Abstract
Exposure to hypoxia and hyperoxia in a rodent model of perinatal ischemia results in delayed cell death and and inflammation. Hyperoxia increases oxidative stress that can trigger inflammatory cascades, neutrophil activation, and brain microvascular injury. Here we show that 100% oxygen resuscitation in our rodent model of perinatal ischemia increases cortical COX-2 protein levels, S-nitrosylated COX-2cys526, PGE2, iNOS and 5-LOX, all components of the prostaglandin and leukotriene inflammatory pathway.
Keywords: Hypoxia ischemia, hyperoxia, cyclooxygenase, nitric oxide Synthase, perinatal ischemia
Introduction
Perinatal hypoxia ischemia (HI) occurs in 0.2-0.4% of all live births (Odding et al., 2006;Platt et al., 2007;de Menezes and Shaw, 2006;Raju, 2006), and up to 60% in preterm (< 37 weeks) or very low birth weight (<1500g) infants (Vannucci et al, 1999; Zanelli et al, 2008). Preterm birth has increased to 12-13% of US births (Allen, 2008; Heron et al., 2009) becoming a major US health problem, with 100% O2 remaining a standard clinical treatment (Bissinger and Ohning, 2006). In developing countries, perinatal asphyxia reports range from 4 to 9 million cases of per year (Moss et al, 2002; Zanelli et al, 2008). Perinatal HI-induced brain injury is a major cause of neonatal morbidity and mortality; often resulting in developmental neurological deficits such as cerebral palsy, and delayed cognitive and behavioral deficits associated with mental retardation (Boichot et al, 2006; Zanelli et al, 2008).
Using the Rice-Vannucci post natal day 7 (P7) rat model of HI (Rice et al., 1981), which consists of unilateral common carotid artery ligation and hypoxia (8%O2), we and others have found a significant increase in apoptotic and necrotic cell death in the hippocampus and cortex of HI-injured neonatal Wistar rats (Hu et al., 2003; 2005; Gill et al., 2008; 2009; Rice et al., 1981).
Increasing evidence indicates that inflammation and the inflammatory consequences of HI-induced cell death with necrotic features contribute substantially to the pathogenesis associated with perinatal HI injury (Bockhorst et al., 2010; Fabian et al., 2008; Gill et al., 2008; 2009; Hedtjarn et al. 2002; Hu et al., 2005; Qiu et al., 2004). We showed that HI + 100%O2 resuscitation (HHI) increased T2-weighted MRI lesion volumes, via increased inflammatory and necrotic signaling, with no amelioration of cortical apoptotic signaling compared to that after HI alone (Gill et al., 2008). Moreover, 100%O2 increased endoplasmic reticulum (ER) calpain activation and ER Bax levels, suggesting that 100% O2 increases HI-induced Bax-mediated activation of ER cell death signaling and increases inflammation (Gill et al., 2008).
Although oxidative stress, including O2− production, is increased during reoxygenation and reperfusion after HI (Saugstad, 2005): there are few data on the optimal use of oxygen, and reports comparing hyperoxic and normoxic resuscitation show mixed results (Bagenholm et al., 1996; Lundstrom et al, 1995; Munkeby et al., 2004; Shimabuku et al., 2005). It is known that periventricular white matter and especially not fully developed oligodendroglia are selectively damaged by HI-generated oxidative stress in humans (Back et al., 2002). HI is known to stimulate inflammatory cytokine release (Hu et al., 2005) that in turn can further increase oxidative stress, which can activate NF-κB and thus stimulate inducible nitric oxide Synthase (iNOS) and cyclooxygenase-2 (COX-2; Hu et al., 2005).”Blockade of interleukin-1beta (IL-1β ) stimulation of nuclear factor kappa B (NFkB) at the IL-1 receptor or transcriptional level decreases HI-induced COX-2 and iNOS (Qiu et al., 2004; Hu et al., 2005).
We have shown in our previous work that HI triggers an inflammatory cascade that is in part mediated by the IL-1 → NFκB → iNOS/COX-2 pathway resulting in significant cell death Hu et al., 2005). Decreasing effective IL-1β levels can be accomplished via anti-IL-1β antibodies (Touzani et al. 1999) or inhibitors of the IL-1 converting enzyme (ICE; Hara et al. 1997). Blockade of this pathway at the level of IL-1/IL-1R binding or NFκB binding to cognate DNA promoters ameliorates cell death after HI (Qiu et al; 2001; 2004; Hu et al., 2003; 2005).
Both IL-1α and IL-1β activate NF-κB, activator protein-1 (AP-1), and other transcription factors,, which increase levels and activity of the free radical generating enzymes COX-2, iNOS and 5-lipooxygenase (5-LOX; Dunn et al., 2002). However, the specific regulation of the signaling mechanisms triggered by HI and the role of oxygen resuscitation in the regulation of these signaling pathways are not well understood.
Cyclooxygenase-2 Prostaglandin H2 Synthase (COX-2) is the rate-limiting enzyme for the conversion of arachidonic acid into prostaglandins. COX-2 is a membrane-bound heme-dependent bis-oxygenase (cyclooxygenase) and hydroperoxidase that catalyzes prostanoid synthesis (Smith et al., 2002). COX is present in the luminal surfaces of the ER and the inner and outer membranes of the nuclear envelope (Morita et al., 1995; Spencer et al., 1999). Cyclooxygenase (COX) is an important enzyme in the inflammatory process. There are two isoforms of COX, designated COX-1 and COX-2. COX-1 is expressed constitutively and appears to be responsible for ongoing physiological function, whereas COX-2 is present only in certain tissues where it is transiently induced by growth factors, inflammatory cytokines, tumor promoters, and bacterial toxins. Prostaglandin E2 (PGE2), a product of the cyclooxygenation of arachidonic acid released from membrane phospholipids, plays a critical role in inflammatory neurodegenerative conditions. iNOS binds and S-nitrosylates COX-2cys526, an activating event associated with COX-2 translocation to cell membranes and COX-2 catalytic activity (Kim et al., 2005). Normally COX-2 is localized in the soma and dendrites of glutaminergic neurons (Kaufmann, et al, 1996) and induced by synaptic activity (Yamagata, et al, 1993). However, increases in COX-2 during CNS trauma are closely related to inflammation. Selective inhibition of COX-2 results in diminished lesion volumes and improved functional outcomes after CNS injury (Resnick, et al, 1998; Hains et al., 2001). IL-1β-induced transcriptional up regulation of COX-2 via p65/p50 NF-κB has been shown in vitro (Newton, et al, 1997), but little is known about such control mechanisms in the in vivo nervous system. However, while COX-2 inhibitors are useful in mechanistic studies of CNS trauma (Hains et al., 2001), their adverse side effects do not make them promising therapeutic agents (Turini and Dubois, 2002; Flower, 2003).
