Abstract
Pacific salmon (Oncorhynchus spp.) returning to streams around the North Pacific Rim provide a nutrient subsidy to these ecosystems. While many species of animals feed directly on salmon carcasses each autumn, salmon-derived nutrients can also be stored in coastal habitats throughout the year. The effects of this storage legacy on vertebrates in other seasons are not well understood, especially in estuaries, which can receive a large portion of post-spawning salmon nutrients. We examine the effects of residual salmon-derived nutrients, forest habitats and landscape features on summer breeding birds in estuary forests. We compared models containing environmental variables and combined chum (Oncorhynchus keta) and pink (Oncorhynchus gorbuscha) salmon biomass to test predictions concerning bird density and diversity. We discovered that total bird, insectivore, golden-crowned kinglet and Pacific wren densities and Shannon's diversity in the summer were strongly predicted by salmon biomass in the autumn. For most metrics, this relationship approaches an asymptote beyond 40 000 kg of salmon biomass. Foliage height diversity, watershed catchment area and estuary area were also important predictors of avian communities. Our study suggests that the legacy of salmon nutrients influences breeding bird density and diversity in estuaries that vary across a wide gradient of spawning salmon biomass.
Keywords: coastal watersheds, ecosystem-based management, biodiversity, wetlands, fisheries, Great Bear Rainforest
1. Introduction
Ecosystem functions are fuelled by interactions between communities of organisms. Some of these processes occur within the defined borders of a system, but cross-boundary nutrient subsidies may also be important by cascading through organisms in complex, far-reaching food webs. External inputs can offset natural in situ nutrient limitations, and contribute to the productivity and resilience of recipient ecosystems [1]. Moreover, nutrients from a peripheral source often filter through multiple trophic levels, thereby having a variety of direct and indirect impacts on communities within ecosystems. For example, in the Amazon basin, ion-containing dust from the Saharan desert fuels tropical rainforest food web productivity [2]. Some ecosystems are supported by such annually repetitive nutrient subsidies (e.g. [3]).
A prime example of the potential importance of repetitive nutrient subsidies involves the annual pulse of salmon (Oncorhynchus spp.), which spawn in coastal ecosystems around the North Pacific Rim. Salmon accrue more than 95 per cent of their nutrients as they grow in the sea [4]. When they return to spawn in their natal streams, the marine-derived nutrients that they have sequestered are transferred to aquatic, terrestrial and coastal marine systems by water movement and by predators such as bears (Ursus spp.) and wolves (Canis lupus) [5]. These nutrients can be tracked through heavier isotope forms of nitrogen (15N), carbon (13C) and sulphur (34S) [6]. Phosphorus, which does not have a stable isotope, can also be elevated. These contributions can be especially important in nutrient-poor watersheds of the North Pacific [7], where much of the terrestrial-derived phosphorus is leached out or bound to soil particles, making these nutrients unavailable to vegetation [8,9]. Such impacts may be counter-balanced by the physical engineering effects of spawning salmon in streams, whereby nutrients and invertebrates may be shifted downstream [10]. Ultimately, this may enhance the productivity of estuaries, which can otherwise be nitrogen limited due to denitrification [11].
Summer breeding birds may benefit from salmon-derived nutrients if these nutrients have a residual effect on their prey or vegetation that lasts beyond the autumn spawning period. Passerine abundances have been shown to respond positively to experimental nitrogen fertilization in northern forests [12], and two small-scale studies have shown that breeding bird densities can be higher beside salmon-bearing streams than beside streams that lack salmon [13,14]. No study has tested for relationships between breeding bird density or diversity across a range of salmon population sizes, nor has any study examined birds breeding in estuaries, which receive much of the nutrients from salmon.
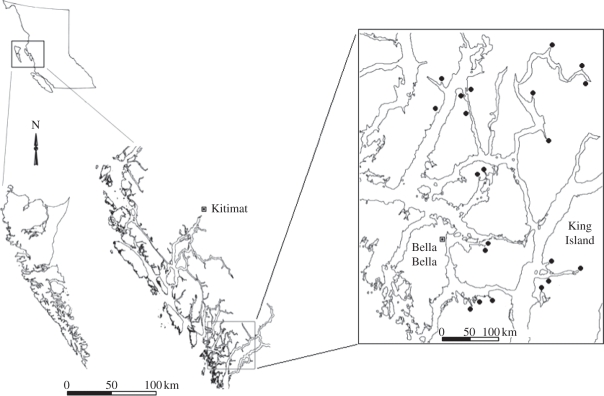
Here, we test the influence of residual salmon-derived nutrients on breeding bird communities by assessing impacts on bird densities and diversities in 21 forests adjacent to estuaries on the Central Coast of British Columbia, Canada (figure 1). Our study sites vary across a wide range of salmon population sizes and watershed and estuary sizes. The objectives of this study were, for the first time, (i) to test whether salmon-derived nutrients affect breeding bird density and diversity, while accounting for effects of forest habitat and landscape variables, and (ii) to determine whether any such relationships reached asymptotes in streams with large numbers of salmon.
Figure 1.
Location of the 21 study estuaries on the Central Coast of British Columbia, Canada.
