Abstract
A fundamental limitation in many climate change experiments is that tests represent relatively short-term ‘shock’ experiments and so do not incorporate the phenotypic plasticity or evolutionary change that may occur during the gradual process of climate change. However, capturing this aspect of climate change effects in an experimental design is a difficult challenge that few studies have accomplished. I examined the effect of temperature and predator climate history in food webs composed of herbaceous plants, generalist grasshopper herbivores and spider predators across a natural 4.8°C temperature gradient spanning 500 km in northeastern USA. In these grasslands, the effects of rising temperatures on the plant community are indirect and arise via altered predator–herbivore interactions. Experimental warming had no direct effect on grasshoppers, but reduced predation risk effects by causing spiders from all study sites to seek thermal refuge lower in the plant canopy. However, spider thermal tolerance corresponded to spider origin such that spiders from warmer study sites tolerated higher temperatures than spiders from cooler study sites. As a consequence, the magnitude of the indirect effect of spiders on plants did not differ along the temperature gradient, although a reciprocal transplant experiment revealed significantly different effects of spider origin on the magnitude of top-down control. These results suggest that variation in predator response to warming may maintain species interactions and associated food web processes when faced with long term, chronic climate warming.
Keywords: adaptation, behaviour, climate change, food web, local climate, predator–prey
1. Introduction
A primary focus in ecology is to understand how climate change may affect species and their interactions within ecological communities [1–3]. Experimental approaches generally manipulate climate variables (e.g. temperature, precipitation, nitrogen deposition, etc.) to simulate conditions predicted by global change models, and measure species responses after a short time period. A limitation of this approach is that relatively short-term ‘shock’ experiments may not accurately test how a long term, chronic effect, such as climate change, will affect ecological communities [4]. For most species, climate change is a gradual process that will span multiple generations. As a consequence, species may exhibit phenotypic plasticity or evolutionary responses, which may alter the effects of climate change within the system [5–7]. Therefore, extrapolating the effects of gradual climate change from short-term experiments that test future climate scenarios on extant systems may be misleading [8–11].
Studies that have addressed long-term responses to climate change within their experimental designs are rare [5,6,12]. Successful designs have used long-term datasets that manipulated variables over decades [8], or microbial systems with short generation times [13]. I report here on a different tack. I examined grassland food webs along a natural temperature gradient to test if food web function can be maintained when species are subjected to long term, chronic differences in climate.
I used food webs composed of herbaceous plants, a generalist grasshopper herbivore (Melanoplus femurrubrum), and a sit-and-wait spider predator (Pisaurina mira). Pisaurina mira causes M. femurrubrum to shift from a grass-dominated diet (P. mira absent) to a herb-dominated diet (P. mira present [14]). Thus, this spider affects the plant community composition by exerting a positive indirect effect on grasses and a negative indirect effect on herbs. Interestingly, experimental warming that shocks the system to levels predicted by climate change models does not directly affect the plant biomass production or magnitude of grasshopper herbivory [15,16]. Instead, warming affects plant biomass via predator-induced changes in herbivore foraging behaviour that cause an indirect effect on competitively dominant herbs. This occurs because spiders and grasshoppers are affected by warming differently. I previously demonstrated that at ambient temperatures these two species overlap spatially within the plant canopy and spiders decrease grasshopper daily feeding time through sublethal risk effects [16]. However, warming causes spiders to seek thermal refuge in the shade of the lower plant canopy where temperatures are cooler [17], whereas grasshopper location is unaffected. Grasshoppers continue to respond to spider presence by consuming a herb-based diet, but also increase daily feeding time as spiders move lower, thereby exacerbating their effect on the competitively dominant herb [16].
