Abstract
The hypothesis that sperm competition should favour increases in sperm size, because it results in faster swimming speeds, has received support from studies on many taxa, but remains contentious for mammals. We suggest that this may be because mammalian lineages respond differently to sexual selection, owing to major differences in body size, which are associated with differences in mass-specific metabolic rate. Recent evidence suggests that cellular metabolic rate also scales with body size, so that small mammals have cells that process energy and resources from the environment at a faster rate. We develop the ‘metabolic rate constraint hypothesis’ which proposes that low mass-specific metabolic rate among large mammals may limit their ability to respond to sexual selection by increasing sperm size, while this constraint does not exist among small mammals. Here we show that among rodents, which have high mass-specific metabolic rates, sperm size increases under sperm competition, reaching the longest sperm sizes found in eutherian mammals. By contrast, mammalian lineages with large body sizes have small sperm, and while metabolic rate (corrected for body size) influences sperm size, sperm competition levels do not. When all eutherian mammals are analysed jointly, our results suggest that as mass-specific metabolic rate increases, so does maximum sperm size. In addition, species with low mass-specific metabolic rates produce uniformly small sperm, while species with high mass-specific metabolic rates produce a wide range of sperm sizes. These findings support the hypothesis that mass-specific metabolic rates determine the budget available for sperm production: at high levels, sperm size increases in response to sexual selection, while low levels constrain the ability to respond to sexual selection by increasing sperm size. Thus, adaptive and costly traits, such as sperm size, may only evolve under sexual selection when metabolic rate does not constrain cellular budgets.
Keywords: sexual selection, sperm competition, metabolic rate, spermatozoa, evolutionary rates, mammals
1. Introduction
Spermatozoa are under strong selective pressures because male reproductive success ultimately relies on their fertilization ability. When females are promiscuous, sperm from rival males compete to fertilize the ova, a process known as sperm competition, which leads to increased sperm numbers [1]. Most theoretical models assume that a constant amount of resources is available to produce an ejaculate, and that the size of gametes is directly proportional to the resources invested in each [2,3]. Thus, ejaculate expenditure is assumed to be the product of the number of sperm and the size of each male gamete, which are expected to trade-off against each other so that the total budget remains invariant. Such models conclude that, under most scenarios, sperm competition should favour an increase in sperm numbers at the expense of a reduction in size [2,3].
An alternative hypothesis proposes that both sperm numbers and size should increase under sperm competition, because an increase in size results in faster swimming speed, which maximizes the chances of winning the race to fertilize the ova [4,5]. Support from empirical studies has grown over the years, with most studies reporting a positive relationship between sperm competition, sperm size and swimming velocity [5–8], although a few negative relationships between sperm size and sperm competition levels have also been found [9]. The debate remains contentious in mammals owing to major inconsistencies between studies [4,10–13] (reviewed in [5]). It is possible that these conflicting results reflect differences between mammalian lineages in the budget available for sperm production and, therefore, in their ability to respond to sexual selection by increasing sperm size.
Mammals are unique in that they show a huge range of variation in body size, with some species achieving the largest sizes of any living animal on Earth. Thus, any size-dependent constraints are likely to be particularly pronounced among mammals. Smaller mammals such as rodents have higher mass-specific metabolic rates and are able to produce more biomass per unit of body mass [14], allowing them to follow a ‘live fast and die young’ strategy [15,16], and to have faster evolutionary rates [17–20]. Recent evidence has revealed unforeseen links between whole-organism size and cellular metabolic rate [21]. The underlying reason is that the properties of cells cannot remain invariant as body size increases, because of the associated scaling of whole-organism metabolic rate, meaning that either cellular metabolic rate or cell size must vary with body size. Different cell types follow different strategies in response to changes in body size depending on their structure and function. In particular, fast-dividing cells follow a strategy in which cellular metabolic rate is body-size dependent [21]. Thus, among small mammals, these cell types have higher cellular metabolic rates and are therefore capable of processing energy and resources at a faster rate than large mammals. This is likely to be the case of spermatogenic cells.