2. Material and methods
(a). Study sites
Our study was conducted in Heiltsuk First Nation traditional territory in the Great Bear Rainforest, one of the largest remaining intact areas of coastal temperate forest in the world [15], on the Central Coast of British Columbia near Bella Bella (figure 1). We surveyed birds, forest vegetation and adjacent landscape habitat characteristics in 21 estuaries on coastal islands and mainland inlets. We defined estuary boundaries by the termination of the sedge (Carex spp.) band that extends from the mouth of the adjacent stream, which is associated with the extent of freshwater along the shore [16]. Our study sites spanned the wide range of spawning salmon abundance typical of the region, from an average of zero to over 59 000 combined chum (Oncorhynchus keta), pink (Oncorhynchus gorbuscha), coho (Oncorhynchus kisutch) and sockeye (Oncorhynchus nerka). These fish spawn from late August to early November. Estuaries ranged from 0.005 to 0.203 km2 (mean = 0.0464 ± 0.0209) at low tide (less than 1.8 m), watershed catchment areas ranged from 0.103 to 4.090 km2 (mean = 1.4673 ± 0.5844), stream lengths ranged from 0.44 to 12.92 km (mean = 3.347 ± 1.432) and stream bankfull widths ranged from 1.8 to 34.1 m (mean = 12.96 ± 3.44) (electronic supplementary material, appendix S1). Estuary and watershed catchment areas were calculated using iMapBC [17]. Our study sites are in the Coastal Western Hemlock Biogeoclimatic Zone, which has high annual precipitation (greater than 3300 mm yr−1), nutrient-poor soils, and dense temperate rainforest canopy of western hemlock (Tsuga heterophylla), western redcedar (Thuja plicata), Sitka spruce (Picea sitchensis) and amabilis fir (Abies amabilis). The understorey is dominated by salmonberry (Rubus spectabilis), red elderberry (Sambucus racemosa), false azalea (Menziesia ferruginea), salal (Gaultheria shallon), blueberries (Vaccinium spp.) and saplings of canopy tree species [18]. Red alder (Alnus rubra) were the only deciduous trees at our sites. Selective logging of spruce was conducted at most sites in the 1940s, two sites had evidence of logging since the 1940s, and only one of our sites had clear-cut logging activity in the upper watershed during our sampling period.
(b). Salmon biomass
Salmon counts were done jointly by the Heiltsuk First Nation, the Canadian Department of Fisheries and Oceans and by our team from Simon Fraser University. Stream surveys are conducted on foot and personnel aim to return to creeks to estimate spawning salmon abundance at least three times over the August–November spawning season (see electronic supplementary material, appendix S1). The salmon term included in models was measured in biomass (kg) rather than abundance because biomass provides a better indicator of salmon nutrient input. We calculated a 3 year mean biomass, which ranged from zero to 38 033 kg (mean = 10 627 ± 4545 kg) for chum and 11 360 kg (mean = 2622 ± 1121 kg) for pink salmon across our study watersheds (electronic supplementary material, appendix S2). Coho and sockeye salmon were excluded from total biomass estimates because they only account for 2 per cent of the total salmon biomass at our study sites and we never observed carcasses of either species in estuaries during autumn carcass surveys.
(c). Breeding birds
We recorded all birds seen and heard up to 50 m into the forest while walking a strip-transect at a constant speed (0.75 km h−1) 5 m out from and parallel to the forest edge along the perimeter of each of the 21 estuaries. The starting point was reversed at each visit. Surveys were conducted within the first 5 h after sunrise and were repeated twice per site from 27 May to 7 July in 2008 and four times per site from 5 May to 27 June in 2009 [19]. Visit days were chosen randomly and R.D.F. was the only observer. Bird surveys were not conducted on days with heavy rainfall or significant wind [20]. A complete list of all bird species observed is presented in electronic supplementary material, appendix S3.
For some analyses, we grouped bird species by foraging guilds, including insectivores, frugivores and generalists. Several of the species appear in more than one guild (electronic supplementary material, appendix S3). Although there are limitations associated with grouping species into guilds, this method allows us to include uncommon species in analyses and to determine how resources influence groups of species with comparable foraging techniques and diets [21,22]. Four species (American robin (Turdus migratorius), golden-crowned kinglet (Regulus satrapa), Pacific-slope flycatcher (Empidonax difficilis) and Pacific wren (Troglodytes pacificus)) were common enough to support individual analyses, as they were not absent from more than four sites over the 2 years. They also happened to encompass a diversity of foraging guilds and habitat-type preferences. Abundance was calculated for all birds together, for each guild separately and for the individual common species by averaging the total number of birds across all within-year surveys. To control for survey effort, bird abundances were divided by forest area surveyed to obtain total-, guild- and species-specific density estimates (number of birds per hectare of forest). Although distance sampling can be useful for estimating densities of organisms that vary in their detection ability with distance from the observer [23], we did not use this method because we were concerned with the relative differences in bird densities among sites, rather than in absolute density estimates, and the structure of the vegetation was similar among sites. Diversity was calculated as: (i) Shannon's index, which emphasizes species richness and rare species in a community, and (ii) Simpson's index (1 − D), which emphasizes evenness and common species [24].
(d). Environmental variables
To account for landscape features, estuary area maps were sketched on air photos of each study site at low tide (less than 1.8 m), and estuary area, surveyed forest area, watershed catchment area and mainstem and tributary lengths were determined using area maps and iMapBC [17]. Stream ‘bankfull’ was measured as the maximum width of a stream without flooding.
Forest canopy and structure were quantified from three to seven forest transects that ran 50 m into the forest perpendicular to the forest edge, with the higher number of forest transects used to characterize the larger estuaries. Diameter at breast height (d.b.h.) and per cent cover by species and by height class (1.3–15, 15–25 and greater than 25 m) of trees were recorded in a 3 m band along the entire length of each transect. Understorey structure was quantified by recording per cent cover of shrub species (including saplings with d.b.h. less than 10 cm) in 1 m2 quadrats at 0, 5, 10, 30 and 50 m along the forest transect. Per cent cover of shrubs was recorded by height class (see below). These sampling protocols were adapted from Meidinger et al. [25] and from Christie & Reimchen [13].
Avian abundance and diversity can be related to the number of deciduous trees, large trees, snags, as well as tree species richness and shrub cover through invertebrate prey availability, foraging and nesting habitat availability, and predator refugia [26–29]. Vertical structural heterogeneity is another important predictor of forest bird abundance and diversity [30,31]. To include this in our study, we split per cent cover estimates of vegetative layers into five height categories representing short shrub (0–0.5 m), shrub (0.5–1.3 m), mid-storey canopy (1.3–15 m), tree canopy (15–25 m) and tree supercanopy (greater than 25 m) cover averaged across all transects at each site (see also [20]). We then calculated foliage height diversity based on MacArthur & MacArthur [30].
