Abstract
Contemporary theory predicts that the degree of mimetic similarity of mimics towards their model should increase as the mimic/model ratio increases. Thus, when the mimic/model ratio is high, then the mimic has to resemble the model very closely to still gain protection from the signal receiver. To date, empirical evidence of this effect is limited to a single example where mimicry occurs between species. Here, for the first time, we test whether mimetic fidelity varies with mimic/model ratios in an intraspecific mimicry system, in which signal receivers are the same species as the mimics and models. To this end, we studied a polymorphic damselfly with a single male phenotype and two female morphs, in which one morph resembles the male phenotype while the other does not. Phenotypic similarity of males to both female morphs was quantified using morphometric data for multiple populations with varying mimic/model ratios repeated over a 3 year period. Our results demonstrate that male-like females were overall closer in size to males than the other female morph. Furthermore, the extent of morphological similarity between male-like females and males, measured as Mahalanobis distances, was frequency-dependent in the direction predicted. Hence, this study provides direct quantitative support for the prediction that the mimetic similarity of mimics to their models increases as the mimic/model ratio increases. We suggest that the phenomenon may be widespread in a range of mimicry systems.
Keywords: Batesian mimicry, female polymorphism, frequency-dependent selection, sexual conflict, signal detection theory
1. Introduction
Mimicry occurs in a wide variety of contexts and has evolved on multiple occasions to allow the signaller to achieve protection from predation or to gain access to prey, hosts, nuptial gifts, mates, pollinators or even parental care [1]. Traditionally, it has been argued that selection always favours mimetic individuals that more closely resemble their model. However, variation in the degree of resemblance is common in nature, with the maintenance of imperfect mimicry now thought to arise through a variety of evolutionary mechanisms, including selective trade-offs (e.g. [2,3]) and mutation–selection balance [4] (see [5] for review). Signal detection theory predicts that once the mimic achieves a certain degree of resemblance to its model, sufficient to reduce the motivation of the signal receiver to respond, then further selection to improve similarity may not be present [4]. This critical degree of resemblance required to afford protection may well depend on a variety of factors, including the local specific mimic/model ratio. For example in the case of Batesian mimicry, when unpalatable models are common relative to their palatable mimics, there will be little motivation for the predator to attack potential prey that even vaguely resemble the model [1,6].
Geographical variation in mimic/model ratios can sometimes be pronounced, providing an excellent opportunity to investigate related variation in phenotypic resemblance [7,8]. Studying a snake mimicry complex, Harper & Pfennig [7] were the first to show that mimetic fidelity increases with the site-specific mimic/model ratio. As noted above, however, in addition to avoiding predation, there are other contexts in which mimicry can evolve, including forms of intraspecific mimicry that have been generated by sexual selection. Perhaps the best-known examples of intraspecific mimicry arise as a component of male polymorphisms, with territorial and female-like males (sneakers), presenting two different sexual strategies [9]. Intraspecific mimicry also occurs in the form of female phenotypes resembling conspecific males (e.g. in butterflies [10], spiders [11] and especially in damselflies [12]). The question then arises as to whether the extent of mimetic fidelity also increases with the population-specific mimic/model ratio, as observed for Batesian mimicry.
The presence of multiple female morphs is generally explained in the context of sexual conflict theory, in which distinct phenotypes coexist as counteradaptations to avoid costly male sexual harassment (e.g. [12–14]). When female polymorphism arises in damselflies, typically one of the female morphs (andromorph) resembles the male in respect of body coloration, whereas the other does not (gynomorph) [15]. Besides body coloration, andromorphs are also similar to males in melanin patterning [8], exhibit male-like behavioural traits [16,17] and show comparable body size and body shape [18–20]. Moreover, previous research suggests that males rely on a combination of phenotypic traits to recognize potential mates both in polymorphic damselflies [21] and in other insects in general [22]. The striking resemblance in multiple traits of andromorphs to conspecific males has therefore prompted repeated proposals that andromorphs may be functional male-mimics [23] (or at least ‘distracters’ [24]). Empirical evidence confirms that andromorphs benefit from resembling males by receiving fewer mating attempts by males, at least when they are rare [25–28]. Indeed, morph-specific harassment levels and associated fitness costs in terms of reduced fecundity have been shown to vary in a negative frequency-dependent manner [29,30].
Wide among-population variation in the relative frequency of mimics (andromorphs) to models (males) has been observed in several female polymorphic damselfly species [31–33]. In populations where the mimic/model ratio is higher, an increased male harassment rate towards andromorphs has been demonstrated [28]. Furthermore, previous research already indicated geographical variation in both male-like melanin patterning [8] and male-like behavioural strategies [34] of andromorphs. However, it is currently unclear how much, if any, of the phenotypic variation arises as a consequence of differential selection on the degree of mimicry, as predicted by general signal detection theory (e.g. [4]).