It remains uncertain, however, whether such exacerbation is maintained when species are exposed to long term, chronic differences in temperature. Current understanding of this system generates the prediction that the indirect effect of P. mira spiders would be stronger in warm climates and weaker in cooler climates [15,16]. However, if P. mira spiders have responded to long term, chronic differences in local climate, it could be predicted that the magnitude of the indirect effect would not differ along a temperature gradient. The thermal tolerances of species across their geographical range may reflect local adaptation to climate. If this is the case, the potential role of adaptation to climate change could be inferred from a space-for-time substitution using a transplant experiment [18]. If populations on the equatorial side of the species range are adapted to warmer climate conditions, then you should see two patterns: (i) in situ populations at different latitudes should function similarly and (ii) transplanting high-latitude populations to lower latitudes should strengthen top-down effects consistent with previous evidence [15,16]. A reciprocal transplant of low-latitude populations to higher latitudes can address whether local adaptation to warmer climates affects function in cooler climates. In other words, are the effects of local adaptation to warmer and cooler climates reciprocal? While this experimental strategy does not test the role of evolution directly, it explicitly accounts for within-species variation arising under different climate histories and how this might influence community-level responses to climate change.
2. Material and methods
I conducted a series of experiments during the summer of 2009 to test if local variation in the physiology and behaviour of P. mira could maintain top-down control by P. mira within grassland food webs facing climate change. I selected three study sites along a north–south temperature gradient spanning 500 km. The study sites were located in northern Vermont (VT), northern Connecticut (CT) and central New Jersey (NJ). The range of mean summer temperature within the plant canopy represented on this gradient (approx. 4.8°C; see §2c) was similar to the increase in temperature predicted by global change models for this region [19]. The sites were established in old-field meadows dominated by the forbs Solidago rugosa and Solidago lancelota, and grasses Poa pratensis and Phleum pretense. To isolate the effects of spider responses, in all experiments I manipulated only spider origin and used grasshoppers and plants endemic to each study site. Laboratory experiments (§§a,b) were conducted at the School of Forestry and Environmental Studies, Yale University, New Haven, CT, USA.
(a). Spider thermal tolerance
I conducted a physiological stress experiment [20] to determine if spiders from warmer locations had a higher tolerance to heat than spiders from cooler locations. I collected adult spiders from each study site and housed them in individual containers at room temperature for 3–5 days before initiating the experiment. I randomly assigned 15 spiders from each study site to numbered Petri dishes inside a low-temperature incubator (Thermo Scientific, Waltham, MA, USA) initially set to 25°C (approximately room temperature), arranged in three rows of 15 dishes. I allowed the spiders to equilibrate in the incubator for 1 h, then initiated the experiment by increasing the temperature 1°C at 5 min intervals [20]. A control group of an additional five spiders from each population were placed in Petri dishes on a laboratory table next to the incubator and subjected to constant room temperature during the course of the experiment. At each 5 min interval, I approached each spider with a metal dissecting teasing needle and observed defensive responses to the approaching needle (e.g. moving away, raising forelimbs or grabbing the needle). While an approaching needle may not exactly indicate a spider's defensive or hunting ability at a given temperature, this technique provides a standardized stimulus useful in comparing responsiveness of spiders from different locations at different temperatures. To maximize the efficiency of this method and minimize the amount of time the incubator door was open, I moved the dissecting needle continuously down the each row, stopping only when a spider failed to respond. Unresponsiveness was confirmed by immediately approaching the spider with the dissecting needle a second time. When an individual did not respond to the approaching needle, it was removed from the incubator and the last temperature at which it responded was recorded as its critical thermal maximum (CTM). The control group was approached at the same intervals as the experimental group, allowing me to isolate the effects of temperature and habituation on observed CTM. After the experiment, spiders were returned to their capture location. This experiment was repeated twice with newly collected individuals. I explored the effect of experimental date and spider origin on CTM using a two-way analysis of variance (ANOVA) followed by a Tukey test.
(b). Behavioural experiment
I conducted behavioural observations [17] to determine the effects of warming and spider origin on grasshopper feeding behaviour. I observed 3rd and 4th instar grasshoppers and adult spiders in terraria constructed from a 30 × 25 cm rectangular plywood base that contained a sod cut from the field at the CT study site. The sod and the vegetation were enclosed by a 75 cm tall piece of an aluminium insect screen that was attached to the base and a 30 × 25 cm removable insect screen lid that prevented arthropods from escaping. I affixed a metric ruler to each enclosure to determine arthropod vertical height in the enclosure. Each terrarium was outfitted with an infrared heat lamp (250 w, Exp-Terra heat Wave Lamp, Mansfield, MA, USA), mounted level with the top of each terrarium and approximately 15 cm from the side and at a 45° angle. This design warms terraria to an average of 3°C above ambient and maintains a vertical temperature gradient similar to that observed under natural field conditions where the top of the plant canopy is 3–4°C warmer than the soil surface [16,17]. I placed a thermometer in the centre of each terrarium to determine the maximum daily temperature (Tmax). The terraria were observed on bench tops in a greenhouse and watered after each day of observations and before stocking arthropods.
