Abstract
Vast numbers of insects and passerines achieve long-distance migrations between summer and winter locations by undertaking high-altitude nocturnal flights. Insects such as noctuid moths fly relatively slowly in relation to the surrounding air, with airspeeds approximately one-third of that of passerines. Thus, it has been widely assumed that windborne insect migrants will have comparatively little control over their migration speed and direction compared with migrant birds. We used radar to carry out the first comparative analyses of the flight behaviour and migratory strategies of insects and birds under nearly equivalent natural conditions. Contrary to expectations, noctuid moths attained almost identical ground speeds and travel directions compared with passerines, despite their very different flight powers and sensory capacities. Moths achieved fast travel speeds in seasonally appropriate migration directions by exploiting favourably directed winds and selecting flight altitudes that coincided with the fastest air streams. By contrast, passerines were less selective of wind conditions, relying on self-powered flight in their seasonally preferred direction, often with little or no tailwind assistance. Our results demonstrate that noctuid moths and passerines show contrasting risk-prone and risk-averse migratory strategies in relation to wind. Comparative studies of the flight behaviours of distantly related taxa are critically important for understanding the evolution of animal migration strategies.
Keywords: seasonal migration, songbirds, Lepidoptera, Autographa gamma, flight speed, orientation
1. Introduction
Long-distance migration has evolved in virtually all major animal groups, but the most prominent examples of flight-borne migrants are found among insects and birds [1–3]. Billions of noctuid moths [4–6] and passerine birds [7–9] perform regular seasonal migrations between summer and winter ranges separated by thousands of kilometres, by undertaking nocturnal flights at altitudes of hundreds or thousands of metres above the ground. Migratory noctuid moths (body mass approx. 0.1–0.4 g, mean airspeed approx. 3–5 m s−1) fly relatively slowly in relation to the surrounding air [4–6], with self-powered airspeeds only approximately one-third that of passerines (body mass approx. 10–50 g, mean airspeed approx. 10–15 m s−1 [10]). Insects and birds also differ dramatically with respect to their sensory capabilities and flight biomechanics, implying that these two groups may be expected to exhibit very different travel speeds and degrees of directional control during their migrations [1,2,11–13]. However, for seasonal long-distance migration to be adaptive, a certain level of flight performance is required, which may lead to the convergence of migratory strategies in distantly related organisms such as insects and birds [14]. Monitoring of high-altitude migration patterns with specially designed entomological [15] and ornithological [16] radars has enabled us to carry out the first comparative study of flight behaviour and migratory performance in these two distantly related taxa.
In this study, we tracked noctuid moths and passerines during nocturnal spring and autumn migrations, over flat rural landscapes in England (moths) and Sweden (birds) between 1999 and 2008. Autographa gamma (silver Y moth) is the most abundant high-altitude migrant moth in the UK [6,17], and we used a well-established methodology to identify the radar-detected moths as A. gamma (see the electronic supplementary material). Our sample of passerines included several species of Old World warblers (Sylvidae), thrushes and chats (Turdidae) and flycatchers (Muscicapidae) (as judged from the composition of nocturnal passerine migrants at the time and place of the radar observations), which breed in Europe in the northern summer, and then over-winter in sub-Saharan Africa and the Mediterranean region. Individual passerines undertake multiple journeys, but they only have one breeding season (with one or two broods in the northern summer) per annual cycle. Silver Y moths, on the other hand, breed continuously throughout the year. Those individuals migrating into northern Europe in the spring are the offspring of individuals that bred throughout the winter in the Mediterranean region. There are one or two generations in northern Europe before the offspring return south in the autumn, and thus in contrast to passerines each individual moth only carries out a single leg of the annual migratory circuit. In common with many continuously breeding migrant moths, the silver Y further differs from passerines, including migratory double breeding species [18], in that it has considerably more breeding opportunities within the annual cycle. The migration patterns of silver Y moths are similar to several other noctuid moth species that carry out high-altitude migration in Europe [6,19] and elsewhere [20,21], and so our results pertaining to silver Y moth migration performance are likely to be representative of a wide range of other highly migratory noctuids.
2. Material and methods
(a). Entomological radar operating procedures and data analysis
We studied the flight behaviour of silver Y moths A. gamma engaged in spring and autumn high-altitude migratory flights, using data collected by two purpose-built, vertical-looking entomological radars (VLRs) situated in inland southern England. The first has been at Rothamsted, Harpenden, Hertfordshire (latitude 51°48′32″ N, longitude 0°21′27″ W) from 1999 to present; the second was at Malvern, Worcestershire (latitude 52°06′04″ N, longitude 2°18′38″ W) from 2000 to 2003, and then at Chilbolton, Hampshire (latitude 51°8′40″ N, longitude 1°26′13″ W) from 2004 to present. The VLR equipment and operating procedures are described in detail elsewhere [15,22,23]. Very briefly, individual targets flying in a given altitude range above the radar (150–1188 m) are interrogated when they pass through the vertically pointing beam within 15 different height-bands. These height-bands are 45 m deep and separated by a 26 m non-sampling interval. Usually, the majority of signals are resolved, and the analysis procedure yields the horizontal speed, displacement direction, body alignment and three radar scattering parameters of each insect (from which body mass and shape factors are calculated). Wind directions and speeds at an appropriate altitude for comparison with moth flight (440 m) were generated by the UK Meteorological Office's numerical weather prediction model, the Unified Model [24]. Directional data (insect flight parameters and wind direction) were analysed as described previously [4–6], using circular statistical procedures [25]. Silver Y tracks were recorded on 72 spring nights and 125 autumn nights during 2000, 2003 and 2006, comprising a total of 77 470 individual moths.
