Abstract
Purpose
Assess dosimetric correlates of long-term dysphagia after chemo-IMRT of oropharyngeal cancer (OPC) sparing parts of the swallowing organs.
Patients and Methods
Prospective longitudinal study: weekly chemotherapy concurrent with IMRT for stages III/IV OPC, aiming to reduce dysphagia by sparing non-involved parts of swallowing-related organs: pharyngeal constrictors (PC), glottic and supraglottic larynx (GSL), and esophagus, as well as oral cavity and major salivary glands. Dysphagia outcomes included patient-reported Swallowing and Eating Domain scores, Observer-based (CTCAEv.2) dysphagia, and videofluoroscopy (VF), before and periodically after therapy through 2 years. Relationships between dosimetric factors and worsening (from baseline) of dysphagia through 2 years were assessed by linear mixed-effects model.
Results
73 patients participated. Observer-based dysphagia was not modeled because at >6 months there were only four grade ≥2 cases (one of whom feeding-tube dependent). PC, GSL, and esophagus mean doses, as well as their partial volume doses (VDs), were each significantly correlated with all dysphagia outcomes. However, the VDs for each organ inter-correlated and also highly correlated with the mean doses, leaving only mean doses significant. Mean doses to each of the parts of the PCs (superior, middle and inferior) were also significantly correlated with all dysphagia measures, with superior PCs demonstrating highest correlations. For VF-based strictures, most significant predictor was esophageal mean doses (48±17 Gy in patients with, vs 27±12 in patients without strictures, p=0.004). Normal tissue complication probabilities (NTCPs) increased moderately with mean doses without any threshold. For increased VF-based aspirations or worsened VF summary scores, TD50 and TD25 were 63Gy and 56Gy for PC, and 56Gy and 39Gy for GSL, respectively. For both PC and GSL, patient-reported swallowing TDs were substantially higher than VF-based TDs.
Conclusions
Swallowing organs mean doses correlated significantly with long-term worsening of swallowing. Different methods assessing dysphagia resulted in different NTCPs, and none demonstrated a threshold.
Keywords: Head neck cancer, oropharyngeal cancer, dysphagia, IMRT, NTCP
Introduction
Dysphagia is a major sequel of chemo-irradiation (CRT) of head and neck (HN) cancer (1,2). In order to reduce its severity it is necessary to understand its pathophysiology as well as its associations with clinical and dosimetric factors. We have previously identified the pharyngeal constrictors (PC) and glottic and supraglottic larynx (GSL) as organs whose dysfunction after CRT is the likely cause of dysphagia, and have assessed the ability of intensity modulated radiotherapy (IMRT) to reduce the doses to the parts of these organs which are outside the targets (3). Subsequently, several studies assessed the correlations between the doses delivered to these organs and various measures of dysphagia (4–11), as well as the clinical correlates of dysphagia (5,6,11,16, 17). All these studies found significant correlations between dysphagia and the doses delivered to the swallowing organs, but almost all were retrospective, included mixtures of therapies, and lacked adjustments to pre-therapy swallowing abnormalities.
We have conducted a prospective study of chemo-IMRT aiming to reduce dysphagia by sparing the parts of the swallowing system which did not overlap with the targets in patients with oropharyngeal cancer. The clinical and functional results of this study have recently been published (17). Longitudinal evaluations of dysphagia included observer-rated, patient-reported, and objective, radiological measurements of swallowing dysfunction, before and periodically through 2 years after therapy. The clinical correlates of dysphagia in the study have previously been assessed (17). We have found that the mean doses to the swallowing structures, as well as several clinical factors, notably the pretherapy outcome scores, T stage, GTV size, and current smoking, were each independently associated with several swallowing outcomes (17). We have previously also reported the dosimetric correlates of early (three months post-therapy) dysphagia in that study (4). However, dysphagia changed significantly from three months through two years post-therapy, with a trend of stabilization between one and two years (17). Therefore, in order to be relevant for long-term dysphagia, dose-effect relationships at one year or later post-therapy need to be assessed.
The current paper analyses the dosimetric correlates of long-term post-CRT worsening of various measures of dysphagia, through two years, compared with the pre-therapy base-line. It strives to find the normal tissue complication probabilities (NTCPs) and establish dose constraints related to long-term dysphagia.