One day prior to observations, I randomly assigned terraria to temperature (ambient or warmed) and trophic-level treatments (2-level grasshopper only or 3-level grasshopper and a single spider from NJ, CT or VT) such that each temperature/trophic-level combination was represented twice. Arthropods were stocked into terraria at densities of one grasshopper · enclosure−1 and one spider · enclosure−1, and heat lamps were turned on in warming treatments. The next day, I observed individuals between 06.00 and 21.00 h. At 30 min intervals, I recorded grasshopper and spider height in the terraria (0–75 cm). I also recorded if grasshoppers were feeding and, if they were, whether they were feeding on grasses or herbs. I calculated the average spider and grasshopper height in the plant canopy. Grasshopper daily feeding time was determined by assuming that each observed feeding bout lasted the entire 30 min interval between observations and multiplying the number of observed feeding bouts by 30 min [21]. I determined daily diet composition for each grasshopper by dividing the number of times I observed it feeding on herbs by the number of times I observed it feeding.
I conducted 12 observations of each treatment combination, using a new complement of individuals during each set of observations. To determine the effect of Tmax on grasshopper and spider behaviour I pooled temperature treatments (ambient and warmed) and conducted a series of linear regressions (n = 24 for each treatment combination) and adjusted the significance level with a sequential Bonferroni correction as appropriate [22]. I tested the effect of Tmax on grasshopper height and daily feeding time when predators were absent and present, Tmax on grasshopper diet composition, Tmax on spider height and spider height on grasshopper feeding time. To determine if magnitude of the effect of the independent variable (Tmax or spider height) on species responses differed among spider origin treatments, I compared the slope (ß) of each linear regression using a t-test.
(c). Reciprocal transplant experiment
I tested the prediction that physiological and behavioural responses to local climate maintained the nature and strength of species' interactions across a temperature gradient in a reciprocal transplant study. I used standard protocols for enclosure experiments evaluating top-down effects [14], which involved systematically altering the number of trophic levels and measuring plant biomass (grasses and herbs) in each trophic treatment at the end of the growing season. Cylinder-shaped enclosures (1 m2 × 0.8 m) were constructed from a vinyl garden fence support frame wrapped in an aluminium insect screen and closed with a removable fibreglass insect screen lid. Enclosures were randomly assigned to one of four treatments: 1 two-trophic-level treatment (local plants and local grasshoppers) and 3 three-trophic-level treatments (local plants, local grasshoppers, and one each of spiders from VT, CT or NJ). These treatments were fully replicated within a block design six times at each of the three sites.
I initiated each experiment when 2nd instar grasshoppers began to appear locally (NJ: 26 June 2009, CT: 2 July 2009 and VT: 8 July 2009). Arthropods were stocked into 2- and 3-level enclosures at densities of 10 grasshoppers × enclosure−1 and two spiders × enclosure−1. At each study site, I placed Hobo temperature data loggers (Onset Corporation, Pocasset, MA, USA) programmed to record temperature at 1 h intervals within three randomly chosen enclosures. Loggers were held approximately 10 cm above the soil surface on a wooden dowel. I terminated each experiment after eight weeks by removing all above-ground vegetation. Vegetation was sorted into two functional groups, grasses and herbs, which were weighed after drying at 60°C for 48 h.
I calculated the mean temperature (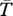 ) at each study location using the mean temperature within each of the three enclosures containing temperature loggers. I calculated the indirect effect magnitude of spiders on grass and herb abundance within each block using the log ratio [ln (Vp+/Vp−)] where Vp+ and Vp− are, respectively, grass or herb biomass in the presence (three-trophic-level treatment) and absence (two-trophic-level treatment) of predators [23]. I first tested the prediction that the effect magnitude of spiders in their local climates would not differ across study sites using a one-way ANOVA. I then examined the effect of spider origin and study site location on the indirect effect magnitude of spiders on grass and herb abundance with a two-way ANOVA followed by a Tukey test whenever a significant difference was detected. Significance tests were adjusted using a sequential Bonferroni method to account for multiple comparisons [22].
