significant interaction effect between condition and period for heart rate variability.
(b). Behavioural observations
There were no significant differences between the pre-treatment and treatment periods for any behaviour during C and CN.
There was a significant interaction effect for preening (Wilks's Lambda = 0.073, F3,11 = 46.334, p = 0.000, partial eta-squared = 0.927), standing alert (Wilks's Lambda = 0.194, F3,11 = 15.276, p = 0.000, partial eta-squared = 0.806), total vocalizations (Wilks's Lambda = 0.487, F3,11 = 3.869, p = 0.041, partial eta-squared = 0.513) and maternal clucking (Wilks's Lambda = 0.641, F3,11 = 7.286, p = 0.018, partial eta-squared = 0.359), with an increase in time spent standing alert and vocalizing, and a decrease in time spent preening between the pre-treatment and treatment periods during both APH and APC. Maternal clucking increased between the pre-treatment and treatment periods exclusively during APC.
There was no significant interaction effect between condition and period for alarm calling, walking, ground pecking, sitting alert or any other behaviour. Similarly, there was no significant interaction effect for total number of behaviours, time spent in different areas of the hen box or movements between the different areas.
(c). Order of testing
The order of testing had no influence on the hens' response to the conditions.
(d). Breed
The hens' breed had no influence on their response to the conditions.
(e). Chick distress vocalizations
For chick distress vocalizations, there was no interaction effect between condition and period. Chick distress calling was not correlated with either maternal vocalizations or vocalizations by the hen in general throughout the conditions.
5. Discussion
During the C condition, no significant changes were observed in hen behaviour or physiology between the pre-treatment and treatment periods. The CN condition similarly had no effect, indicating that extraneous stimuli associated with the air puff (e.g. sight of apparatus and noise of puffing action) did not, by themselves, have any manifest effect on the hens, either directly or associatively via a learned connection with the air puff sensation itself. It is therefore likely that the response of the hens to APC was directly relevant to the chicks' situation, rather than simply being a response to a peripheral feature of the air puff. The air puff was selected for the use as a putative stressor during APH and APC, and the behavioural and physiological changes occurring in response to APH indicate that it fulfilled its role, to the extent that it induced changes consistent with ‘emotional stress’ [18,19] and behavioural responses observed in negatively valenced situations [20].
Hens showed a similar set of behavioural and physiological changes to both APH and APC. During the hens' exposure to the air puff (APH), eye and comb temperature decreased, time spent preening decreased and time spent standing alert increased. During the chicks' exposure to the air puff (APC), hens showed a decrease in eye temperature and time spent preening, and an increase in heart rate and time spent standing alert. Increased standing alert and decreased preening therefore occurred during both APH and APC, and it seems likely that the two changes are related. Standing alert facilitates a number of functions; it might allow hens to pay attention to stimuli such as conspecifics, predators or potential food sources. In domestic chickens, time spent standing alert is associated with higher levels of fear in response to novel object, open field and predator tests [21]. Lower levels of preening have been observed in negatively valenced situations such as behaviourally restrictive housing [22,23]. Hens also selectively avoid environments associated with high levels of standing alert and low levels of preening [16]. In the present study it seems likely that hens increased their vigilance by decreasing preening and increasing time spent standing alert in response to both an air puff directed towards themselves and towards their brood of chicks.
Eye temperature decreased during both APH and APC, and comb temperature decreased exclusively during APH. The phenomenon of SIH is thought to occur in numerous species and is characterized by an increase in core body temperature of between 0.5 and 1.5°C within 10 min of the onset of emotional stress [24], occurring irrespective of any physical activity [25]. During SIH, vasoconstriction in the peripheries causes a decrease in surface body temperature. It has been suggested that this mechanism might have two functions; to redistribute blood to more important organs (e.g. the muscles and brain) and to prevent blood loss owing to potential injuries in the peripheries [26]. Decreased surface body temperature has been found in response to apparent emotional stress in sheep [27] and cattle [18]. Similarly, rats show a decrease in tail temperature in response to restraint in a restraining tube [26]. Very little has been published on the effects of relevant stressors on eye or comb temperature in chickens, but the patterns of change seen in the current study are consistent with the features of SIH.
