Abstract
Cuticular hydrocarbons provide arthropods with the chemical equivalent of the visually extravagant plumage of birds. Their long chain length, together with the number and variety of positions in which methyl branches and double bonds occur, provide cuticular hydrocarbons with an extraordinary level of information content. Here, we demonstrate phenotypic plasticity in an individual's cuticular hydrocarbon profile. Using solid-phase microextraction, a chemical technique that enables multiple sampling of the same individual, we monitor short-term changes in cuticular hydrocarbon profiles of individual crickets, Teleogryllus oceanicus, in response to a social challenge. We experimentally manipulate the dominance status of males and find that dominant males, on losing fights with other dominant males, change their hydrocarbon profile to more closely resemble that of a subordinate. This result demonstrates that cuticular hydrocarbons can be far more responsive to changes in social dominance than previously realized.
Keywords: social status, dominance, solid-phase microextraction, gas chromatography
1. Introduction
Male–male aggressive behaviour is a complex social interaction that is widespread in the animal kingdom. In many species, dominant males display ‘badges’ of high status in order to minimize conflict escalation over resources or serve as signals of resource-holding potential or aggressiveness. Such badges may also be used by females in mate choice as a reliable indication of male quality. For example, visual signals such as plumage in birds are often involved in dominance contests as a badge of status used to assess individual fighting ability (e.g. [1,2]). Pheromones are another prominent badge often used in agonistic communication in animal societies. Differences in pheromones produced by dominant and subordinate individuals have been reported in a wide range of animal taxa including mammals (e.g. [3]), crustaceans (e.g. [4,5]), fish (e.g. [6]) and insects (e.g. [7,8]). While the differences in pheromone components between dominant and subordinate males have now been well studied, the reliability of such chemical cues has received considerably less attention.
From an evolutionary perspective, stable communication systems require signals that convey, on average, reliable information; otherwise, receivers will be unable to gain fitness benefits and will stop attending to those signals [9]. Male dominance status can be frequently challenged and is therefore a potentially unstable trait (e.g. [10]). For pheromones to honestly reflect a male's current social status, they would be required to mirror such changes in status. For these reasons, the relationship between pheromone components and social status may be far more complicated than previously thought. Although there is some evidence to suggest that pheromones can change in response to a change in dominance status [7], direct evidence on how pheromones function in rank formation is still lacking and debate about the reliability of such signals continues.
Cuticular hydrocarbons are chemical compounds found on the cuticle of most terrestrial arthropods. Although the original function of cuticular hydrocarbons is thought to be protection against desiccation, it is now widely accepted that these chemicals are also used in short-range communication [11]. In females, changes in cuticular hydrocarbons have been widely reported, with mated females more often than not displaying a significantly different cuticular hydrocarbon profile than their unmated counterparts [12]. In males, it has recently been demonstrated that the social environment can influence the expression of cuticular hydrocarbons [13], and that changes in cuticular hydrocarbon expression can occur rapidly [14]. Here, for the first time in any species, we determine whether cuticular hydrocarbon profiles of males undergo short-term changes in response to a forced experimental change in dominance status.
We use the Australian field cricket Teleogryllus oceanicus, in which dominance status is associated with male mating and competitive fertilization success [8,15]. Although males cannot force copulations, dominance status does determine a male's ability to attract females via acoustic signals; dominant males will produce calling and/or courtship songs in the presence of subordinate males, whereas subordinate males are suppressed in their courtship song through intermittent attacks by the nearby dominant male [15]. Subordinate males appear to compensate for this disadvantage by displaying a relatively larger proportion of certain cuticular hydrocarbons that have previously been shown to increase male attractiveness to females [16]. Here, we determine if differences in cuticular hydrocarbon profiles between dominant and subordinate T. oceanicus males are fixed, or whether they can display different cuticular hydrocarbon phenotypes depending on the social environment (phenotypic plasticity), in this case a change in social status.
2. Materials and methods
Experimental animals were from an outbred laboratory stock derived from Carnarvon, north Western Australia. Individuals were separated into individual containers (7 × 7 × 5 cm) as late instar nymphs and left for 14 ± 4 days post-maturation before being used in experiments. All crickets were maintained, and experiments conducted, in a constant temperature room, at 25°C with a 12 L : 12 D cycle. Food and water were provided ad libitum.