Nitric oxide Synthase (NOS).
There is evidence that NO is an important mediator of ischemic and neurodegenerative pathology in the CNS (Mizushima et al. 2002; Brown and Bal-Price 2003). NO is endogenously produced as a byproduct of arginine metabolism by different NOS isoforms: Neuronal (nNOS), endothelial (eNOS), and inducible NOS (iNOS). Both nNOS and eNOS are constitutively expressed, whereas iNOS is expressed in response to a variety of stimuli and there is a relationship between iNOS induction and ischemic lesions in brain (Almeida and Bolanos, 2001; Hara et al. 1996;; Iadecola et al., 1995; Mizushima et al. 2002; Niwa et al. 2001). It is thought that the increased production of NO by iNOS is partly responsible for the propagation of HI injury through the toxic effects of NO reaction products, particularly peroxynitrites (Rodrigo et al., 2001). For example, brain ischemia in the adult rat significantly stimulates nNOS after 6h, whereas eNOS levels in microvessel endothelia increase by 72h, as does iNOS, which also increases in activated astrocytes, macrophages and microglia by 72h (Niwa et al., 2001). iNOS is also found after HI in the immature rat brain (Ikeno et al., 2000). Importantly, administration of IL-1β to cultured brain endothelial cells and hippocampal neurons induces expression of iNOS mRNA and increases NO release (Bonmann et al. 1997; Serou et al. 1999). NO is a cause for cerebral ischemic damage (Bolanos et al., 1999; Iadecola et al., 1995; Niwa et al. 2001) via energy depletion, lipid and protein peroxidation, protein nitrosylation and DNA damage (Almeida and Bolanos, 2001). Studies of eNOS protein and/or mRNA expression and NO production in HI have shown mixed results (Cai et al., 1999; Kutzsche et al., 1999; Lubec et al., 1999; Van den Tweel et al., 2005; Zhang et al., 1993). Inhibition of nNOS and iNOS has been found to be protective in a rat model of cerebral HI (Hamada et al., 1994; Hains et al., 2001; Resnick, et al, 1998; Trifiletti, 1992). These observations, and evidence showing that induction of iNOS is regulated by NFκB p65/p50 (Teng et al. 2002), also support our hypothesis that activation of p65/p50 by IL-1 after HI may further stimulate iNOS synthesis and NO formation, which will in turn trigger cell death.
Besides regulation at the transcriptional level, there are interactions among the NO synthases and COX-2 that play an important role in regulating blood flow and apoptosis/necrosis after HI. For example, eNOS can be activated by Akt phosphorylation at Ser-1177 and Ser-614, and protein kinase A (PKA) can also phosphorylate eNOS at both Ser-1177 and Ser-633.
Materials and Methods
All procedures involving animals were approved by the Animal Care and Use Committee at UTMB. E15 pregnant Wistar rat dams (Charles Rivers Laboratories, Wilmington, MA) were housed upon arrival in 12h light-dark cycle with access to food and water ad libitum and fed a standard laboratory diet.
P7 HI model.
The day of birth is P0; on P1, Wistar rat litters were culled to 10 pups and the pups mixed among dams to minimize litter effects. On P7 all pups are removed from dams, sexed, weighed and randomly assigned to experimental groups. Multiple dams were ordered and housed as described above, except that on P1 after culling to ten pups, the pups were mixed among the dams to minimize potential, confounding litter effects among the multiple groups. Surgical protocols (Hypoxia-ischemia). Hypoxia-ischemia (HI) insult involving a unilateral, permanent ligation of the common carotid was performed as described elsewhere (Hu et al., 2005; Vannucci et al., 1999) with minor modifications. Briefly, anesthesia was induced in P7 rat pups by 7-minute incubation in 5% isoflurane, balanced with blood-gas grade 100% O2, in a 37°C E-Z anesthesia chamber (Euthanex Corp., Palmer, PA) with maintenance of anesthesia at 2.0% isoflurane. As prolonged neuroprotective effects have been observed with isoflurane incubations of greater than 1h (Loepke et al., 2006), care was taken to ensure that no pup was anesthetized for longer than 0.75h. After a mid-neckline incision, the left common carotid was isolated and ischemia was induced by electrocauterization at two points, one rostral and one caudal, along the isolated artery using a ball-end cauterizer. Double electrocauterization cut the left common carotid in the majority of surgeries (>95%). In those cases in which the blood vessel did not sever, the artery was cut between the two cauterization points using micro-spring scissors to prevent reperfusion across cauterization points. Furthermore, care was taken not to damage the vagal nerve which runs parallel and dorsal (underneath) the common carotid artery. After cauterization, the mid-neckline incision was sutured with 5-0 P3 surgical silk and the pup was returned to a normoxic (20.8% O2) 37°C chamber until the effects of the anesthesia dissipated (5-10 min). Sham pups were anesthetized, given the mid-neckline incision and, then, sutured. Isolation of the left common carotid was not performed on sham pups as isolation of the artery can produce minor (<1-2s) ischemia-reperfusion events (unreported observational finding). The total time for performing ischemia surgery on a litter of pups was approximately 1h. After the last pup recovered from anesthesia, the pups were returned to the dam for a 2.5h recovery period to prevent any potential isoflurane-mediated respiratory complications during the hypoxia component of the HI injury.
After the 2.5h recovery period, all pups were removed from the dam and placed either in a normoxic 37°C chamber (sham) or hypoxic (8.0% O2 balanced with blood-gas grade nitrogen) 37°C chamber for 1.5h. A small pet intensive care unit (ICU) (Harvard Apparatus, Boston, MA) was used for the hypoxia chamber as its larger volume promoted greater inertia of oxygen levels and its heated internal water bath reduced temperature variations commonly observed with small chambers wrapped in heated water pads (unreported observational finding). After the 1.5h period, designated 0h survival, pups were returned to the dam until their randomly assigned survival time point. Cortices were collected from sham (0h) and HI-injured pups at 3h, 1, 3, 7, 21days survival times after HI.
Western Blot Analysis.
