Abstract
The Lepidoptera represent one of the most successful radiations of plant-feeding insects, which predominantly took place within angiosperms beginning in the Cretaceous period. Angiosperm colonization is thought to underlie the evolutionary success of the Lepidoptera because angiosperms provide an enormous range of niches for ecological speciation to take place. By contrast, the basal lepidopteran lineage, Micropterigidae, remained unassociated with angiosperms since Jurassic times but nevertheless achieved a modest diversity in the Japanese Archipelago. We explored the causes and processes of diversification of the Japanese micropterigid moths by performing molecular phylogenetic analysis and extensive ecological surveying. Phylogenetic analysis recovered a monophyletic group of approximately 25 East Asian endemic species that feed exclusively on the liverwort Conocephalum conicum, suggesting that niche shifts hardly played a role in their diversification. Consistent with the low flying ability of micropterigid moths, the distributions of the Conocephalum specialists are each localized and allopatric, indicating that speciation by geographical isolation has been the major process shaping the diversity of Japanese Micropterigidae. To our knowledge, this is the largest radiation of herbivorous insects that does not accompany any apparent niche differentiation. We suggest that the significance of non-ecological speciation during the diversification of the Lepidoptera is commonly underestimated.
Keywords: Eriocraniidae, non-glossatan moths, Japan, molecular dating
1. Introduction
The Lepidoptera is one of the most species-rich groups of plant-feeding insects [1]. The successful radiation of Lepidoptera is often linked to their association with angiosperms [2–4], because angiosperms by far predominate the host plants of modern lepidopteran species [5]. Common explanation for the high diversity of Lepidoptera posits that angiosperm toxins and insect countermeasures have led to an increased level of host specialization [6–8], allowing Lepidoptera to diversify along the enormous taxonomic diversity of angiosperms. In addition, architectural complexity of flowering plants allows Lepidoptera to specialize in different plant parts (leaves, stems, flowers and seeds) and evolve different feeding modes (external feeding, leaf mining and galling), providing multiple axes of ecological niche along which diversification can occur (e.g. [9]). In fact, phylogenetic comparative studies have found that ecological opportunities facilitated by host diversity and niche heterogeneity are probably the cause, rather than the consequence, of herbivorous insect diversity [10,11]. These findings are consistent with the preposition that angiosperm colonization has played a major role in the evolutionary success of the Lepidoptera.
By contrast, recent comparative studies in several herbivore groups indicate that non-ecological, geographical modes of speciation may better characterize many recent speciation events [12–14]. For example, Nyman et al. [12] found that nearly half of the speciation events in nematine sawflies do not involve apparent niche shifts, suggesting that the importance of ecological speciation in herbivorous insect diversification may at present be overestimated. Such non-ecological modes of speciation may also partly underlie host conservatism, or the tendency of rapidly radiating lineages to remain associated with the same genus or the family of host plants, which is evidently common in Lepidoptera [2,5,15]; however, phylogeny-based tests of non-ecological speciation in Lepidoptera are still lacking. Comparative insight from the evolutionary history of non-angiosperm-feeding, ancestral lepidopteran lineage may, therefore, enhance our understanding of the historical importance of ecological specialization, and that of angiosperm colonization, in diversification of the Lepidoptera.
Micropterigidae is the most basal family of the extant Lepidoptera, a relationship that is consistently supported by morphological, molecular and fossil evidence [16–18]. It is one of the three basal lepidopteran families (Micropterigidae, Agathiphagidae and Heterobathmiidae) that do not possess the lepidopteran proboscis and retain functional mandibles. Micropterigid moths emerged well before the mid-Cretaceous angiosperm radiation; the earliest definitive fossil of Micropterigidae appears in the Lower Cretaceous [19], while the family as a whole is thought to have originated in the Jurassic [1]. Despite their antiquity, there is no ecological or palaeontological evidence of angiosperm colonization by micropterigids, and modern species are either detritivores or bryophyte-feeders as larvae [20–22]. Thus, the micropterigids remained apart from the mainstream of lepidopteran radiation that took place within angiosperms; however, the moderate amount of diversity found in the present day Micropterigidae raises the question of how they have achieved their current diversity. In the Japanese Archipelago, 17 micropterigid species have been recorded [21]. Most of these species feed on the liverwort Conocephalum conicum, and are highly specialized in riverine moist environments where diverse bryophytes and ferns prosper (figure 1).
Figure 1.
Habitats and habits of (a–g) micropterigid and (h–j) eriocraniid moths in Japan. (a) Moist riverine habitat of Neomicropteryx nipponensis at Akame, Mie Prefecture. (b) Damp cliff habitat of N. nipponensis at Takeda-gawa Valley, Fukui Pref. (c) Adult of Neomicropteryx matsumurana. (d) Larva of Issikiomartyria bisegmentata feeding on C. conicum at Sekikawa, Yamagata Pref. (e) Larva of Neomicropteryx nipponensis feeding on C. conicum at Mt. Nabejiri, Shiga Pref. (f) Adult of Issikiomartyria akemiae at Kiyotsu Valley, Niigata Pref. (g) Adult of Micropterix aureatella at Shibunoyu, Nagano Pref. (h) Larva of Eriocrania sp. mining a leaf of Fagus crenata at Makino, Shiga Pref. (i) Mine of Eriocrania semipurpurella on a leaf of Betula platyphylla collected at Abashiri, Hokkaido. (j) Adult of Eriocrania komaii associated with Sorbus japonica.