(b). Ornithological radar tracking and data analysis
Nocturnal passerine migrants were recorded by a tracking radar (200 kW peak power, 0.25 µs pulse duration, 504 Hz pulse repetition frequency, 1.5° beam width, X-band) on the roof of the Ecology Building in Lund (55°42′ N, 13°12′ E), 91.5 m.a.s.l. Data were obtained from four spring seasons (13–27 April 1999, 28 April–25 May 2004, 2 May–7 June 2006 and 6 May–10 June 2008) and three autumn seasons (22 September–11 October 1999, 25 July–31 August 2006 and 8–26 August 2008). All tracks were collected during dark hours, approximately 3–4 h either side of midnight. Because of disturbance from ground echoes, birds flying below approximately 500 m are probably underestimated in our samples. The radar operator was searching for echoes from migrating birds by scanning manually at changing antenna elevations between approximately 5° and 40°. After finding a target at distances most often between 2 and 6 km, the radar was switched into automatic tracking mode and readings of azimuth, elevation and range were transferred to a computer every 2 s. Only targets that were considered to be single individual passerines, indicated by the characteristic radar echo signature pattern (discrete Fourier transform analysis was applied to the echo signature data) associated with bounding flight, were included in this study. Minimum tracking time for each target was 30 s with mean tracking time about 1 min. Wind data were measured within 2 h of all bird tracks, by releasing and tracking helium balloons with reflectors. Airspeed and heading direction were calculated by the subtraction of the wind vector at the altitude where the bird was flying (with a maximal time difference between wind and bird data of 2 h) from the bird's track and ground speed vector. Overall mean speeds (ground speed, airspeed, vertical speed and wind speed) and directions (track direction, heading direction and wind direction) as well as mean altitude (above the radar) were calculated for each night. The radar operating procedures and data handling have been described in further detail elsewhere [16]. Passerine tracks were recorded on 63 spring nights and 37 autumn nights, comprising a total of 2992 individual birds.
(c). Data analysis
The radar observations produced very large sample sizes (particularly for the silver Y moths), but some of the individual migratory parameters cannot be considered to be completely independent. For example, the movement speeds and directions of individuals flying during the same night and at the same altitude will be affected by the same winds. Thus to control for any problems of pseudoreplication that may confound the analysis of individual tracks, our primary analysis compared migratory characteristics of moths and birds based on calculations of nightly mean values (table 1) rather than separate data points from all individuals tracked. All nights with at least one track were ranked according to the number of individuals tracked during each night, but only the nights with substantial numbers of observations (encompassing 90% cumulatively of the seasonal total sample of individual moths and birds) were included in further analyses (table 1). This resulted in sample sizes of 31 and 41 spring nights, and 59 and 25 autumn nights, for moths and birds, respectively. Nights with only a few tracks were omitted to avoid the risk of possible bias to the results arising from a few individuals migrating under atypical conditions. Differences in the seasonal means of the various flight parameters of each taxon were tested for with t-tests for linear variables (speeds and altitudes), and Watson's U2-test for circular variables (directions) [25]. In addition to the comparisons of nightly mean values, we also carried out comparisons using all individual tracks (see the electronic supplementary material); the two analyses showed virtually the same pattern (compare nightly means in table 1 with individual means in electronic supplementary material, table S1), albeit with less variation in the nightly mean comparison.
Table 1.
Comparisons of nocturnal migratory flight characteristics of silver Y moths and passerines, based on nightly means. Altitude is measured above the radar. Means and concentrations of directional data were calculated as mean vector direction and length, respectively. Mean vector length r (in parentheses) is a measure of the concentration of circular data (inversely related to angular scatter) ranging from 0 (uniform distribution in all directions) to 1 (all observations in the same direction). Wind direction is the direction from which the wind is blowing. Differences between characteristics of moth and bird migration were analysed on the basis of the nightly mean values by t-tests (equal variances not assumed) for ground speed, wind speed and altitude and by Watson's U2-tests for the remaining circular variables [25]. Two-tailed significance levels from these tests are given. The two right-hand columns show overlap values of the frequency distributions of nightly mean values for moths and birds, respectively (in intervals of 2 m s−1 for speeds, 200 m for altitude and 10° for directions).