Proteins determined by bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). 50μg of protein separated by SDS PAGE and transferred overnight at 4°C to PDVF membranes (Millipore, Billerica, MA). PDVF membranes then blocked 1h rt in blocking buffer (BB) and washed 2X in wash buffer (WB). Membranes then incubated overnight with primary antibody. Western Blot Analysis. Proteins determined by bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). 50μg of protein separated by SDS PAGE and transferred overnight at 4°C to PDVF membranes (Millipore, Billerica, MA). PDVF membranes then blocked 1h rt in blocking buffer (BB) and washed 2X in wash buffer (WB). Membranes then incubated overnight with primary antibody. Primary antibodies were mouse anti-β-actin [1:15000] (Sigma-Aldrich, Saint Louis, MO), anti COX-2 rabbit antibody (Cayman Chemical) 1:500 and anti iNOS rabbit (Cayman Chemical) 1:1000; mouse anti-poly-ADP-ribose polymerase 1 (PARP-1) (clone C-2-10) [1:500], and rabbit anti-IL-1β [1:500] Millipore, Billerica, MA). After incubation with primary antibody, membranes were washed 6X/10min in WB and incubated 1h/rt with secondary antibody. Secondary antibodies were goat horseradish conjugated anti-mouse (anti-mouse-POD), rat adsorbed, and goat anti-rabbit F(ab’)2-POD, human and mouse adsorbed (Southern Biotech, Birmingham, AL). Secondary antibodies were diluted 1:3000. Membranes were then washed 6X 10min in WB and developed for enhanced chemiluminescence (ECL) system (GE Healthcare, Piscataway, NJ). Films were scanned and densitometric analyses performed with AlphaEase software (Alpha Innotech, San Leandro, CA). Sample outcomes were normalized to β-actin.
Cytosolic oligonucleosome ELISA.
Cytosolic oligonucleosome (DNA+histone complexes due to cleavage at interlinker regions between histones on DNA) presence is a measure of apoptosis detected with Cell Death Detection ELISA® (Roche Applied Sciences, Indianapolis, IN) at rt. Briefly, 96-well microplates are coated for 1.5h with mouse monoclonal antibody diluted in coating buffer (clone H11-4 to the histones H2A, H2B, H3 and H4). Plates are incubated in incubation buffer 0.5h and 50μg cytosolic protein loaded in triplicate wells and incubated 1.5h. Then microplates are washed 3X with wash buffer and incubated 1.5h in peroxidase-conjugated antibody to DNA in incubation buffer. Microplates are washed 3X in wash buffer, incubated 5min to 1h in 2,2-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) substrate solution on rotary shaker (250rpm) with multiple readings at 405nm with Dynex MRX™ microplate reader (Dynex Technologies, Chantilly, VA).
Biotin Switch:
S-nitrosylation will be determined using the biotin switch method. Samples are homogenized with HEN buffer (25mM Hepes, ph7.7/0.1mM EDTA/0.01 mM necuproine). The supernatant containing membrane fragments and the cytosolic protein recovered. The samples are incubated for 30 min at 40° C with blocking solution containing HEN buffer, 0.1% SDS, and 20mM N-ethylmaleimide (NEM) to block free thiols. Lysates are centrifuged at 16,000X g for 10 min at 40°C. Cold acetone is added to precipitate the proteins. The pellets are resuspended in HEN buffer with 1% SDS, adding 20mM sodium ascorbate to break the SNO bonds. The resulting free thiols in the sample are reacted with 0.05 mm biotinylating agent [N-(3-malemidylpropionyl) biocytin (MPB)] for 30min at room temperature. The excess MPB is removed by additional protein precipitation in cold acetone, immunoprecipitated with specific polyclonal Abs; immunoprecipitates are washed with HEN buffer and resuspended in 50μl of HEN containing Laemmli sample buffer, boiled at 95°C for 5min, and electrophoresed (SDSPAGE).
Statistical analyses are performed with GraphPad Prism 5 software (GraphPad Software, San Diego, CA). For analyses involving 2 groups, a student’s t-test is performed and p-value less of than 0.05 considered significant. If variances for 2 groups are deemed significantly different, a Welsh correction is added to student’s t-test. For analyses of 2+ groups, a one-way analysis of variance (ANOVA) is performed with a Tukey-Kramer post-hoc test to compare groups. A p-value less than 0.05 is deemed significant.
Results
HI increases cell death with apoptotic fatures and inflammation.
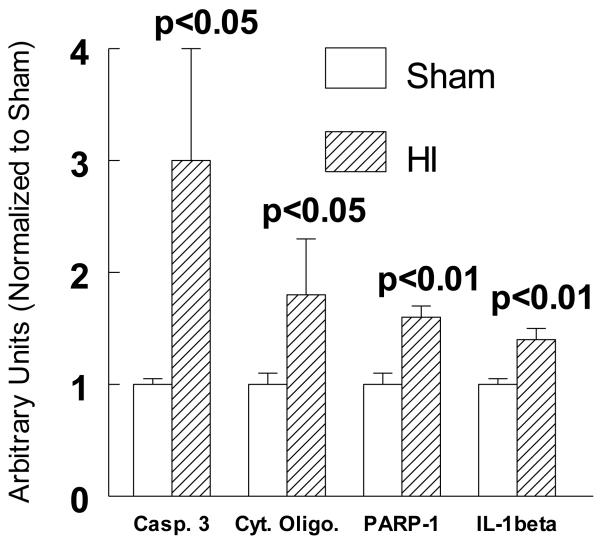
We used a modification of the Rice-Vannucci P7 rat model of hypoxia ischemia (Rice et al., 1981), which consists of unilateral common carotid artery ligation and hypoxia (8%O2), a clinically relevant hypoxia/ischemia (HI) rodent model that mimics the targeted infant patient population. We showed that there is significantly increased cell death with both apoptotic and necrotic features in the ipsilateral cortex by several criteria as early as 3h after HI (Figure 1). Thus, HI-induced a 3 fold increase in caspase-3 cleavage and a 75% increase in the presence of histone-DNA complexes in the cytoplasm both established outcomes associate with imminent cell death with apopototic feataures (Figure 1). There was also a 60% increase in nuclear cleavage of PARP and a 45% increase in IL-1β cleavage consistent with the appearance of an inflammatory response (Figure 1).
Figure 1.
Increased ipsilalteral cortical cell death 3h after HI as measured by caspase 3 cleavage, cytoplasmic cytochrome c levels, nuclear PARP-cleavage, and IL-1β cleaved protein levels normalized to sham-treated cortices. Sham values for each smaple normalized to actin protein levels. Means ± SD. One way ANOVA, Tukey’s multiple comparison test. p values are compared to shams.