In the current study, we studied phenotypic variation in mimetic similarity in the polymorphic damselfly Nehalennia irene, a species with known high geographical variation in proportion of andromorph females [33,35]. We specifically selected six populations with contrasting mimic/model ratios and conducted morphometric analyses of males and female morphs repeated over a 3 year study period. Our aims were twofold: (i) to investigate whether andromorphs resemble males morphologically and whether this pattern varies across populations (cf. [19]); and (ii) to examine the relationship between the phenotypic similarity of mimics (andromorphs) to models (males) and the among-population variation in the mimic/model ratio (cf. [7,8]). Our prediction was that mimic–model similarity would be highest in populations with a high mimic/model ratio, because signal receivers (males) should use a correspondingly higher threshold when deciding to attack the male-like object [4]. The intraspecific mimicry system in damselflies is well suited for this purpose because it simultaneously allows us to evaluate phenotypic similarity between the model and the non-mimetic morph (gynomorph) as a form of control.
2. Material and methods
(a). Study species
The sedge sprite, N. irene, is a small damselfly (Zygoptera; Odonata) that inhabits marshy or boggy waters and is common throughout most of Canada and the northern parts of the United States [36]. The reproductive season starts in early June and lasts until mid-August, with population numbers peaking from mid-June to mid-July. Nehalennia irene exhibits a clear polymorphism restricted to the female sex, with morphs being easily classified into andromorphs and gynomorphs based on their body coloration [37] (electronic supplementary material, appendix S1). Adult andromorph females resemble the conspecific male's blue body coloration and pattern by having a triangular patch of pale blue on the anteriodorsal part of segment eight and large paired dark spots on the dorsum of segment nine [8]. Gynomorph females, in contrast, are more yellowish in body coloration and lack the triangular patch and paired spots (for a more detailed description, see [37]; for colour drawings, see [38]). Although the genetic basis of the polymorphism has yet to be evaluated in N. irene, laboratory crossing experiments in several related coenagrionid damselfly species are consistent with the polymorphism being determined at a single autosomal locus with female-limited expression (reviewed in [31]).
(b). Study sites and sampling procedures
Past research with N. irene documented considerable spatial variation in proportion of andromorph females [33,35]. Based on these previous findings, six populations were selected that differed largely in relative abundance of andromorphs to study variation in mimetic similarity (table 1). Previous experimental work in these populations has shown that male harassment rates (i.e. male-mating attempts) towards andromorphs increase with the mimic/model ratio [28].
Table 1.
Estimates of the mimic/model ratio (andromorph/male, A/M) per site, averaged across years (mean ± s.e.). n refers to the minimum and maximum total number of adult individuals counted per year on which these estimates are based.
| site (province) | lat./long. | n | A/M |
|---|---|---|---|
| Jack's Marsh | 44°34′/−76°20′ | 179–326 | 0.02 ± 0.01 |
| Barb's Marsh | 44°31′/−76°22′ | 122–333 | 0.03 ± 0.01 |
| Otter Marsh | 44°33′/−76°22′ | 141–1129 | 0.10 ± 0.03 |
| Quebec City | 46°46′/−70°58′ | 96–131 | 0.54 ± 0.14 |
| Airpark Road | 54°00′/−123°02′ | 119–438 | 0.62 ± 0.07 |
| Summit Lake | 54°10′/−122°41′ | 117–265 | 0.74 ± 0.12 |
To account for possible seasonal variation in body size [39], each of the selected populations was sampled during the reproductive season for 3 subsequent years from 2007 to 2009. Before collecting individuals, site-specific abundance of andromorphs was determined in a standardized manner as described by Van Gossum et al. [35]. This method is based on counts of adult individuals captured with an insect net while walking slowly in a standardized manner through the reproductive area, sweeping ‘figures of eight’. Numbers of adult males and females of N. irene were recorded and marked prior to their release to exclude multiple counts. In doing so, andromorph frequency (proportion of andromorph females) and the mimic/model ratio (andromorph/male ratio) can be calculated for each site and year (table 1). Both frequency estimates are strongly correlated (Spearman correlation: r = 0.92; n = 18; p < 0.0001). Analyses using andromorph frequency gave similar results as analyses using the mimic/model ratio, such that we only report the latter in what follows. For morphological measurements, we aimed to collect 25 individuals of each adult type (male, andromorph, gynomorph) in each population for each year. All individuals were stored for further measurements in 95 per cent ethanol immediately after capture. A total of 1296 individuals were measured, comprising 422 males, 438 andromorphs and 436 gynomorphs.