| moths |
birds |
difference moths versus birds |
overlap moths versus birds |
|||||
|---|---|---|---|---|---|---|---|---|
| spring | autumn | spring | autumn | spring | autumn | spring | autumn | |
| ground speed (m s−1), mean (s.d.) | 15.7 (4.6) | 13.0 (3.5) | 13.1 (3.2) | 12.1 (3.7) | * | n.s. | 0.83 | 0.92 |
| wind speed (m s−1), mean (s.d.) | 8.7 (4.4) | 8.2 (3.3) | 7.8 (4.0) | 8.3 (4.1) | n.s. | n.s. | 0.81 | 0.88 |
| altitude (m), mean (s.d.) | 550 (107) | 486 (95) | 1094 (319) | 1050 (280) | *** | *** | 0.12 | 0.02 |
| track direction, mean vector (r) | 344° (0.70) | 179° (0.48) | 15° (0.92) | 172° (0.84) | ** | * | 0.65 | 0.71 |
| wind direction, mean vector (r) | 171° (0.56) | 351° (0.42) | 256° (0.09) | 264° (0.59) | *** | *** | 0.44 | 0.46 |
| heading minus wind, mean vector (r) | 178° (0.77) | −164° (0.83) | 26° (0.24) | −37° (0.85) | *** | *** | 0.16 | 0.17 |
| heading direction, mean vector (r) | 347° (0.79) | 200° (0.62) | 22° (0.91) | 209° (0.95) | n.s. | *** | 0.68 | 0.69 |
| track minus heading, mean vector (r) | −6° (0.93) | −15° (0.93) | 6° (0.79) | −55° (0.68) | n.s. | ** | 0.78 | 0.48 |
| number of nights | 31 | 59 | 41 | 25 | ||||
***p < 0.001.
**p < 0.01.
*p < 0.05.
n.s., p > 0.05.
We also produced frequency distributions of speeds, altitudes and directions to examine the degree of overlap between silver Y moths and passerines, using both the nightly means (table 1) and the individual data points (figure 1 and electronic supplementary material, table S1). To calculate the degree of similarity between moths and birds of the various migration parameters within each season, we used the overlap index:
where m and b are the frequency distributions of silver Y moths and passerines, respectively, in different intervals (i). This technique provides a measure of similarity ranging from 0 for non-overlapping distributions to 1 for identical distributions [26].
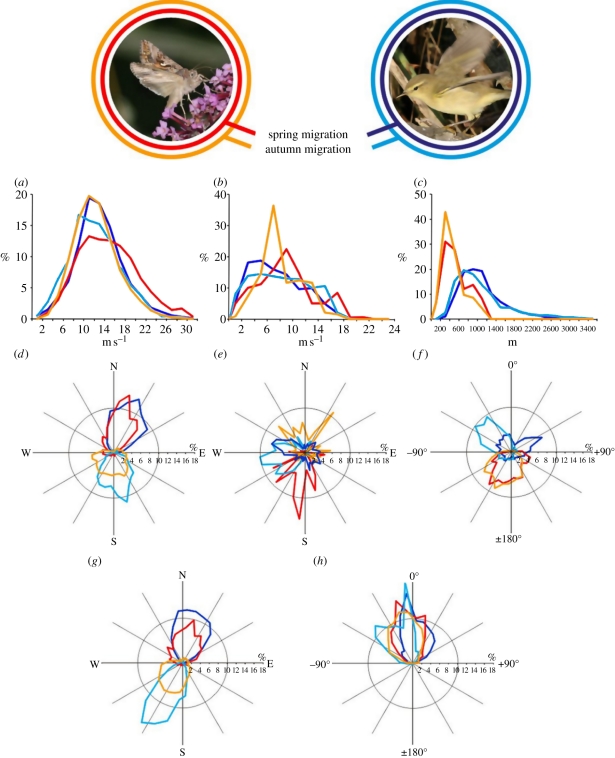
Figure 1.
Comparisons of frequency distributions of migratory flight characteristics of Autographa gamma noctuid moths and passerines (exemplified by willow warbler Phylloscopus trochilus in photo) during spring and autumn. Frequency distributions (based on individuals) are represented by straight lines connecting percentage of observations in different frequency bins. (a) Ground speed, (b) wind speed, (c) altitude, (d) track direction, (e) wind direction, (f) heading relative to wind, (g) heading direction, and (h) track relative to heading. Intervals for speed distributions (a,b) are 2 m s−1, for altitude distributions (c) 200 m and for directional distributions (d–h) 10° sectors. Distributions (d,e,g) show geographical compass directions with north = 0° and south = 180°. Distributions (f) and (h) are calculated as heading minus wind direction and track minus heading direction, respectively. Wind direction is the direction from which the wind is blowing, meaning that 0° in (f) designates a heading straight into the wind direction and 180° a heading in the due downwind direction (photos by T. A.).