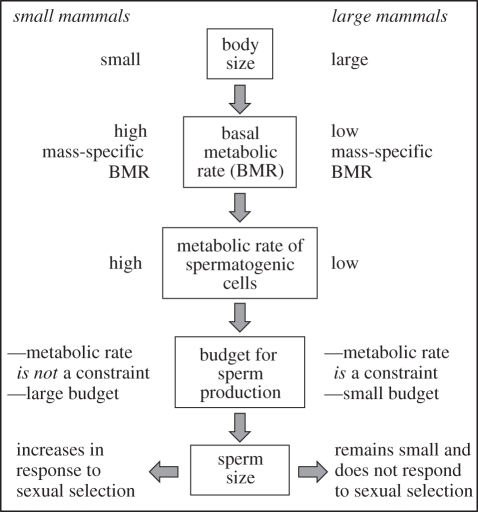
Here we propose and test the ‘metabolic rate constraint hypothesis’ (figure 1) which states that body-size-dependent differences between mammalian lineages in mass-specific and cellular metabolic rates will determine the budget available for spermatogenic cells to respond to sperm competition by increasing sperm size. Among small mammals, high mass-specific metabolic rates will be associated with high cellular budgets, allowing spermatogenic cells to respond to increased levels of sperm competition by increasing sperm size. By contrast, large mammals with low mass-specific metabolic rates will have less metabolically efficient spermatogenic cells, which could explain the observation made some time ago that large mammals do not produce long sperm [22]. Under limited budgets, spermatogenic cells will not be able to increase sperm size as sexual selection intensifies.
Figure 1.
The metabolic rate constraint hypothesis. Metabolic rate scales nonlinearly with body mass, so small mammals (such as rodents) have high mass-specific metabolic rates. Body size also influences cellular metabolic rate. Mammals with small body size have high mass-specific metabolic rates and presumably more metabolically efficient germ cells. As sexual selection intensifies, resources are turned into sperm rapidly enough to increase sperm size. By contrast, large mammals are metabolically less efficient. As a consequence, sperm size does not increase when sexual selection intensifies.
2. Material and methods
Data on body mass (g), testes mass (g) and total sperm length (µm) were obtained from the literature for 215 species of eutherian mammals (see electronic supplementary material, table S1). We were able to obtain data on the length (µm) of sperm components (head, midpiece, principal piece and total flagellum) for 190 of these species (see electronic supplementary material, table S1). Data on basal metabolic rate (mlO2 h−1) were obtained for a subset of 77 species (see electronic supplementary material, table S1). We tested the influence of sperm competition on sperm dimensions using multiple regression analyses with total sperm length, as well as the length of different sperm components, as dependent variables, and both log10-transformed testes mass and body mass as predictors. We also tested the association between sperm size and mass-specific metabolic rate, modifying the previous model to include basal metabolic rate as an additional predictor.
To test the effect of mass-specific metabolic rate as a constraint on the maximum size that sperm can achieve, and therefore on the degree of variability between species of similar mass-specific metabolic rate, we classified our dataset into six consecutive categories of mass-specific metabolic rates (mlO2 h−1 g−1), each of which had an equal range. We then performed linear regression analyses using, for each category of mass-specific metabolic rate, the mean mass-specific metabolic rate of the corresponding category as an independent variable. The dependent variables were the maximum value of sperm length for each category, as well as the range between minimum and maximum sperm length values. The same analyses were carried out for the length of each sperm component.
To control for phylogenetic effects in the dataset we used a generalized least-squares (GLS) approach in a phylogenetic framework. This method estimates a phylogenetic scaling parameter lambda (λ), which represents the transformation that makes the data fit the Brownian motion evolutionary model. Owing to the unavailability of a complete phylogeny for all species analysed, a phylogenetic reconstruction was used (see electronic supplementary material, figure S1). All statistical analyses were conducted with R v. 2.8.1 (R Foundation for Statistical Computing 2009).
3. Results
(a). Differences between mammalian lineages in body size, mass-specific metabolic rate, sperm size and levels of sperm competition
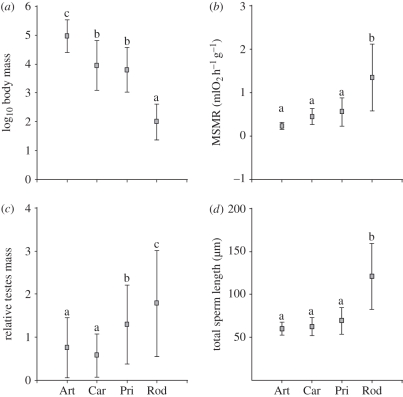
We first compared different mammalian lineages in terms of body size, mass-specific metabolic rate, relative testes mass—a reliable index of sperm competition levels [23]—and sperm dimensions. We analysed four orders for which data were available for a sufficient number of species (see electronic supplementary material, table S1). Rodents had small body sizes, while artiodactyls, carnivores and primates had intermediate to high values (figure 2a and electronic supplementary material, table S2). Associated with these differences in body size there were opposite patterns between lineages in mass-specific metabolic rate: high in rodents, with a large degree of variation, and low in artiodactyls, carnivores and primates (figure 2b). Relative testes mass was higher among rodents, which also showed the greatest range, intermediate in primates and low in artiodactyls and carnivores (figure 2c and electronic supplementary material, table S2). Thus, some rodent species had relative testes mass that was two to three times the maximum values found in artiodactyls and carnivores, respectively. The most dramatic differences between rodents and other taxa were in terms of sperm size. The comparative analyses revealed that rodents had the largest range of sperm sizes, with both the minimum and maximum sperm sizes found in our study sample. In addition, the longest sperm size among rodents was around three times the maximum size for the other three orders. By contrast, Artiodactyla, Carnivora and Primates had short sperm and little variation between species (figure 2d and electronic supplementary material, table S2).