In this paper, we examined the causes and processes of diversification of the Japanese micropterigid moths through molecular phylogenetic analysis and extensive surveying of larval host plants and distribution ranges. We focused primarily on the five micropterigid genera endemic to East Asia that together comprise 24 described species. Considering the great age of the Micropterigidae, it is possible that the richness of the modern species simply reflects the fact that the group is ancient. We addressed this possibility by performing the same analysis in the Eriocraniidae, an angiosperm-feeding moth family representing another early-branching lineage within the Lepidoptera [18]. Its broadly overlapping distribution with Micropterigidae in Japan allows them to serve as a good comparative model to investigate how host plant associations affect patterns of diversification while controlling for clade age and geography. Based on these analyses, we illustrate the pattern and history of diversification of the Japanese micropterigid moths and discuss how our results can be used to understand the overall diversification process of the Lepidoptera.
2. Material and methods
(a). Study organisms
Micropterigidae currently consist of 148 named species in 17 genera from all faunal regions [23]. Their global distribution is patchy, with the highest concentrations of species found in Japan and Taiwan (greater than 25 spp.), New Caledonia (greater than 20 spp.), New Zealand (20 spp.) and Madagascar (ca 15 spp.) [21,23–26]. Micropterigid larvae live in the soil and feed either on bryophytes [21,22] or decomposing litter and/or fungal hyphae [20]. Soil detritophagy is considered the ancestral feeding type of the Lepidoptera [27]. The adults of Sabatinca and Austromartyria feed on fern spores using the mandibles [20,23], while those of Micropterix, Sabatinca, Paramartyria and Tasmantrix have been collected feeding pollen on a range of angiospermous flowers [21,23,28,29].
In the Japanese Archipelago and nearby Taiwan, there are five endemic micropterigid genera (Paramartyria, Palaeomicroides, Issikiomartyria, Kurokopterix and Neomicropterix) that together contain 24 described species [21,30]. There is also a widespread genus, Micropterix, which occurs throughout Eurasia, from Europe to Japan. The larvae of the above five East Asian endemic genera are known to feed on liverworts, including Makinoa crispata, Heteroscyphus coalitus and C. conicum, which are used by the species of Paramartyria [21], while species of the other four genera are so far only recorded on C. conicum (figure 1; [21]). The life cycle of Japanese micropterigid moths is completed in a year; adults emerge in spring when they mate and oviposit, and hatching larvae continue feeding until early the following spring when they pupate and emerge as adults. Adults are active during the day, but their flight is weak and do not last long. Adults of the Japanese species do not feed on pollen or fern spores, except those of Micropterix and Paramartyria that have been collected on flowers [21].
Eriocraniidae is another basal family of Lepidoptera and is considered the earliest branching lineage of moths that possess the proboscis (namely, Glossata). Two genera (Eriocrania and Issikiocrania) are recognized from cool temperate regions in Japan; Eriocrania is also widespread throughout the Holarctic region [31]. Eriocraniid larvae are leaf miners on three angiosperm families (Betulaceae, Fagaceae and Rosaceae), and each species feeds on one or two closely related hosts in the same plant family. Larvae mine newly opened leaves in early spring (figure 1), and pupate in the soil after vacating the leaves. Adult moths emerge the following spring.
(b). Sampling
To determine the phylogenetic relationships and estimate divergence times of the Japanese micropterigid moths, we first sampled micropterigids extensively from 46 sites covering a wide range within the Japanese Archipelago (electronic supplementary material, figure S1). Specimens were collected by searching for adult moths, or larvae feeding on liverworts. In this way, we sampled all 16 Japanese micropterigid species belonging to the East Asian endemic genera and identified a number of new species, including a putative new genus. These undescribed taxa were generally readily recognizable based on male genital morphology and wing colour/pattern. However, Kurokopterix, Paramartyria and Micropterix contained specimens that were difficult to classify into species, based on the morphology and/or molecular data. While a detailed taxonomic study is clearly needed, we tentatively included three to six specimens from each of these genera in the analysis, which represented the wide range of molecular and morphological variations found within each genus. We also sampled a species of Palaeomicroides, a genus endemic to Taiwan, and Sabatinca viettei in New Caledonia.
Eriocraniid moths were collected primarily by searching for leaf-mining larvae. Sampling was conducted at 16 sites in Japan. We sampled two of the four described Japanese eriocraniid species and several new species of Eriocrania. Additionally, we sampled five species representing four higher lepidopteran families (Nepticulidae, Opostegidae, Heliozelidae and Elachistidae) in order to facilitate divergence time estimation (see below). We also collected three caddisfly species from each of the three Trichoptera suborders (Annulipalpia, Spicipalpia and Integripalpia) to root the entire Lepidoptera tree.
Specimens were either larvae or adults, and stored in 99 per cent alcohol prior to DNA extraction. In total, 70 moths and three caddisflies were newly sampled in the present study. Details of the specimens included in this study are provided in the electronic supplementary material, table S1.