(a). Male dominance status
(i). Day 1—initial dominance ranking
Initial male fighting ability was ranked using methods similar to that of Savage et al. [17], Shackleton et al. [18] and Thomas & Simmons [8] (see electronic supplementary material, figure S1). We tested males in 22 blocks, with eight males in each block. Within each block, males were tested in three rounds of fights. In the first round of fights, males were haphazardly assigned a competitor and dominance status recorded. This generated four males who won (W) and four males who lost (L) their first round of contests. In round two, winners were paired against winners, and losers against losers resulting in four categories (WW, LL, WL and LW). In round three, we only used males who had won or lost both their fights (WW or LL). Thus, individual crickets with the same fight history were competed against each another. At the end of round three, we were again left with four categories (WWW, WWL, LLW, LLL). Only those males who had lost (LLL) or won (WWW) all three of their contests were used in the social challenge experiment, thereby maximizing the difference in fighting ability between male pairs. These males are subsequently referred to as subordinate (S) or dominant (D), respectively. This experimental design controlled for any potential confounding effects that fight history may have on the outcome of subsequent fights [17], because males were only fought against crickets who had the same fight history.
(ii). Day 2—social challenge
On the day following the initial dominance ranking, we socially challenged males in order to determine whether their cuticular hydrocarbon profiles changed according to their social status. This was done by swapping males across blocks so that two dominant (D) or two subordinate (S) males fought together. This manipulation imposed a change of status on one of the two males, creating four equally represented social phenotypes: males who remained dominant across the manipulation (DD), males who were dominant and became subordinate (DS), males who were subordinate and became dominant (SD) and males who remained subordinate (SS). We then repeated this social challenge with the same male pairs to ensure that one male retained his dominance over the other. In only two cases did the two trials yielded a different dominant cricket. When this occurred, we fought these males until one male lost two consecutive battles. In both cases this took three rounds of battles.
In all dominance contests (initial ranking and social challenge), males were tested in arenas (17 × 12 × 6 cm) and left together for a period of 10 min. Dominance status was usually established within the first few minutes. Dominance status was easy to measure because subordinate males would display avoidance behaviour of the other male [15], while dominant males usually produced an aggressive song as a victory display [19]. Male age difference within each block was ±4 days. During contests, males within each block were individually marked with a small dot of water-based acrylic paint to allow discrimination between opponents. This mark was placed on the middle leg to avoid contamination during cuticular hydrocarbon extraction. Animals were re-isolated for between 10 and 30 min between fights. We measured the weight and pronotum width of each cricket used in the experiments. Dominance status was not related to male size in this experiment (paired t-tests; weight, t = −0.459, d.f. = 22, p = 0.651; pronotum width, t = −0.636, d.f. = 22, p = 0.531), consistent with previous results for this species [8].
(b). Cuticular hydrocarbon analysis
Cuticular hydrocarbon profiles of individuals were measured twice using solid-phase microextraction (SPME); once following the initial dominance ranking (day 1), and once following the social challenge (82 ± 50 min) (day 2). SPME involves solvent-less recovery and concentration of substances on silica fibres, and can therefore be repeated on the same animal. SPME was performed using a polydimethylsioxane (PDMS) 100 µm Supelco fibre. Prior to sampling, the fibre was cleaned twice by injection into gas chromatography (GC) at 250°C for 5 min using the splitless mode. Following cold anaesthetization of crickets (30 min at 4°C), the full length of the fibre was rubbed softly one way along the principal parts of the cricket's body (head, thorax, wings, abdomen). This was repeated 10 times with the fibre being slightly rotated between rubs. Animals were unaffected by this treatment. Similar methods have been used to study hydrocarbons in other insects [20,21]. Individual males were sampled at the same time of day on day 1 and day 2 of hydrocarbon sampling [13,22].
SPME samples were analysed by gas chromatography and mass spectrometry (GCMS, Agilent GC-6890N, MS-5975 with inert Mass Selective Detector). The GCMS was operated in the split mode (ratio 30 : 1) and fitted with a Stabilwax column (Restek) of 30 m × 0.25 mm internal diameter using helium as a carrier gas (flow 1 ml min−1). The column temperature profile began at a temperature of 150°C for 1 min and was ramped at 8°C min−1 to 250°C for 10 min. The transfer line from the GC to the mass spectrometer was set at 250°C. We analysed 96 profiles derived from 48 individuals. We analysed hydrocarbons only from those males who were used in the social challenge experiment.
(c). Data analysis
For data analysis of cuticular hydrocarbon profiles, peaks were labelled by peak number, which corresponded to their retention times (table 1). Hydrocarbon profiles of each male consisted of the relative abundances (peak areas) of 23 individual compounds. This compositional dataset was transformed to log-contrasts (using peak 3 as the divisor), as described previously for T. oceanicus cuticular hydrocarbon data [8,16]. A principal component analysis was conducted to determine if there was a difference in cuticular hydrocarbon profiles between dominant and subordinate males prior to the social challenge. To determine if individuals who changed their social status also changed their hydrocarbon profiles to reflect their current social status, we conducted a second principal component analysis using the difference in each peak area of each individual before and after the social challenge.
Table 1.