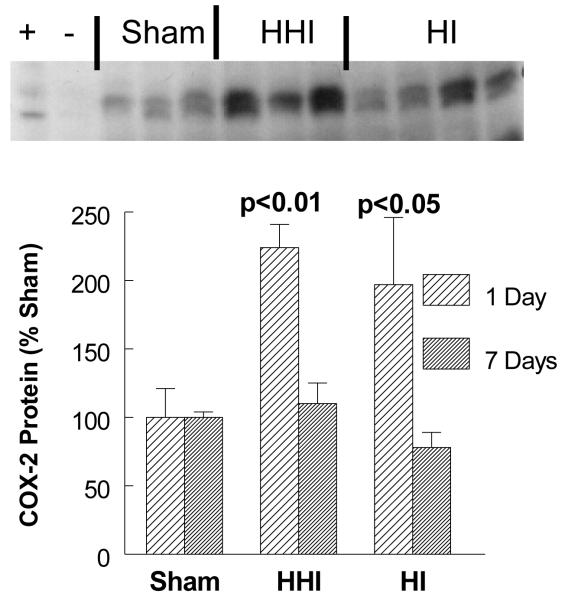
Hyperoxia treatment of rat pups exposed to HI (HHI), or HI alone, increased COX-2 protein levels.
In order to determine the inflammatory consequences of 100% oxygen resuscitation (HHI) after HI, we measured COX-2 protein levels by Western immunoblot assays in three groups of pups: sham-treated (SHAM; N = 3); hypoxia ischemia alone (HI; N = 3); and HI pups treated with 100% oxygen after HI (HHI; N = 4). When rat pups were exposed to HHI or HI alone, there was a significant two fold increase in COX-2 protein levels one day after the HHI or the HI treatment. By day 7 after both treatments there was return fo COX-2 protein to sham levels (Figure 2).
Figure 2.
Increased ipsilateral cortical levels of COX-2 protein at one and seven days after HHI or HI alone normalized to sham-treated cortices. Sham values for each sample normalized to actin protein levels. Sham and HI N = 3 and HHI N = 4. Means ± SD. One way ANOVA, Tukey’s multiple comparison test. p values are compared to seven days post insult.
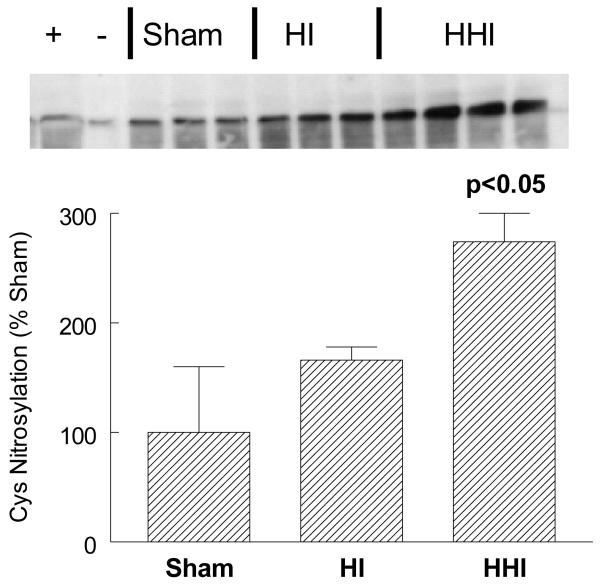
Hyperoxia treatment of rat pups exposed to HI (HHI) stimulated COX-2 activation via Cysteine nitrosylation.
In order to assess activation of COX-2 via iNOS after 100% oxygen resuscitation (HHI) after HI, we measured COX-2 cysteine nitrosylation using the biotin switch assay in three groups of pups: sham-treated (SHAM; N = 3); hypoxia ischemia alone (HI; N = 3); and HI pups treated with 100% oxygen after HI (HHI; N = 4). HHI, but not HI alone, increased COX-2 nitrosylation more than 2 fold after 24 hours. Although the HI sample displayed 50% more COX-2 nitrosylation, the increase was not significant (p<0.10; Figure 3).
Figure 3.
Increased ipsilateral cortical levels of nitrosylated COX-2-cys at 24 hours after HHI but not HI normalized to Shams. +, − lanes contained a positive and negative control sample using cis-platin stimulated cell extracts. Sham and HI N = 3 and HHI N = 4. Sham values for each smaple normalized to actin protein levels. p values are compared to shams and HI animals.
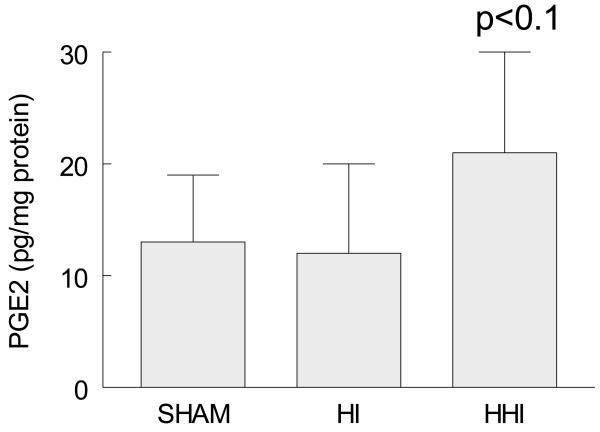
Hyperoxia treatment of rat pups exposed to HI (HHI) stimulated PGE2 levels.
Given that prostaglandin E2 (PGE2) is a product of the cyclooxygenation activity of COX-2, we measured PGE2 levels after HHI and HI alone. HHI but not HI treatment resulted in increased levels of PGE2 (pg/mg protein) after 24 hours although the increases were not significant (Figure 4; p<0.1).
Figure 4.
Increased ipsilateral cortical levels of PGE2 at 24 hours after HHI but not HI normalized to Shams. Sham and HI N = 3 and HHI N = 4. p values are compared to shams and HI animals.
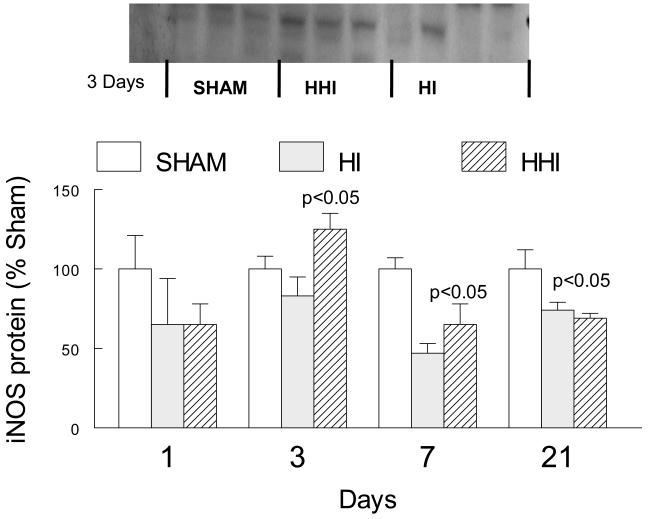
Hyperoxia treatment of rat pups exposed to HI (HHI) stimulated iNOS protein levels.