3. Results and discussion
Distributions of nightly mean ground speeds were surprisingly similar between silver Y moths and passerines (table 1), and the majority of individuals (62–76% of both taxa; figure 1a) had travel speeds between 8 and 18 m s−1 (approx. 30–65 km h−1). Mean ground speeds of silver Y moths and passerines were not significantly different in the autumn (moths: 13.0 m s−1; passerines: 12.1 m s−1; p > 0.05; table 1), but during spring migration the moths travelled significantly faster (moths: 15.7 m s−1; passerines: 13.1 m s−1; p < 0.05; table 1). This unexpected result (i.e. moths travelling as fast as, or faster than, passerines) occurred despite the fact that the passerines have airspeeds that are approximately three times faster than those of moths: mean airspeeds of the migrating passerines were 12.8 ± 2.6 and 12.2 ± 2.6 m s−1 during spring and autumn, respectively, while migrating noctuids have typical airspeeds of 3–5 m s−1 [4,6]. The vast majority of individual silver Y moths (99%) achieved ground speeds greater than their airspeed (figure 1a), and thus gained a considerable degree of wind assistance. In contrast, only approximately 50 per cent of individual passerines travelled at a ground speed that exceeded their airspeed (figure 1a), and thus many individuals received little or no wind assistance.
In order to travel at such great speeds compared with their self-powered airspeed, silver Y moths clearly exploited favourable winds very efficiently. Our data demonstrate that silver Y moths have a number of mechanisms that facilitate their efficient exploitation of winds. Firstly, they flew in airstreams that were not significantly different in speed to those encountered by the birds (overlap = 0.81 and 0.88 during spring and autumn, respectively; figure 1b and table 1). Silver Y moths travelled at significantly lower flight altitudes in comparison to passerines, with very little overlap (0.12 and 0.02 during spring and autumn, respectively; figure 1c and table 1), with the majority of moths migrating in the range 200–800 m above the ground (71% in spring and 80% in autumn). Passerines migrated at greater altitudes and over a wider altitudinal range: mostly between 400 and 1400 m, but up to 3600 m (figure 1c). Noctuid moths are typically constrained to fly below 1000 m in the relatively cool climate of northwest Europe [24,27], but this has the advantage of concentrating the noctuids in the fast, warm airstreams associated with the presence of nocturnal temperature inversions [28], resulting in correspondingly fast ground speeds in comparison to their airspeed (figure 1a). Passerines by contrast are more flexible with regard to flight altitude than insects because they can maintain flight in higher altitude (and thus colder) airstreams [29], which may impart significant aerodynamic [30] and physiological [31] benefits. Furthermore, passerines did not restrict their migration solely to nights when there were strong tailwinds aligned with their preferred seasonal migration direction (see below), as the silver Y moths did, but additionally migrated on nights with a wide range of wind directions (including cross- and head-winds). A large degree of flexibility in flight altitude selection allows passerines to obtain the best conditions with respect to flight physiology, but with a concomitant reduction in travel speed compared with moths that preferentially migrate in relatively strong tailwinds.
Owing to the relatively slow airspeeds of noctuid moths, they frequently fly in airstreams that exceed their self-powered flight speed: indeed, in the present study, only 15 and 9 per cent of the silver Y moths migrated in wind currents that were slower than their airspeed in the spring and autumn, respectively (figure 1b). By contrast, passerines typically migrated in wind currents that were slower than their airspeed (87% and 80% in spring and autumn, respectively; figure 1b). Silver Y moths, therefore, seemingly expose themselves to the risk of frequent displacement in highly disadvantageous directions—a ‘risk-prone’ strategy with respect to control over the migratory direction [32]—while passerines that fly in wind currents with speeds lower than their airspeed will have a much lower risk of unfavourable displacement (a ‘risk-averse’ strategy). The range of possible track directions that an individual can achieve is restricted to a sector around the downwind direction equal to ±arcsin (a), where (a) is the ratio of the animal's airspeed to the wind speed [33]. With mean wind speeds approximately twice their airspeed, migrating silver Y moths' directional scope was thus limited to ±30° from the downwind direction. The great majority of moths were therefore only able to have a very limited effect on their track direction through the action of their flight heading. By contrast, the great majority of passerines were in full control of their resulting migration direction, although they would of course make slow progress when struggling against head- and cross-winds. These different behaviours are probably related to whether or not migrants are heading for a very specific destination area (typical of passerines) or are more flexible with respect to the ultimate location (typical of most insects).