(c). Measurements of mimic–model similarity
Six morphological characteristics were measured, namely individual mass, wing surface and four length measures (see below), which together provide general information on body size and shape of individuals. We chose not to evaluate colour characteristics because specimens can lose their colours after death even when preserved. Therefore, these sets of traits were not considered reliable in a multi-annual, multi-site study of this kind. Before determining mass, individuals were placed on a sheet of absorbent paper for exactly 2 min to allow standardized evaporation and absorption of most of the ethanol. Individuals were then weighed on a Kern & Sohn GmbH 870 balance (accuracy 0.1 mg). A digital picture was taken (Nikon D70; Tamron macro lens, 90 mm, 1 : 2.8) of each specimen while it was laterally positioned. Using ImageJ 1.38x [40], measurements were made of the right hind wing and the abdomen. Wing length was determined from the second antenodal cross vein to the stigma and wing surface was defined based on a polygon using five landmarks where main veins meet the wing margin (electronic supplementary material, appendix S2). Furthermore, total abdomen length (from the first abdominal segment to the tip of the abdomen), length and width of the fourth abdominal segment (S4) were measured. The rationale for measuring these traits is the overall high values of additive genetic variance based on parent–offspring regressions found in a common laboratory environment in the closely related damselfly species Ischnura elegans [41]. Based on our measurements, it was possible to calculate several morphological parameters, including overall body size and shape (principal component analysis, PCA; see below), volume of S4 (1/2 × width S4 × π × length S4) and two estimates of manoeuvrability: aspect ratio (wing length2/wing surface) and wing load (individual mass/(4 × wing surface)).
To evaluate how reliable our measurements were, repeatability was calculated to determine the variation in the data caused by measurement errors in the same standard conditions. A set of randomly chosen individuals within each population and year (total n = 121–150) were weighed or measured twice. Between repeated measures, individuals were replaced in the tube with ethanol followed by identical weight and measure procedures. Repeatability was then calculated as the proportion of the variation between individuals to the total variation (i.e. between and within individuals [42]). All our measurements were highly repeatable (≥0.89), indicating limited measurement error.
(d). Statistical analyses
First, we examined whether andromorphs are more similar to males compared with gynomorphs and whether these patterns of similarity vary across populations. Detecting geographical variation in similarity is necessary to be able to use populations as replicates when testing our second prediction (see below). To obtain a measure of the most important variation in our dataset, we performed a PCA with six of the morphological parameters (mass, abdomen length, width and length of S4, wing length and surface). Only the first two principal components were used, since together they explained more than 85 per cent of the variation in our dataset (PC1: 60.9%; PC2: 27.1%). All factor loadings had positive and large values in PC1 (electronic supplementary material, appendix S3) and can thus be interpreted as an overall estimate of body size. PC2, however, indicated variation in abdomen shape, since factor loadings were large and positive for the length of the abdomen and S4, and also large but negative for the width of S4 (electronic supplementary material, appendix S3). More specifically, the shape of the abdomen ranged from long and narrow (high PC2) to short and wide (low PC2). Next, a two-way ANOVA was performed to test for differences between types (males, andromorphs and gynomorphs) and sites. Several analyses were carried out with PC1, PC2, volume of S4, aspect ratio or wing load as dependent variables, while adding type, site and their interactions as explanatory variables (all fixed categorical variables). Since annual variation in the mimic/model ratio was limited relative to the variation across sites [33], we treated year as a random variable to control for multiple sampling. All post hoc paired comparisons between type means per site were Bonferroni-corrected.
Our second aim was to relate phenotypic similarity between andromorphs and males to the among-population variation in the mimic/model ratio. As a control, the similarity between gynomorphs and males in relation to the mimic/model ratio is also evaluated. We used a single similarity index that combines values of all measured traits. This similarity index is characterized by Mahalanobis distances (MDs) between defined groups, such as type and site in every year, and takes into account the variance and covariance among the measures. In all analyses, we controlled for sample size in each site and year, and added a repeated-site statement because every site is sampled for 3 years. The appropriate covariance structure was determined for each regression model based on BIC values and turned out to be the autoregressive one in all cases. In addition to the MD as a proxy for integrated morphology, we performed similar analyses with individual phenotypic aspects as dependent variables, such as the mean differences in aspect ratio, wing load, PC1 (size) and PC2 (shape) between males and both female morphs. All analyses were carried out in SAS 9.1.3 (SAS Institute Inc.), except calculating MDs, which was done in R 2.8.0 (R Development Core Team).