(e). Data analysis
We used an information theoretic approach (Akaike's information criterion adjusted for small sample sizes (AICc) [32]) to determine the ability of each forest habitat variable to predict each of our response variables. We used variance inflation factor (VIF) scores to measure how much the variance of an estimated regression coefficient is increased by collinearity among predictors in an ordinary least-squares regression. A measure of tolerance for the ith predictor is 1 minus the proportion of variance it shares with other predictors in the model (1 − Ri2), and the VIF is measured as 1/(1 − Ri2). The VIF scores were less than 3 for all habitat variables, which indicates an acceptably small amount of covariance among predictors [33]. The sum of each predictor's AICc weight (wi), which is the relative likelihood of a model, was used to rank each forest parameter. To avoid over-fitting the models in our main analysis owing to sample size constraints, we chose a cut-off for top-variable parameter likelihood of 0.4 and retained the top-ranked habitat variable for each response variable.
We used an exploratory analysis to choose between potential metrics of salmon biomass. We compared: (i) the influence of historically accumulated nutrients using 3 year mean salmon biomass estimates, (ii) the influence of nutrients on avian abundance and diversity metrics using single-year spawning biomass estimates from 2 years prior to bird surveys, and (iii and iv) both salmon biomass terms divided by estuary area (km2) to account for the potential dilution of the influence of salmon nutrients as estuaries increase in size. Based on AICc scores, 3 year mean salmon biomass, consisting of combined chum and pink biomass, was the best predictor of bird metrics, so we retained this simple and more readily available metric for the main analysis (electronic supplementary material, appendix S2).
In the main analysis, the effects of residual salmon nutrients on bird densities and diversities were also tested using AICc model comparison. A set of seven or 15 linear regression candidate models was created for each bird response variable, depending on whether a forest habitat variable was retained in the exploratory AICc step for the response variable of interest (see above). The influence of salmon biomass was compared with estuary area, catchment area and the top-ranked forest habitat variable. Salmon terms were transformed (log10[salmon + 1]) to avoid using over-parametrized nonlinear models, and estuary area was transformed (log10[estuary area]) to improve assumptions about residual distributions. Year was included as a two-level factor (2008 and 2009) in all candidate models to control for potential inter-annual variability. None of the variables had VIF scores greater than 3, indicating an acceptable amount of covariance among predictors [33]. We calculated AICc weights (wi) and ΔAICc values, which are the difference between AICc values of the top model and subsequent models [34]. Model averaging was also conducted for each variable to obtain the weighted parameter estimates and the unconditional standard error (s.e.), which aid in interpretation of model ranking and individual predictor importance (electronic supplementary material, appendix S4 [35]). We also hypothesized that a positive asymptotic relationship existed between bird metrics and untransformed salmon biomass. We tested this using a nonlinear asymptotic exponential model. Assumptions of residual distributions were met. All statistical analyses were computed using R [36].
3. Results
Salmon biomass proved to be a significant predictor of Shannon's diversity, which highlights richness and rare species, and total bird, insectivore, frugivore, golden-crowned kinglet and Pacific wren densities. For these bird metrics, models that included salmon biomass provided better fits to the data than models that included habitat variables alone. We observed similar results in a post hoc analysis, which divided insectivores into aerial, foliage and ground foragers. Post hoc analyses also revealed that chum biomass was a more important predictor than total or pink biomass alone for most of our bird metrics. Although salmon biomass was in top-ranked models, the low model R2 and insignificant p-values (greater than 0.094) for frugivore, generalist and American robin densities across all candidate models suggest that none of the models were particularly good descriptors of these species' densities.
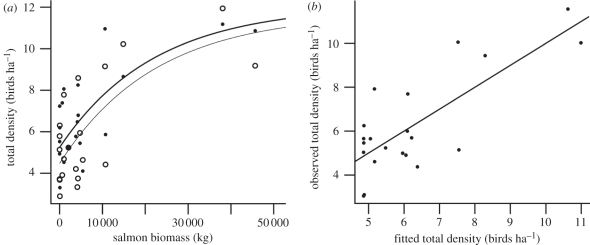
The data supported our prediction of a positive asymptotic relationship between bird communities and untransformed salmon biomass (e.g. figure 2a). This asymptote was reached beyond approximately 40 000 kg of salmon biomass for Shannon's index of diversity and total bird and insectivore density. A graphical illustration of nonlinear asymptotic exponential model fit for total bird density predicted by untransformed salmon biomass is presented in figure 2b.
Figure 2.
(a) Total bird density in 2008 (open circles and thin line) and 2009 (filled circles and thick line) (number of birds per hectare of forest) in relation to untransformed salmon biomass (kg) fitted by nonlinear asymptotic exponential models; (b) observed total bird density versus fitted total bird density of the nonlinear model with untransformed salmon biomass as the only predictor. Model for combined years: total bird density = 11.89 – 7.034e−0.00004495 × salmon biomass. Here, we omitted ‘year’ as a predictor by averaging bird metrics across both years.
Shrub cover was the most common top-ranked habitat feature and, contrary to our predictions, it negatively predicted total bird, insectivore, frugivore and Pacific-slope flycatcher densities (electronic supplementary material, appendix S3). Foliage height diversity was the second most commonly top-ranked habitat feature and positively predicted Shannon's index of diversity and generalist and American robin densities (table 1). However, foliage height diversity described a significant amount of variation in the top-ranked model only for Shannon's diversity (p < 0.001). When a forest habitat metric together with salmon biomass were in top-ranked models, salmon biomass models with these forest habitat variables excluded show little differences in R2 values, suggesting that these habitat variables only accounted for a small amount of additional variation in total bird, insectivore, golden-crowned kinglet and Pacific wren densities compared with salmon biomass alone.
Table 1.