The mean difference in individual peaks (day 2 − day 1) ± s.e. (n = 12) following the social challenge. Values represent logcontrasts (×10−2). Negative value indicates a decrease in the relative proportion of cuticular hydrocarbons, and a positive value represents an increase in the relative proportion. Peaks that were found to differ significantly in PC1 for dominant males are indicated by bold print. DD, dominant males who remained dominant; DS, dominant males who became subordinate; SS, subordinate males who remained subordinate; SD, subordinate males who became dominant.
| peak | retention time | hydrocarbon | DD | DS | SS | SD |
|---|---|---|---|---|---|---|
| 1 | 11.310 | C29:1 | −2.10 ± 1.18 | 4.90 ± 0.77 | 2.58 ± 1.37 | −1.02 ± 1.58 |
| 2 | 11.670 | unresolved | 0.28 ± 0.53 | 3.20±2.24 | 1.74 ± 0.97 | 1.25 ± 0.72 |
| 4 | 12.010 | C31:1 | −1.27 ± 0.85 | 0.50 ± 0.70 | 0.98 ± 0.42 | −0.07 ± 0.52 |
| 5 | 12.170 | C31:1 | 0.85 ± 0.74 | 1.23 ± 0.75 | 0.32 ± 0.40 | 1.40 ± 0.67 |
| 6 | 12.470 | C31:1 | −1.96 ± 3.45 | 3.72 ± 3.15 | 0.55 ± 1.76 | −0.98 ± 1.78 |
| 7 | 12.520 | C31:2 | −2.08 ± 1.48 | 2.79 ± 1.51 | 0.71 ± 1.61 | 1.04 ± 1.59 |
| 8 | 12.580 | C31:2 | −0.75 ± 1.35 | 1.94 ± 1.03 | −1.69 ± 0.90 | −0.69 ± 1.14 |
| 9 | 12.630 | C31:2 | 1.32 ± 2.11 | 6.37 ± 2.19 | 6.20 ± 2.49 | 0.73 ± 1.63 |
| 10 | 12.730 | C31:2 | −4.32 ± 4.18 | 12.13 ± 3.43 | 7.53 ± 4.31 | −1.60 ± 3.73 |
| 11 | 12.840 | C31:2 | −2.97 ± 2.64 | 5.80 ± 2.64 | 3.68 ± 2.56 | −2.04 ± 1.57 |
| 12 | 12.880 | x-meC33 | 0.92 ± 0.65 | −0.69 ± 0.96 | 0.12 ± 0.83 | 0.61 ± 0.45 |
| 13 | 13.310 | unresolved | −0.37 ± 0.41 | 0.56 ± 0.30 | 0.29 ± 0.37 | 0.21 ± 0.49 |
| 14 | 13.530 | C33:1 | 1.66 ± 1.42 | −1.34 ± 1.20 | 0.63 ± 1.64 | 1.13 ± 1.39 |
| 15 | 13.690 | C33:1 | 0.22 ± 0.40 | 1.41 ± 0.36 | 0.77 ± 0.41 | 0.83 ± 0.35 |
| 16 | 13.780 | C33:1 | 0.41 ± 1.83 | 5.50 ± 1.47 | 2.98 ± 1.35 | 2.23 ± 1.95 |
| 17 | 14.120 | C33:1 | 0.08 ± 1.03 | 0.75 ± 0.45 | 0.73 ± 1.21 | 0.39 ± 0.60 |
| 18 | 14.170 | C33:2 | −0.95 ± 0.69 | 1.56 ± 1.09 | −0.93 ± 0.82 | 0.45 ± 0.98 |
| 19 | 14.230 | C33:2 | −1.10 ± 1.41 | 2.48 ± 1.08 | 0.36 ± 1.41 | −0.59 ± 1.37 |
| 20 | 14.330 | C33:2 | 2.29 ± 3.26 | 9.67 ± 2.49 | 4.27 ± 1.73 | 6.88 ± 3.07 |
| 21 | 14.420 | C33:2 | −0.14 ± 5.34 | 13.39 ± 3.15 | 5.55 ± 2.25 | 4.55 ± 3.64 |
| 22 | 14.550 | C33:2 | −0.67 ± 1.73 | 2.06 ± 0.74 | 1.73 ± 0.65 | −0.13 ± 1.07 |
| 23 | 16.540 | C35:2 | 0.05 ± 0.61 | 1.01 ± 0.59 | −0.06 ± 0.31 | 0.50 ± 0.54 |
3. Results
Consistent with previous studies of T. oceanicus that used solvent-based extraction methods ([8,16,23], electronic supplementary material, table S1), we were able to distinguish 23 peaks on male crickets that ranged in chain length from 29 to 35 carbons (electronic supplementary material, table S2). We first investigated whether there was a difference between subordinate and dominant male cuticular hydrocarbons after the initial round of contests. To do this, we analysed only the hydrocarbon profiles extracted from males prior to the social challenge (day 1). Using a principal component analysis, this dataset was reduced to five components. These components had eigenvalues greater than 1 [24] and collectively explained 79.22 per cent of the variance in cuticular hydrocarbon blends (electronic supplementary material, table S3). The percentage of variance for components 1–5 were 32.41, 17.44, 16.23, 8.45 and 4.68 per cent, respectively. Matched-pairs analysis revealed that only principal component 1 (PC1) showed a significant difference between dominant and subordinate males in the initial dominance rankings (PC1, t = 2.092, d.f. = 22, p = 0.048). To examine the relative contribution of each peak to PC1, we examined correlations between the original variables and the PC scores (electronic supplementary material, table S3). Eight peaks were found to have contributed significantly to PC1; peaks 13, 15, 16, 18–22. Examination of the raw concentrations of these eight peaks revealed that the strongest component was peak 21, representing 26.4 per cent of the total cuticular hydrocarbons present on subordinates, and 24.7 per cent of the total cuticular hydrocarbons present on dominate males (electronic supplementary material, table S2).