3. Results
(a). Variation in morphology
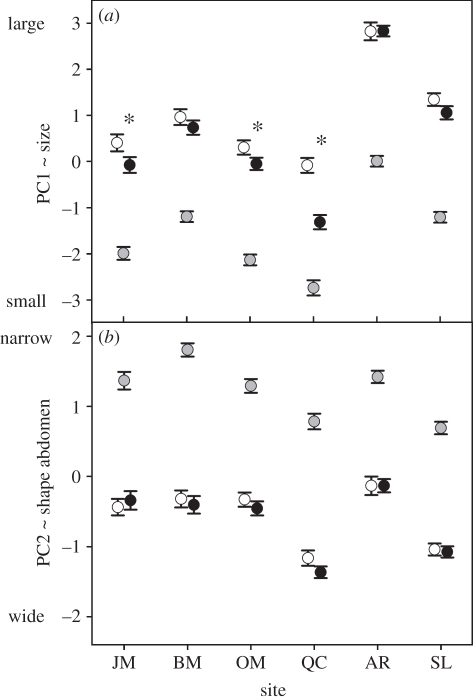
All of the studied phenotypic traits showed significant differences between andromorphs, gynomorphs and males, but the patterns differed across study sites as illustrated by the significant site × type interactions (summarized in table 2). Finding geographical variation in similarity between andromorphs and males (based on post hoc test for the site × type interaction) was required for using these populations as replicates when evaluating mimic–model similarity against the mimic/model ratio (see below). Specifically, males were consistently smaller (PC1; figure 1a) and had a narrower abdomen (PC2; figure 1b), lower abdomen volume, lower aspect ratio and lower wing load (electronic supplementary material, appendix S4) than both female morphs in all study sites. Furthermore, significant differences in several of these traits were detected between andromorphs and gynomorphs, but only at some of the study sites. In such cases, values for andromorphs were repeatedly more similar to males than the ones for gynomorphs. Indeed, post hoc tests revealed that andromorphs were significantly smaller than gynomorphs in three out of six populations, and a tendency for this pattern was observed in two other populations (figure 1a). Likewise, the volume of S4 was significantly smaller in andromorphs compared with gynomorphs in four out of six populations (electronic supplementary material, appendix S4a). Also, wing load was higher in gynomorphs than in andromorphs in one population (electronic supplementary material, appendix S4c). By contrast, no differences were found in abdominal shape (PC2) between female morphs (all p > 0.1; figure 1d). Also, for aspect ratio, no clear pattern was observed, since wings of andromorphs and gynomorphs differed significantly in two populations, but results were in opposite directions (table 2; electronic supplementary material, appendix S4b). Overall, andromorphs had a smaller body size (PC1) and smaller abdomen volume than gynomorphs (post hoc tests for the main effect type: t1170 = −5.10, p < 0.0001 for body size; t1272 = −4.73, p < 0.0001 for abdomen volume). In contrast, for aspect ratio, wing load and body shape (PC2), overall differences between female morphs were not significant (all p > 0.3).
Table 2.
Results of ANOVA analyses testing for differences in all studied traits among types (males, andromorphs and gynomorphs) and across sites. All analyses are controlled for annual variation by adding ‘year’ as a random variable. d.f. 1 and 2 refer to, respectively, numerator and denominator degrees of freedom.
| effect | F | d.f. 1 | d.f. 2 | p |
|---|---|---|---|---|
| PC1 ∼ size | ||||
| type | 494.8 | 2 | 1170 | <0.0001 |
| site | 178.2 | 5 | 1170 | <0.0001 |
| site × type | 3.71 | 10 | 1170 | <0.0001 |
| PC2 ∼ abdomen shape | ||||
| type | 611.2 | 2 | 1170 | <0.0001 |
| site | 51.4 | 5 | 1170 | <0.0001 |
| site × type | 1.99 | 10 | 1170 | 0.031 |
| volume S4 | ||||
| type | 1238.0 | 2 | 1172 | <0.0001 |
| site | 27.8 | 5 | 1172 | <0.0001 |
| site × type | 3.81 | 10 | 1172 | <0.0001 |
| aspect ratio | ||||
| type | 18.9 | 2 | 1212 | <0.0001 |
| site | 7.75 | 5 | 1212 | <0.0001 |
| site × type | 2.30 | 10 | 1212 | 0.011 |
| wing load | ||||
| type | 157.7 | 2 | 1178 | <0.0001 |
| site | 6.72 | 5 | 1178 | <0.0001 |
| site × type | 2.38 | 10 | 1178 | 0.009 |
Figure 1.
Geographical variation in (a) PC1 (size) and (b) PC2 (abdominal shape) between males, andromorphs and gynomorphs, with sites being ordered by increasing proportion of andromorphs: Jack's Marsh (JM), Barb's Marsh (BM), Otter Marsh (OM), Quebec City (QC), Airpark Road (AR), Summit Lake (SL). An asterisk indicates significant Bonferroni-corrected differences between andromorphs and gynomorphs in pairwise post hoc comparisons for the site × type interaction. Differences between males and both female morphs were always highly significant and are therefore not illustrated. Black circles, andromorph; white circles, gynomorph; grey circles, male.