(c). DNA sequencing, phylogenetic analyses and divergence time estimation
We extracted genomic DNA using the NucleoSpin Tissue Kit (Macherey-Nagel, Düren, Germany). The head capsule of the larva or the head, wings and abdomen of the adult were stored as vouchers. We sequenced fragments of the mitochondrial cytochrome oxidase subunit I (COI) and nuclear 18S rRNA and elongation factor 1-alpha (EF-1α) genes. Laboratory protocols, as well as the PCR primers used, are detailed in Kawakita et al. [32] and Kawakita & Kato [33].
Alignment of sequences was straightforward for the protein-coding COI and EF-1α genes. For 18S rRNA, we aligned the sequences using the program Clustal X under default settings and manually corrected obvious misalignments. The resulting dataset contained 585, 729 and 549 base pairs of COI, 18S rRNA and EF-1α, respectively, for 55 in-group Lepidoptera. All sequences generated for this study were deposited in the DNA Data Bank of Japan, and COI sequences in the Barcode of Life database together with collection records, specimen images and PCR primer information (see the electronic supplementary material, table S1 for accession numbers).
We constructed phylogenetic trees by maximum-parsimony (MP), maximum-likelihood (ML) and Bayesian methods, using the programs PAUP* (v. 4.0b10; [34]), Treefinder [35] and MrBayes (v. 3.1.2; [36]), respectively. The three gene regions were analysed simultaneously, because initial analyses of individual genes suggested no strongly conflicting phylogenetic signals among the genes. Obtained phylogenetic trees and the sequence alignment used to produce the phylogeny were deposited in the TreeBASE database under study accession number 11158.
To determine the age of the Japanese micropterigid species and to compare the timing of micropterigid and eriocraniid radiation, we estimated divergence times using the above sequence data and phylogenetic trees. Divergence dates were estimated under the penalized-likelihood approach, using the program ‘r8s’ (v. 1.7; [37]), and two alternative Bayesian relaxed-clock methods, using Multidivtime [38] and BEAST (v. 1.5.3; [39]).
Basal Lepidoptera are relatively rich in the Mesozoic fossil record (electronic supplementary material, table S2). We used the following two non-redundant fossils to constrain node ages in the penalized-likelihood analysis, and to place priors for the Bayesian analyses. First, the minimum age of the stem group Micropterigidae was set to 110 Ma, based on Parasabatinca aftimacrai, an adult micropterigid moth preserved in Lebanese amber that has the affinity to extant Sabatinca [40]. Second, the minimum age of the stem group Nepticulidae was placed at 99 Ma, based on trace fossils of characteristic leaf mines appearing at the Dakota Formation [15,41]. Because there is no a priori information for the age of root node based on palaeontological evidence, we used a stepwise exploratory approach to determine the effect of root node age on overall results. We tested dates ranging from 130 to 170 Ma in steps of 10 Myr as constraints/priors at root node, confirming that our inference of the age of the Japanese micropterigid radiation is not greatly affected by root node age.
For full detail of the methods used in phylogenetic and dating analyses, see the electronic supplementary material.
(d). Analysis of larval diet breadth
Previous observational records indicate that micropterigid larvae of the four Japanese endemic genera feed on liverworts [21]. However, the degree of larval host specificity of the Japanese micropterigids has not been studied quantitatively. Moreover, recent taxonomic studies using molecular data indicated that the liverwort C. conicum, the primary food plant of the Japanese micropterigids, consists of several cryptic species [42,43], raising the possibility that micropterigid species may separate niches by specializing on alternative ‘species’ of C. conicum. To investigate the potential role of larval diet specialization in micropterigid speciation, we conducted a thorough survey of the larval host plant in all the liverwort-feeding species sampled for the phylogenetic analysis. We further made quantitative assessment of the level of specialization to different liverwort species and the cryptic taxa of C. conicum, in seven micropterigid species representing Issikiomartyria, Kurokopterix and Neomicropterix. Detailed surveying procedures are provided in the electronic supplementary material.