Considering the above, it is therefore rather surprising that the distributions of silver Y moth and passerine track directions were relatively similar (table 1), and that both taxa routinely achieved migratory tracks in well-defined seasonally beneficial directions (figure 1d). Migration was approximately northwards in spring (silver Y moths: α = 344°, r = 0.70; passerines: α = 15°, r = 0.92; overlap: 0.65), and approximately southwards in autumn (silver Y moths: α = 179°, r = 0.48; passerines: α = 172°, r = 0.84; overlap: 0.71). Silver Y moths selected winds blowing from highly beneficial directions for their migratory flights (spring winds: α = 171°, r = 0.56; autumn winds: α = 351°, r = 0.42; figure 1e), resulting in windborne displacement in seasonally favourable directions. Passerines, on the other hand, showed very little selectivity for advantageous winds: in spring, they flew in winds from a very wide range of directions, as indicated by the extremely small r-value (α = 256°, r = 0.09), and in autumn, most migration occurred on westerly winds (α = 264°, r = 0.59; figure 1e). The distributions of wind directions used by moths and passerines were significantly different in both spring and autumn (p < 0.001 in both cases, table 1), indicating that silver Y moths exhibited a much greater degree of selectivity. As a consequence of their low degree of selectivity for favourable wind directions, passerines avoid long and unpredictable delays waiting for migratory opportunities with favourably directed tailwinds. Consequently, they will maintain a high degree of spatio-temporal control over their migratory progress, but with increased metabolic costs associated with making headway into head- or cross-winds, and slower travel speeds. These increased flight costs are offset, however, by a reduction in costs associated with waiting out periods of unfavourable weather at stopover sites, especially at low ambient temperatures [34], which will be exacerbated if foraging and fuel deposition cannot take place during the waiting time [35]. The relatively weak flight ability of noctuid moths prevents them from adopting similarly undiscriminating selectivity in relation to wind direction, as rapid progress in seasonally advantageous directions is entirely dependent on tailwind assistance. Shorebirds, which typically complete their migration by a few long flights, are often highly prone to exploit tailwinds for their migratory bouts [32,36] in the same way as migrant moths [4–6].
Migratory noctuids are known to orient their flight headings close to the downwind direction, so that they maximize their displacement speed [4–6,21]. This was indeed the case in the present study, with the mean difference between the silver Y moths' flight headings and downwind directions being only 2° (r = 0.77) in spring and 16° (r = 0.83) in autumn (figure 1f and table 1). As a result of their proficiency in selecting favourably directed tailwinds, their mean flight headings were consequently rather close to their preferred seasonal migration directions of north in spring and south in autumn (spring: α = 347°, r = 0.79; autumn: α = 200°, r = 0.62; figure 1g). Passerines maintained flight headings in their seasonally beneficial directions (spring: α = 22°, r = 0.91; autumn: α = 209°, r = 0.95; figure 1g), irrespective of the wind direction, and there was a relatively high degree of overlap between the moths and birds in both seasons (table 1). Given the prevailing wind conditions in the study period, and the absence of selectivity, passerines often travelled into cross-winds and even head-winds, and as a consequence there were considerably larger discrepancies between flight heading and the downwind direction among passerines than observed in the moths (p < 0.001; table 1 and figure 1f).
Owing to the effects of wind, the resulting track direction of individual migrants often differed from their heading direction by up to ±60° with a similar distribution for moths and passerines (figure 1h and table 1). The angle between track and heading was kept within these limits by the silver Y moths' selection of wind directions in rough agreement with their preferred migratory directions, and by the passerines' faster airspeed restricting the effect of the wind vector. Cases where track and heading directions differed by more than 90° (in either direction), reflecting situations where individuals were overpowered by the winds (and thus displaced sideways or even backwards) were rare: only 2–3% of passerines exhibited this and it has not been observed in moths.
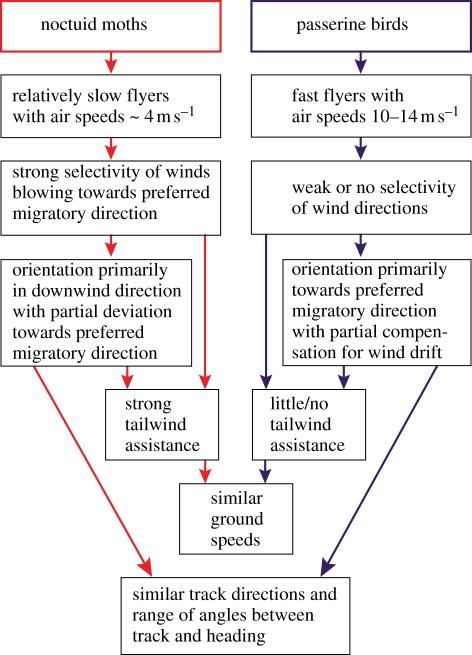
We conclude that silver Y moths and passerines achieved extremely similar levels of travel speed and orientation performance during their nocturnal migratory flights in the northern temperate zone by way of contrasting risk-prone and risk-averse behavioural strategies, respectively, in relation to the wind, as summarized in figure 2. The similarities in migratory performance between silver Y moths and passerines were clearly demonstrated by the relatively large overlap values for the frequency distributions of ground speed and track direction (table 1), while the differences in strategy were manifested by great differences between the frequency distributions of wind direction during the flights, of orientation in relation to the wind and of flight altitudes (p < 0.001 in all six pair-wise comparisons; table 1). The similarities and differences were also clearly manifested in the results from analyses based on individual tracks rather than nightly means (see the electronic supplementary material, table S1). Our comparative analysis of the flight strategies employed by insects and birds raises new perspectives and questions about the ecology and evolution of migration, and stresses the key role of responses to wind conditions (selectivity of timing and flight altitude, as well as orientation responses) for the evolution of long-distance migration among animals with markedly different flight capabilities. A key question arising from this research is how actively migrating moths are able to select the fastest high-altitude airstreams moving in seasonally beneficial directions [28,37]. It seems likely that they do this by detection of wind-related factors rather than a visually mediated mechanism [28,38,39], but although the field of insect responses to wind-induced motion is progressing rapidly [40,41], the precise sensory mechanisms used by migrant moths remain unresolved.