To determine if individuals showed a change in cuticular hydrocarbon profiles with a forced change in dominance status, we calculated the change in the area of each peak before and after the social challenge for each individual. We then performed a second separate principal component analysis on these differences in peak areas. This principal component analysis resulted in six eigenvalues greater than 1, which collectively explained 77.62 per cent of the variation. The percentages of variance explained were 33.47, 15.73, 9.24, 7.01, 6.31 and 5.86 for components 1–6, respectively. In order to determine if a forced change in social status was mirrored by a change in cuticular hydrocarbon profiles, we analysed dominant and subordinate males in separate analysis of variances using these six PCs. We used separate ANOVAs because subordinate and dominate males were not independent of each other owing to our paired experimental design.
We found that dominant males showed a significant change in PC1 following the social challenge (F1,22 = 9.03, p = 0.007; figure 1); males who remained dominant (DD) showed a decrease in PC1, reflecting a decrease in the compounds contributing to this PC, whereas males who became subordinate (DS) displayed an increase in PC1, reflecting an increase in compounds contributing to PC1. To examine the relative contribution of each peak to PC1, we examined the correlations between the original variables and PC1 (electronic supplementary material, table S4). We found that six peaks contributed significantly to this PC, peaks 1, 15, 16, 20, 21 and 22. Peaks 15, 16, 20, 21 and 22 are five of the original eight peaks that were found to contribute significantly to the differences between dominant and subordinate males (electronic supplementary material, table S3). The relative proportion of these hydrocarbons increased following the reversal in dominance status (table 1). In contrast to PC1, PCs 2–6 showed no significant change following the social challenge in dominant males (Fs < 1.884, ps > 0.184). In subordinate males we found that, irrespective of whether males became dominant (SD) or remained subordinate (SS), males showed no significant alteration in PC1 (F1,22 = 0.450, p = 0.509) or any of the other PCs (Fs ≤ 3.182, ps > 0.089). Subordinate males did not alter their hydrocarbon profile following a reversal in status (table 1).
Figure 1.
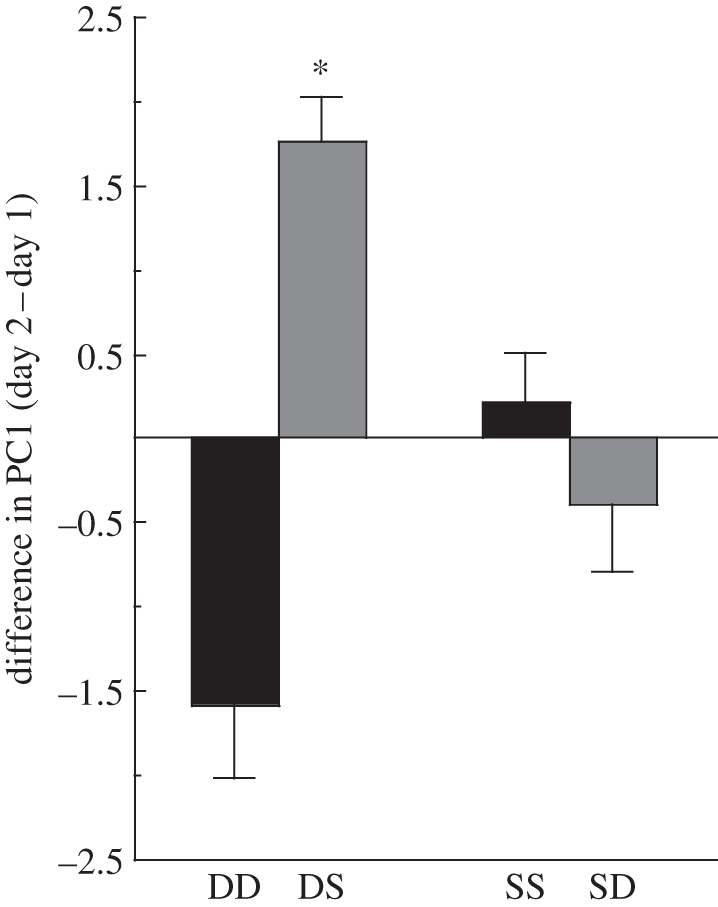
Males with the same fighting history (dominant or subordinate) were paired together to force one male to change their social status. Cuticular hydrocarbon profiles of males were analysed before and after the social challenge. Data represent differences in values of PC1 derived from logcontrasts of cuticular hydrocarbon data. Error bars represent s.e. and n = 12 for each category. Black bars represent males who did not alter their social status: DD, dominant males who remained dominant; SS, subordinate males who remained subordinate; grey bars represent males who did alter their social status: DS, dominant males who became subordinate; SD, subordinate males who became dominant. Asterisks indicate significant p-value between pairs.