) at each study location using the mean temperature within each of the three enclosures containing temperature loggers. I calculated the indirect effect magnitude of spiders on grass and herb abundance within each block using the log ratio [ln (Vp+/Vp−)] where Vp+ and Vp− are, respectively, grass or herb biomass in the presence (three-trophic-level treatment) and absence (two-trophic-level treatment) of predators [23]. I first tested the prediction that the effect magnitude of spiders in their local climates would not differ across study sites using a one-way ANOVA. I then examined the effect of spider origin and study site location on the indirect effect magnitude of spiders on grass and herb abundance with a two-way ANOVA followed by a Tukey test whenever a significant difference was detected. Significance tests were adjusted using a sequential Bonferroni method to account for multiple comparisons [22].
3. Results
(a). Spider thermal tolerance
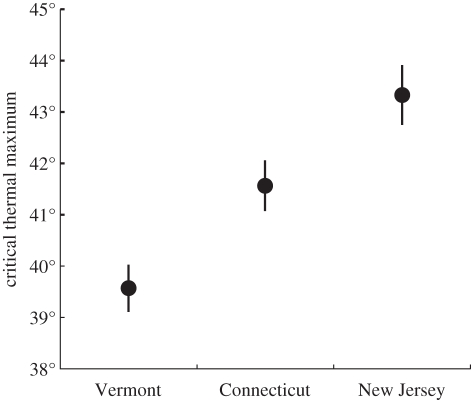
Spiders within the control group always responded to the approaching dissecting needle, indicating that spiders did not habituate to this stimulus. Spider CTM differed among study sites (two-way ANOVA F2,84 = 13.45, p < 0.001; figure 1). CTM was highest for NJ spiders (43.3°C ± 0.60), followed by CT spiders (41.6°C ± 0.49) and VT spiders (39.6°C ± 0.46; Tukey test, p's < 0.05). There was not a significant effect of experiment date (F1,84 = 0.02, p = 0.881) or a date × origin interaction (F2,84 = 2.13, p < 0.125).
Figure 1.
Critical thermal maximum differed among spiders from different study sites. Spiders from warmer locations continued to respond to stimuli at higher temperatures than spiders from cooler locations (two-way ANOVA F2,84 = 13.45, p < 0.001, Tukey test all p's < 0.05). Points represent mean ± s.e.
(b). Behavioural experiment
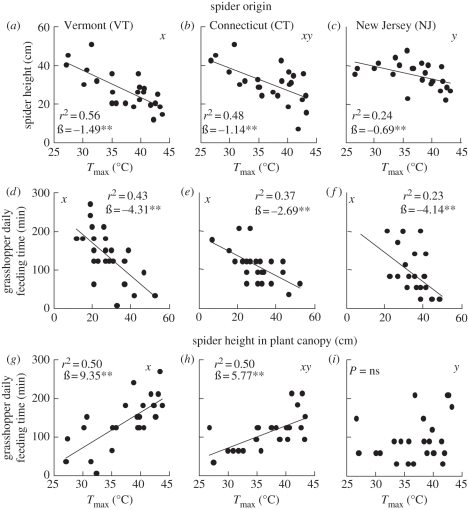
Warming had no direct effect on grasshopper height in the plant canopy (no spider treatments, linear regression, F1,22 = 0.56, p = 0.44) or daily feeding time (no spider treatments, linear regression, F1,22 = 0.003, p = 0.96). Consistent with previous results [17], warming segregated grasshoppers and spiders spatially by causing spiders to move lower in the plant canopy (linear regression, F1,22 ≥ 6.81, p's ≤ 0.032, r2 = 0.24–0.56; figure 2a–c). However, the magnitude of the effect of 1°C increase in Tmax on spider position more than doubled between spiders from the warmer NJ site (ß = −0.69) and those from the cooler VT site (ß = −1.49, t-test, t = 2.06, d.f. = 46, p = 0.045; figure 2). As a result, NJ spiders remained higher on average than VT spiders (One-way ANOVA, F2,69 = 4.35, p = 0.032, Tukey test, NJ versus VT spiders p = 0.013, all other pairwise comparisons p ≥ 0.18).