Figure 2.
Schematic of how noctuid moths and passerine birds achieve similar speed and orientation performances by contrasting behavioural strategies in relation to the wind.
The evolution of long-range seasonal migration is comparatively rare among the Noctuidae compared with passerines: only 3 per cent of noctuids found in northwest Europe are regular long-range migrants from the Mediterranean, compared with 44 per cent of songbirds (see the electronic supplementary material), which implies that it may be a difficult strategy for insects to evolve. However, once it has arisen, it is clearly a highly beneficial strategy as many long-range migrant Lepidoptera are undergoing rapid population increases in northwest Europe [42]. These changes are in contrast to the fortunes of many migratory passerines in the same region, which have undergone severe declines in recent decades [8,9,43]. These contrasting fortunes may be partly explained by the highly efficient migration strategies of noctuid moths, in combination with their enormous breeding potential and highly adaptable life-history strategies.
Acknowledgements
We are grateful to C.-G. Carlsson and Ulf Olsson (Aerotech Telub), Bertil Larsson in Sweden and Darcy Ladd (Chilbolton Observatory) in the UK for technical radar support. Statistical advice was provided by Dr Suzanne Clark at Rothamsted and Dr Ola Olsson at Lund University, and information about moth species in Sweden by Dr Lars Pettersson, Lund University. We also thank two anonymous referees and members of the editorial board for extremely useful comments. Rothamsted Research receives grant-aided support from the UK Biotechnology and Biological Sciences Research Council. Authors from Lund are associated with the Center for Animal Movement Research at Lund University. The radar work in Sweden was supported by grants from the Swedish Research Council (to T.A.).
References
- 1.Alerstam T. 2006. Conflicting evidence about long-distance animal navigation. Science 313, 791–794 10.1126/science.1129048 (doi:10.1126/science.1129048) [DOI] [PubMed] [Google Scholar]
- 2.Holland R. A., Wikelski M., Wilcove D. S. 2006. How and why do insects migrate? Science 313, 794–796 10.1126/science.1127272 (doi:10.1126/science.1127272) [DOI] [PubMed] [Google Scholar]
- 3.Newton I. 2008. The migration ecology of birds. London, UK: Academic Press, Elsevier [Google Scholar]
- 4.Chapman J. W., Reynolds D. R., Mouritsen H., Hill J. K., Riley J. R., Sivell D., Smith A. D., Woiwod I. P. 2008. Wind selection and drift compensation optimise migratory pathways in a high-flying moth. Curr. Biol. 18, 514–518 10.1016/j.cub.2008.02.080 (doi:10.1016/j.cub.2008.02.080) [DOI] [PubMed] [Google Scholar]
- 5.Chapman J. W., Reynolds D. R., Hill J. K., Sivell D., Smith A. D., Woiwod I. P. 2008. A seasonal switch in compass orientation in a high-flying migrant moth. Curr. Biol. 18, R908–R909 10.1016/j.cub.2008.08.014 (doi:10.1016/j.cub.2008.08.014) [DOI] [PubMed] [Google Scholar]
- 6.Chapman J. W., Nesbit R. L., Burgin L. E., Reynolds D. R., Smith A. D., Middleton D. R., Hill J. K. 2010. Flight orientation behaviors promote optimal migration trajectories in high-flying insects. Science 327, 682–685 10.1126/science.1182990 (doi:10.1126/science.1182990) [DOI] [PubMed] [Google Scholar]
- 7.Thorup K., Bisson I. A., Bowlin M. S., Holland R. A., Wingfield J. C., Ramenofsky M., Wikelski M. 2007. Evidence for a navigational map stretching across the continental U.S. in a migratory songbird. Proc. Natl Acad. Sci. USA 104, 18 115–18 119 10.1073/pnas.0704734104 (doi:10.1073/pnas.0704734104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Møller A. P., Rubolini D., Lehikoinen E. 2008. Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc. Natl Acad. Sci. USA 105, 16 195–16 200 10.1073/pnas.0803825105 (doi:10.1073/pnas.0803825105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahn S., Bauer S., Liechti F. 2009. The natural link between Europe and Africa—2.1 billion birds on migration. Oikos 118, 624–626 10.1111/j.1600-0706.2008.17309.x (doi:10.1111/j.1600-0706.2008.17309.x) [DOI] [Google Scholar]