Figure 2.
Variation in body mass, mass-specific metabolic rate, relative testes mass and total sperm length in four orders of mammals. Squares represent mean values. Error bars are standard deviations. (a) Log10 body mass, ANOVA p < 0.0001, F = 259.74; (b) mass-specific metabolic rate (MSMR), ANOVA p < 0.0001, F = 15.19; (c) relative testes mass, ANOVA p < 0.0001, F = 15.92; (d) total sperm length, ANOVA p < 0.0001, F = 73.01. Different letters indicate significant differences (α < 0.05) between orders in a post hoc LSD test. Abbreviations: Art, Artiodactyla; Car, Carnivora; Pri, Primates; Rod, Rodentia.
(b). Relationship between sperm size and sperm competition in different mammalian lineages
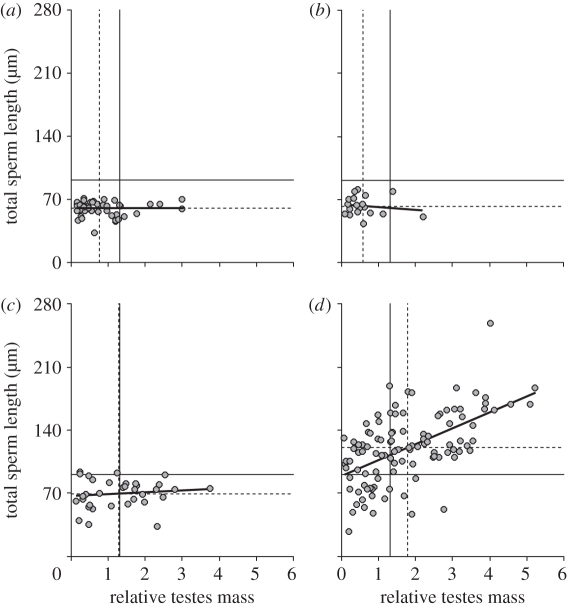
Next we analysed whether sperm competition influences sperm dimensions in all mammalian orders. Using phylogenetically controlled (see electronic supplementary material, figure S1) multiple regression analyses, we found that testes mass after correcting for body mass (thereafter, relative testes mass) was related to sperm size in rodents but not in the other orders (table 1). When each order was compared with the average values for mammals (figure 3), we found that among Artiodactyla and Carnivora, the average relative testes mass was lower than for the whole sample of mammals, and the same was true for total sperm length (figure 3a,b). Among Primates, the average relative testes mass was similar to that of the whole mammalian sample, but the average sperm length was lower (figure 3c). Finally, rodents had larger average relative testes mass and total sperm length than the whole sample of mammals (figure 3d). More importantly, at intermediate values of relative testes mass, artiodactyls, carnivores and primates show no associated increases in sperm size, while among rodents, sperm size already increases at these levels of sperm competition and continues to do so as they increase even further.
Table 1.
Sperm length in relation to body mass and testes mass in four mammalian orders. Phylogenetically controlled multiple regressions. The superscripts following the λ value indicate significance levels (n.s. = p > 0.05; *p < 0.05) in a likelihood ratio test against models with λ = 0 (first position) and λ = 1 (second position). The effect size r calculated from the F values and its non-central 95% confidence limits (CLs) are presented. Confidence intervals excluding 0 indicate statistically significant relationships. The p-values and CL that indicate statistical significance are shown in bold.
| taxon | dependent variable | predictor | slope | F | p | λ | r | CLs | n |
|---|---|---|---|---|---|---|---|---|---|
| Artiodactyla | total sperm length | body mass | −3.08 | 3.38 | 0.0722 | 0.9013*,n.s. | 0.25 | −0.02 to 0.54 | 52 |
| testes mass | −0.51 | 0.04 | 0.8499 | 0.03 | −0.25 to 0.31 | ||||
| Carnivora | total sperm length | body mass | −7.95 | 6.98 | 0.0177 | 0.0001n.s.,* | 0.55 | 0.13 to 1.11 | 19 |
| testes mass | 2.61 | 0.19 | 0.6724 | 0.11 | −0.38 to 0.60 | ||||
| Primates | total sperm length | body mass | −4.29 | 1.24 | 0.273 | <0.0001n.s.,* | 0.20 | −0.15 to 0.55 | 34 |
| testes mass | 1.29 | 0.04 | 0.853 | 0.03 | −0.32 to 0.39 | ||||
| Rodentia | total sperm length | body mass | −13.97 | 0.25 | 0.6163 | 0.9999*,n.s. | 0.05 | −0.14 to 0.24 | 106 |
| testes mass | 23.91 | 9.01 | 0.0034 | 0.28 | 0.10 to 0.48 |
Figure 3.