It is interesting to note that C33 alkenes and alkadienes made up a higher proportion of a subordinate's total cuticular hydrocarbon profile than a dominant male's (mean ± s.d., C33 alkenes and alkadienes; DD = 0.20 ± 1.14, SS = 1.78 ± 2.08; matched paired t-test, t = 2.46, d.f. = 8, p = 0.039, table 1). Moreover, the relative abundance of both C31 and C33 alkenes and alkadienes increased on day 2 in dominant crickets that became subordinate (mean ± s.d.; C31 alkenes and alkadienes—DD = −2.64 ± 3.93, DS = 8.56 ± 11.03; C33 alkenes and alkadienes—DD = 0.20 ± 1.14, DS = 3.94 ± 4.75). However, this relationship was significant only for C33 alkenes and alkadienes (paired t-test, t = 2.40, d.f. = 8, p = 0.04; table 1) and not for C31 alkenes and alkadienes (paired t-test, t = 2.15, d.f. = 7, p = 0.07).
4. Discussion
Our results support the idea that cuticular hydrocarbons can provide individuals with information as to the current social status of their dominant competitor. We found that following defeat (DS), males will increase the relative proportions of a number of cuticular compounds (notably C33 alkenes and alkadienes) to more closely resemble the hydrocarbon profile of a subordinate, but continue to decrease the relative contribution of these compounds following victory (DD). This is consistent with previous work that has shown dominant males to invest differentially in the relative proportions of these C33 alkenes and alkadienes compared with subordinate males ([8]; see also electronic supplementary material, table S1). Dominant males also invest more in their ejaculates and thereby achieve a greater share of paternity when competing for fertilizations than their subordinate counterparts [8]. Such differential investment by dominant males in reproductive traits may be adaptive, given that female preference for males who win fights has been demonstrated in many species of crickets [25–27], including T. oceanicus [15], although this may not be the case in all field crickets [28].
Sexual selection has recently been shown to play an important role in the evolution of male cuticular hydrocarbons; female mate choice appears to be driving cuticular hydrocarbons to a single most attractive peak [16]. Males who have relatively greater proportions of five of the eight hydrocarbons (peaks 16, 18–21) that were found to differ between dominant and subordinate males require a shorter duration of courtship to persuade females to mount ([16], see electronic supplementary material, table S1 for corresponding peaks). These males also obtain a greater number of successful matings [16]. Here, we found that males increased the relative proportion of three of these ‘attractive’ compounds (peaks 16, 20 and 21) with a change in social status from dominant to subordinate. Taken together, these results suggest that subordinate males invest relatively more in compounds that are attractive to females during mate choice when they are unsuccessful in competition with dominant males. We have suggested elsewhere that because acoustic signals attract aggressive attacks from dominant males [15], subordinate males may adopt the alternative tactics of silently searching for females, relying more heavily on olfactory signalling to induce them to mate [8].
Why do we not see a similar reversal of hydrocarbon profiles in subordinate males that become dominant? Dominant males, but not subordinate, often display phenotypic plasticity in response to an experimental manipulation of social status [29]. This pattern suggests that males of different social competitiveness are either predisposed to specific patterns of fluctuations, or that social experience can strongly influence a male's decision in subsequent interactions. The rapid alteration in hydrocarbon profiles of dominant crickets to a change in social status suggests that cuticular hydrocarbons do not merely signify a generic behavioural process. This is supported by data in Drosophila where the genes that influence female condition directly have been shown to influence male cuticular hydrocarbon expression indirectly [14]. Here also, males respond with a quantitative, rather than a qualitative, change in cuticular hydrocarbon profiles, suggesting that regulation of these phenotypically plastic changes in cuticular hydrocarbon profiles is at the transport and deposition level [11].