3. Results and discussion
(a). Phylogeny and the evolution of diet breadth in East Asian Micropterigidae
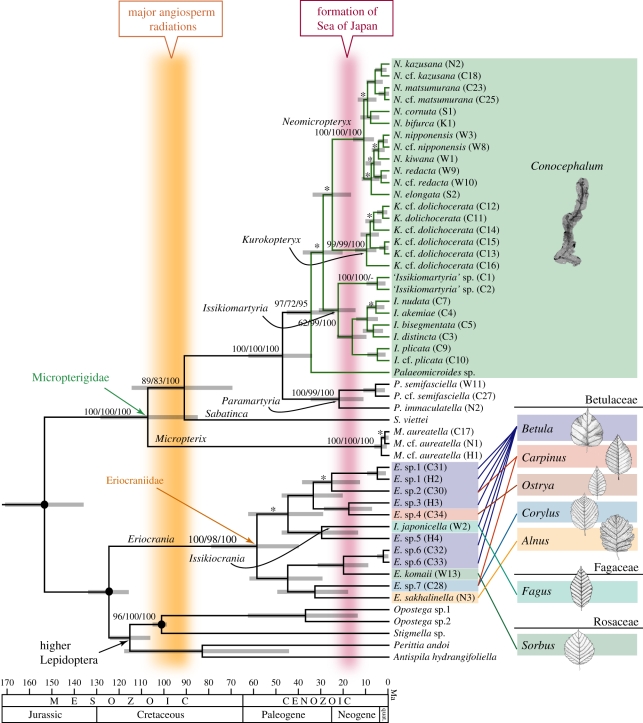
Phylogenetic analyses based on 1863 bp of the combined COI + 18S rRNA + EF-1α dataset produced phylogenies that are generally congruent across different methods of tree reconstruction (MP, ML and Bayesian methods; figure 2 and electronic supplementary material, figure S2). Monophyly of families and genera was recovered with high support values, except for Eriocrania, which was non-monophyletic with respect to the embedded Issikiocrania. The six micropterigid genera endemic to the East Asian islands, including the putative new genus (indicated as ‘Issikiomartyria’ (with quotations) in figure 2), were recovered as a monophyletic group. This clade consisted of the basal-most Paramartyria, which are polyphagous on the liverworts, M. crispata, H. coalitus and C. conicum [21], and a large clade that comprises the remaining five genera (Palaeomicroides, Neomicropterix, Kurokopterix, Issikiomartyria and the new genus). Our extensive field survey indicated that, as advanced previously [21], the latter five genera are in fact true specialists of C. conicum. No larvae of any species in these five genera were found feeding on bryophytes other than C. conicum throughout our survey (electronic supplementary material, table S3), despite the fact that as many as 14 other bryophyte species commonly co-occur in our study sites (electronic supplementary material, table S3). We also found that micropterigid larvae do not discriminatively feed on the cryptic species of C. conicum; after all, of the three C. conicum types known to occur in Japan, one (namely J-type sensu [42]) predominated each local bryophyte flora and was usually the food plant of micropterigid larvae at all study sites, while the other two C. conicum types were also used whenever they occurred in the habitat (electronic supplementary material, table S3). Micropterigid larvae feed on C. conicum by grazing the surface of the thalli (figure 1), and there were no apparent differences in feeding mode among species of the five genera. These findings, therefore, indicate that the Conocephalum-feeding micropterigids of East Asia constitute a large radiation of approximately 25 species that has not been accompanied by any niche differentiation.
Figure 2.
Phylogenetic relationships and divergence times of basal Lepidoptera. Node heights represent mean ages estimated by the BEAST program. Grey bars denote 95% high posterior densities. The phylogeny is rooted with three caddisfly species, which are not shown in the figure. Conocephalum specialists form a clade as indicated in green. Numbers noted at branches are maximum-parsimony bootstrap values, followed by maximum-likelihood bootstrap values and Bayesian posterior probabilities, for selected nodes. Posterior probabilities are greater than 0.95 unless marked with asterisks. Locality codes are given in parentheses after terminal labels, which correspond to those in the electronic supplementary material, table S1 and figure S1. Host plant genera are shown on the right. Divergence times were estimated using 150 ± 10 Ma as an arbitrarily-selected prior root node age; thus, caution is required when interpreting deep-node age estimates.
The results of the divergence time estimation suggest that the high species richness of East Asian Conocephalum-feeding micropterigids is not explained by clade age (figure 2). Although slightly different estimates were obtained by the three methods of analysis (r8s, Multidivtime and BEAST; electronic supplementary material, table S4), the Conocephalum specialists were estimated to have diverged since ca 35 Ma (figure 2). The majority of speciation events within the Conocephalum specialists involve within-genus radiations, which took place even more recently during the last 15–25 Myr. These dates are considerably recent with respect to the antiquity of Micropterigidae as a whole, which dates back at least to 110 Ma by fossil evidence [19]. The recentness of the East Asian radiation contrasts markedly with the estimated age of eriocraniids, which have started diversifying during the Paleocene/Eocene, predating the age of Conocephalum-feeding micropterigids by roughly 20 Myr. These data indicate that the present-day diversity of micropterigids in Japan cannot be attributed to the great age of the family and that diversification without niche shift can occur in a relatively short period as 15–25 Ma, comparable to other herbivorous-insect radiations (e.g. [15]).