- 10.Alerstam T., Rosén M., Bäckman J., Ericson P. G. P., Hellgren O. 2007. Flight speeds among bird species: allometric and phylogenetic effects. PLoS Biol. 5, 1656–1662 10.1371/journal.pbio.0050197 (doi:10.1371/journal.pbio.0050197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Åkesson S., Hedenström A. 2007. How migrants get there: migratory performance and orientation. Bioscience 57, 123–133 10.1641/B570207 (doi:10.1641/B570207) [DOI] [Google Scholar]
- 12.Cardé R. T. 2008. Insect migration: do migrant moths know where they are heading? Curr. Biol. 18, R472–R473 10.1016/j.cub.2008.04.018 (doi:10.1016/j.cub.2008.04.018) [DOI] [PubMed] [Google Scholar]
- 13.Cardé R. T. 2008. Animal migration: seasonal reversals of migrant moths. Curr. Biol. 18, R1007–R1009 10.1016/j.cub.2008.09.013 (doi:10.1016/j.cub.2008.09.013) [DOI] [PubMed] [Google Scholar]
- 14.Wikelski M., Moskowitz D., Adelman J. S., Cochran J., Wilcove D. S., May M. L. 2006. Simple rules guide dragonfly migration. Biol. Lett. 2, 325–329 10.1098/rsbl.2006.0487 (doi:10.1098/rsbl.2006.0487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman J. W., Reynolds D. R., Smith A. D. 2003. High-altitude insect migration monitored with vertical-looking radar. Bioscience 53, 503–511 10.1641/0006-3568(2003)053[0503:VRANTF]2.0.CO;2 (doi:10.1641/0006-3568(2003)053[0503:VRANTF]2.0.CO;2) [DOI] [Google Scholar]
- 16.Bäckman J., Alerstam T. 2003. Orientation scatter of free-flying nocturnal passerine migrants: components and causes. Anim. Behav. 65, 987–996 10.1006/anbe.2003.2119 (doi:10.1006/anbe.2003.2119) [DOI] [Google Scholar]
- 17.Chapman J. W., Reynolds D. R., Smith A. D., Smith E. T., Woiwod I. P. 2004. An aerial netting study of insects migrating at high-altitude over England. Bull. Entomol. Res. 94, 123–136 10.1079/BER2004287 (doi:10.1079/BER2004287) [DOI] [PubMed] [Google Scholar]
- 18.Rohwer S., Hobson K. A., Rohwer V. G. 2009. Migratory double breeding in Neotropical migrant birds. Proc. Natl Acad. Sci. USA 106, 19 050–19 055 10.1073/pnas.0908121106 (doi:10.1073/pnas.0908121106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood C. R., Reynolds D. R., Wells P. M., Barlow J. F., Woiwod I. P., Chapman J. W. 2009. Flight periodicity and the vertical distribution of high-altitude moth migration over southern Britain. Bull. Entomol. Res. 99, 525–535 10.1017/S0007485308006548 (doi:10.1017/S0007485308006548) [DOI] [PubMed] [Google Scholar]
- 20.Showers W. B. 1997. Migratory ecology of the black cutworm. Annu. Rev. Entomol. 42, 393–425 10.1146/annurev.ento.42.1.393 (doi:10.1146/annurev.ento.42.1.393) [DOI] [PubMed] [Google Scholar]
- 21.Feng H. Q., Wu X. F., Wu B., Wu K. M. 2009. Seasonal migration of Helicoverpa armigera (Lepidoptera: Noctuidae) over the Bohai Sea. J. Econ. Entomol. 102, 95–104 10.1603/029.102.0114 (doi:10.1603/029.102.0114) [DOI] [PubMed] [Google Scholar]
- 22.Chapman J. W., Smith A. D., Woiwod I. P., Reynolds D. R., Riley J. R. 2002. Development of vertical-looking radar technology for monitoring insect migration. Comput. Electron. Agric. 35, 95–110 10.1016/S0168-1699(02)00013-3 (doi:10.1016/S0168-1699(02)00013-3) [DOI] [Google Scholar]
- 23.Reynolds D. R., Smith A. D., Chapman J. W. 2008. A radar study of emigratory flight and layer formation by insects at dawn over southern Britain. Bull. Entomol. Res. 98, 35–52 10.1017/S0007485307005470 (doi:10.1017/S0007485307005470) [DOI] [PubMed] [Google Scholar]
- 24.Wood C. R., Chapman J. W., Reynolds D. R., Barlow J. F., Smith A. D., Woiwod I. P. 2006. The influence of the atmospheric boundary layer on nocturnal layers of moths migrating over southern Britain. Int. J. Biometeorol. 50, 193–204 10.1007/s00484-005-0014-7 (doi:10.1007/s00484-005-0014-7) [DOI] [PubMed] [Google Scholar]
- 25.Batschelet E. 1981. Circular statistics in biology. London, UK: Academic Press [Google Scholar]
- 26.Horn H. S. 1966. Measurement of overlap in comparative ecological studies. Am. Nat. 100, 419–424 10.1086/282436 (doi:10.1086/282436) [DOI] [Google Scholar]