(b). Mimic–model similarity versus mimic/model ratio
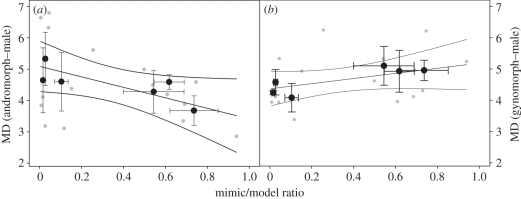
Combining all measured traits into one similarity index (i.e. MDs) allowed evaluating variation in phenotypic similarity against variation in the mimic/model ratio. Specifically, the similarity between andromorphs (mimics) and males (models) increased (decreasing values of MD) with increasing ratio of andromorphs relative to males (F1,9 = 8.53, p = 0.017; figure 2a). As a control, MDs were calculated between gynomorphs and males, but showed no relationship with the mimic/model ratio (F1,7 = 2.82, p = 0.13; figure 2b). Furthermore, none of the individual morphological aspects showed a significant relationship with the mimic/model ratio (all p > 0.1), although all estimated slopes were in the predicted direction. This indicates that our index for similarity is not driven by one specific morphological trait, but that all traits combined function as an integrated phenotype. Similar tendencies were found when relating abdominal melanin pattern, as a single trait, with site-specific andromorph frequency [8].
Figure 2.
The morphological similarity between (a) andromorphs and males increases with the site-specific mimic/model ratio, whereas no such pattern was found for (b) gynomorphs. Similarity is characterized by Mahalanobis distances (MD), with high values of MD indicating poor similarity and vice versa. Regression fit and 95% confidence interval are based on MD in every site and year (grey dots). Black dots represent mean values per site, with vertical standard error bars indicating annual variation in MD and horizontal ones representing annual variation in the mimic/model ratio. (a) p = 0.017; (b) p = 0.13.
4. Discussion
Frequency-dependent selection has been recognized as one of the most common explanations for the maintenance of genetic polymorphisms [43,44]. This frequency dependence may not only affect morph-specific fitness [29,30] but also the phenotype [4]. Indeed, in the present study, we demonstrated that phenotypic similarity of mimetic female morphs (andromorphs) to the model (males) increases with the mimic/model ratio in a damselfly showing intraspecific mimicry. This fundamental prediction, based primarily on signal detection theory, had previously only once been tested (and supported), by analyses of phenotypic variation in a snake mimicry complex [7]. Thus, our results indicate that the phenomenon applies not just to examples of classical Batesian mimicry, but also to cases in which mimicry has been suggested to have evolved through sexual selection.
The logic relating to changes in the mimic phenotype is as follows: when the ratio of andromorphs (mimics) to males (models) is high, then only andromorphs that closely resemble the male should receive any protection against harassing males. In contrast, selection for mimicry is different when the mimic/model ratio is low, because the majority of male-like individuals are indeed males [23], so mate-searching males may do best by avoiding any individual that even vaguely resembles a male. As such, in geographical areas where males are more common, andromorphs with a relatively poor resemblance may also be protected from male harassment and hence are not selected to invest in a full arsenal of male-like traits (see also [4,7]).
So far, we have argued that mimics should evolve towards the model's phenotype. However, one may also consider selection favouring a model's phenotype to diverge from its mimics, resulting in an evolutionary chase to reduce encounters with the signal receivers [45,46]. Specifically, males may be expected to evolve a distinct phenotype to minimize unprofitable male–male sexual interactions [47]. Our results illustrate that mimetic similarity of andromorphs increased with the mimic/model ratio. However, the similarity relationship of gynomorphs to males was absent, or showed a slight reverse tendency. Thus, although this pattern indicates the presence of selection for mimicry in andromorphs, it is not possible to fully exclude selection on male divergence. In other words, even though selection on mimics for phenotypic convergence may be stronger than on males for phenotypic divergence, an evolutionary chase between mimics and models cannot completely be ruled out based on our current results [5].
Another interesting finding of this study is that andromorphs are overall smaller than gynomorphs and, owing to allometric relationships, also have a smaller volume of the abdomen. This pattern corresponds with that for I. elegans, a species in which fecundity of andromorphs is overall lower but also dependent on the levels of male harassment [19,26]. Thus, the frequency-dependent advantage of reduced male harassment through mimicry may in part be counterbalanced by a reduced fecundity, a question that is currently being studied in detail for N. irene (A. Iserbyt 2007–2009, unpublished results). Together, a complex interplay of morph-specific costs and benefits may enable multiple female morphs to coexist in natural populations [12,14,26].
5. Conclusion
We have presented empirical evidence that mimetic fidelity increases with the mimic/model ratio in an intraspecific mimicry system. Thus, our findings are in line with predictions of signal detection theory, thereby suggesting its applicability to a wide variety of mimicry systems. An interesting direction for future research would be to investigate whether body coloration, behavioural and/or morphological aspects act together as an integrated set of male-like traits to increase mimetic similarity. Indeed, so far no studies have been conducted that use a combination of morphometric, behavioural and spectrophotometric data when studying variation in phenotypic similarity for an intra- or interspecific mimicry system.