Relations between total sperm length (µm) and relative testes mass in four orders of eutherian mammals. (a) Artiodactyla (n = 52); (b) Carnivora (n = 19); (c) Primates (n = 34); (d) Rodentia (n = 106). Solid lines indicate the mean values in total sperm length and relative testes mass for the complete dataset, and dotted lines indicate the mean values for these two variables in each group.
These findings show that, among rodents, increased levels of sperm competition are associated with increases in sperm size, while this is not the case among the other mammalian lineages. Furthermore, when the different sperm components (i.e. head, midpiece, principal piece and flagellum length) are analysed separately, levels of sperm competition are associated with increases in all sperm components in rodents, but not in the other mammalian lineages (see electronic supplementary material, table S3).
(c). Testing the metabolic rate constraint hypothesis
According to the metabolic rate constraint hypothesis, the differences between rodents and the other mammalian lineages may have to do with their most striking feature: their small body size. We suggest that rodents are able to respond to sexual selection because their higher mass-specific metabolic rates allow them to invest enough resources to produce longer sperm, while other mammalian taxa are constrained by the low mass-specific metabolic rates associated with large body size. To test our hypothesis, we analysed jointly the effects of body mass, metabolic rate and testes mass upon sperm size on a sample of mammals for which data on all traits was available. Since artiodactyls, carnivores and primates showed no major differences in any of the traits examined, we analysed these orders together as ‘non-rodents’ to increase sample size. As predicted, among rodents relative testes mass had a positive effect upon sperm size, while body size and metabolic rate did not have any effects (table 2). By contrast, among non-rodents, after controlling for body size, metabolic rate had a negative effect upon sperm size while testes size did not have a significant effect (table 2). We conclude that among non-rodents, species metabolic rate (corrected for body size) constrains their ability to process enough resources to produce long sperm as sperm competition increases.
Table 2.
Sperm length in relation to body mass, metabolic rate and testes mass. Phylogenetically controlled multiple regressions. The superscripts following the λ value indicate significance levels (n.s. = p > 0.05; *p < 0.05) in a likelihood ratio test against models with λ = 0 (first position) and λ = 1 (second position). The effect size r calculated from the F values and its non-central 95% confidence limits (CLs) are presented. Confidence intervals excluding 0 indicate statistically significant relationships. The p-values and CL that indicate statistical significance are shown in bold. Abbreviations: BMR, basal metabolic rate (mlO2 h−1).
| taxon | dependent variable | predictor | slope | F | p | λ | r | CLs | n |
|---|---|---|---|---|---|---|---|---|---|
| Rodents | total sperm length | body mass | −11.02 | 0.09 | 0.7641 | 0.9999*,n.s. | 0.05 | −0.28 to 0.38 | 39 |
| BMR | −21.37 | 0.40 | 0.4679 | 0.11 | −0.22 to 0.43 | ||||
| testes mass | 35.42 | 6.44 | 0.0158 | 0.39 | 0.09 to 0.74 | ||||
| Non Rodents | total sperm length | body mass | 12.60 | 1.11 | 0.2985 | 0.9999n.s.,n.s. | 0.18 | −0.15 to 0.51 | 38 |
| BMR | −13.74 | 7.44 | 0.0100 | 0.42 | 0.12 to 0.78 | ||||
| testes mass | −5.13 | 1.64 | 0.2084 | 0.21 | −0.11 to 0.55 |
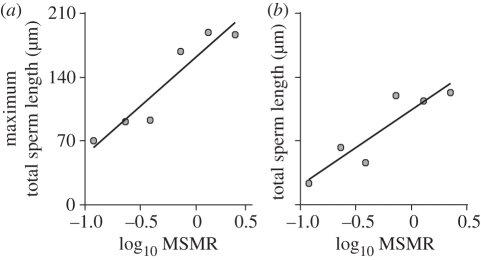
Since rodents and non-rodents differ in many life-history traits, and this single comparison does not allow us to disentangle the effects of higher levels of sperm competition and higher mass-specific metabolic rates among rodents, we classified the whole dataset of eutherian mammals in groups of species according to their mass-specific metabolic rates. This allowed us to increase our sample size from two (rodents versus non-rodents) to six categories of mass-specific metabolic rates. The metabolic rate constraint hypothesis predicts that species with low levels of mass-specific metabolic rates will be under a major constraint and will produce uniformly small sperm. By contrast, species with high levels of mass-specific metabolic rates will not suffer any constraints, so there will be a much larger degree of variation: species with low levels of sperm competition will produce small sperm, while those with high levels of sperm competition will produce large sperm. A simple distribution of data for all species considered clearly shows that as mass-specific metabolic rate increases, there is an