AICc model selection analysis of linear regression models (top models presented: ΔAICc < 3 to a maximum of five models) describing bird density and diversity response variables predicted by salmon biomass and environmental variables. K = number of parameters, ΔAICc = difference between the model AICc and the top model AICc, wi = model AICc weight, R2 = model regression coefficient, salmon = summed chum + pink salmon biomass and FHD = foliage height diversity. Salmon and estuary area were log10-transformed. Year was included as a variable in all models but is excluded in the table for clarity of presentation.
| response variable | model | K | ΔAICc | wi | R2 |
|---|---|---|---|---|---|
| total bird density | salmon + estuary area | 5 | 0 | 0.40 | 0.43 |
| salmon + shrub cover + estuary area | 6 | 2.06 | 0.14 | 0.44 | |
| salmon + estuary area + catchment area | 6 | 2.22 | 0.13 | 0.44 | |
| Shannon's diversity | salmon + FHD + catchment area | 6 | 0 | 0.27 | 0.53 |
| salmon + FHD + estuary area + catchment area | 7 | 0.42 | 0.22 | 0.56 | |
| salmon + FHD + estuary area | 6 | 0.69 | 0.19 | 0.52 | |
| FHD + catchment area | 5 | 1.27 | 0.14 | 0.48 | |
| FHD + estuary area + catchment area | 6 | 1.31 | 0.14 | 0.52 | |
| Simpson's diversity (1 − D) | estuary area | 4 | 0 | 0.26 | 0.25 |
| salmon | 4 | 0.14 | 0.24 | 0.25 | |
| catchment area | 4 | 0.20 | 0.24 | 0.25 | |
| salmon + estuary area | 5 | 2.22 | 0.09 | 0.26 | |
| salmon + catchment area | 5 | 2.44 | 0.08 | 0.26 | |
| insectivore density | salmon + estuary area | 5 | 0 | 0.43 | 0.45 |
| salmon + shrub cover + estuary area | 6 | 2.09 | 0.15 | 0.46 | |
| salmon + estuary area + catchment area | 6 | 2.47 | 0.12 | 0.46 | |
| frugivore density | catchment area | 4 | 0 | 0.17 | 0.11 |
| salmon | 4 | 0.03 | 0.17 | 0.11 | |
| shrub cover | 4 | 0.70 | 0.12 | 0.09 | |
| salmon + catchment area | 5 | 1.32 | 0.09 | 0.14 | |
| salmon + shrub cover | 5 | 1.41 | 0.09 | 0.13 | |
| generalist density | FHD | 4 | 0 | 0.20 | 0.08 |
| FHD + catchment area | 5 | 0.53 | 0.15 | 0.12 | |
| salmon + FHD | 5 | 0.80 | 0.13 | 0.12 | |
| FHD + estuary area | 5 | 0.91 | 0.13 | 0.11 | |
| estuary area | 4 | 2.02 | 0.07 | 0.03 | |
| American robin density | FHD | 4 | 0 | 0.32 | 0.11 |
| FHD + estuary area | 5 | 2.00 | 0.12 | 0.13 | |
| FHD + catchment area | 5 | 2.26 | 0.10 | 0.12 | |
| salmon + FHD | 5 | 2.38 | 0.10 | 0.12 | |
| golden-crowned kinglet density | salmon + estuary area | 5 | 0 | 0.34 | 0.40 |
| salmon + deciduous trees + estuary area | 6 | 2.07 | 0.12 | 0.41 | |
| salmon + catchment area | 5 | 2.16 | 0.12 | 0.37 | |
| salmon + estuary area + catchment area | 6 | 2.40 | 0.10 | 0.41 | |
| salmon | 4 | 2.95 | 0.08 | 0.32 | |
| Pacific-slope flycatcher density | estuary area | 4 | 0 | 0.34 | 0.18 |
| estuary area + catchment area | 5 | 1.75 | 0.14 | 0.19 | |
| shrub cover + estuary area + catchment area | 5 | 2.12 | 0.12 | 0.19 | |
| salmon + estuary area | 5 | 2.48 | 0.10 | 0.18 | |
| shrub cover + estuary area + catchment area | 6 | 2.59 | 0.09 | 0.23 | |
| Pacific wren density | salmon + tree richness | 5 | 0 | 0.28 | 0.21 |
| salmon | 4 | 0.67 | 0.20 | 0.15 | |
| salmon + catchment area | 5 | 2.19 | 0.09 | 0.17 | |
| salmon + estuary area | 5 | 2.41 | 0.08 | 0.17 | |
| salmon + tree richness + estuary area | 6 | 2.49 | 0.08 | 0.22 |
Estuary area was the most commonly top-ranked landscape feature and was a significant positive predictor in top-ranked models for total bird, insectivore, golden-crowned kinglet and Pacific-slope flycatcher densities (p < 0.05). Although estuary area was in the top-ranked model for Simpson's index of diversity, year was the only significant predictor of this metric, demonstrated by the similarity in R2 values across all candidate models (table 1). Catchment area was a significant positive predictor in top-ranked models for Shannon's index of diversity and frugivore densities (p < 0.05).
4. Discussion
Salmon biomass was an important predictor of several measures of breeding bird density and diversity. Our findings demonstrate that breeding birds may benefit from residual salmon-derived nutrients in landscapes adjacent to spawning grounds and that this trend extends beyond stream riparian zones to estuarine riparian forests, and well beyond the salmon spawning season.
Total bird density, Shannon's index of diversity (which emphasizes richness and rare species) and the densities of insectivorous birds, golden-crowned kinglets and Pacific wrens were correlated strongly with salmon biomass. Nutrients from salmon probably affect breeding birds through several indirect pathways, including increased availability of emerged adult aquatic insect prey. Adults of aquatic invertebrates are found throughout forested habitats and are consumed by insectivorous birds in multiple foraging guilds [37]. However, aquatic invertebrates are highly variable in marine-derived nutrient enrichment owing to the variety of diet pathways between invertebrates and salmon tissues [38]. Salmon flesh and eggs are consumed both by aquatic invertebrates directly, and indirectly through grazing of nitrogen-enriched benthic algae and microbial decomposers [39]. Large biomasses of certain species of aquatic invertebrates, such as chironomid midges (Diptera: Chironomidae) and Zapada (Plecoptera: Nemouridae), use in-stream carcasses as sources of nutrients for over-wintering pupae, and emerge and disperse throughout the terrestrial environment in spring [40]. Some bird species may opportunistically subsidize their regular diets with emerging aquatic insects prior to the peak of terrestrial insect prey biomass during the early portion of the breeding season [41]. Pacific wrens frequently consume aquatic invertebrates [37], and isotopic analyses of their tissues have confirmed that they consume salmon nitrogen-enriched prey items [42].