Apart from its role as an activator of COX-2, iNOS catalyzes the formation of nitric oxide, which together with oxyl radicals generates the very toxic peroxinitrite (Figure 7). We therefore measured iNOS protein levels by Western immunoblot assays in three groups of pups: sham-treated (SHAM; N = 3); hypoxia ischemia alone (HI; N = 3); and HI pups treated with 100% oxygen after HI (HHI; N = 4). HHI, but not HI alone, increased iNOS protein levels significantly after 3 days (Figure 5). At later times, iNOS protein level values for both HHI and HI alone were significantly lower than sham values.
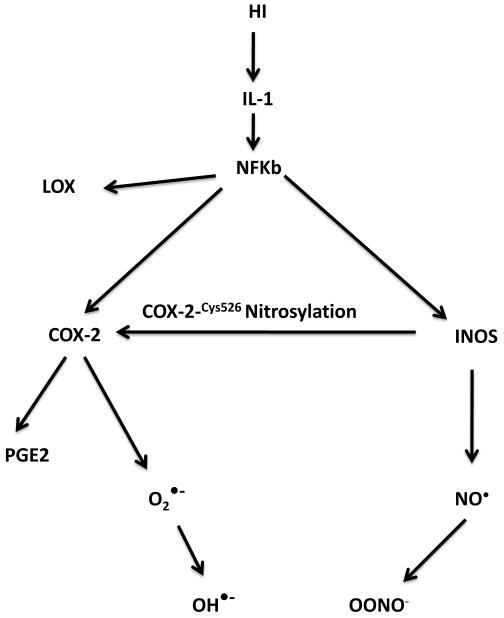
Figure 7.
Scheme of inflammatory responses to HI and HHI.
Figure 5.
Increased ipsilateral cortical levels of iNOS only at 3 days after HHI but not HI normalized to Shams. N = 3 for Sham, HI and HHI. Sham values for each sample normalized to actin protein levels. p values at three days are compared to sham or HI animals, at seven days compared to sham animals, at 21 days both HI and HHI were significantly different from sham animals.
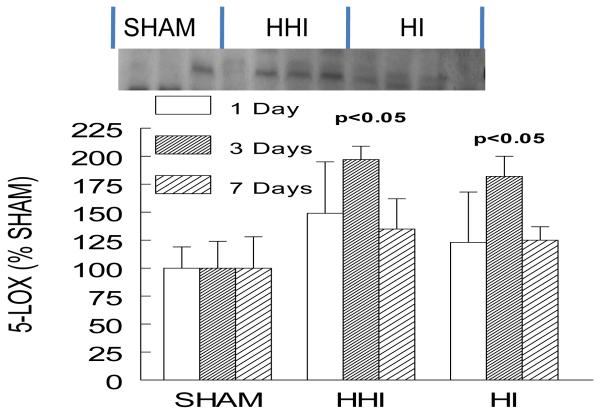
Hyperoxia treatment of rat pups exposed to HI (HHI), or HI alone, increased 5-LOX protein levels.
Five-lipooxygenase (5-LOX) is a complementary inflammatory enzyme in leukotriene biosynthesis. In order to obtain a broader determination of the inflammatory response to 100% oxygen resuscitation (HHI) after HI, we measured 5-LOX protein levels by Western immunoblot assays in three groups of pups: sham-treated (SHAM; N = 3); hypoxia ischemia alone (HI; N = 3); and HI pups treated with 100% oxygen after HI (HHI; N = 4). Exposing rat pups to both HHI or HI alone resulted in an almost 2 fold increase in 5-LOX three days after treatment with a return to sham levels 7 days after either HHI or HI (Figure 6).
Figure 6.
Increased ipsilateral cortical levels of 5-LOX at 1, 3 and 7 days after both HHI and HI alone normalized to Shams. Sham and HI N = 3 and HHI N = 4. Sham values for each sample normalized to actin protein levels. p values are compared only to HI animals.
Discussion
We showed that there is significantly increased cell death with both apoptotic and necrotic features in the ipsilateral cortex by several criteria as early as 3h after HI (Figure 1), peaking at 12h after HI and persisting until 48h post-HI, then decreasing to sham levels by 72h after injury (Hu et al.,2003;2005; Gill et al., 2008). We also observed a prompt decrease in anti-apoptotic signaling via Bcl-XL (Hu et al., 2003), consistent with the literature (Szaflarski et.al., 1995).
When we measured cytosolic oligonucleosome levels and caspase 3 cleavage over time as indicative of apoptotic cell death, and IL-1β and PARP-1 as measures of documented inflammatory responses (Martin et al., 2005) (Fig. 1), our results showed that HI induced inflammatory signaling and cell death.
Not surprisingly, both hypoxia and hyperoxia significantly increased COX-2 levels (Fig. 2) although HHI did not appear to augment the HI effect suggesting that the major contribution to COX-2 stimulation is by HI alone. Thus, hyperoxia treatment of pups exposed to HI had no significant effect on the HI-induced increases in COX-2, consistent with hyperoxia having no beneficial effects on inflammation and in agreement with earlier studies that showed a similar lack of an effect of hyperoxia on edema formation (Ferrari et al., 2010a;b). The opposite was the case for HI and HHI effects on COX-2 cys-nitrosylation levels (Fig. 3). While the HI-induced increase in COX-2 cys-nitrosylation levels was not significant, the HHI-induced increase was significant. Levels of the COX-2 product PGE2 were also selectively stimulated by hyperoxia treatment of the HI-exposed pups, consistent with a general increase of COX-2 activity (Fig.5). Given that iNOS generates NO and also uncouples eNOS via tyrosine nitrosylation (Fabian et al., 2008) and activates COX-2 via cysteine nitrosylation (Kim et al., 2005), the simultaneous HHI-induced increases in COX-2, iNOS (Fig. 4) and COX-2 cys-nitrosylation together with uncoupling of eNOS (Fabian et al., 2008) may explain the absence of any beneficial effect or even deleterious effects of hyperoxia treatment of HI.
The selective increases in 5-LOX after HHI but not HI treatment alone (Fig. 6-7) are consistent with the hypothesis that although increased expression of enzymes with major roles in free radical-generating pathways are important in hypoxia, the HHI-mediated increased recruitment of the free radical generating pathway (5-LOX) and stimulaton of post-translational modification of COX-2cys526 are to be considered in oxygen resuscitation of the newborn (Fig. 7). These findings are also consistent with our earlier work on effects of HI on NOS uncoupling, which also increases free radical generation (Fabien et al., 2008).