increase in the range of sperm sizes produced (see electronic supplementary material, figure S2). Further analyses support these predictions since there is a very strong positive relationship between levels of mass-specific metabolic rate and maximum sperm length (figure 4a; GLS: p = 0.0075, R2 = 0.83). Furthermore, as mass-specific metabolic rate increases, so does the range of sperm sizes (figure 4b; GLS: p = 0.0113, R2 = 0.79). Interestingly, the effect of mass-specific metabolic rate upon both the maximum size and the range of sizes is strong for all sperm components (see electronic supplementary material, figures S3–S4 and table S4). The six categories of mass-specific metabolic rates do not merely re-classify our sample size into several categories of rodents and several non-rodents since all of the categories contain a mixture of lineages, except for the category that corresponds to the lowest values of mass-specific metabolic rate, which does not contain rodents, and the category that corresponds to the highest values of mass-specific metabolic rate, which only contains rodents (see electronic supplementary material, figure S5). When the category that contains only rodents is removed from the analyses, the relationships between mass-specific metabolic rate upon maximum sperm size (GLS: p = 0.0108, R2 = 0.89) and the range of sperm sizes (GLS: p = 0.0366, R2 = 0.75) remain significant. However, when all rodents are excluded from the analyses, the relationships are no longer significant, probably because the proportional representation of rodents in each category increases as mass-specific metabolic rate increases.
Figure 4.
Relations between sperm dimensions and mass-specific metabolic rate (MSMR) in eutherian mammals. (a) Maximum total sperm length; (b) total sperm length range. Each point represents one of six MSMR categories. MSMR values are log10 transformed.
4. Discussion
Our findings show that while rodents respond to increasing levels of sperm competition by increasing sperm size, this is not the case among mammalian lineages with larger body sizes. As a result, some rodents have the longest sperm sizes found among eutherian mammals, while large mammals tend to have small sperm. In addition, among rodents all sperm components (head, midpiece, principal piece and flagellum) increase in length as levels of sperm competition increase, suggesting that all of them contribute jointly to enhance sperm competitive ability. By contrast, artiodactyls, carnivores and primates show no association between sperm length and levels of sperm competition, nor do they respond by increasing the dimensions of any particular sperm component. As a result, these lineages produce sperm that are smaller in size and show less variation between species. These findings suggest that contradictory results between previous studies on mammals may simply reflect a different representation of mammalian lineages in the study sample. Thus, comparative studies will be more likely to find an effect of sperm competition on sperm size if they include a large proportion of rodents than if they do not.
Our analyses suggest that such major differences in the way mammalian lineages respond to sexual selection are due to major differences in mass-specific metabolic rates between small (rodents) and large mammals. Thus, among large mammals (with low mass-specific metabolic rates), sperm size is influenced by metabolic rate (corrected for body mass) but not by levels of sperm competition. By contrast, as a result of the high mass-specific metabolic rates that rodents have, sperm size is not constrained by metabolic rate (corrected for body size) and increases as sperm competition levels intensify.
When we analyse all eutherian mammals jointly, we are able to show that as mass-specific metabolic rate increases so does the maximum size that sperm achieve. Furthermore, species with low mass-specific metabolic rates produce small sperm of similar size, while species with high mass-specific metabolic rates show major differences in sperm size, presumably because they have the capacity of increasing sperm size in response to more intense sexual selection, but sperm remain small among species with no sperm competition. When all rodents are excluded from the analyses, the relationships between mass-specific metabolic rate and maximum sperm size, as well as the range of sperm sizes, are no longer significant, suggesting that the relationship is largely driven by rodents, which tend to have higher mass-specific metabolic rates than other mammalian lineages. These findings suggest that mass-specific metabolic rate acts as a constraint on sperm size, which implies that sperm size is a costly trait.