Acknowledgements
This research was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) to T.N.S., Queens University Biological Station, the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen research grant) to J.B. and A.I., the Fund for Scientific Research-Flanders (postdoctoral FWO research grant) to H.V.G. and J.B., and a research grant from the University of Antwerp (BOF-NOI, project number 2401) to H.V.G. We are grateful F. P. Jvostov for field assistance, to M. Op De Beeck, G. De Winter and J. Boeye for data collection, to S. Lindgren for technical support and to the Zwozdesky family for local support in Prince George, BC. Two anonymous referees and K. Abbott provided valuable comments on earlier versions of the manuscript.
References
- 1.Ruxton G. D., Sherratt T. N., Speed M. P. 2004. Avoiding attack; the evolutionary ecology of crypsis, warning signals & mimicry, 1st edn. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Edmunds M. 2000. Why are there good and poor mimics? Biol. J. Linn. Soc. 70, 459–466 10.1006/bijl.1999.0425 (doi:10.1006/bijl.1999.0425) [DOI] [Google Scholar]
- 3.Johnstone R. A. 2002. The evolution of inaccurate mimics. Nature 418, 524–526 10.1038/nature00845 (doi:10.1038/nature00845) [DOI] [PubMed] [Google Scholar]
- 4.Sherratt T. N. 2002. The evolution of imperfect mimicry. Behav. Ecol. 13, 821–826 10.1093/beheco/13.6.821 (doi:10.1093/beheco/13.6.821) [DOI] [Google Scholar]
- 5.Gilbert F. 2005. The evolution of imperfect mimicry. In Insect evolutionary ecology (eds Fellowes M., Holloway G., Rolff J.), 1st edn. London, UK: Royal Entomological Society [Google Scholar]
- 6.Pfennig D. W., Harcombe W. R., Pfennig K. S. 2001. Frequency-dependent Batesian mimicry—predators avoid look-alikes of venomous snakes only when the real thing is around. Nature 410, 323–323 10.1038/35066628 (doi:10.1038/35066628) [DOI] [PubMed] [Google Scholar]
- 7.Harper G. R., Pfennig D. W. 2007. Mimicry on the edge: why do mimics vary in resemblance to their model in different parts of their geographical range? Proc. R. Soc. B 274, 1955–1961 10.1098/rspb.2007.0558 (doi:10.1098/rspb.2007.0558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Gossum H., Robb T., Forbes M. R., Rasmussen L. 2008. Female-limited polymorphism in a widespread damselfly: morph frequencies, male density, and phenotypic similarity of andromorphs to males. Can. J. Zool. 86, 1131–1138 10.1139/Z08-091 (doi:10.1139/Z08-091) [DOI] [Google Scholar]
- 9.Shuster S. M., Wade M. J. 2003. Mating systems and strategies, 1st edn. Princeton, NJ: Princeton University Press [Google Scholar]
- 10.Cook S. E., Vernon J. G., Bateson M., Guilford T. 1994. Mate choice in the polymorphic African swallowtail butterfly, Papilio dardanus: male-like females may avoid sexual harassment. Anim. Behav. 47, 389–397 10.1006/anbe.1994.1053 (doi:10.1006/anbe.1994.1053) [DOI] [Google Scholar]
- 11.Oxford G. S., Gunnarsson B. 2006. Spatial variation in colour morph, spotting and allozyme frequencies in the candy-stripe spider, Enoplognatha ovata (Theridiidae) on two Swedish archipelagos. Genetica 128, 51–62 10.1007/s10709-005-5367-7 (doi:10.1007/s10709-005-5367-7) [DOI] [PubMed] [Google Scholar]
- 12.Van Gossum H., Sherratt T. N., Cordero-Rivera A. 2008. The evolution of sex-limited colour polymorphisms. In Dragonflies: model organisms for ecological and evolutionary research (ed. Córdoba-Aguilar A.). Oxford, UK: Oxford University Press [Google Scholar]
- 13.Härdling R., Bergsten J. 2006. Nonrandom mating preserves intrasexual polymorphism and stops population differentiation in sexual conflict. Am. Nat. 167, 401–409 10.0003-0147/2006/16703-40921 (doi:10.0003-0147/2006/16703-40921) [DOI] [PubMed] [Google Scholar]
- 14.Svensson E. I., Abbott J. K., Gosden T. P., Coreau A. 2009. Female polymorphisms, sexual conflict and limits to speciation processes in animals. Evol. Ecol. 23, 93–108 (doi:10.1007/s10682-007-9208-2) [Google Scholar]