- 27.Reynolds D. R., Chapman J. W., Edwards A. S., Smith A. D., Wood C. R., Barlow J. F., Woiwod I. P. 2005. Radar studies of the vertical distribution of insects migrating over southern Britain: the influence of temperature inversions on nocturnal layer concentrations. Bull. Entomol. Res. 95, 259–274 10.1079/BER2004358 (doi:10.1079/BER2004358) [DOI] [PubMed] [Google Scholar]
- 28.Reynolds A. M., Reynolds D. R., Smith A. D., Chapman J. W. 2010. A single wind-mediated mechanism explains high-altitude ‘non-goal oriented’ headings and layering of nocturnally-migrating insects. Proc. R. Soc. B 277, 765–772 10.1098/rspb.2009.1221 (doi:10.1098/rspb.2009.1221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liechti F., Schaller E. 1999. The use of low-level jets by migrating birds. Naturwissenschaften 86, 549–551 10.1007/s001140050673 (doi:10.1007/s001140050673) [DOI] [PubMed] [Google Scholar]
- 30.Kerlinger P., Moore F. R. 1989. Atmospheric structure and avian migration. In Current ornithology, vol. 6 (ed. Power D. M.), pp. 109–142 New York, NY: Plenum Press [Google Scholar]
- 31.Klaassen M., Kvist A., Lindströ Å. 1999. How body water and fuel stores affect long-distance flight in migrating birds. In Proc. of the 22nd Int. Ornithol. Congress (eds Adamse N. J., Slotow R. H.), pp. 1450–1467 Johannesburg, Republic of South Africa: Birdlife South Africa [Google Scholar]
- 32.Green M. 2004. Flying with the wind—spring migration of Arctic-breeding waders and geese over southern Sweden. Ardea 92, 145–160 [Google Scholar]
- 33.Alerstam T. 1978. Analysis and a theory of visible migration. Oikos 30, 273–349 10.2307/3543483 (doi:10.2307/3543483) [DOI] [Google Scholar]
- 34.Wikelski M., Tarlow E. M., Raim A., Diehl R. H., Larkin R. P., Visser G. H. 2003. Costs of migration in free-flying songbirds. Nature 423, 704. 10.1038/423704a (doi:10.1038/423704a) [DOI] [PubMed] [Google Scholar]
- 35.Thorup K., Alerstam T., Hake M., Kjellén N. 2006. Travelling or stopping of migrating birds in relation to wind: an illustration for the osprey. Behav. Ecol. 17, 497–502 10.1093/beheco/arj054 (doi:10.1093/beheco/arj054) [DOI] [Google Scholar]
- 36.Gill R. E., Jr, et al. 2009. Extreme endurance flights by landbirds crossing the Pacific Ocean: ecological corridor rather than barrier? Proc. R. Soc. B 276, 447–457 10.1098/rspb.2008.1142 (doi:10.1098/rspb.2008.1142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chapman J. W., Drake V. A., Reynolds D. R. 2011. Recent insights from radar studies of insect flight. Annu. Rev. Entomol. 56, 337–356 10.1146/annurev-ento-120709-144820 (doi:10.1146/annurev-ento-120709-144820) [DOI] [PubMed] [Google Scholar]
- 38.Reynolds A. M., Reynolds D. R., Riley J. R. 2009. Does a ‘turbophoretic’ effect account for layer concentrations of insects migrating in the stable night-time atmosphere? J. R. Soc. Interface 6, 87–95 10.1098/rsif.2008.0173 (doi:10.1098/rsif.2008.0173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynolds A. M., Reynolds D. R., Smith A. D., Chapman J. W. 2010. Orientation cues for high-flying nocturnal insect migrants: do turbulence-induced temperature and velocity fluctuations indicate the mean wind flow? PLoS ONE 5, e15758. 10.1371/journal.pone.0015758 (doi:10.1371/journal.pone.0015758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sane S. P., Dieudonne A., Willis M. A., Daniel T. L. 2007. Antennal mechanosensors mediate flight control in moths. Science 315, 863–866 10.1126/science.1133598 (doi:10.1126/science.1133598) [DOI] [PubMed] [Google Scholar]
- 41.Combes S. A., Dudley R. 2009. Turbulence-driven instabilities limit insect flight performance. Proc. Natl Acad. Sci. USA 106, 9105–9108 10.1073/pnas.0902186106 (doi:10.1073/pnas.0902186106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sparks T. H., Roy D. B., Dennis R. L. H. 2005. The influence of temperature on migration of Lepidoptera into Britain. Glob. Change Biol. 11, 507–514 10.1111/j.1365-2486.2005.00910.x (doi:10.1111/j.1365-2486.2005.00910.x) [DOI] [Google Scholar]
- 43.Both C., Van Turnhout C. A. M., Biljsma R. G., Siepel H., Van Strien A. J., Foppen R. P. B. 2010. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc. R. Soc. B 277, 1259–1266 10.1098/rspb.2009.1525 (doi:10.1098/rspb.2009.1525) [DOI] [PMC free article] [PubMed] [Google Scholar]