The available evidence suggests that sperm size and numbers are costly in terms of both resources and time. The high energetic costs associated with sperm production [24,25] result in limited sperm supplies, leading to substantial declines in the number of sperm per ejaculate in successive copulations [26,27]. In natural populations, limited sperm availability constrains male reproductive success [28,29]. The level of resources required for sperm production are such that they are traded-off against body growth [30], immunity [31,32], lifespan [33], the size of other metabolically expensive organs such as the brain [34] and the size of sexual characters [35]. Increases in sperm size are costly too in terms of resources and also result in evolutionary trade-offs [36–38]. In addition, producing longer sperm takes more time, so that spermatogenesis takes longer in species with longer sperm [39]. Given that sperm competition selects both for increased sperm numbers and size, spermatogenic cells are under the conflicting demands of producing more sperm per unit time (shorter spermatogenic cycle) and longer sperm (longer spermatogenic cycle). Thus, species with high levels of sperm competition tend to have shorter spermatogenic cycles, but this relationship is only apparent after controlling for a positive relationship between the duration of spermatogenesis and sperm length [39].
The key issue is whether spermatogenic cells have the capacity to process resources efficiently enough to increase simultaneously the size of each sperm cell and the rate at which they produce sperm per unit time. There is some preliminary evidence suggesting that mass-specific metabolic rate constrains the rate of spermatogenesis, since species with low mass-specific metabolic rates take longer to produce sperm [40]. Our findings suggest that mass-specific metabolic rate, through its presumed effects on the metabolic rate of spermatogenic cells, leads to major differences between mammalian species in their ability to process resources efficiently enough to respond to sexual selection by increasing both sperm size and numbers (figure 1). Our empirical results therefore challenge one of the fundamental assumptions of theoretical models: that the budget available for sperm production is constant [2,3]. Among small body-sized mammals with high mass-specific metabolic rates, germ cells will have the capacity to process the resources available fast enough to produce more and longer sperm when these traits are advantageous under sperm competition. This could explain why, among eutherian mammals, rodents produce not only the longest, but also the most morphologically complex sperm [41]. By contrast, low cellular metabolic rates among large mammals limit the turnover of resources into sperm and these species are unable to respond to increased levels of sperm competition by increasing sperm size. Because some mammalian species have large body sizes compared with other taxa, it is possible that the effect of metabolic rate acting as a constraint on the rate at which resources can be turned into sperm production is particularly pronounced in this group. We conclude that differences in mass-specific metabolic rates, associated with body mass, may explain why lineages differ in the ability to respond to selective forces at the cellular level.
Acknowledgements
We are grateful to Becky Colley for her preliminary work compiling data from the literature and to Rob Asher for his advice on mammalian phylogenies. M.T. enjoyed a Boehringer Ingelheim Travel Grant and a postdoctoral fellowship of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina. M.T. is currently a postdoctoral researcher funded by the Programa Nacional de Movilidad de Recursos Humanos de Investigación of the Spanish Ministry of Education. This research was supported by the Spanish Ministry of Science and Innovation.
References
- 1.Parker G. A. 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 525–567 10.1111/j.1469-185X.1970.tb01176.x (doi:10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 2.Parker G. A. 1993. Sperm competition games: sperm size and sperm number under adult control. Proc. R. Soc. Lond. B 253, 245–254 10.1098/rspb.1993.0110 (doi:10.1098/rspb.1993.0110) [DOI] [PubMed] [Google Scholar]
- 3.Parker G. A. 1998. Sperm competition and the evolution of ejaculates: towards a theory base. In Sperm competition and sexual selection (eds Birkhead T. R., Møller A. P.), pp. 3–54 San Diego, CA: Academic Press [Google Scholar]