Given the broad spectrum of HI outcomes and evolving modifications to the clinical use of oxygen resuscitation, it is unlikely that COX-2/iNOS levels alone are the only targets for intervention. The central role played by IL-1 and perhaps NFkB as gatakeepers of inflammation would suggest that a thorough knowledge of oxidative pathways downstream may be clinically relevant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited[utmb1]
- Allen MC. Neurodevelopmental Outcomes of Preterm Infants. Curr Opin Neurol. 2008;218:123–28. doi: 10.1097/WCO.0b013e3282f88bb4. [DOI] [PubMed] [Google Scholar]
- Almeida A, Bolanos JP. A transient inhibition of mitochondrial ATP synthesis by nitric oxide synthase activation triggered apoptosis in primary cortical neurons. J Neurochem. 2001;77:676–690. doi: 10.1046/j.1471-4159.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- Back SA, Han BH, Luo NL, Chrichton CA, Tam J, Xanthoudakis S, Arvin KL, Holtzman DM. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagenholm R, Hagberg H, Kjellmer I. Impact of reoxygenation with oxygen and air on the extent of the brain damage after hypoxia-ischaemia in neonatal rats. Acta Paediatr. 1996;85:1228–1231. doi: 10.1111/j.1651-2227.1996.tb18234.x. [DOI] [PubMed] [Google Scholar]
- Bissinger RL, Ohning BL. Neonatal Resuscitation. 2006 http://www.emedicine.com/ped/topic2598.htm.
- Bockhorst KH, Narayana PA, Dulin J, Liu R, Rea HC, Wosik HK, Perez-Polo JR. Normobaric hyperoximia increases hypoxia-induced cerebral injury: DTI study in rats. J. Neurosci Res. 2010;88:1146–56. doi: 10.1002/jnr.22273. PMCID:PMC Journal – In Process. [DOI] [PubMed] [Google Scholar]
- Boichot C, Walker PM, Durand C, Grimaldi M, Chapuis S, Gouyon JB, Brunotte F. Term Neonate Prognoses After Perinatal Asphyxia: Contributions of Mr Imaging, Mr Spectroscopy, Relaxation Times, and Apparent Diffusion Coefficients. Radiology. 2006;239:839–48. doi: 10.1148/radiol.2393050027. [DOI] [PubMed] [Google Scholar]
- Bolanos JP, Almeida A. Roles of nitric oxide in brain hypoxia-ischemia. Biochim. Biophys. Acta. 1999;1411:415–436. doi: 10.1016/s0005-2728(99)00030-4. [DOI] [PubMed] [Google Scholar]
- Bonmann E, Suschek C, Spranger M, Kolb-Bachofen V. The dominant role of exogenous or endogenous interleukin-1 beta on expression and activity of inducible nitric oxide synthase in rat microvascular brain endothelial cells. Neurosci Lett. 1997;230:109–112. doi: 10.1016/s0304-3940(97)00485-0. [DOI] [PubMed] [Google Scholar]
- Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol. 2003;27:325–355. doi: 10.1385/MN:27:3:325. [DOI] [PubMed] [Google Scholar]
- Cai Z, Xiao F, Lee B, Paul IA, Rhodes PG. Prenatal hypoxia-ischemia alters expression and activity of nitric oxide synthase in the young rat brain and causes learning deficits. Brain Res Bull. 1999;49:359–365. doi: 10.1016/s0361-9230(99)00076-3. [DOI] [PubMed] [Google Scholar]
- Calvert JW, Cahill J, Yamguchi-Okada M, Zhang JH. Oxygen treatment after experimental hypoxia-ischemia in neonatal rats alters the expression of HIF-1α and downstream target genes. J. Appl. Physiol. 2006;101:853–865. doi: 10.1152/japplphysiol.00268.2006. [DOI] [PubMed] [Google Scholar]
- deMenezes MS, Shaw DW. Hypoxic-Ischemic Brain Injury in the Newborn. 2006 http://www.emedicine.com/neuro/topic696.htm.
- Dunn SL, Young EA, Hall MD, McNulty S. Activation of astrocyte intracellular signaling pathways by interleukin-1 in rat primary striatal cultures. Glia. 2002;37:31–42. doi: 10.1002/glia.10010. [DOI] [PubMed] [Google Scholar]
- Fabian RH, Perez-Polo JR, Kent TA. Electrochemical monitoring of Superoxide anion production and cerebral blood flow: Effect of Interleukin-1β Pre-treatment in a model of focal ischemia and reperfusion. J. Neurosci. Res. 2006;60:795–803. doi: 10.1002/1097-4547(20000615)60:6<795::AID-JNR12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Fabian RH, Perez-Polo JR, Kent TA. A decoy oligonucleotide inhibiting NF-κB binding to the IgGκB consensus site reduces cerebral injury and apoptosis in neonatal hypoxic-ischemic encephalopathy. J.Neurosci. Res. 2007;85(7):1420–6. doi: 10.1002/jnr.21253. [DOI] [PubMed] [Google Scholar]
- Fabian RH, Perez-Polo JR, Kent TA. Perivascular nitric oxide and superoxide in neonatal cerebral hypoxia-ischemia. Amer. J.Physiol. 2008;295:H1809–H1814. doi: 10.1152/ajpheart.00301.2007. PMCID:PMC2593505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari DC, Nesic-Taylor OB, Perez-Polo JR. Oxygen resuscitation does not ameliorate neonatal hypoxia/ischemia-induced cerebral edema. J. Neurosci. Res. 2010;88:2056–2065. doi: 10.1002/jnr.22358. PMCID:PMC Journal – In Process. [DOI] [PubMed] [Google Scholar]
- Ferrari DC, Nesic O, Perez-Polo JR. Perspectives on Neonatal Hypoxia/Ischemia-Induced Edema Formation. Neuruochem. Res. 2010;35:1957–1965. doi: 10.1007/s11064-010-0308-y. DOI 10.1007/s11064-010-0308-y. [DOI] [PubMed] [Google Scholar]
- Flower RJ. The development of COX2 inhibitors. Nat. Drug Discov. 2003;2:179. doi: 10.1038/nrd1034. [DOI] [PubMed] [Google Scholar]
- Formigli L, Papucci L, Tani A, Schiavone N, Tempestini A, Orlandini GE, Capaccioli S, Orlandini SZ. Aponecrosis: morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis. J Cell Physiol. 2000;182:41–49. doi: 10.1002/(SICI)1097-4652(200001)182:1<41::AID-JCP5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gill, Martin KH, Bockhorst PA, Narayana JR, Perez-Polo Bax shuttling after neonatal hypoxia- ischemia: hyperoxia effects. J. Neurosci. Res. 2008 Dec;86(16):3584–604. doi: 10.1002/jnr.21795. PMCID:PMC2585158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill M, Perez-Polo JR. Bax shuttling after rotenone treatment of neuronal primary cultures: effects on cell death phenotypes. J Neurosic Res. 2009;87:2047–65. doi: 10.1002/jnr.22019. PMCID: PMC Journal – In Process. [DOI] [PubMed] [Google Scholar]
- Hains BC, Yucra JA, Hulsebosch CE. Reduction of pathological and behavioral deficits following spinal cord contusion injury with the selective cycloooxygenase-2 inhibitor NS-398. J. Neurotrauma. 2001;18:409–423. doi: 10.1089/089771501750170994. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Hayakawa T, Hattori H, Mikawa H. Inhibitor of nitric oxide synthesis reduces hypoxic-ischemic brain damage in the neonatal rat. Pediatr Res. 1994;35:10–14. doi: 10.1203/00006450-199401000-00003. [DOI] [PubMed] [Google Scholar]