Patients and Methods
This was a prospective longitudinal study of chemo-IMRT for head and neck cancer aiming to reduce dysphagia by sparing the parts of the swallowing structures not included in the targets: pharyngeal constrictors (PCs), glottic and supraglottic larynx (GSL), and esophagus, as well as the major salivary glands and oral cavity. The study was approved by the Institutional Review Board of the University of Michigan. Details of the outlining of the swallowing structures, including the PCs and their schematic division into 3 parts: Superior (SPC), middle (MPC), and inferior (IPC), and the GSL, as well as the details of IMRT objectives, planning and delivery, have been published elsewhere (4, 17). All patients received concurrent chemotherapy – weekly carboplatin and paclitaxel – as detailed elsewhere (4, 17). Feeding tubes (FTs) were inserted only if weight loss during therapy approached 10%.
Objective assessment of swallowing was made by videofluoroscopy (VF) (18). VFs were performed pre-therapy and at 3, 12 and 24 months post-therapy. VF summary score was calculated using the Swallowing Performance Scale (19), detailed elsewhere (4, 17). Aspirations were defined as events on VF in which the food/liquid bolus passed past the level of the glottis (17). Patient-reported dysphagia (PRD) was assessed with the Eating Domain of the Head and Neck Quality of Life questionnaire (HNQOL) (20) and the Swallowing Question from the University of Washington Head and Neck-related QOL questionnaire (UWQOL) (21). The scoring of the eating Domain and the Swallowing Question is detailed elsewhere (4, 17). The patients filled the questionnaires pre-therapy and at follow-up clinical visits. Observer-rated dysphagia (ORD) was scored 0–4 at consultation and at each follow-up visit based on Common Terminology Criteria for Adverse Events (CTCAE)v2.0, as was detailed elsewhere (4, 17).
In order to take into account the potential effects of different fraction doses on organ function, the 2 Gy/fraction-equivalent organ dose distributions (EQD2) were calculated, by converting each point in the 3D dose distributions to normalized isoeffective doses, using the linear-quadratic model, assuming α/β ratio of 3 Gy for late effects, or 10 Gy for early effects (which may be relevant if late dysphagia is a consequential effect of acute mucositis) (23). The nominal mean doses, the mean EQD2s, and various partial organ volumes receiving 40, 45, 50, 55, 60, 65, or 70 Gy (VDs), were then calculated for each organ in each patient.
Statistical Methods
To assess the unadjusted effect of baseline parameters on the dysphagia outcomes (worsened aspiration relative to baseline and impaired swallowing based on VF scores, HNQOL Eating Domain, and UW Swallowing scores), we modeled each outcome variable measured longitudinally during post-therapy using the linear mixed-effects model for continuous outcomes, such as Eating domain scores, or generalized linear mixed-effects model with logit link, for dichotomized outcomes such as aspiration. The unadjusted effects of the parameters (univariate estimates) were each obtained using a model adjusted only to time since therapy. Differences in mean organ doses between patients with and without worsening of swallow (“events”) were tested by two-tailed independent t-tests.
Parameters (TD50 and m) for a normal tissue complication probability (NTCP) versus mean dose model were computed using maximum likelihood methods as described previously (24). In brief, this model assumes, for a population of patients, a normal distribution of complications as a function of mean dose. The distribution is characterized by a mean TD50 and a standard deviation m·TD50. Summation of that distribution with increasing dose produces the cumulative NTCP for the population (sigmoid-shaped curve). The maximum likelihood NTCP method requires binary definition of complications rather than continuous parameters. The definition of “complication” in the VF-based aspiration was determined as any increase in aspiration based on VF ≥ 12 months post-therapy compared with pre-therapy. A “complication” related to the VF summary score was defined as an increase from 0–4 (no or mild dysphagia) pre-therapy to ≥5 (moderate or severe dysphagia) at ≥12 months. Defining a “complication” in the patient-reported scores was based on an observation of the longitudinal trend of the scores at 12, 18, and 24 months compared to baseline. For the UWQOL Swallow scores, an increase (worsening) of ≤20 (on a 0–100 scale) at any of these time points was in most cases non-reproducible, with most patients reporting improvement to base-line scores at the other time points. In comparison, any worsening by >20 points was associated with worse scores compared to the baseline scores at all other 12–24 months time points. Therefore, worsening of UWQOL Swallow score by >20 points at any time ≥12 months was considered a “complication”. Similar assessment of the longitudinal trend of the HNQOL Eating Domain scores showed that an increase (worsening) of scores from ≤30 at baseline to ≥50 at any time point between 12–24 months was reproducible in all the other time points, therefore this change was considered a “complication” for the related NTCP calculation. An observer-based CTCAE “complication” was defined as worsening from grade 0–1 pre-therapy to grade ≥2 or from grade 2 to ≥3.
Results
Seventy three patients participated in the study. Patient and tumor characteristics are detailed in Table 1. HPV status was available for 49 patients (67%). Of the 49, 13 (27%) were HPV− (all were previous/current smokers) and 36 (73%) were HPV+, of whom 11 (31%) had <10 pack-year smoking history. Thus, 11/49 (22%) of the patients with known HPV status had both HPV+ cancer and non/minimal smoking history.