- 4.Gomendio M., Roldan E. R. S. 1991. Sperm competition influences sperm size in mammals. Proc. R. Soc. Lond. B 243, 181–185 10.1098/rspb.1991.0029 (doi:10.1098/rspb.1991.0029) [DOI] [PubMed] [Google Scholar]
- 5.Gomendio M., Roldan E. R. S. 2008. Implications of diversity in sperm size and function for sperm competition and fertility. Int. J. Dev. Biol. 52, 439–447 10.1387/ijdb.082595mg (doi:10.1387/ijdb.082595mg) [DOI] [PubMed] [Google Scholar]
- 6.Fitzpatrick J. L., Montgomerie R., Desjardins J. K., Stiver K. A., Kolm N., Balshine S. 2009. Female promiscuity promotes the evolution of faster sperm in cichlid fishes. Proc. Natl Acad. Sci. USA 106, 1128–1132 10.1073/pnas.0809990106 (doi:10.1073/pnas.0809990106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lüpold S., Calhim S., Immler S., Birkhead T. R. 2009. Sperm morphology and sperm velocity in passerine birds. Proc. R. Soc. B 276, 1175–1181 10.1098/rspb.2008.1645 (doi:10.1098/rspb.2008.1645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tourmente M., Gomendio M., Roldan E. R. S., Giojalas L., Chiaraviglio M. 2009. Sperm competition and reproductive mode influence sperm dimensions and structure among snakes. Evolution 63, 2513–2524 10.1111/j.1558-5646.2009.00739.x (doi:10.1111/j.1558-5646.2009.00739.x) [DOI] [PubMed] [Google Scholar]
- 9.Immler S., Birkhead T. R. 2007. Sperm competition and sperm midpiece size: no consistent pattern in passerine birds. Proc. R. Soc. B 274, 561–568 10.1098/rspb.2006.3752 (doi:10.1098/rspb.2006.3752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosken D. J. 1997. Sperm competition in bats. Proc. R. Soc. Lond. B 264, 385–392 10.1098/rspb.1997.0055 (doi:10.1098/rspb.1997.0055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson M. J., Dixson A. F. 2002. Sperm competition: motility and the midpiece in primates. Nature 416, 496. 10.1038/416496a (doi:10.1038/416496a) [DOI] [PubMed] [Google Scholar]
- 12.Gage M. J. G., Freckleton R. 2003. Relative testis size and sperm morphometry across mammals: no evidence for an association between sperm competition and sperm length. Proc. R. Soc. Lond. B 270, 625–632 10.1098/rspb.2002.2258 (doi:10.1098/rspb.2002.2258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson M. J., Nyholt J., Dixson A. F. 2005. Sperm competition and the evolution of sperm midpiece volume in mammals. J. Zool. 267, 135–145 10.1017/S0952836905007284 (doi:10.1017/S0952836905007284) [DOI] [Google Scholar]
- 14.Clarke A., Rothery P., Isaac N. J. B. 2010. Scaling of basal metabolic rate with body mass and temperature in mammals. J. Anim. Ecol. 79, 610–619 10.1111/j.1365-2656.2010.01672.x (doi:10.1111/j.1365-2656.2010.01672.x) [DOI] [PubMed] [Google Scholar]
- 15.Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 10.1890/03-9000 (doi:10.1890/03-9000) [DOI] [Google Scholar]
- 16.Sibly R. M., Brown J. H. 2007. Effects of body size and lifestyle on evolution of mammal life histories. Proc. Natl Acad. Sci. USA 104, 17 707–17 712 10.1073/pnas.0707725104 (doi:10.1073/pnas.0707725104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bromham L., Penny D. 2003. The modern molecular clock. Nat. Rev. Genet. 4, 216–224 10.1038/nrg1020 (doi:10.1038/nrg1020) [DOI] [PubMed] [Google Scholar]
- 18.Gillooly J. L., Allen A. P., West G. B., Brown J. H. 2005. The rate of DNA evolution: effects of body size and temperature on the molecular clock. Proc. Natl Acad. Sci. USA 102, 140–145 10.1073/pnas.0407735101 (doi:10.1073/pnas.0407735101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikolaev S. I., Montoya-Burgos J. I., Popadin K., Parand L., Margulies E. H., Antonarakis S. E. & National Institutes of Health Intramural Sequencing Center Comparative Sequencing Program 2007. Life-history traits drive the evolutionary rates of mammalian coding and noncoding genomic elements. Proc. Natl Acad. Sci. USA 104, 20 443–20 448 10.1073/pnas.0705658104 (doi:10.1073/pnas.0705658104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bromham L. 2009. Why do species vary in their rate of molecular evolution? Biol. Lett. 5, 401–404 10.1098/rsbl.2009.0136 (doi:10.1098/rsbl.2009.0136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savage V. M., Allen A. P., Brown J. H., Gillooly J. F., Herman A. B., Woodruff W. H., West G. B. 2007. Scaling of number, size, and metabolic rate of cells with body size in mammals. Proc. Natl Acad. Sci. USA 104, 4718–4723 10.1073/pnas.0611235104 (doi:10.1073/pnas.0611235104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummins J. M., Woodall P. F. 1985. On mammalian sperm dimensions. J. Reprod. Fert. 75, 153–175 10.1530/jrf.0.0750153 (doi:10.1530/jrf.0.0750153) [DOI] [PubMed] [Google Scholar]