The influence of social experience on the outcome of dominance interactions is widely recognized. A recent meta-analysis demonstrated that a previous winning experience doubles the chances of winning a subsequent interaction, whereas a previous losing experience decreases the chance of winning by five times [30]. This asymmetry in winner/loser effects is further confounded by loser effects frequently lasting longer in time than winner effects [30]. The mechanisms underlying the winner and loser effects are still poorly understood, however, changes induced by social experience in brain neuromodulators and hormonal responses to social interactions have been suggested to contribute to this phenomenon [31–33]. Certainly, one of the most studied hormonal responses in relation to social challenge has been testosterone, a hormone that is generally considered to moderate aggression associated with reproduction in vertebrates, including the establishment and maintenance of territories. The ‘challenge hypothesis’ has been proposed as a conceptual framework for the responsiveness of this hormone to social stimuli in vertebrates [34] and several studies have documented such a response in a variety of vertebrate taxa (e.g. mice [32], fish [6,35] and birds [36]).
Recently the challenge hypothesis has been extended to insects [37] and could provide a physiological mechanism underlying the hydrocarbon changes found in this system. Although insects do not have testosterone, there is some evidence to suggest that equivalent hormones in insects can respond to social stimuli in a similar manner as testosterone in vertebrates [37]. For example, juvenile hormone (JH) has been shown as a factor mediating aggression in several insect species [10,38,39] and this hormone is known to induce pheromone production in Blattodea and Coleoptera [40]. More recently, JH has been shown to influence the composition of cuticular hydrocarbons in the ant Myrmicaria eumenoides [41]. Further investigation is required to determine if the changes in hydrocarbon profiles reported in this study are adaptive or simply by-products of physiological mechanisms. But whatever the mechanism, our study demonstrates that cuticular hydrocarbons can provide arthropods with an amazing response in real time to others in the group.
Acknowledgements
We thank Ricarda Fenske and Matthew Timmins from Metabolomics Australia for help with SPME method development and running of the GCMS/SPME. Thanks to Maxine Beveridge for assistance with experiments and animal husbandry. This project was funded by the Australian Research Council, the University of Western Australia and the West Australian Center of Excellence in Science and Innovation Programme.
References
- 1.Edler A. U., Friedl T. W. P. 2010. Plumage colouration, age, testosterone and dominance in male red bishops (Euplectes orix): a laboratory experiment. Ethology 116, 806–820 10.1111/j.1439-0310.2010.01799.x (doi:10.1111/j.1439-0310.2010.01799.x) [DOI] [Google Scholar]
- 2.Galvan I., Sanz J. J. 2009. Cheek plumage uniformity as a social status signal in great tits. Ann. Zool. Fenn. 46, 271–282 [Google Scholar]
- 3.Setchell J. M., Vaglio S., Moggi-Cecchi J., Boscaro F., Calamai L., Knapp L. A. 2010. Chemical composition of scent-gland secretions in an old world monkey (Mandrillus sphinx): influence of sex, male status, and individual identity. Chem. Senses 35, 205–220 10.1093/chemse/bjp105 (doi:10.1093/chemse/bjp105) [DOI] [PubMed] [Google Scholar]
- 4.Shabani S., Kamio M., Derby C. D. 2009. Spiny lobsters use urine-borne olfactory signaling and physical aggressive behaviors to influence social status of conspecifics. J. Exp. Biol. 212, 2464–2474 10.1242/jeb.026492 (doi:10.1242/jeb.026492) [DOI] [PubMed] [Google Scholar]
- 5.Skog M., Chandrapavan A., Hallberg E., Breithaupt T. 2009. Maintenance of dominance is mediated by urinary chemical signals in male European lobsters, Homarus gammarus. Mar. Freshw. Behav. Physiol. 42, 119–133 10.1080/10236240902833729 (doi:10.1080/10236240902833729) [DOI] [Google Scholar]