Figure 2.
Results of behavioural experiments show that the effect of warming on grasshopper daily feeding time was indirect and mediated by spider response to temperature. (a–c) Spider vertical location within the plant canopy was negatively affected by increasing temperature. (d–f) Grasshopper daily feeding time increased as spider height in the plant canopy decreased. (g–i) Warming indirectly increased grasshopper daily feeding time when VT and CT spiders were present, but had no effect when NJ spiders were present. Statistical significance of linear regressions is reported as *p < 0.05, **p < 0.01 (p-values adjusted with sequential Bonferroni). Different letters (x and y) indicate significantly different slopes among spider origins according to t-tests.
Grasshopper daily feeding time increased as spider height in the plant canopy decreased (linear regressions, F1,22 ≥ 6.69, p's ≤ 0.048, r2 = 0.23–0.43, figure 2d–f). The magnitude of this relationship did not differ among spider origin treatments (t-tests, |t| ≤ 1.23, d.f. = 46, p's ≥ 0.23) indicating that grasshoppers responded directly to spider location within the plant canopy independently of spider origin (figure 2d–f). Therefore, the effect of warming on grasshopper feeding time was indirect and differed among spider populations. Warming increased grasshopper daily feeding time when spiders from VT and CT were present (linear regressions, F1,22 ≥ 14.82, p's ≤ 0.009, r2 = 0.40–0.50), but had no effect when NJ spiders were present (linear regression F1,22 = 1.68, p = 0.21, figure 2g–i). The proportion of herbs consumed was unaffected by temperature in all spider treatments (linear regressions, F1,22 ≤ 1.58, p's ≥ 0.22).
(c). Reciprocal transplant experiment
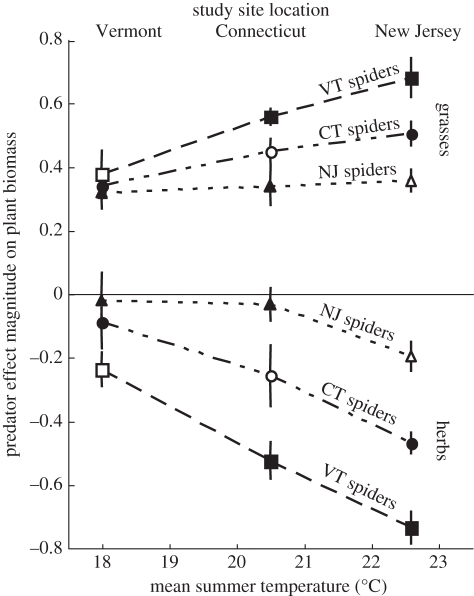
Average temperatures (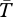 ) within mesocosms in VT, CT and NJ were 18.1°C, 20.8°C and 22.9°C, respectively. Comparing only local spiders at their respective study sites (figure 3, open markers), there was no difference in the magnitude of the indirect effect of spiders on grasses (one-way ANOVA, F2,15 = 0.30, p = 1.00) or herbs (one-way ANOVA, F2,15 = 0.017, p = 0.98) among study sites. However, the transplant experiment revealed different effects of climate on spiders from different study sites (figure 3, closed markers). The strength of the indirect effect of spiders on grasses (two-way ANOVA, F2,45 = 6.00, p < 0.012, Tukey test p ≤ 0.043; figure 3) and herbs (two-way ANOVA, F2,45 = 13.38, p < 0.004, Tukey test p ≤ 0.0097; figure 3) was weakest at the VT study site, but the CT and NJ study sites did not differ (Tukey test p's ≥ 0.091). Spider origin affected the magnitude of the indirect effect of spiders on grasses (two-way ANOVA, F2,45 = 20.22, p < 0.004; figure 3) and herbs (two-way ANOVA, F2,45 = 23.30, p < 0.003; figure 3). The magnitude of the indirect effect of VT spiders was largest, followed by CT spiders and NJ spiders (Tukey tests, grasses p's ≤ 0.015, herbs p's ≤ 0.007). There was no interaction effect of the study site and spider origin on spider effect magnitude on grasses (two-way ANOVA, F2,45 = 1.35, p = 0.27) or herbs (two-way ANOVA, F2,45 = 0.69, p = 1.00).