- 23.Soulsbury C. D. 2010. Genetic patterns of paternity and testes size in mammals. PLoS ONE 5, e9581. 10.1371/journal.pone.0009581 (doi:10.1371/journal.pone.0009581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dewsbury D. A. 1982. Ejaculate cost and male choice. Am. Nat. 279, 601–610 10.1086/283938 (doi:10.1086/283938) [DOI] [Google Scholar]
- 25.Olsson M., Madsen T., Shine R. 1997. Is sperm really so cheap? Costs of reproduction in male adders, Vipera berus. Proc. R. Soc. Lond. B 264, 455–459 10.1098/rspb.1997.0065 (doi:10.1098/rspb.1997.0065) [DOI] [Google Scholar]
- 26.Pierce J. D., Jr, Ferguson B., Salo A. L., Sawrey D. K., Shapiro L. E., Taylor S. A., Dewsbury D. A. 1990. Patterns of sperm allocation across successive ejaculates in four species of voles (Microtus). J. Reprod. Fert. 88, 141–149 10.1530/jrf.0.0880141 (doi:10.1530/jrf.0.0880141) [DOI] [PubMed] [Google Scholar]
- 27.Amann R. P. 2009. Considerations in evaluating human spermatogenesis on the basis of total sperm per ejaculate. J. Androl. 30, 626–641 10.2164/jandrol.108.006817 (doi:10.2164/jandrol.108.006817) [DOI] [PubMed] [Google Scholar]
- 28.Nakatsuru K., Kramer D. L. 1982. Is sperm cheap? Limited male fertility and female choice in the lemon tetra (Pisces, Characidae). Science 216, 753–755 10.1126/science.216.4547.753 (doi:10.1126/science.216.4547.753) [DOI] [PubMed] [Google Scholar]
- 29.Preston B. T., Stevenson I. R., Pemberton J. M., Wilson K. 2001. Dominant rams lose out by sperm depletion. Nature 409, 681–682 10.1038/35055617 (doi:10.1038/35055617) [DOI] [PubMed] [Google Scholar]
- 30.Sella G., Lorenzi M. C. 2003. Increased sperm allocation delays body growth in a protandrous simultaneous hermaphrodite. Biol. J. Linn. Soc. 78, 149–154 10.1046/j.1095-8312.2003.00167.x (doi:10.1046/j.1095-8312.2003.00167.x) [DOI] [Google Scholar]
- 31.Hosken D. J. 2001. Sex and death: microevolutionary trade-offs between reproductive and immune investment in dung flies. Curr. Biol. 11, R379–R380 10.1016/S0960-9822(01)00211-1 (doi:10.1016/S0960-9822(01)00211-1) [DOI] [PubMed] [Google Scholar]
- 32.Simmons L. W., Roberts B. 2005. Bacterial immunity traded for sperm viability in male crickets. Science 309, 2031. 10.1126/science.1114500 (doi:10.1126/science.1114500) [DOI] [PubMed] [Google Scholar]
- 33.Van Voorhies W. A. 1992. Production of sperm reduces nematode life-span. Nature 360, 456–458 10.1038/360456a0 (doi:10.1038/360456a0) [DOI] [PubMed] [Google Scholar]
- 34.Pitnick S., Jones K. E., Wilkinson G. S. 2006. Mating system and brain size in bats. Proc. R Soc. B 273, 719–724 10.1098/rspb.2005.3367 (doi:10.1098/rspb.2005.3367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simmons L. W., Emlen D. J. 2006. Evolutionary trade-off between weapons and testes. Proc. Natl Acad. Sci. USA 103, 16 346–16 351 10.1073/pnas.0603474103 (doi:10.1073/pnas.0603474103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitnick S., Markow T. A., Spicer G. S. 1995. Delayed male maturity is a cost of producing large sperm in Drosophila. Proc. Natl Acad. Sci. USA 92, 10 614–10 618 10.1073/pnas.92.23.10614 (doi:10.1073/pnas.92.23.10614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitnick S. 1996. Investment in testes and the cost of making long sperm in Drosophila. Am. Nat. 148, 57–80 10.1086/285911 (doi:10.1086/285911) [DOI] [Google Scholar]
- 38.Joly D., Luck N., Dejonghe B. 2008. Adaptation to long sperm in Drosophila: correlated development of the sperm roller and sperm packaging. J. Exp. Zool. B 310, 167–178 10.1002/jez.b.21167 (doi:10.1002/jez.b.21167) [DOI] [PubMed] [Google Scholar]
- 39.Ramm S. A., Stockley P. 2010. Sperm competition and sperm length influence the rate of mammalian spermatogenesis. Biol. Lett. 6, 219–221 10.1098/rsbl.2009.0635 (doi:10.1098/rsbl.2009.0635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parapanov R., Nusslé S., Hausser J., Vogel P. 2008. Relationships of basal metabolic rate, relative testis size and cycle length of spermatogenesis in shrews (Mammalia, Soricidae). Reprod. Fertil. Dev. 20, 431–439 10.1071/RD07207 (doi:10.1071/RD07207) [DOI] [PubMed] [Google Scholar]
- 41.Roldan E. R. S., Gomendio M., Vitullo A. D. 1992. The evolution of eutherian spermatozoa and underlying selective forces: female selection and sperm competition. Biol. Rev. 67, 551–593 10.1111/j.1469-185X.1992.tb01193.x (doi:10.1111/j.1469-185X.1992.tb01193.x) [DOI] [PubMed] [Google Scholar]