Terrestrial invertebrates provide another potential source of salmon nutrients to summer breeding birds. Salmon carcasses that have been transferred to adjacent terrestrial zones by bears, wolves and other species are quickly colonized by terrestrial invertebrates, which transfer these nutrients to terrestrial food webs. For example, large biomasses of flies accumulate on and deposit their eggs on these carcasses, leaving their offspring to consume the salmon tissues and over-winter as pupae in surrounding soils [43]. Flies have high marine-derived 13C and 15N signatures [42], and could benefit birds such as flycatchers and some warblers. It should be noted that, like aquatic invertebrates, terrestrial invertebrates vary widely in the amount of marine-derived 15N that they retain [44]; therefore, additional research is needed to determine the extent to which these invertebrates provide salmon-derived nutrients to avian consumers.
Plants can grow more quickly when they are fertilized by salmon nutrients [45] and, since insects generally target fast-growing plants with higher levels of nitrogen, this could lead to higher abundances of herbivorous insects [46]. Moreover, nitrogen-rich indicator species, such as salmonberry (R. spectabilis) [47], are common around our salmon-bearing streams and, owing to reduced production of defensive phenolic metabolites in nitrogen-enriched vegetation, may also harbour an increased number of insect herbivores [48]. This could benefit foliage-gleaning birds such as the golden-crowned kinglet, whose densities were well predicted by salmon biomass in our study. Shrub fruit productivity is also increased by nitrogen fertilization [49]. However, contrary to our prediction, frugivore densities were not related to salmon biomass.
Post hoc analysis revealed that chum salmon, which comprise 81 per cent of the total salmon biomass in our study sites, have stronger relationships to avian density and diversity than total salmon biomass and smaller bodied pink salmon for the majority of bird metrics. This finding matches a study of salmon-derived 15N enrichment in riparian plants and invertebrates [50]. This could be because bears, which are the main wildlife vector for transport of salmon to forests, prefer the larger bodied chum [51]. It should also be noted that pink salmon generally spawn in stream reaches closer to the sea than chum [4], and we have observed large numbers of wolf-killed pink carcasses in estuaries in the autumn. Therefore, pink salmon may contribute large amounts of nutrients to some estuaries.
The influence of forest habitat and landscape features on birds was variable. The negative association of shrub cover with birds was contrary to our prediction. However, similar results to ours were observed by Shirley [29] who found that birds placed in a ‘riparian specialist’ guild were also negatively predicted by shrub cover. Insectivorous aerial foragers and hovering foliage gleaners can benefit from sparse shrub cover [52,53], and this may have contributed to the pattern we found. Other than foliage height diversity, the remaining forest habitat variables were less frequently shown to be top predictors. Large catchment areas provide greater terrestrial nutrient input to estuaries [54], and larger estuaries potentially have an increased availability of food for breeding birds in meadows, mudflats and intertidal zones. Other features, such as watershed red alder composition [45], climate, soil fertility, successional stage and plant species makeup [55], may mediate availability of nutrients to breeding birds. Moreover, variation in salmon nutrient retention by streams owing to in-stream organic debris, freshets, carnivore scavenging [56,57] and complexity of stream habitat [58] may influence the amount of nutrients from salmon that become available to birds in the estuary.
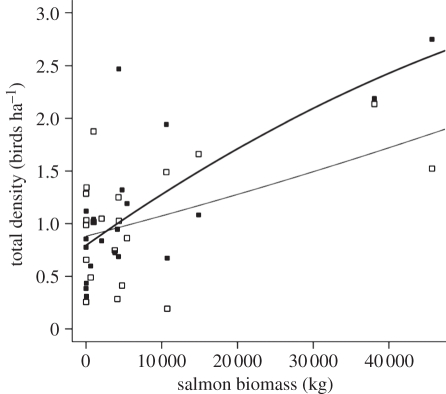
The curvilinear increases that we observed in density and diversity of birds across sites (e.g. figure 2a) suggest that residual salmon nutrients may be important for breeding birds even at sites with relatively few fish. There has been some uncertainty around this issue, as the studies reviewed by Janetski et al. [59] suggested that low densities of salmon (0.1–1.0 kg m−2) did not influence stream ecosystem response variables. Current fisheries management practices are limited in their ability to set goals for salmon population sizes that support the nutritional requirements of watersheds [60]. Our study shows that, for this region, the relationship between salmon and the diversity and density of birds generally approaches an asymptote beyond 40 000 kg of salmon biomass, after which there is no additional effect of salmon perhaps owing to avian territory size limitations or other habitat limitations. However, some species, such as golden-crowned kinglets and Pacific-slope flycatchers, exhibited a positive relationship with salmon that did not approach an asymptote at a biomass within our sampling range (figure 3). Thus, objectives would need to be specified clearly when managing salmon populations to achieve ‘ecosystem values’. Some caution is warranted when interpolating results within our asymptotic relationship (figure 2) and especially towards much larger inland river systems where the proportion of nutrients reaching estuaries may be quite different and nutrients may be diluted across a larger coastal landscape.
Figure 3.
Golden-crowned kinglet (filled squares and thick line) and Pacific-slope flycatcher (open squares and thin line) densities (number of birds per hectare of forest) in relation to untransformed salmon biomass (kg).
In conclusion, our study suggests that salmon have ecological influences on breeding bird populations, probably through the long-term cycling of salmon nutrients through coastal watershed food webs. This demonstrates that the importance of salmon extends to species whose presence in these ecosystems is asynchronous with salmon. Understanding how nutrients from salmon are important to recipient ecosystems is a vital step towards informed conservation and ecosystem-based management of wild salmon [61], and contributes to a more holistic appreciation of ecological phenomena.