- Hara H, Fink K, Endres M, Friedlander RM, Gagliardini V, Yuan J, Moskowitz MA. Attenuation of transient focal cerebral ischemic injury in transgenic mice expressing a mutant ICE inhibitory protein. J Cereb Blood Flow Metab. 1997;17:370–375. doi: 10.1097/00004647-199704000-00002. [DOI] [PubMed] [Google Scholar]
- Hara H, Huang PL, Panahian N, Fishman MC, Moskowitz MA. Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. J Cereb Blood Flow Metab. 1996;16:605–611. doi: 10.1097/00004647-199607000-00010. [DOI] [PubMed] [Google Scholar]
- Hedtjarn M, Leverin AL, Eriksson K, Blomgren K, Mallard C, Hagberg H. Interleukin-18 involvement in hypoxic-ischemic brain injury. J Neurosci. 2002;22:5910–5919. doi: 10.1523/JNEUROSCI.22-14-05910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, et al. National Vital Statistics Reports. 14. Vol. 57. U.S. Centers for Disease Control and Prevention; Atlanta: 2009. Deaths: Final data for 2006. [PubMed] [Google Scholar]
- Hu X, Qiu J, Grafe MR, Rea HC, Rassin DK, Perez-Polo JR. Bcl-2 family members make different contributions to cell death in hypoxia and/or hyperoxia in rat cerebral cortex. Int.J. Dev. Neurosci. 2003;21:371–378. doi: 10.1016/s0736-5748(03)00089-3. [DOI] [PubMed] [Google Scholar]
- Hu X, Nesic-Taylor O, Qiu J, Rea HC, Fabian R, Rassin DK, Perez-Polo JR. Activation of nuclear factor-κB signaling pathway by interleukin-1 after hypoxia/ischemia in neonatal rat hippocampus and cortex. J. Neurochem. 2005;93:26–37. doi: 10.1111/j.1471-4159.2004.02968.x. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang FY, Xu S, Casey R, Ross ME. Inducible nitric oxide synthase gene expression in brain following cerebral ischemia. J. Cer. Blood Flow Metab. 1995;15:378–384. doi: 10.1038/jcbfm.1995.47. [DOI] [PubMed] [Google Scholar]
- Ikeno S, Nagata N, Yoshida S, Takahashi H, Kigawa J, Terakawa N. Immature brain injury via peroxynitrite production induced by inducible nitric oxide synthase after hypoxia-ischemia in rats. J. Obstet. Gynaecol. Res. 2000;26:227–234. doi: 10.1111/j.1447-0756.2000.tb01316.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc. Natl. Acad. Sci. U. S. A. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966–70. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- Kutzsche S, Kirkeby OJ, Rise IR, Saugstad OD. Effects of hypoxia and reoxygenation with 21% and 100%-oxygen on cerebral nitric oxide concentration and microcirculation in newborn piglets. Biol Neonate. 1999;76:153–167. doi: 10.1159/000014155. [DOI] [PubMed] [Google Scholar]
- Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Ståhlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M. Protective Role of Reactive Astrocytes in Brain Ischemia. J Cereb Blood Flow Metab. 2008;28:468–81. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- Loepke AW, McCann JC, Kurth CD, McAuliffe JJ. The physiologic effects of isoflurane anesthesia in neonatal mice. Anesth Analg. 2006;102:75–80. doi: 10.1213/01.ANE.0000181102.92729.B8. [DOI] [PubMed] [Google Scholar]
- Lubec B, Kozlov AV, Krapfenbauer K, Berger A, Hoeger H, Herrera-Marschitz M, Nohl H, Koeck T, Lubec G. Nitric oxide and nitric oxide synthase in the early phase of perinatal asphyxia of the rat. Neuroscience. 1999;93:1017–1023. doi: 10.1016/s0306-4522(99)00256-0. [DOI] [PubMed] [Google Scholar]
- Lundstrom KE, Pryds O, Greisen G. Oxygen at birth and prolonged cerebral vasoconstriction in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1995;73:F81–F86. doi: 10.1136/fn.73.2.f81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SS, Perez-Polo JR, Noppens KM, Grafe MR. Biphasic changes in the levels of poly(ADP-ribose) polymerase-1 and caspase 3 in the immature brain following hypoxia-ischemia. Int J Dev Neurosci. 2005;23:673–686. doi: 10.1016/j.ijdevneu.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Mizushima H, Zhou CJ, Dohi K, Horai R, Asano M, Iwakura Y, Hirabayashi T, Arata S, Nakajo S, Takaki A, Ohtaki H, Shioda S. Reduced postischemic apoptosis in the hippocampus of mice deficient in interleukin-1. J Comp Neurol. 2002;448:203–216. doi: 10.1002/cne.10262. [DOI] [PubMed] [Google Scholar]
- Morita I, Schindler M, Regier MK, Otto JC, Hori T, DeWitt DL, et al. Different intracellular locations for prostaglandin endoperoxidase H synthase-1 and -2. J. Biol. Chem. 1995;270:10902–8. doi: 10.1074/jbc.270.18.10902. [DOI] [PubMed] [Google Scholar]
- Moss W, Darmstadt GL, Marsh DR, Black RE, Santosham M. Research Priorities for the Reduction of Perinatal and Neonatal Morbidity and Mortality in Developing Country Communities. J. Perinatology. 2002:484–95. doi: 10.1038/sj.jp.7210743. [DOI] [PubMed] [Google Scholar]
- Munkeby BH, Borke WB, Bjornland K, Sikkeland LI, Borge GI, Halvorsen B, Saugstad OD. Resuscitation with 100% O2 increases cerebral injury in hypoxemic piglets. Pediatr Res. 2004;56:783–790. doi: 10.1203/01.PDR.0000141988.89820.E3. [DOI] [PubMed] [Google Scholar]
- Newton R, Kuitert LM, Bergmannn M, et al. Evidence for involvement of NF-κB in transcriptional control of COX-2 gene expression by IL-1β. Biochem. Biophys. Res. Comm. 1997;237:28–32. doi: 10.1006/bbrc.1997.7064. [DOI] [PubMed] [Google Scholar]
- Niwa M, Inao S, Takayasu M, et al. Time course of expression of three nitric oxide synthase isoforms after transient middle cerebral artery occlusion in rats. Neurol Mol Chir (Tokyo) 2001;41:63–73. doi: 10.2176/nmc.41.63. [DOI] [PubMed] [Google Scholar]
- Odding E, Roebroeck ME, Stam HJ. The epidemiology of cerebral palsy: incidence, impairments and risk factors. Disabil Rehabil. 2006;28:183–191. doi: 10.1080/09638280500158422. [DOI] [PubMed] [Google Scholar]
- Platt MJ, Cans C, Johnson A, Surman G, Topp M, Torrioli MG, Krageloh-Mann I. Trends in cerebral palsy among infants of very low birthweight (<1500 g) or born prematurely (<32 weeks) in 16 European centres: a database study. Lancet. 2007;369:43–50. doi: 10.1016/S0140-6736(07)60030-0. [DOI] [PubMed] [Google Scholar]
- Qiu J-X, Grafe MR, Schmura SM, Glasgow J, Kent TA, Rassin DK, Perez-Polo JR. Differential NF-κB regulation of bcl-x gene expression in Hippocampus and basal forebrain in response to hypoxia. J. Neurosci. Res. 2001;64:223–234. doi: 10.1002/jnr.1070. [DOI] [PubMed] [Google Scholar]
- Qiu J, Hu X, Nesic O, Grafe MR, Rassin DK, Perez-Polo JR. Effects of NF-κB Oligonucleotide “Decoys” on gene expression in p7 rat hippocampus after hypoxia/ischemia. J. Neurosci. Res. 2004;77:108–18. doi: 10.1002/jnr.20156. [DOI] [PubMed] [Google Scholar]
- Raju TNK. Hypoxic Ischemic Encephalopathy. 2006 http://www.emedicine.com/ped/topic149.htm.