) within mesocosms in VT, CT and NJ were 18.1°C, 20.8°C and 22.9°C, respectively. Comparing only local spiders at their respective study sites (figure 3, open markers), there was no difference in the magnitude of the indirect effect of spiders on grasses (one-way ANOVA, F2,15 = 0.30, p = 1.00) or herbs (one-way ANOVA, F2,15 = 0.017, p = 0.98) among study sites. However, the transplant experiment revealed different effects of climate on spiders from different study sites (figure 3, closed markers). The strength of the indirect effect of spiders on grasses (two-way ANOVA, F2,45 = 6.00, p < 0.012, Tukey test p ≤ 0.043; figure 3) and herbs (two-way ANOVA, F2,45 = 13.38, p < 0.004, Tukey test p ≤ 0.0097; figure 3) was weakest at the VT study site, but the CT and NJ study sites did not differ (Tukey test p's ≥ 0.091). Spider origin affected the magnitude of the indirect effect of spiders on grasses (two-way ANOVA, F2,45 = 20.22, p < 0.004; figure 3) and herbs (two-way ANOVA, F2,45 = 23.30, p < 0.003; figure 3). The magnitude of the indirect effect of VT spiders was largest, followed by CT spiders and NJ spiders (Tukey tests, grasses p's ≤ 0.015, herbs p's ≤ 0.007). There was no interaction effect of the study site and spider origin on spider effect magnitude on grasses (two-way ANOVA, F2,45 = 1.35, p = 0.27) or herbs (two-way ANOVA, F2,45 = 0.69, p = 1.00).
Figure 3.
The indirect effect of spiders on grasses and forbs did not differ among treatments matching spider origin and study site (open markers). However, the reciprocal transplant experiment revealed that climate did not affect spiders from different study sites equally (closed markers). Transplanting spiders from VT and CT altered the magnitude of the indirect effect of spiders on grasses and herbs. However, the effect magnitude of NJ spiders did not differ among study sites. Points represent mean ± s.e.
4. Discussion
Previous research has shown that experimental warming by as little as 2.5°C can significantly increase the magnitude of the indirect effect of P. mira spiders on grasses and herbs [15]. The strength of the indirect effect of spiders on plants is largely a function of the degree of spatial overlap between spider predators and grasshopper herbivores [16,17]. As temperatures rise, thermal stress causes spiders to seek cooler temperatures lower in the plant canopy and thereby reduces the effects of predation risk on grasshopper daily feeding time (figure 2). Therefore, one may predict the top-down effects of predation risk to differ among populations from different climates. However, this study demonstrates spider thermal tolerance varied across a 4.8°C temperature gradient such that the magnitude of top-down control was conserved across all study sites.
Examining the effects of warming on VT spiders is akin to most experimental climate change research, in which future climate scenarios are simulated within extant systems [15,17]. Experimental warming altered food web interactions by shocking the system with temperatures near or above the CTM for VT spiders. As a result, warming had significant effects on food web dynamics and it could be inferred that similar effects will occur if global temperatures continue to rise at the projected rate. By contrast, examining the effect of warming on NJ spiders allowed me to test the effects of long-term chronic levels of increased temperatures, something rarely done with an experimental approach. Spiders from NJ had higher CTM, most probably a result of their warmer climate. Consequently, experimental warming never exceeded CTM and had little to no effect on food webs. Thus, it appears that P. mira has the capacity to tolerate higher temperatures than is currently demonstrated throughout much of its range, and such adaptive capacity may mitigate the effects of climate warming on these grassland food webs.
By conducting experiments on food webs across a temperature gradient, I illuminated several important ideas that would not be readily apparent from traditional, in situ warming manipulations. First, my approach demonstrated considerable within-species variation in spider responses to temperature and that these responses had measurably different effects within the grassland community (figure 3). During the transplant experiments, spiders from different locations had different effects within the same plant communities. However, in situ populations at different latitudes functioned similarly despite relatively large differences in mean temperatures. Such a result is particularly noteworthy because mean summer temperature has considerable influence on the magnitude of top-down control in these grassland food webs [15]. Thus, the conservation of the magnitude of top-down control across this temperature gradient is compelling evidence that within-species variation and adaptive responses to temperature can influence the way in which climate change manifests in ecological systems.