Table 1.
Baseline patient and tumor characteristics
| Characteristic | Number (%) |
|---|---|
| Age in years | |
| Median (range) | 55 (50–78) |
| Gender | |
| Male | 65 (89) |
| Female | 8 (11) |
| Tumor Location | |
| Tonsil | 35 (48) |
| Base of tongue | 38 (52) |
| Gross tumor volume, mL | |
| Median (range) | 110 (19–378) |
| T stage | |
| 1 | 9 (12) |
| 2 | 29 (40) |
| 3 | 17 (23) |
| 4 | 18 (25) |
| N stage | |
| 0 | 6 (8) |
| 1 | 6 (8) |
| 2 | 55 (75) |
| 3 | 6 (8) |
| AJCC stage | |
| III | 9 (12) |
| IVA | 58 (80) |
| IVB | 6 (8) |
| Smoking status | |
| Never* | 26 (36) |
| Previous | 31 (42) |
| Current | 16 (22) |
Abbreviations: RT=radiation therapy, AJCC=American Joint Committee on Cancer
Patients who never smoked reported less than ten pack-year cigarettes or equivalent life-time tobacco use.
Sixty-eight (93%) received at least six of the planned seven cycles of weekly concurrent chemotherapy and the remaining five received 5 cycles each. All patients received the prescribed total RT dose with no interruptions apart from holidays.
Details of the longitudinal results of the various dysphagia assessments are provided elsewhere (17). During therapy, 21 patients (29%) required FTs and 6–7% still require supplemental FT feeding at 3–6 months. One patient was FT dependent after 6 months (observer-rated grade 3). This patient presented pre-therapy with dysphagia grade 2. At ≥12 months 94% of patients had observer-rated dysphagia grade 0–1. No modeling of observer-rated post-therapy dysphagia was performed due to the small number (four) of patients with grades ≥2 after 6 months.
For all dysphagia endpoints, mean PC, GSL, and esophageal doses were highly significant after adjusting for clinical variables (p<0.001). Dosimetric parameters including the mean doses and selected VDs for the whole PCs as well as the superior, middle and inferior PCs, the GSL, and esophagus, are presented in Table 2. Strong, highly statistically significant correlations were observed among all the VDs for each of the organs: PCs and each of the parts of the PCs (r>0.9), GSL (r>0.88), and esophagus (r>0.9, except for volumes received by high doses to the esophagus, which were rare). Furthermore, highly significant correlations were observed between each VD and the mean dose to the respective organ, with Spearman correlation coefficients r ≥0.8 (p<0.001) in almost all cases. The correlation coefficients for the PCs are demonstrated in Table 3, and similar results were obtained for each of the parts of the PCs as well as GSL and esophagus. These high correlations resulted in a redundancy of the inclusion of any VD in the dose-response models when the organ mean dose was included. Therefore, the VDs were excluded from further dose-response modeling and the mean doses served as the sole dosimetric variables.
Table 2.
Dosimetric parameters: mean doses and partial volumes receiving specified doses (VDs) for the swallowing related organs: Means ±standard deviations
| TnQTable2V70 (%) | 33 ±21 | 44 ±25 | 26 ± 28 | 7 ± 20 | 13 ± 21 | 4 ± 16 |
PC: pharyngeal constrictors. GSL: Glottic and supraglottic larynx.
Table 3.
Correlations among the pharyngeal constrictors (PCs) mean dose and the partial volumes of the PCs receiving specified doses (VDs). Pearson’s correlation coefficients (r) are provided. All correlations were highly statistically significant.