- 6.Oliveira R. F., Silva A., Canario A. V. M. 2009. Why do winners keep winning? Androgen mediation of winner but not loser effects in cichlid fish. Proc. R. Soc. B 276, 2249–2256 10.1098/rspb.2009.0132 (doi:10.1098/rspb.2009.0132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kou R., Chang H. W., Chen S. C., Ho H. Y. 2009. Suppression pheromone and cockroach rank formation. Naturwissenschaften 96, 691–701 10.1007/s00114-009-0522-0 (doi:10.1007/s00114-009-0522-0) [DOI] [PubMed] [Google Scholar]
- 8.Thomas M. L., Simmons L. W. 2009. Male dominance influences pheromone expression, ejaculate quality, and fertilization success in the Australian field cricket, Teleogryllus oceanicus. Behav. Ecol. 20, 1118–1124 10.1093/beheco/arp105 (doi:10.1093/beheco/arp105) [DOI] [Google Scholar]
- 9.Maynard-Smith J. M., Harper D. 2003. Animal signals. Oxford, UK: Oxford University Press [Google Scholar]
- 10.Kou R., Chou S. Y., Chen S. C., Huang Z. Y. 2009. Juvenile hormone and the ontogeny of cockroach aggression. Horm. Behav. 56, 332–338 10.1016/j.yhbeh.2009.06.011 (doi:10.1016/j.yhbeh.2009.06.011) [DOI] [PubMed] [Google Scholar]
- 11.Blomquist G. J., Bagnères A. G. 2010. Insect hydrocarbons: biology, biochemistry, and chemical ecology. Cambridge, UK: Cambridge University Press [Google Scholar]
- 12.Thomas M. L. 2010. Detection of female mating status using chemical signals and cues. Biol. Rev. 86, 1–13 10.1111/j.1469-185X.2010.00130.x (doi:10.1111/j.1469-185X.2010.00130.x) [DOI] [PubMed] [Google Scholar]
- 13.Kent C., Azanchi R., Smith B., Formosa A., Levine I. D. 2008. Social context influences chemical communication in D. melanogaster males. Curr. Biol. 18, 1384–1389 10.1016/j.cub.2008.07.088 (doi:10.1016/j.cub.2008.07.088) [DOI] [PubMed] [Google Scholar]
- 14.Petfield D., Chenoweth S. F., Rundle H. D., Blows M. W. 2005. Genetic variance in female condition predicts indirect genetic variance in male sexual display traits. Proc. Natl Acad. Sci. USA 102, 6045–6050 10.1073/pnas.0409378102 (doi:10.1073/pnas.0409378102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burk T. 1983. Male aggression and female choice in a field cricket (Teleogryllus oceanicus): the importance of courtship song. In Orthopteran mating systems: sexual competition in a diverse group of insects (eds Gwynne D. T., Morris G. K.), pp. 97–119 CO: Westview Press [Google Scholar]
- 16.Thomas M. L., Simmons L. W. 2009. Sexual selection on cuticular hydrocarbons in the Australian field cricket, Teleogryllus oceanicus. BMC Evol. Biol. 9, 162–173 10.1186/1471-2148-9-162 (doi:10.1186/1471-2148-9-162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savage K. E., Hunt J., Jennions M. D., Brooks R. 2005. Male attractiveness covaries with fighting ability but not with prior fight outcome in house crickets. Behav. Ecol. 16, 196–200 10.1093/beheco/arh143 (doi:10.1093/beheco/arh143) [DOI] [Google Scholar]
- 18.Shackleton M. A., Jennions M. D., Hunt J. 2005. Fighting success and attractiveness as predictors of male mating success in the black field cricket, Teleogryllus commodus: the effectiveness of no-choice tests. Behav. Ecol. Sociobiol. 58, 1–8 10.1007/s00265-004-0907-1 (doi:10.1007/s00265-004-0907-1) [DOI] [Google Scholar]
- 19.Logue D. M., Abiola I. O., Rains D., Bailey N. W., Zuk M., Cade W. H. 2010. Does signalling mitigate the cost of agonistic interactions? A test in a cricket that has lost its song. Proc. R. Soc. B 277, 2571–2575 10.1098/rspb.2010.0421 (doi:10.1098/rspb.2010.0421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musteata F. M., Pawliszyn J. 2007. In vivo sampling with solid phase microextraction. J. Biochem. Biophys. Methods 70, 181–193 10.1016/j.jbbm.2006.07.006 (doi:10.1016/j.jbbm.2006.07.006) [DOI] [PubMed] [Google Scholar]
- 21.Everaerts C., Farine J.-P., Cobb M., Ferveur F. 2010. Drosophila cuticular hydrocarbons revisited: mating status alters cuticular profiles. PLoS ONE 5, e9607. 10.1371/journal.pone.0009607 (doi:10.1371/journal.pone.0009607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kent C., Azanchi R., Smith B., Chu A., Levine J. 2007. A model-based analysis of chemical and temporal patterns of cuticular hydrocarbons in male Drosophila melanogaster. PLoS ONE 2, e962. 10.1371/journal.pone.0000962 (doi:10.1371/journal.pone.0000962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas M. L., Simmons L. W. 2008. Sexual dimorphism in cuticular hydrocarbons of the Australian field cricket Teleogryllus oceanicus (Orthoptera: Gryllidae). J. Insect Physiol. 54, 1081–1089 10.1016/j.jinsphys.2008.04.012 (doi:10.1016/j.jinsphys.2008.04.012) [DOI] [PubMed] [Google Scholar]