Incorporating experimentation and a space-for-time substitution to address the long-term effects of climate change on ecological communities overcomes many of the limitations associated with using either method independently [24], however, my approach is not without limitations. My results show that within-species variation in response to temperatures exists, but cannot directly address the selective pressures that caused this variation. Inherent in these results is the assumption that adaptation can occur at a rate similar to that of climate warming, but this assumption remains an unknown [6,7,12]. The Earth's climate is changing at an unprecedented rate [19], and although this study demonstrates that species may have the capacity to adapt to warmer climates, it remains unclear if the rate of climate change will preclude such adaptation.
This study demonstrates that physiological and behavioural responses of predators to local climates can maintain food web function across a range of temperatures similar to that predicted by global change models. This conclusion differs markedly from the predominant view in the literature that species responses may alter, but not attenuate, the effects of climate change [2,5,6,12] (but see also [25]). Few studies have addressed the long-term effects of climate change on species, but those that have ubiquitously demonstrate the assumption that climate effects will remain constant through time is invalid [8–11]. Even fewer have explored the effects of evolution or phenotypic plasticity in shaping future species interactions [26]. Thus, it is the responsibility of ecologists to design experiments that explicitly test how gradual climate change will affect future systems, or else risk-making unrealistic and misleading conclusions.
Acknowledgements
This research was supported by a grant from the National Science Foundation (DEB-0910047). I thank O. Schmitz for invaluable feedback on experimental design and previous drafts of this manuscript, P. Morin, M. Battin and K. Johnson for accommodations and access to study sites, and F. Douglas and K. Freund for field assistance. J. Harmon, D. Hoekman, A. Ives, E. Larson, N. Rafferty, P. Raymond, D. Skelly, M. Smith and two anonymous reviewers provided constructive comments that greatly improved this manuscript.
References
- 1.Harrington R., Woiwod I., Sparks T. 1999. Climate change and trophic interactions. Trends Ecol. Evol. 14, 146–150 10.1016/S0169-5347(99)01604-3 (doi:10.1016/S0169-5347(99)01604-3) [DOI] [PubMed] [Google Scholar]
- 2.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 10.1146/annurev.ecolsys.37.091305.110100 (doi:10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 3.Tylianakis J. M., Didham R. K., Bascompte J., Wardle D. A. 2008. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363 10.1111/j.1461-0248.2008.01250.x (doi:10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- 4.Walther G. R. 2007. Tackling ecological complexity in climate impact research. Science 315, 606–607 10.1126/science.1138574 (doi:10.1126/science.1138574) [DOI] [PubMed] [Google Scholar]
- 5.Deutsch C. A., Tewksbury J. J., Huey R. B., Sheldon K. S., Ghalambor C. K., Haak D. C., Martin P. R. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672 10.1073/pnas.0709472105 (doi:10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gienapp P., Teplitsky C., Alho J. S., Mills J. A., Merilä J. 2008. Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178 10.1111/j.1365-294X.2007.03413.x (doi:10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- 7.Skelly D. K., Joseph L. N., Possingham H. P., Freidenburg L. K., Farrugia T. J., Kinnison M. T., Hendry A. P. 2007. Evolutionary responses to climate change. Conserv. Biol. 21, 1353–1355 10.1111/j.1523-1739.2007.00764.x (doi:10.1111/j.1523-1739.2007.00764.x) [DOI] [PubMed] [Google Scholar]
- 8.Clark C. M., Tilman D. 2008. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 451, 712–715 10.1038/nature06503 (doi:10.1038/nature06503) [DOI] [PubMed] [Google Scholar]