(b). Patterns of distribution and speciation in East Asian Micropterigidae
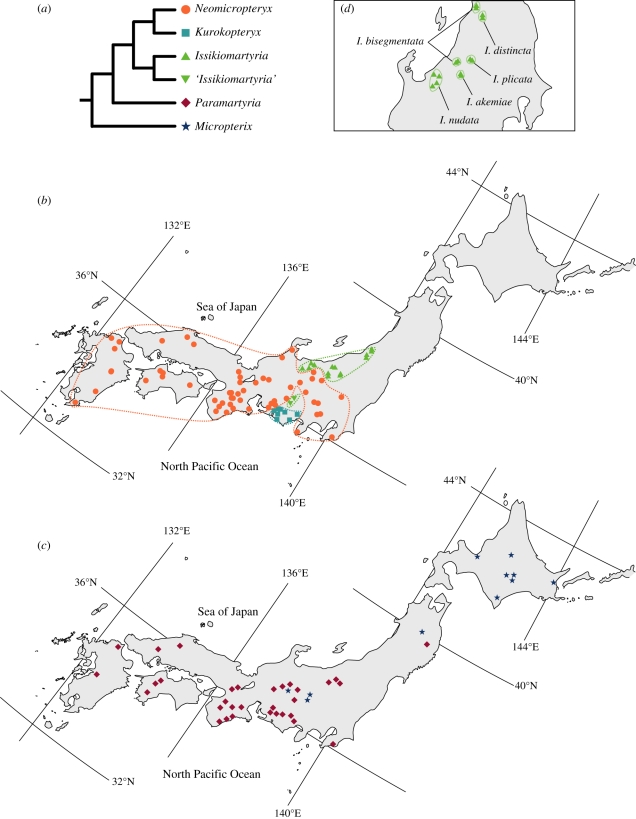
In figure 3, we reconstructed the distribution of each of the six micropterigid genera occurring in Japan, based on specimen localities of our samples and those of Hashimoto [21]. This indicates that the genera of Conocephalum specialists (figure 3a) show localized distributions that are clearly allopatric to each other. For example, the distribution of Issikiomartyria is confined to the snow-rich area facing the Sea of Japan, whereas that of Kurokopteryx is restricted to southern central Japan, facing the northwest Pacific Ocean. Furthermore, the distribution of species within each genus is also very narrow and allopatric to one another (figures 3d), despite the observation that micropterigids occur in very high densities within each population. For example, each of the five Issikiomartyria species occurring in northern central Japan is distributed in a very small area and does not co-occur with other Issikiomartyria species or the nearby Neomicropterix species (figure 3), although species of Paramartyria occasionally occur in some of these populations. The mechanism allowing the coexistence of Paramartyria and other genera is unknown, but temporal separation of the mating period between Paramartyria and other genera [44] may serve as an allochronic isolation of the two species. Overall, the distribution pattern of the Japanese micropterigid moths suggests that allopatric speciation has played a major role in shaping the current diversity and distribution of the Japanese micropterigid moths.
Figure 3.
Distribution of micropterigid moths in the Japanese Archipelago. (a) Phylogenetic relationships of micropterigid genera inferred from the present study. (b) Known sampling records for the three-derived genera, Kurokopteryx, Neomicropteryx and Issikiomartyria, which are all endemic to Japan. Areas enclosed by green, blue and orange lines are distribution limits of Issikiomartyria, Kurokopteryx and Neomicropteryx, respectively. (c) Sampling records of the first two basal clades, Micropterix and Paramartyria, are plotted similarly, as above. Information is based on Hashimoto [21] and the present study. (d) Sampling records of Issikiomartyria in northern central Japan.
The allopatric mode of speciation in the Japanese micropterigids is most probably attributable to their low dispersal ability. Micropterigid moths are generally weak and do not fly long distances, even when disturbed. For example, Tuskes & Smith [45] showed that the Californian micropterigid, Epimartyria pardella, fly very sporadically, and when they do, disperse a mere 21 cm per flight. Their reduced flying ability is probably owing to their strong association with Conocephalum liverworts, which are limited to continuously moist microhabitats along streams. In fact, adult moths are only found in such humid environments, and larvae and eggs are highly susceptible to drought stress [46].
Formation of many, allopatrically distributed species in Japan is paralleled in Mitella plants [47] and Onychodactylus salamanders [48], which are also strongly associated with similar humid microenvironments and exhibit limited dispersal ability. For example, the distribution range of Issikiomartyria largely overlaps with that of Mitella koshiensis that is endemic to this region, and patterns of species distribution within Neomicropterix (electronic supplementary material, figure S3) more or less agrees with lineage boundaries observed in Mitella and Onychodactylus. The complex topographical characteristics of the Japanese Archipelago, including a high density of mountains and valleys, coupled with continuously high rainfall, are probably responsible for extensive allopatric speciation in these organisms. The radiation of the Conocephalum-feeding micropterigids during the last 30 Myr is also consistent with the geological history of the region; the uplift of the Japanese landmass and the resulting formation of the Sea of Japan started 20 Ma [49], which coincides with the age at which the Japanese Conocephalum-feeding species began diversifying rapidly (figure 2).
(c). Non-ecological speciation and evolution of resource use in herbivorous insects
The Conocephalum-feeding micropterigids in the Japanese Archipelago is, to our knowledge, the largest radiation of herbivorous insects that took place in a single host taxon. This contrasts markedly with traditional explanations of herbivorous insect diversity [2,4], which posit that evolution of larval diet has played a major role in generating species diversity. However, recent studies in many herbivorous insect groups have shown that, while shifts in resource use are often essential for macroevolutionary events, it is probably of less importance for more recent speciation [5,12]. For example, Nyman et al. [12] found that nearly half of recent speciation events in nematine sawflies do not involve niche shifts, undermining the common, ecology-based explanation of herbivorous insect diversification. Similarly, Condon et al. [13] showed that Blepharoneura fruitflies, when sampled across South America, exhibit much higher diversity than would be expected from the sum of available niches (host plant diversity and plant parts), suggesting that non-ecological, geographical processes have contributed significantly to the evolution of their diversity. Therefore, allopatric, non-ecological modes of speciation, as found in Micropterigidae, is probably an important mechanism generating herbivorous insect diversity, but is commonly overlooked because the geographical scale at which it operates differs greatly depending on the dispersal ability of the insect. Difference in dispersal ability probably partly explains why Eriocraniidae is less diverse than Micropterigidae in Japan despite having a broader ecological niche and a longer time since initial divergence (figure 2). Eriocraniids are capable of flying among host trees and have broader distribution ranges, and thus, speciation by allopatry, if it ever occurs, is less likely to contribute to the richness of regional fauna (e.g. Japan).