Acknowledgements
We thank D. Esler and R. Ydenberg for constructive input during this study and we thank everyone who assisted us in the field, including D. Brown, M. Chung, F. Definney, J. Harding, P. Harrington, M. Hocking, L. Honka, J. Linton, R. Midgley, M. Nelson, H. Recker, M. Segal, M. Spoljaric, M. Stubbs and N. Swain. We appreciate analytical advice from D. Braun, A. Cooper, N. Dulvy, J. Harding, M. Hocking, J. Linton, M. Nelson, W. Palen, N. Swain and J. Verspoor. We thank the National Sciences and Engineering Research Council of Canada (NSERC), the Rix Family Scholarship Fund and the Tom Buell BC Leadership Chair endowment for financial support, and the Heiltsuk First Nation for allowing our study to be conducted in their traditional territory.
R.D.F. and J.D.R. designed the study and wrote the paper, and R.D.F. did the fieldwork and analysed the data.
References
- 1.Polis G. A., Strong D. R. 1996. Food web complexity and community dynamics. Am. Nat. 147, 813–846 10.1086/285880 (doi:10.1086/285880) [DOI] [Google Scholar]
- 2.Swap R., Garstang M., Greco S., Talbot R., Kallberg P. 1992. Saharan dust in the Amazon Basin. Tellus B 44, 133–149 10.1034/j.1600-0889.1992.t01-1-00005.x (doi:10.1034/j.1600-0889.1992.t01-1-00005.x) [DOI] [Google Scholar]
- 3.Ward J. V. 1989. The 4-dimensional nature of lotic ecosystems. J. N. Am. Benthol. Soc. 8, 2–8 10.2307/1467397 (doi:10.2307/1467397) [DOI] [Google Scholar]
- 4.Groot C., Margolis L. 1991. Pacific salmon life histories. Vancouver, Canada: UBC Press [Google Scholar]
- 5.Reimchen T. E., Mathewson D. D., Hocking M. D., Moran J., Harris D. 2003. Isotopic evidence for enrichment of salmon-derived nutrients in vegetation, soil, and insects in Riparian zones in coastal British Columbia. In Nutrients in salmonid ecosystems: sustaining production and biodiversity, vol. 34 (ed. Stockner J. G.), pp. 59–69 Herndon, VA: American Fisheries Society [Google Scholar]
- 6.Naiman R. J., Bilby R. E., Schindler D. E., Helfield J. M. 2002. Pacific salmon, nutrients, and the dynamics of freshwater and riparian ecosystems. Ecosystems 5, 399–417 10.1007/s10021-001-0083-3 (doi:10.1007/s10021-001-0083-3) [DOI] [Google Scholar]
- 7.Gresh T., Lichatowich J., Schoonmaker P. 2000. An estimation of historic and current levels of salmon production in the Northeast Pacific ecosystem: evidence of a nutrient deficit in the freshwater systems of the Pacific Northwest. Fisheries 25, 15–21 (doi:10.1577/1548-8446(2000)025<0015:AEOHAC>2.0.CO;2) [DOI] [Google Scholar]
- 8.Sidle R. C., Shaw C. G. 1983. Evaluation of planting sites common to a southeast Alaska clear-cut. 1. Nutrient status. Can. J. Forest. Res. 13, 1–8 10.1139/x83-001 (doi:10.1139/x83-001) [DOI] [Google Scholar]
- 9.Willson M. F., Gende S. M., Marston B. H. 1998. Fishes and the forest. Bioscience 48, 455–462 10.2307/1313243 (doi:10.2307/1313243) [DOI] [Google Scholar]
- 10.Moore J. W. 2006. Animal ecosystem engineers in streams. Bioscience 56, 237–246 10.1641/0006-3568(2006)056[0237:AEEIS]2.0.CO;2 (doi:10.1641/0006-3568(2006)056[0237:AEEIS]2.0.CO;2) [DOI] [Google Scholar]
- 11.Haycock N., Burt T., Goulding K., Pinay G. 1996. Buffer zones: their processes and potential in water protection. Harpenden, UK: Quest Environmental [Google Scholar]
- 12.Folkard N. F. G., Smith J. N. M. 1995. Evidence for bottom up effects in the boreal forest: do passerine birds respond to large scale experimental fertilization? Can. J. Zool. 73, 2231–2237 10.1139/z95-264 (doi:10.1139/z95-264) [DOI] [Google Scholar]
- 13.Christie K. S., Reimchen T. E. 2008. Presence of salmon increases passerine density on Pacific Northwest streams. Auk 125, 51–59 10.1525/auk.2008.125.1.51 (doi:10.1525/auk.2008.125.1.51) [DOI] [Google Scholar]
- 14.Gende S. M., Willson M. F. 2001. Passerine densities in riparian forests of southeast Alaska: potential effects of anadromous spawning salmon. Condor 103, 624–629 10.1650/0010-5422(2001)103[0624:PDIRFO]2.0.CO;2 (doi:10.1650/0010-5422(2001)103[0624:PDIRFO]2.0.CO;2) [DOI] [Google Scholar]
- 15.Shaw K. 2004. The global/local politics of the Great Bear Rainforest. Environ. Polit. 13, 373–392 10.1080/0964401042000209621 (doi:10.1080/0964401042000209621) [DOI] [Google Scholar]
- 16.Stevens M., Hoag C. 1996. Plant guide: slough sedge, pp. 1–5 Aberdeen, MD: USDA NRCS Idaho Plant Materials Center and the National Plant Data Center [Google Scholar]
- 17.Government of British Columbia: Integrated Land Management Bureau iMapBC. See http://www.webmaps.gov.bc.ca/imfx/imf.jsp?site=imapbc (Retrieved 15 August 2008)
- 18.Pojar J., Klinka K., Meidinger D. V. 1987. Biogeoclimatic ecosystem classification in British Columbia. For. Ecol. Manage. 22, 119–154 10.1016/0378-1127(87)90100-9 (doi:10.1016/0378-1127(87)90100-9) [DOI] [Google Scholar]