- 24.Norman G. R., Streiner D. L. 1994. Biostatistics: the bare essentials. St Louis, MO: Mosby [Google Scholar]
- 25.Rantala M. J., Kortet R. 2004. Male dominance and immunocompetence in a field cricket. Behav. Ecol. 15, 187–191 10.1093/beheco/arg103 (doi:10.1093/beheco/arg103) [DOI] [Google Scholar]
- 26.Simmons L. W. 1986. Intermale competition and mating success in the field cricket, Gryllus bimaculatus (De Geer). Anim. Behav. 34, 567–579 10.1016/S0003-3472(86)80126-9 (doi:10.1016/S0003-3472(86)80126-9) [DOI] [Google Scholar]
- 27.Wedell N., Tregenza T. 1999. Successful fathers sire successful sons. Evolution 53, 620–625 10.2307/2640798 (doi:10.2307/2640798) [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez-Muñoz R., Bretman A., Slate J., Walling C. A., Tregenza T. 2010. Natural and sexual selection in a wild insect population. Science 328, 1269–1272 10.1126/science.1188102 (doi:10.1126/science.1188102) [DOI] [PubMed] [Google Scholar]
- 29.Pizzari T., Cornwallis C. K., Froman D. P. 2007. Social competitiveness associated with rapid fluctuations in sperm quality in male fowl. Proc. R. Soc. B 274, 853–860 10.1098/rspb.2006.0080 (doi:10.1098/rspb.2006.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rutte C., Taborsky M., Brinkhof M. W. G. 2006. What sets the odds of winning and losing? Trends Ecol. Evol. 21, 16–21 10.1016/j.tree.2005.10.014 (doi:10.1016/j.tree.2005.10.014) [DOI] [PubMed] [Google Scholar]
- 31.Huber R., Delago A. 1998. Serotonin alters decisions to withdraw in fighting crayfish, Astacus astacus: the motivational concept revisited. J. Comp. Physiol. A 182,573–583 10.1007/s003590050204 (doi:10.1007/s003590050204) [DOI] [Google Scholar]
- 32.Oyegbile T. O., Marler C. A. 2005. Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Horm. Behav. 48, 259–267 10.1016/j.yhbeh.2005.04.007 (doi:10.1016/j.yhbeh.2005.04.007) [DOI] [PubMed] [Google Scholar]
- 33.Winberg S., Nilsson G. E. 1993. Roles of brain monoamine neurotransmitters in agonistic behaviour and stress reactions, with articular reference to fish. Comp. Biochem. Physiol. 106C, 597–614 [Google Scholar]
- 34.Wingfield J. C., Hegner R. E., Dufty A. M., Ball G. F. 1990. The challenge hypothesis: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136, 829–846 10.1086/285134 (doi:10.1086/285134) [DOI] [Google Scholar]
- 35.Clotfelter E. D., Paolino A. D. 2003. Bystanders to contest between conspecifics are primed for increased aggression in male fighting fish. Anim. Behav. 66, 343–347 10.1006/anbe.2003.2227 (doi:10.1006/anbe.2003.2227) [DOI] [Google Scholar]
- 36.Wingfield J. C., Hahn T. P. 1994. Testosterone and territorial behaviour in sedentary and migratory sparrows. Anim. Behav. 47, 77–89 10.1006/anbe.1994.1009 (doi:10.1006/anbe.1994.1009) [DOI] [Google Scholar]
- 37.Scott M. P. 2006. Resource defense and juvenile hormone: the ‘challenge hypothesis’ extended to insects. Horm. Behav. 49, 276–281 10.1016/j.yhbeh.2005.07.003 (doi:10.1016/j.yhbeh.2005.07.003) [DOI] [PubMed] [Google Scholar]
- 38.Choe S.-Y., Huang Z. Y., Chen S.-C., Yang R.-L., Kou R. 2007. Antenna contact and agonism in the male lobster cockroach Nauphoeta cinerea. Horm. Behav. 52, 252–260 10.1016/j.yhbeh.2007.04.013 (doi:10.1016/j.yhbeh.2007.04.013) [DOI] [PubMed] [Google Scholar]
- 39.Scott M. P. 2006. The role of juvenile hormone in competition and cooperation by burying beetles. J. Insect Physiol. 52, 1005–1011 10.1016/j.jinsphys.2006.04.006 (doi:10.1016/j.jinsphys.2006.04.006) [DOI] [PubMed] [Google Scholar]
- 40.Tillman J. A., Seybold S. J., Jurenka R. A., Blomquist G. J. 1999. Insect pheromones: an overview of biosynthesis and endocrine regulation. Insect Biochem. Mol. Biol. 29, 481–514 10.1016/S0965-1748(99)00016-8 (doi:10.1016/S0965-1748(99)00016-8) [DOI] [PubMed] [Google Scholar]
- 41.Lengyel F., Westerlund S., Kaib M. 2007. Juvenile hormone III influences task-specific cuticular hydrocarbon profile changes in the ant Myrmicaria eumenoides. J. Chem. Ecol. 33, 167–181 10.1007/s10886-006-9185-x (doi:10.1007/s10886-006-9185-x) [DOI] [PubMed] [Google Scholar]