Finally, we note that large radiations on single host plant taxa are not necessarily exceptions but rather the case in many Lepidoptera lineages [5]. For example, Nepticulidae leaf miners of the genera Stigmella and Ectoedemia have diverged into 19 and 26 species, respectively, on Quercus alone, with different species overlapping in distribution and host breadth to a varying extent [50,51]. Although the exact mechanism responsible for such diversity patterns is not clear, a possible scenario is that the initial stage of allopatric speciation is followed by, or accompanied with, minor divergences in host choice, resulting in regional moth fauna consisting of species with slightly different ecological requirements. Future studies should, therefore, investigate how different mechanisms involved in the speciation process, including allopatry, host shift and ecological reinforcement, combine to generate the present pattern of resource use and diversity in herbivorous insects [52].
Acknowledgements
We thank T. Kato and R. Goto for specimens; Y. Imada, R. Iritani and S. Furukawa for field assistance; T. Hikida for the topographic map; and A. Katayama for advice on phylogenetic analysis. This work was supported by a grant from the Japan Society for the Promotion of Science to M.K. We also thank two anonymous reviewers for constructive comments on the manuscript.
References
- 1.Grimaldi D., Engel M. S. 2005. Evolution of the insects. Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Ehrlich P. R., Raven P. H. 1964. Butterflies and plants: a study in coevolution. Evolution 18, 586–608 (doi:10.2307/2406212) [Google Scholar]
- 3.Jermy T. 1984. Evolution of insect/host plant relationships. Am. Nat. 124, 609–630 (doi:10.1086/284302) [Google Scholar]
- 4.Mitter C., Farrell B., Wiegmann B. 1988. The phylogenetic study of adaptive zones: has phytophagy promoted insect diversification? Am. Nat. 132, 107–128 (doi:10.1086/284840) [Google Scholar]
- 5.Menken S. B. J., Boomsma J. J., Van Nieukerken E. J. 2009. Large-scale evolutionary patterns of host plant associations in the Lepidoptera. Evolution 64, 1098–1119 (doi:10.1111/j.1558-5646.2009.00889.x) [DOI] [PubMed] [Google Scholar]
- 6.Jaenike J. 1990. Host specialization in phytophagous insects. Annu. Rev. Ecol. Syst. 21, 243–273 (doi:10.1146/annurev.es.21.110190.001331) [Google Scholar]
- 7.Futuyma D. J. 1991. Evolution of host specificity in herbivorous insects: genetic, ecological, and phylogenetic aspects. In Plant–animal interactions: evolutionary ecology in tropical and temperate regions (eds Price P. W., Lewinsohn T. M., Fernandes G. W., Benson W. W.), pp. 431–454 New York, NY: John Wiley & Sons [Google Scholar]
- 8.Thompson J. N. 1994. The coevolutionary process. Chicago, IL: Chicago University Press [Google Scholar]
- 9.Pellmyr O., Balcázar-Lara M., Althoff M., Segraves K. A., Leebens-Mack J. 2005. Phylogeny and life history evolution of Prodoxus yucca moths (Lepidoptera: Prodoxidae). Syst. Entomol. 31, 1–20 (doi:10.1111/j.1365-3113.2005.00301.x) [Google Scholar]
- 10.Janz N., Nylin S., Wahlberg N. 2006. Diversity begets diversity: host expansions and the diversification of plant-feeding insects. BMC Evol. Biol. 6, 4 (doi:10.1186/1471-2148-6-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyman T., Farrell B. D., Zinovjev A. G., Vikberg V. 2006. Larval habits, host-plant associations, and speciation in nematine sawflies (Hymenoptera: Tenthredinidae). Evolution 60, 1622–1637 (doi:10.1554/05-674.1) [PubMed] [Google Scholar]
- 12.Nyman T., Vikberg V., Smith D. R., Boevé J. L. 2010. How common is ecological speciation in plant-feeding insects? A ‘higher’ Nematinae perspective. BMC Evol. Biol. 10, 266 (doi:10.1186/1471-2148-10-266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Condon M. A., Scheffer S. J., Lewis M. L., Swensen S. M. 2008. Hidden Neotropical diversity: greater than the sum of its parts. Science 320, 928–931 (doi:10.1126/science.1155832) [DOI] [PubMed] [Google Scholar]
- 14.Winkler I. S., Mitter C. 2008. The phylogenetic dimension of insect/plant interactions: a summary of recent evidence. In Specialization, speciation, and radiation: the evolutionary biology of herbivorous insects (ed. Tilmon K.), pp. 240–263 Berkeley, CA: University of California Press [Google Scholar]