- 9.Suttle K. B., Thomsen M. A., Power M. E. 2007. Species interactions reverse grassland responses to changing climate. Science 315, 640–642 10.1126/science.1136401 (doi:10.1126/science.1136401) [DOI] [PubMed] [Google Scholar]
- 10.Chapin F. S., Shaver G. R., Giblin A. E., Nadelhoffer K. J., Laundre J. A. 1995. Responses of Arctic tundra to experimental and observed changes in climate. Ecology 76, 694–711 10.2307/1939337 (doi:10.2307/1939337) [DOI] [Google Scholar]
- 11.Körner C. 2006. Plant CO2 responses: an issue of definition, time and resource supply. New Phytol. 172, 393–411 10.1111/j.1469-8137.2006.01886.x (doi:10.1111/j.1469-8137.2006.01886.x) [DOI] [PubMed] [Google Scholar]
- 12.Pertoldi C., Bach L. A. 2007. Evolutionary aspects of climate-induced changes and the need for multidisciplinarity. J. Therm. Biol. 32, 118–124 10.1016/j.jtherbio.2007.01.011 (doi:10.1016/j.jtherbio.2007.01.011) [DOI] [Google Scholar]
- 13.Petchey O. L., McPhearson P. T., Casey T. M., Morin P. J. 1999. Environmental warming alters food-web structure and ecosystem function. Nature 402, 69–72 10.1038/47023 (doi:10.1038/47023) [DOI] [Google Scholar]
- 14.Schmitz O. J. 2004. From mesocosms to the field: the role and value of cage experiments in understanding top-down effects in ecosystems. In Insects and ecosystem function (eds Weisser W. W., Siemann E.), pp. 277–302 Berlin, Germany: Springer series in Ecological Studies [Google Scholar]
- 15.Barton B. T., Beckerman A. P., Schmitz O. J. 2009. Climate warming strengthens indirect interactions in an old-field food web. Ecology 90, 2346–2351 10.1890/08-2254.1 (doi:10.1890/08-2254.1) [DOI] [PubMed] [Google Scholar]
- 16.Barton B. T. 2010. Climate warming and predation risk during herbivore ontogeny. Ecology 91, 2811–2818 10.1890/09-2278.1 (doi:10.1890/09-2278.1) [DOI] [PubMed] [Google Scholar]
- 17.Barton B. T., Schmitz O. J. 2009. Experimental warming transforms multiple predator effects in a grassland food web. Ecol. Lett. 12, 1317–1325 10.1111/j.1461-0248.2009.01386.x (doi:10.1111/j.1461-0248.2009.01386.x) [DOI] [PubMed] [Google Scholar]
- 18.Fukami T., Wardle D. A. 2005. Long-term ecological dynamics: reciprocal insights from natural and anthropogenic gradients. Proc. R. Soc. B 272, 2105–2115 10.1098/rspb.2005.3277 (doi:10.1098/rspb.2005.3277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NERA 2001. New England regional assessment. In Preparing for a changing climate: the potential consequences of climate variability and change (ed. U. S. G. C. R. Program). Washington, DC: US Global Change Research Program; Editorp [Google Scholar]
- 20.Schmalhofer V. R. 1999. Thermal tolerances and preferences of the crab spiders Misumenops asperatus and Misumenoides formosipes (Araneae, Thomisidae). J. Arachnol. 27, 470–480 [Google Scholar]
- 21.Belovsky G. E., Slade J. B. 1986. Time budgets of grassland herbivores—body size similarities. Oecologia 70, 53–62 10.1007/BF00377110 (doi:10.1007/BF00377110) [DOI] [PubMed] [Google Scholar]
- 22.Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70 [Google Scholar]
- 23.Schmitz O. J., Hamback P. A., Beckerman A. P. 2000. Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants. Am. Nat. 155, 141–153 10.1086/303311 (doi:10.1086/303311) [DOI] [PubMed] [Google Scholar]
- 24.Dunne J. A., Saleska S. R., Fischer M. L., Harte J. 2004. Integrating experimental and gradient methods in ecological climate change research. Ecology 85, 904–916 10.1890/03-8003 (doi:10.1890/03-8003) [DOI] [Google Scholar]
- 25.Wilmers C. C., Getz W. M. 2005. Gray wolves as climate change buffers in Yellowstone. PLoS Biol. 3, 571–576 10.1371/journal.pbio.0030092 (doi:10.1371/journal.pbio.0030092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harmon J. P., Moran N. A., Ives A. R. 2009. Species response to environmental change: impacts of food web interactions and evolution. Science 323, 1347–1350 10.1126/science.1167396 (doi:10.1126/science.1167396) [DOI] [PubMed] [Google Scholar]