- 15.Corbet P. S. 1999. Dragonflies: behaviour and ecology of Odonata. Essex, UK: Harley Books [Google Scholar]
- 16.Forbes M. R. L., Schalk G., Miller J. G., Richardson J. M. L. 1997. Male–female morph interactions in the damselfly Nehallenia irene (Hagen). Can. J. Zool. 75, 253–260 10.1139/z97-032 (doi:10.1139/z97-032) [DOI] [Google Scholar]
- 17.Van Gossum H., Stoks R., De Bruyn L. 2001. Frequency-dependent male mate harassment and intra-specific variation in its avoidance by females of the damselfly Ischnura elegans. Behav. Ecol. Sociobiol. 51, 69–75 10.1007/s002650100418 (doi:10.1007/s002650100418) [DOI] [Google Scholar]
- 18.Abbott J. K., Svensson E. I. 2008. Ontogeny of sexual dimorphism and phenotypic integration in heritable morphs. Evol. Ecol. 22, 103–121 10.1007/s10682-007-9161-0 (doi:10.1007/s10682-007-9161-0) [DOI] [Google Scholar]
- 19.Abbott J. K., Gosden T. P. 2009. Correlated morphological and colour differences among females of the damselfly Ischnura elegans. Ecol. Entomol. 34, 378–386 10.1111/j.1365-2311.2009.01087.x (doi:10.1111/j.1365-2311.2009.01087.x) [DOI] [Google Scholar]
- 20.Bots J., Breuker C. J., Van Kerkhove A., Van Dongen S., De Bruyn L., Van Gossum H. 2009. Variation in flight morphology in a female polymorphic damselfly: intraspecific, intrasexual, and seasonal differences. Can. J. Zool. 87, 86–94 10.1139/Z08-141 (doi:10.1139/Z08-141) [DOI] [Google Scholar]
- 21.Gorb S. N. 1998. Visual cues in mate recognition by males of the damselfly, Coenagrion puella (L) (Odonata: Coenagrionidae). J. Insect Behav. 11, 73–92 10.1023/A:1020818617066 (doi:10.1023/A:1020818617066) [DOI] [Google Scholar]
- 22.Jennions M. D., Petrie M. 1997. Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. Camb. Phil. Soc. 72, 283–327 10.1017/S0006323196005014 (doi:10.1017/S0006323196005014) [DOI] [PubMed] [Google Scholar]
- 23.Sherratt T. N. 2001. The evolution of female-limited polymorphisms in damselflies: a signal detection model. Ecol. Lett. 4, 22–29 10.1046/j.1461-0248.2001.00184.x (doi:10.1046/j.1461-0248.2001.00184.x) [DOI] [Google Scholar]
- 24.Fincke O. M. 2004. Polymorphic signals of harassed female odonates and the males that learn them support a novel frequency-dependent model. Anim. Behav. 67, 833–845 10.1016/j.anbehav.2003.04.017 (doi:10.1016/j.anbehav.2003.04.017) [DOI] [Google Scholar]
- 25.Cordero Rivera A. C., Sanchéz-Guillén R. A. 2007. Male-like females of a damselfly are not preferred by males even if they are the majority morph. Anim. Behav. 74, 247–252 10.1016/j.anbehav.2006.06.023 (doi:10.1016/j.anbehav.2006.06.023) [DOI] [Google Scholar]
- 26.Gosden T. P., Svensson E. I. 2009. Density-dependent male mating harassment, female resistance, and male mimicry. Am. Nat. 173, 709–721 10.1086/598491 (doi:10.1086/598491) [DOI] [PubMed] [Google Scholar]
- 27.Van Gossum H., Bots J., Van Heusden J., Hammers M., Huyghe K., Morehouse N. I. 2011. Reflectance spectra and mating patterns support intraspecific mimicry in the colour polymorphic damselfly Ischnura elegans. Evol. Ecol. 25, 139–154 10.1007/s10682-010-9388-z (doi:10.1007/s10682-010-9388-z) [DOI] [Google Scholar]
- 28.Ting J. J., Bots J., Jvostov F. P., Van Gossum H., Sherratt T. N. 2009. Effects of extreme variation in female morph frequencies on the mating behaviour of male damselflies. Behav. Ecol. Sociobiol. 64, 225–236 10.1007/s00265-009-0839-x (doi:10.1007/s00265-009-0839-x) [DOI] [Google Scholar]
- 29.Svensson E. I., Abbott J., Härdling R. 2005. Female polymorphism, frequency dependence and rapid evolutionary dynamics in natural populations. Am. Nat. 165, 567–576 10.0003-0147/2005/16505-40626 (doi:10.0003-0147/2005/16505-40626) [DOI] [PubMed] [Google Scholar]
- 30.Takahashi Y., Yoshimura J., Morita S., Watanabe M. 2010. Negative frequency-dependent selection in female color polymorphism of a damselfly. Evolution 64, 3620–3628 10.1111/j.1558-5646.2010.01083.x (10.1111/j.1558-5646.2010.01083.x) [DOI] [PubMed] [Google Scholar]