It is also interesting to consider the differences in response to APH and APC. Hens' heart rate increased following their chicks' exposure to the air puff (APC), and not following APH. An increased heart rate is a general feature of the sympathetic nervous system response, and is commonly associated with physical exertion or preparation for such exertion. However, physically demanding behaviours, such as walking or flapping wings, were not features of the hens' response to APC. Indeed, maternal clucking was the only behaviour to occur exclusively in response to APC and, as this does not require significant body movement, it is unlikely to have been the cause of the heart rate change. It could also be argued that the heart rate response to APC might have been caused by a change in posture; hens spent more time standing alert (with head and neck upright) and less time preening (with head and neck in a downward position). However, these changes were also observed in response to APH, during which heart rate did not increase significantly. Therefore, it is unlikely that the heart rate increase occurred in response to physical activity or changes in posture, and may be more indicative of a preparatory state (aroused readiness to act) in response to the chicks' situation.
Maternal clucking occurred exclusively in response to APC. Maternal clucking, also termed the ‘follow me call’ [28], is emitted by the hen to stimulate following behaviour in the chicks [17,28,29]. It has also been found to enhance memory retention during discriminative learning in chicks [28]. The authors suggest that the acoustic qualities of this type of call stimulate chicks to store information about events occurring around the time it is emitted. It seems plausible in the current study that mother hens would call their chicks towards them and away from the perceived danger of the air puff, despite them being physically separated during testing. Additionally, enhancement of the chicks' memory about what to do if this event occurred again in the absence of the mother would prove adaptive. Interestingly, maternal clucking was not a feature of the response to APH, so its presence in APC may be relevant to the hens' perception of the two situations (i.e. that the air puff to the chicks required a different response from the air puff directed at the hens themselves).
A more parsimonious possibility is that the increased maternal clucking by the hens during the APC treatment period was simply an automatic response to distress calling by the chicks. However, the data do not support this; during APC there was no significant increase in chick distress vocalizations between the pre-treatment and treatment period, and there was no correlation between chick distress calls and vocalizations made by the hens. Other aspects of chick behaviour were not recorded, so we cannot speculate as to the exact cues that stimulated the hens' responses, but the feature of their chicks' distress-induced behaviour that the hens are sensitive to remains an interesting topic for further study.
It is important to consider whether the behavioural and physiological responses in this study are indicative of a valenced response (i.e. a positive or negative response), as would be necessary to label it as ‘emotional’ and thereby ‘empathic’. Some of the physiological and behavioural changes discussed have previously been used as signs of an emotional response in animals [19,20], and we have shown independently that adult hens will work to avoid environments associated with some of the behavioural changes that were observed during the APC and APH conditions in this experiment [16]. However, direct evidence would be required to conclude that the hens' response to APC was negative, as it might simply indicate a non-valenced, and thus non-emotional, reaction (akin, for example, to ‘arousal’, ‘interest’ or ‘heightened attention’) to the behaviour of their chicks. Future research should focus on methods to determine the valence of the responses of farmed animals to perceived conspecific distress.
6. Conclusion
The presence of specific behavioural and physiological changes in hens observing their chicks being exposed to a mildly aversive stimulus indicates a responsive capacity that is distinguishable from the hens' own experiences of the same stimulus. It can therefore be concluded that adult female birds possess at least one of the essential underpinning attributes of ‘empathy’: the ability to be affected by, and share, the emotional state of another [1].
However, it is not possible from this study to conclusively differentiate between a non-evaluative behavioural and physiological response (akin, for example, to ‘interest’ or ‘heightened attention’) and one that is accompanied by a valenced, emotional component1 (i.e. positive/reinforcing or negative/punishing [30]), the latter being an important requirement for the demonstration of ‘emotional contagion’. Despite this, the current study provides an important platform to investigate the possible empathic responsiveness of chickens using methods to determine both the valence of the response and further cognitive features of the state generated while observing conspecific distress.
Acknowledgements
This project was funded by the BBSRC Animal Welfare Initiative. This project was carried out following university ethical approval.