- Resnick DK, Graham SH, Dixon CE, Marion DW. Role of cyclooxygenase 2 in acute spinal cord injury. J Neurotrauma. 1998;15:1005–13. doi: 10.1089/neu.1998.15.1005. [DOI] [PubMed] [Google Scholar]
- Rice JE, VAnnucci RC, Brieley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Rodrigo J, Alonso D, Fernandez AP, Serrano J, Richart A, Lopez JC, Santacana M, Martinez-Murillo R, Bentura ML, Ghiglione M, Uttenthal LO. Neuronal and inducible nitric oxide synthase expression and protein nitration in rat cerebellum alter oxygen and glucose deprivation. Brain Res. 2001;909:20–45. doi: 10.1016/s0006-8993(01)02613-0. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ. Cytokines- killers in the brain? J. Physiol. 1999;514.1:3–17. doi: 10.1111/j.1469-7793.1999.003af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck RP, Bull P, Takamiya M, et al. RelB, a new Rel family transcription activator that can interact with p50-NF-kappa B. Mol. Cell Biol. 1992;12(2):674–684. doi: 10.1128/mcb.12.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugstad OD. Oxidative stress in the newborn--a 30-year perspective. Biol Neonate. 2005;88:228–3631. doi: 10.1159/000087586. [DOI] [PubMed] [Google Scholar]
- Serou MJ, DeCoster MA, Bazan NG. Interleukin-1 beta activates expression of cyclooxygenase-2 and inducible nitric oxide synthase in primary hippocampal neuronal culture: platelet-activating factor as a preferential mediator of cyclooxygenase-2 expression. J Neurosci Res. 1999;58:593–598. [PubMed] [Google Scholar]
- Shimabuku R, Ota A, Pereyra S, Veliz B, Paz E, Nakachi G, More M, Oliveros M. Hyperoxia with 100% oxygen following hypoxia-ischemia increases brain damage in newborn rats. Biol. Neonate. 2005;88:168–71. doi: 10.1159/000086206. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular and molecular biology. Ann. Rev. Biochem. 2002;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Spencer AG, Thuresson E, Otto JC, Song I, Smith T, DeWitt DL, et al. The membrane binding domains of prostaglandin endoperoxidase H synthases 1 and 2. Peptide mapping and mutational analysis. J. Biol. Chem. 1999;274:32936–42. doi: 10.1074/jbc.274.46.32936. [DOI] [PubMed] [Google Scholar]
- Szaflarski J, Burtrum D, Silverstein FS. Cerebral hypoxia-ischemia stimulates cytokine gene expression in perinatal rats. Stroke. 1995;26:1093–1100. doi: 10.1161/01.str.26.6.1093. [DOI] [PubMed] [Google Scholar]
- Teng X, Zhang H, Snead C, Catravas JD. Molecular mechanisms of iNOS induction by IL-1 beta and IFN-gamma in rat aortic smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:C144–C152. doi: 10.1152/ajpcell.2002.282.1.C144. [DOI] [PubMed] [Google Scholar]
- Touzani O, Boutin H, Chuquet J, Rothwell N. Potential mechanisms of interleukin-1 involvement in cerebral ischaemia. J Neuroimmunol. 1999;100:203–215. doi: 10.1016/s0165-5728(99)00202-7. [DOI] [PubMed] [Google Scholar]
- Trifiletti RR. Neuroprotective effects of NG-nitro-L-arginine in focal stroke in the 7-day old rat. Eur J Pharmacol. 1992;218:197–198. doi: 10.1016/0014-2999(92)90168-4. [DOI] [PubMed] [Google Scholar]
- Turini ME, DuBois RN. Cyclooxygenase 2: a therapeutic target. Annu. Rev. Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- Van den Tweel ER, Nijboer C, Kavelaars A, Heijnen CJ, Groenendaal F, van Bel F. Expression of nitric oxide synthase isoforms and nitrotyrosine formation after hypoxia-ischemia in the neonatal rat brain. J Neuroimmunol. 2008;167:64–71. doi: 10.1016/j.jneuroim.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Connor JR, Mauger DT, Palmer C, Smith MB, Towfighi J, Vannucci SJ. Rat Model of Perinatal Hypoxic-Ischemic Brain Damage. J Neurosci Res. 1999;55:158–63. doi: 10.1002/(SICI)1097-4547(19990115)55:2<158::AID-JNR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–86. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Zanelli S, Stanley DP, Kaufman D. Hypoxic-Ischemic Encephalopathy. 2008 http://emedicine.medscape.com/article/973501-overview.
- Zhang ZG, Chopp M, Zaloga C, Pollock JS, Forstermann U. Cerebral endothelial nitric oxide synthase expression after focal cerebral ischemia in rats. Stroke. 1993;24:2016–2022. doi: 10.1161/01.str.24.12.2016. [DOI] [PubMed] [Google Scholar]