- 31.Sánchez-Guillèn R. A., Van Gossum H., Cordero-Rivera A. 2005. Hybridization and the inheritance of female colour polymorphism in two ischnurid damselflies (Odonata: Coenagrionidae). Biol. J. Linn. Soc. 85, 471–481 10.1111/j.1095-8312.2005.00506.x (doi:10.1111/j.1095-8312.2005.00506.x) [DOI] [Google Scholar]
- 32.Hammers M., Van Gossum H. 2008. Variation in female morph frequencies and mating frequencies: random, frequency-dependent harassment or male mimicry? Anim. Behav. 76, 1403–1410 10.1016/j.anbehav.2008.06.021 (doi:10.1016/j.anbehav.2008.06.021) [DOI] [Google Scholar]
- 33.Iserbyt A., Bots J., Ting J. J., Jvostov F. P., Forbes M. R., Sherratt T. N., Van Gossum H. 2009. Multi-annual variation in female morph frequencies of the polymorphic damselfly, Nehalennia irene, at continental and regional scales. Anim. Biol. 59, 313–326 10.1163/157075609X454944 (doi:10.1163/157075609X454944) [DOI] [Google Scholar]
- 34.Iserbyt A., Van Gossum H. 2009. Unexpected absence of behavioural differences between female damselfly colour morphs. Anim. Behav. 78, 1463–1469 10.1016/j.anbehav.2009.09.031 (doi:10.1016/j.anbehav.2009.09.031) [DOI] [Google Scholar]
- 35.Van Gossum H., Beirinckx K., Forbes M. R., Sherratt T. N. 2007. Do current hypotheses explain continental and seasonal variation in female morph frequencies of the damselfly, Nehalennia irene? Biol. J. Linn. Soc. 90, 501–508 10.1111/j.1095-8312.2007.00740.x (doi:10.1111/j.1095-8312.2007.00740.x) [DOI] [Google Scholar]
- 36.Walker E. M. 1953. The Odonata of Canada and Alaska, 1st edn. Toronto, Canada: University of Toronto Press [Google Scholar]
- 37.Forbes M. R. L., Richarson J. M. L., Baker R. L. 1995. Frequency of female morphs is related to an index of male density in the damselfly, Nehalennia irene (Hagen). Ecoscience 2, 28–33 [Google Scholar]
- 38.Lam E. 2004. Damselflies of the northeast. A guide to the species of eastern Canada and the northeastern United States, 1st edn. New York, NY: Biodiversity Books [Google Scholar]
- 39.Wong-Muñoz J., Córdoba-Aguilar A., Cueva del Castillo R., Serrano-Meneses M. A., Payne J. 2011. Seasonal changes in body size, sexual size dimorphism and sex ratio in relation to mating system in an adult odonate community. Evol. Ecol. 25, 59–75 10.1007/s10682-010-9379-0 (doi:10.1007/s10682-010-9379-0) [DOI] [Google Scholar]
- 40.Abramoff M. D., Magelhaes P. J., Ram S. J. 2004. Image processing with ImageJ. Biophoton. Int. 11, 36–42 [Google Scholar]
- 41.Abbott J. K., Svensson E. I. 2010. Morph-specific variation in intersexual genetic correlations in an intra-specific mimicry system. Evol. Ecol. Res. 12, 105–118 [Google Scholar]
- 42.Lessells C. M., Boag P. T. 1987. Unrepeatable repeatabilities—a common mistake. Auk 104, 116–121 [Google Scholar]
- 43.Punzalan D., Rodd F. H., Hughes K. A. 2005. Perceptual processes and the maintenance of polymorphism through frequency-dependent predation. Evol. Ecol. 19, 303–320 10.1007/s10682-005-2777-z (doi:10.1007/s10682-005-2777-z) [DOI] [Google Scholar]
- 44.Trotter M. V., Spencer H. G. 2008. The generation and maintenance of genetic variation by frequency-dependent selection: constructing polymorphisms under the pairwise interaction model. Genetics 180, 1547–1557 10.1534/genetics.108.088880 (doi:10.1534/genetics.108.088880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gavrilets S., Hastings A. 1998. Coevolutionary chase in two-species systems with applications to mimicry. J. Theor. Biol. 191, 415–427 10.1006/jtbi.1997.0615 (doi:10.1006/jtbi.1997.0615) [DOI] [PubMed] [Google Scholar]
- 46.Holmgren N. M. A., Enquist M. 1999. Dynamics of mimicry evolution. Biol. J. Linn. Soc. 66, 145–158 10.1111/j.1095-8312.1999.tb01880.x (doi:10.1111/j.1095-8312.1999.tb01880.x) [DOI] [Google Scholar]
- 47.Sherratt T. N., Forbes M. R. 2001. Sexual differences in coloration of Coenagrionid damselflies (Odonata): a case of intraspecific aposematism? Anim. Behav. 62, 653–660 10.1006/anbe.2001.1789 (doi:10.1006/anbe.2001.1789) [DOI] [Google Scholar]