- 15.Lopez-Vaamonde C., Wikström N., Labandeira C., Godfray H. C. J., Goodman S. J., Cook J. M. 2006. Fossil-calibrated molecular phylogenies reveal that leaf-mining moths radiated millions of years after their host plants. J. Evol. Biol. 19, 1314–1326 (doi:10.1111/j.1420–9101.2005.01070.x) [DOI] [PubMed] [Google Scholar]
- 16.Mackay M. R. 1970. Lepidoptera in Cretaceous amber. Science 167, 379–380 (doi:10.1126/science.167.3917.379) [DOI] [PubMed] [Google Scholar]
- 17.Wiegmann B. M., Mitter C., Regier J. C., Friedlander T. P., Wagner D. M., Nielsen E. S. 2000. Nuclear genes resolve Mesozoic-aged divergences in the insect order Lepidoptera. Mol. Phylogenet. Evol. 15, 242–259 (doi:10.1006/mpev.1999.0746) [DOI] [PubMed] [Google Scholar]
- 18.Kristensen N. P., Scoble M. J., Karsholt O. 2007. Lepidoptera phylogeny and systematics: the state of inventorying moth and butterfly diversity. Zootaxa 1668, 699–747 [Google Scholar]
- 19.Kristensen N. P., Skalski A. W. 1999. Phylogeny and paleontology. In Lepidoptera: moths and butterflies, 1: evolution, systematics and biogeography, handbook of zoology (ed. Kristensen N. P.), pp. 7–25 Berlin, Germany: Walter de Gruyter [Google Scholar]
- 20.Kristensen N. P. 1999. The Homoneurous Glossata. In Lepidoptera: moths and butterflies, 1: evolution, systematics and biogeography, handbook of zoology (ed. Kristensen N. P.), pp. 51–63 Berlin, Germany: Walter de Gruyter [Google Scholar]
- 21.Hashimoto S. 2006. A taxonomic study of the family Micropterigidae (Lepidoptera, Micropterigoidea) of Japan, with the phylogenetic relationships among the Northern Hemisphere genera. Bull. Kitakyushu Mus. Nat. Hist. Hum. Hist. Ser. A 4, 39–109 [Google Scholar]
- 22.Powell J. A., Opler P. A. 2009. Moths of western North America. Berkeley, CA: University of California Press [Google Scholar]
- 23.Gibbs G. W. 2010. Micropterigidae (Lepidoptera) of the Southwestern Pacific: a revision with the establishment of five new genera from Australia, New Caledonia and New Zealand. Zootaxa 2520, 1–48 [Google Scholar]
- 24.Gibbs G. W. 1983. Evolution of Micropterigidae (Lepidoptera) in the SW Pacific. GeoJournal 7, 505–510 (doi:10.1007/BF00218523) [Google Scholar]
- 25.Dugdale J. S. 1988. Lepidoptera: annotated catalogue, and keys to family-group taxa. In Fauna of New Zealand, Number 14 (ed. Duval C. T.), pp. 52–53 Auckland, New Zealand: Manaaki Whenua Press [Google Scholar]
- 26.Lees D. C., Stonis J. R. 2007. The first record of Tischeriidae (Insecta: Lepidoptera) from Madagascar, with description of Coptotriche alavelona sp. n. and an updated distributional checklist of Afrotropical Tischeriidae. Zootaxa 1645, 35–45 [Google Scholar]
- 27.Stekolnikov A. A., Korzeev A. I. 2007. The ecological scenario of Lepidopteran evolution. Entomol. Rev. 87, 830–839 (doi:10.1134/S0013873807070056) [Google Scholar]
- 28.Thien L. B., Bernhardt P., Gibbs G. W., Pellmyr O., Bergström G., Groth I., McPherson G. 1985. The pollination of Zygogynum (Winteraceae) by a moth, Sabatinca (Micropterigidae): an ancient association? Science 227, 540–543 (doi:10.1126/science.227.4686.540) [DOI] [PubMed] [Google Scholar]
- 29.Kozlov M. V., Zvereva E. L. 2006. Aggregation of Micropterix maschukella moths on inflorescences of common elder: mating at foraging sites (Lepidoptera Micropterigidae). Ethol. Ecol. Evol. 18, 147–158 (doi:10.1080/08927014.2006.9522719) [Google Scholar]
- 30.Hashimoto S. 1996. Description of a new species and redescriptions of two species of the genus Palaeomicroides (Lepidoptera, Micropterigidae) from Taiwan. Trans. Lepid. Soc. Jpn 47, 93–99 [Google Scholar]
- 31.Mizukawa H., Hirowatari T., Hashimoto S. 2006. A new species of the genus Eriocrania (Lepidoptera, Eriocraniidae) from Japan. Trans. Lepid. Soc. Jpn 57, 149–155 [Google Scholar]
- 32.Kawakita A., Takimura A., Terachi T., Sota T., Kato M. 2004. Cospeciation analysis of an obligate pollination mutualism: have Glochidion trees (Euphorbiaceae) and pollinating Epicephala moths (Gracillariidae) diversified in parallel? Evolution 58, 2201–2214 (doi:10.1111/j.0014–3820.2004.tb01598.x) [DOI] [PubMed] [Google Scholar]
- 33.Kawakita A., Kato M. 2006. Assessment of the diversity and species specificity of the mutualistic association between Epicephala moths and Glochidion trees. Mol. Ecol. 15, 3567–3581 (doi:10.1111/j.1365–294X.2006.03037.x) [DOI] [PubMed] [Google Scholar]