- 19.Manuwal D. A., Carey A. B. 1991. Wildlife-habitat relationships: sampling procedures for Pacific Northwest vertebrates—methods for measuring populations of small, diurnal forest birds (eds Carey A. B., Ruggiero L. F.), pp. 1–21 Portland, OR: USDA Forest Service [Google Scholar]
- 20.Lock P. A., Naiman R. J. 1998. Effects of stream size on bird community structure in coastal temperate forests of the Pacific Northwest, USA. J. Biogeogr. 25, 773–782 10.1046/j.1365-2699.1998.2540773.x (doi:10.1046/j.1365-2699.1998.2540773.x) [DOI] [Google Scholar]
- 21.Verner J. 1984. The guild concept applied to management of bird populations. Environ. Manage. 8, 1–13 10.1007/BF01867868 (doi:10.1007/BF01867868) [DOI] [Google Scholar]
- 22.Block W., Brennan L., Gutierrez R. 1986. Wildlife 2000: modeling habitat relationships of terrestrial vertebrates In The use of guilds and guild-indicator species for assessing habitat suitability. CA, USA: University of Wisconsin Press [Google Scholar]
- 23.Thomas L., Buckland S. T., Burnham K. P., Anderson D. R., Laake J. L., Borchers D. L., Strindberg S. 2002. Encyclopedia of environmetrics. In Distance sampling. Chichester, UK: John Wiley & Sons [Google Scholar]
- 24.Nagendra H. 2002. Opposite trends in response for the Shannon and Simpson indices of landscape diversity. Appl. Geogr. 22, 175–186 10.1016/S0143-6228(02)00002-4 (doi:10.1016/S0143-6228(02)00002-4) [DOI] [Google Scholar]
- 25.Meidinger D., Trowbridge R., Macadam A., Tolkamp C. 1998. Field manual for describing terrestrial ecosystems. Victoria, Canada: Crown Publications Inc [Google Scholar]
- 26.Stauffer D. F., Best L. B. 1980. Habitat selection by birds of riparian communities—evaluating effects of habitat alterations. J. Wildlife Manage. 44, 1–15 10.2307/3808345 (doi:10.2307/3808345) [DOI] [Google Scholar]
- 27.James F. C., Wamer N. O. 1982. Relationships between temperate forest bird communities and vegetation structure. Ecology 63, 159–171 10.2307/1937041 (doi:10.2307/1937041) [DOI] [Google Scholar]
- 28.Green R. N., Klinka K. 1994. A field guide to site identification and interpretation for the Vancouver forest region. Victoria, Canada: Research Branch—Ministry of Forests [Google Scholar]
- 29.Shirley S. 2004. The influence of habitat diversity and structure on bird use of riparian buffer strips in coastal forests of British Columbia, Canada. Can. J. Forest Res. 34, 1499–1510 10.1139/x04-038 (doi:10.1139/x04-038) [DOI] [Google Scholar]
- 30.MacArthur R., MacArthur J. W. 1961. On bird species diversity. Ecology 42, 594–598 10.2307/1932254 (doi:10.2307/1932254) [DOI] [Google Scholar]
- 31.Willson M. F. 1974. Avian community organization and habitat structure. Ecology 55, 1017–1029 10.2307/1940352 (doi:10.2307/1940352) [DOI] [Google Scholar]
- 32.Anderson D. R. 2008. Model based inferences in the life sciences, New York, NY: Springer [Google Scholar]
- 33.Zuur A. F., Ieno E. N., Elphick C. S. 2009. A protocol for data exploration to avoid common statistical problems. Method. Ecol. Evol. 1, 3–14 10.1111/j.2041-210X.2009.00001.x (doi:10.1111/j.2041-210X.2009.00001.x) [DOI] [Google Scholar]
- 34.Burnham K. P., Anderson D. R. 2002. Model selection and multimodel inference. New York, NY: Springer-Verlag [Google Scholar]
- 35.Anderson D. R., Burnham K. P. 2002. Avoiding pitfalls when using information-theoretic methods. J. Wildlife Manage. 66, 912–918 10.2307/3803155 (doi:10.2307/3803155) [DOI] [Google Scholar]
- 36.R Development Core Team 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 37.Murakami M., Nakano S. 2001. Species-specific foraging behavior of birds in a riparian forest. Ecol. Res. 16, 913–923 10.1046/j.1440-1703.2001.00448.x (doi:10.1046/j.1440-1703.2001.00448.x) [DOI] [Google Scholar]
- 38.Chaloner D. T., Martin K. M., Wipfli M. S., Ostrom P. H., Lamberti G. A. 2002. Marine carbon and nitrogen in southeastern Alaska stream food webs: evidence from artificial and natural streams. Can. J. Fish. Aquat. Sci. 59, 1257–1265 10.1139/f02-084 (doi:10.1139/f02-084) [DOI] [Google Scholar]
- 39.Kline T. C., Goering J. J., Mathisen O. A., Poe P. H., Parker P. L. 1990. Recycling of elements transported upstream by runs of Pacific salmon. I. Delta-N-15 and delta-C-13 evidence in Sashin Creek, southeastern Alaska. Can. J. Fish. Aquat. Sci. 47, 136–144 10.1139/f90-014 (doi:10.1139/f90-014) [DOI] [Google Scholar]
- 40.Chaloner D. T., Wipfli M. S., Caouette J. P. 2002. Mass loss and macroinvertebrate colonisation of Pacific salmon carcasses in south-eastern Alaskan streams. Freshw. Biol. 47, 263–273 10.1046/j.1365-2427.2002.00804.x (doi:10.1046/j.1365-2427.2002.00804.x) [DOI] [Google Scholar]