Endnote
References
- 1.Preston S. D., de Waal F. B. M. 2002. Empathy: its ultimate and proximate bases. Behav. Brain Sci. 25, 1–2 10.1017/S0140525X02000018 (doi:10.1017/S0140525X02000018) [DOI] [PubMed] [Google Scholar]
- 2.de Waal F. B. M. 2003. On the possibility of animal empathy. In Feelings and emotions: the Amsterdam symposium (eds Manstead A. S. R., Frijda N. H., Fisch A.), ch. 22, pp. 377–399 Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.de Waal F. B. M. 2008. Putting the altruism back into altruism: the evolution of empathy. Annu. Rev. Psychol. 59, 279–300 10.1146/annurev.psych.59.103006.093625 (doi:10.1146/annurev.psych.59.103006.093625) [DOI] [PubMed] [Google Scholar]
- 4.Singer T. 2006. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci. Biobehav. Rev. 30, 855–863 10.1016/j.neubiorev.2006.06.011 (doi:10.1016/j.neubiorev.2006.06.011) [DOI] [PubMed] [Google Scholar]
- 5.Wascher C. A. F., Isabella Scheiber I. B. R., Kotrschal K. 2008. Heart rate modulation in bystanding geese watching social and non-social events. Proc. R. Soc. B 275, 1653–1659 10.1098/rspb.2008.0146 (doi:10.1098/rspb.2008.0146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser O. N., Bugnyar T. 2010. Do ravens show consolation? Responses to distressed others. PLoS ONE 5, e10605. 10.1371/journal.pone.0010605 (doi:10.1371/journal.pone.0010605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeon D., Kim S., Chetana M., Jo D., Ruley H. E., Lin S. Y., Rabah D., Kinet J. P., Shin H. S. 2010. Observational fear learning involves affective pain system and Ca(v)1.2 Ca2+ channels in ACC. Nat. Neurosci. 13, 482–488 10.1038/nn.2504 (doi:10.1038/nn.2504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker C.-D., Kudreikis K., Sherrard A., Johnston C. C. 2003. Repeated neonatal pain influences maternal behavior, but not stress responsiveness in rat offspring. Brain Res. Dev. Brain Res. 140, 253–261 10.1016/S0165-3806(02)00611-9 (doi:10.1016/S0165-3806(02)00611-9) [DOI] [PubMed] [Google Scholar]
- 9.Wilkins L. J., Pope S., Leeb C., Glen E., Phillips A., Zimmerman P., Nicol C., Brown S. N. 2005. Fracture rate in laying-strain hens at the end of the rearing period and the end of the laying period. Anim. Sci. Pap. Rep. 23, 189–194 [Google Scholar]
- 10.Knowles T. G., Kestin S. C., Haslam S. M., Brown S. N., Green L. E., Butterworth A., Pope S. J., Pfeiffer D., Nicol C. J. 2008. Leg disorders in broiler chickens: prevalence, risk factors and prevention. PLoS ONE 6, e1545. (doi:10.1371/journal.pone.0001545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wauters A. M., Richard-Yris M. A. 2003. Maternal food calling in domestic hens: influence of feeding context. C. R. Biol. 326, 677–686 10.1016/s1631-0691(03)00128-8 (doi:10.1016/s1631-0691(03)00128-8) [DOI] [PubMed] [Google Scholar]
- 12.Roden C., Wechsler B. 1998. A comparison of the behaviour of domestic chicks reared with or without a hen in enriched pens. Appl. Anim. Behav. Sci. 55, 317–326 10.1016/S0168-1591(97)00073-7 (doi:10.1016/S0168-1591(97)00073-7) [DOI] [Google Scholar]
- 13.Shimmura T., Kamimura E., Azuma T., Kansaku N., Uetake K., Tanaka T. 2010. Effect of broody hens on behaviour of chicks. Appl. Anim. Behav. Sci. 126, 125–133 10.1016/j.applanim.2010.06.011 (doi:10.1016/j.applanim.2010.06.011) [DOI] [Google Scholar]
- 14.Nicol C. J., Pope S. J. 1996. The maternal feeding display of domestic hens is sensitive to perceived chick error. Anim. Behav. 52, 767–774 10.1006/anbe.1996.0221 (doi:10.1006/anbe.1996.0221) [DOI] [Google Scholar]
- 15.Lowe J. C., Abeyesinghe S. M., Demmers T. G. M., Wathes C. M., McKeegan D. E. F. 2007. A novel telemetric logging system for recording physiological signals in unrestrained animals. Comput. Electron. Agric. 57, 74–79 10.1016/j.compag.2007.02.003 (doi:10.1016/j.compag.2007.02.003) [DOI] [Google Scholar]
- 16.Nicol C. J., Caplen G., Edgar J., Browne W. J. 2009. Associations between welfare indicators and environmental choice in laying hens. Anim. Behav. 78, 413–424 10.1016/j.anbehav.2009.05.016 (doi:10.1016/j.anbehav.2009.05.016) [DOI] [Google Scholar]