- 34.Swofford D. L. 2002. PAUP*, phylogenetic analysis using parsimony (*and other methods), version 4.0. Sutherland, MA: Sinauer Associates [Google Scholar]
- 35.Jobb G. 2008. Treefinder, version of October 2008. Munich: Germany; Program distributed by the author (http://www.treefinder.de/) [Google Scholar]
- 36.Ronquist F., Huelsenbeck J. P. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 (doi:10.1093/bioinformatics/btg180) [DOI] [PubMed] [Google Scholar]
- 37.Sanderson M. J. 2002. Estimating absolute rates of molecular evolution and divergence times: a penalized likelihood approach. Mol. Biol. Evol. 19, 101–109 [DOI] [PubMed] [Google Scholar]
- 38.Thorne J. L., Kishino H. 2002. Divergence time and evolutionary rate estimation with multilocus data. Syst. Biol. 51, 689–702 (doi:10.1080/10635150290102456) [DOI] [PubMed] [Google Scholar]
- 39.Drummond A. J., Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 (doi:10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whalley P. E. S. 1978. New taxa of fossil and Recent Micropterigidae with a discussion of their evolution and a comment on the origin of the Lepidoptera. Ann. Trans. Mus. 31, 71–86 [Google Scholar]
- 41.Labandeira C. C., Dilcher D. L., Davis D. R., Wagner D. L. 1994. Ninety-seven million years of angiosperm–insect association: paleobiological insights into the meaning of coevolution. Proc. Natl Acad. Sci. USA 91, 12 278–12 282 (doi:10.1073/pnas.91.25.12278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akiyama H., Hiraoka T. 1994. Allozyme variability within and divergence among populations of the liverwort Conocephalum conicum (Marchantiales: Hepaticae) in Japan. J. Plant Res. 107, 307–320 (doi:10.1007/BF02344259) [Google Scholar]
- 43.Miwa H., et al. 2009. Adaptive evolution of rbcL in Conocephalum (Hepaticae, Bryophytes). Gene 441, 169–175 (doi:10.1016/j.gene.2008.11.020) [DOI] [PubMed] [Google Scholar]
- 44.Hashimoto S. 1998. The most archaic moth: Micropterigidae. In Biology of Microlepidoptera (eds Yasuda T., Hirowatari T., Ishii M.), pp. 146–152 Osaka, Japan: Bunkyo Shuppan. [In Japanese] [Google Scholar]
- 45.Tuskes P. M., Smith N. J. 1984. The life history and behavior of Epimartyria pardella (Micropterigidae). J. Lepid. Soc. 38, 40–46 [Google Scholar]
- 46.Kobayashi Y., Ando H. 1981. The embryonic-development of the primitive moth, Neomicropteryx nipponensis Issiki (Lepidoptera, Micropterigidae): morphogenesis of the embryo by external observation. J. Morphol. 169, 49–59 (doi:10.1002/jmor.1051690105) [DOI] [PubMed] [Google Scholar]
- 47.Okuyama Y., Kato M. 2009. Unveiling cryptic species diversity of flowering plants: successful biological species identification of Asian Mitella using nuclear ribosomal DNA sequences. BMC Evol. Biol. 9, 105 (doi:10.1186/1471-2148-9-105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshikawa N., Matsui M., Nishikawa K., Kim J. B., Kryukov A. 2008. Phylogenetic relationships and biogeography of the Japanese clawed salamander, Onychodactylus japonicus (Amphibia: Caudata: Hynobiidae), and its congener inferred from the mitochondrial cytochrome b gene. Mol. Phylogenet. Evol. 49, 249–259 (doi:10.1016/j.ympev.2008.07.016) [DOI] [PubMed] [Google Scholar]
- 49.Tada R. 1994. Paleoceanographic evolution of the Japan Sea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 108, 487–508 (doi:10.1016/0031-0182(94)90248-8) [Google Scholar]
- 50.Nieukerken E. J., Van Johansson R. 2003. The Quercus feeding Stigmella species of the West Palaearctic: new species, key and distribution (Lepidoptera: Nepticulidae). Tijdschr. Entomol. 146, 307–370 [Google Scholar]
- 51.Nieukerken E. J., Van Laštůvka A., Laštůvka Z. 2010. Western Palaearctic Ectoedemia (Zimmermannia) Hering and Ectoedemia Busck s. str. (Lepidoptera, Nepticulidae): five new species and new data on distribution, hostplants and recognition. Zookeys 32, 1–82 (doi:10.3897/zookeys.32.282) [Google Scholar]
- 52.Singer M. C., McBride C. S. 2010. Multitrait, host-associated divergence among sets of butterfly populations: implications for reproductive isolation and ecological speciation. Evolution 64, 921–933 (doi:10.1111/j.1558–5646.2009.00866.x) [DOI] [PubMed] [Google Scholar]