- 41.Nakano S., Murakami M. 2001. Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc. Natl Acad. Sci. USA 98, 166–170 10.1073/pnas.98.1.166 (doi:10.1073/pnas.98.1.166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christie K. S., Hocking M. D., Reimchen T. E. 2008. Tracing salmon nutrients in riparian food webs: isotopic evidence in a ground-foraging passerine. Can. J. Zool. 86, 1317–1323 10.1139/Z08-110 (doi:10.1139/Z08-110) [DOI] [Google Scholar]
- 43.Hocking M. D., Reimchen T. E. 2006. Consumption and distribution of salmon (Oncorhynchus spp.) nutrients and energy by terrestrial flies. Can. J. Fish. Aquat. Sci. 63, 2076–2086 10.1139/F06-110 (doi:10.1139/F06-110) [DOI] [Google Scholar]
- 44.Hocking M. D., Reimchen T. E. 2002. Salmon-derived nitrogen in terrestrial invertebrates from coniferous forests of the Pacific Northwest. BMC Ecol. 2, 4. 10.1186/1472-6785-2-4 (doi:10.1186/1472-6785-2-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helfield J. M., Naiman R. J. 2001. Effects of salmon-derived nitrogen on riparian forest growth and implications for stream productivity. Ecology 82, 2403–2409 10.1890/0012-9658(2001)082[2403:EOSDNO]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[2403:EOSDNO]2.0.CO;2) [DOI] [Google Scholar]
- 46.Price P. W. 1991. The plant vigor hypothesis and herbivore attack. Oikos 62, 244–251 10.2307/3545270 (doi:10.2307/3545270) [DOI] [Google Scholar]
- 47.Mathewson D. D., Hocking M. D., Reimchen T. E. 2003. Nitrogen uptake in riparian plant communities across a sharp ecological boundary of salmon density. BMC Ecol. 3, 4–15 10.1186/1472-6785-3-4 (doi:10.1186/1472-6785-3-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witzell J., Shevtsova A. 2004. Nitrogen-induced changes in phenolics of Vaccinium myrtillus—implications for interaction with a parasitic fungus. J. Chem. Ecol. 30, 1937–1956 10.1023/B:JOEC.0000045587.75128.a4 (doi:10.1023/B:JOEC.0000045587.75128.a4) [DOI] [PubMed] [Google Scholar]
- 49.Penney B. G., McRae K. B., Bishop G. A. 2003. Second-crop N fertilization improves lowbush blueberry (Vaccinium angustifolium Ait.) production. Can. J. Plant Sci. 83, 149–155 [Google Scholar]
- 50.Hocking M. D., Reimchen T. E. 2009. Salmon species, density and watershed size predict magnitude of marine enrichment in riparian food webs. Oikos 118, 1307–1318 10.1111/j.1600-0706.2009.17302.x (doi:10.1111/j.1600-0706.2009.17302.x) [DOI] [Google Scholar]
- 51.Reimchen T. E. 2000. Some ecological and evolutionary aspects of bear-salmon interactions in coastal British Columbia. Can. J. Zool. 78, 448–457 10.1139/cjz-78-3-448 (doi:10.1139/cjz-78-3-448) [DOI] [Google Scholar]
- 52.Robinson S. K., Holmes R. T. 1984. Effects of plant-species and foliage structure on the foraging behaviour of forest birds. Auk 101, 672–684 [Google Scholar]
- 53.Sakai H. F., Noon B. R. 1991. Nest-site characteristics of Hammond and Pacific-slope flycatchers in northwestern California. Condor 93, 563–574 10.2307/1368188 (doi:10.2307/1368188) [DOI] [Google Scholar]
- 54.Bridge J. S. 2003. Rivers and floodplains: forms, processes, and sedimentary record. Oxford, UK: Blackwell [Google Scholar]
- 55.Vitousek P. M., Sanford R. L. 1986. Nutrient cycling in moist tropical forest. Ann. Rev. Ecol. Syst. 17, 137–167 10.1146/annurev.es.17.110186.001033 (doi:10.1146/annurev.es.17.110186.001033) [DOI] [Google Scholar]
- 56.Cederholm C. J., Houston D. B., Cole D. L., Scarlett W. J. 1989. Fate of coho salmon (Oncorhynchus kisutch) carcasses in spawning streams. Can. J. Fish. Aquat. Sci. 46, 1347–1355 10.1139/f89-173 (doi:10.1139/f89-173) [DOI] [Google Scholar]
- 57.Cederholm C. J., Peterson N. P. 1985. The retention of coho salmon (Oncorhynchus kisutch) carcasses by organic debris in small streams. Can. J. Fish. Aquat. Sci. 42, 1222–1225 10.1139/f85-150 (doi:10.1139/f85-150) [DOI] [Google Scholar]
- 58.Meehan E. P., Seminet-Reneau E. E., Quinn T. P. 2005. Bear predation on Pacific salmon facilitates colonization of carcasses by fly maggots. Am. Midl. Nat. 153, 142–151 10.1674/0003-0031(2005)153[0142:BPOPSF]2.0.CO;2 (doi:10.1674/0003-0031(2005)153[0142:BPOPSF]2.0.CO;2) [DOI] [Google Scholar]
- 59.Janetski D. J., Chaloner D. T., Tiegs S. D., Lamberti G. A. 2009. Pacific salmon effects on stream ecosystems: a quantitative synthesis. Oecologia 159, 583–595 10.1007/s00442-008-1249-x (doi:10.1007/s00442-008-1249-x) [DOI] [PubMed] [Google Scholar]
- 60.Bilby R. E., Fransen B. R., Walter J. K., Scarlett W. J. 2001. Preliminary evaluation of the use of nitrogen stable isotope ratios to establish escapement levels for pacific salmon. Fisheries 26, 6–14 (doi:10.1577/1548-8446(2001)026<0006:PEOTUO>2.0.CO;2) [DOI] [Google Scholar]
- 61.Willson M. F., Halupka K. C. 1995. Anadromous fish as keystone species in vertebrate communities. Conserv. Biol. 9, 489–497 10.1046/j.1523-1739.1995.09030489.x (doi:10.1046/j.1523-1739.1995.09030489.x) [DOI] [Google Scholar]