- 17.Collias N., Joos M. 1953. The spectrographic analysis of sound signals of the domestic fowl. Behaviour 5, 175–188 10.1163/156853953X00104 (doi:10.1163/156853953X00104) [DOI] [Google Scholar]
- 18.Schaefer A. L., Stewart M., Webster J. R., Cook N. J., Colyn J. J., Lepage P., Church J. S., Haley D. B. 2006. Objective measurement of pain and fear in cattle using infrared thermography. In Proc. of the Int. Society of Applied Ethology, North American Regional Meeting, Vancouver, Canada. Wageningen, The Netherlands: Wageningen Academic Publishers. [Google Scholar]
- 19.Cabanac M., Aizawa S. 2000. Fever and tachycardia in a bird (Gallus domesticus) after simple handling. Physiol. Behav. 69, 541–545 10.1016/S0031-9384(00)00227-4 (doi:10.1016/S0031-9384(00)00227-4) [DOI] [PubMed] [Google Scholar]
- 20.Reefmann N., Wechsler B., Gygax L. 2009. Behavioural and physiological assessment of positive and negative emotion in sheep. Anim. Behav. 78, 651–659 10.1016/j.anbehav.2009.06.015 (doi:10.1016/j.anbehav.2009.06.015) [DOI] [Google Scholar]
- 21.Campler M., Jongren M., Jensen P. 2009. Fearfulness in red junglefowl and domesticated white leghorn chickens. Behav. Process. 81, 39–43 10.1016/j.beproc.2008.12.018 (doi:10.1016/j.beproc.2008.12.018) [DOI] [PubMed] [Google Scholar]
- 22.Fortomaris P., Arsenos G., Tserveni-Gousi A., Yannakopoulos A. 2007. Performance and behaviour of broiler chickens as affected by the housing system. Arch. Geflugelkd 71, 97–104 [Google Scholar]
- 23.Pohle K., Cheng H. W. 2009. Furnished cage system and hen well-being: comparative effects of furnished cages and battery cages on behavioral exhibitions in White Leghorn chickens. Poult. Sci. 88, 1559–1564 10.3382/ps.2009-00045 (doi:10.3382/ps.2009-00045) [DOI] [PubMed] [Google Scholar]
- 24.Bouwknecht J. A., Olivier B., Paylor R. E. 2007. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci. Biobehav. Rev. 31, 41–59 10.1016/j.neubiorev.2006.02.002 (doi:10.1016/j.neubiorev.2006.02.002) [DOI] [PubMed] [Google Scholar]
- 25.Bakken M., Moe R. O., Smith A. J., Selle G. M. E. 1999. Effects of environmental stressors on deep body temperature and activity levels in silver fox vixens (Vulpes vulpes). Appl. Anim. Behav. Sci. 64, 141–151 10.1016/S0168-1591(99)00022-2 (doi:10.1016/S0168-1591(99)00022-2) [DOI] [Google Scholar]
- 26.Busnardo C., Tavares R. F., Resstel L. B. M., Elias L. L. K., Correa F. M. A. 2010. Paraventricular nucleus modulates autonomic and neuroendocrine responses to acute restraint stress in rats. Auton. Neurosci. 158, 51–57 10.1016/j.autneu.2010.06.003 (doi:10.1016/j.autneu.2010.06.003) [DOI] [PubMed] [Google Scholar]
- 27.Lowe T. E., Cook C. J., Ingram J. R., Harris P. J. 2005. Changes in ear-pinna temperature as a useful measure of stress in sheep (Ovis aries). Anim. Welf. 14, 35–42 [Google Scholar]
- 28.Field S. E., Rickard N. S., Toukhsati S. R., Gibbs M. E. 2007. Maternal hen calls modulate memory formation in the day-old chick: the role of noradretialine. Neurobiol. Learn Mem. 88, 321–330 10.1016/j.nim.2007.04.001 (doi:10.1016/j.nim.2007.04.001) [DOI] [PubMed] [Google Scholar]
- 29.Collias N. E. 1987. The vocal repertoire of the red junglefowl—a spectrographic classification and the code of communication. Condor 89, 510–524 10.2307/1368641 (doi:10.2307/1368641) [DOI] [Google Scholar]
- 30.Rolls E. T. 2005. Emotion explained. Oxford, UK: Oxford University Press; (doi:10.1093/acprof:oso/9780198570035.001.0001) [Google Scholar]
- 31.Mendl M., Burman O. H. P., Paul E. S. 2010. An integrative and functional framework for the study of animal emotion and mood. Proc. R. Soc. B 277, 2895–2904 10.1098/rspb.2010.0303 (doi:10.1098/rspb.2010.0303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berridge K. C., Winkielman P. 2003. What is an unconscious emotion? (The case for unconscious ‘liking’). Cogn. Emotion 17, 181–211 10.1080/02699930244000273 (doi:10.1080/02699930244000273) [DOI] [PubMed] [Google Scholar]