| PC Mean Dose | PC V40 | PC V45 | PC V50 | PC V55 | PC V60 | PC V65 | PC V70 | |
|---|---|---|---|---|---|---|---|---|
| PC Mean Dose | 0.94 | 0.96 | 0.97 | 0.97 | 0.96 | 0.93 | 0.83 | |
| PC V40 | 0.94 | 0.98 | 0.96 | 0.92 | 0.88 | 0.79 | 0.64 | |
| PC V45 | 0.96 | 0.98 | 0.98 | 0.96 | 0.92 | 0.84 | 0.70 | |
| PC V50 | 0.97 | 0.96 | 0.98 | 0.98 | 0.95 | 0.88 | 0.75 | |
| PC V55 | 0.97 | 0.92 | 0.96 | 0.98 | 0.98 | 0.92 | 0.79 | |
| PC V60 | 0.96 | 0.88 | 0.92 | 0.95 | 0.98 | 0.96 | 0.83 | |
| PC V65 | 0.93 | 0.79 | 0.84 | 0.88 | 0.92 | 0.96 | 0.90 | |
| PC V70 | 0.83 | 0.94 | 0.70 | 0.75 | 0.79 | 0.83 | 0.90 |
Next, we assessed whether the mean doses to any part of the PCs (the SPC, MPC, and IPC) were stronger predictors of outcomes compared with the mean dose to the whole PC. For increases in aspirations and worsening VF summary scores, PC and SPC mean doses had similar predictive value (OR 1.13 and 1.17, respectively; p=0.001 each) while MPC and IPC, though highly significant, had slightly weaker association (OR 1.11 and 1.06, respectively, p=0.004 and 0.005, respectively). For patient-reported dysphagia, PC and SPC mean doses were most significant predictors of the UWQOL Swallow (p=0.001 each) though the mean doses to the IPC and MPC were also significant predictors (p=0.03 and p=0.02, respectively), while the PC and IPC mean doses (p=0.001 each) were most significantly associated with the HNQOL Eating Domain scores, followed by the SPC and MPC (p=0.004 and 0.03 respectively).
For the small number (five) of VF-based strictures, PC mean doses were highly associated with their risk (mean 68±4 Gy in patients with vs 55±9 Gy in patients without strictures, p=0.008), as well as the mean doses to each of the individual constrictors, and GSL mean doses (57±14 Gy in patients with strictures vs 40±15 Gy in patients without strictures, p=0.027). The most significant predictor of strictures was esophageal mean doses (48±17 Gy in patients with vs 27±12 Gy in patients without strictures, p=0.004).
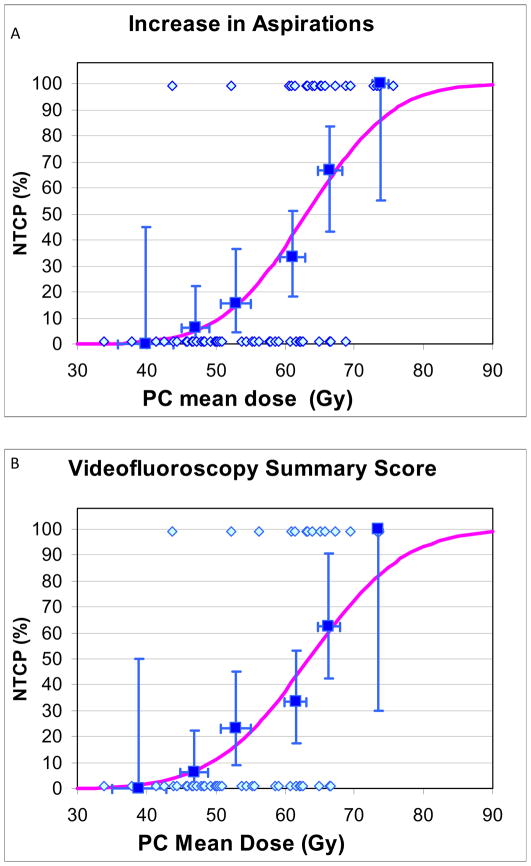
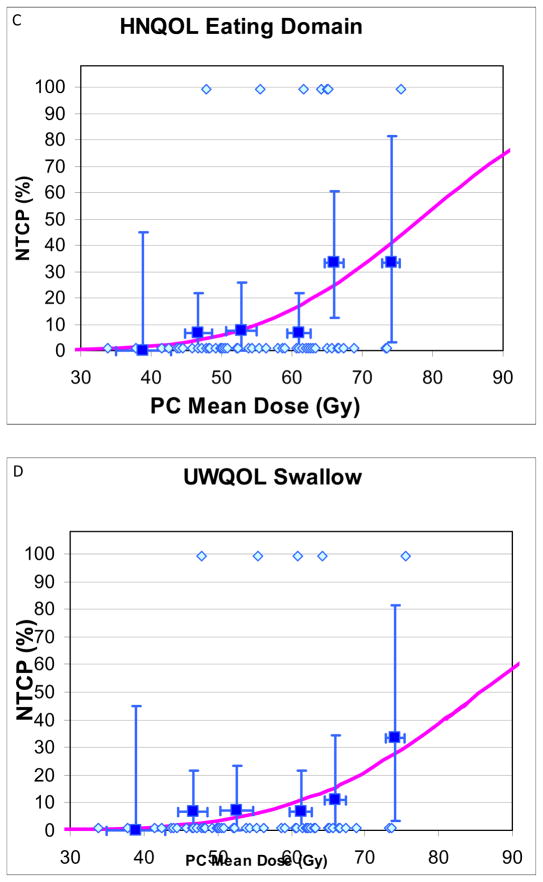
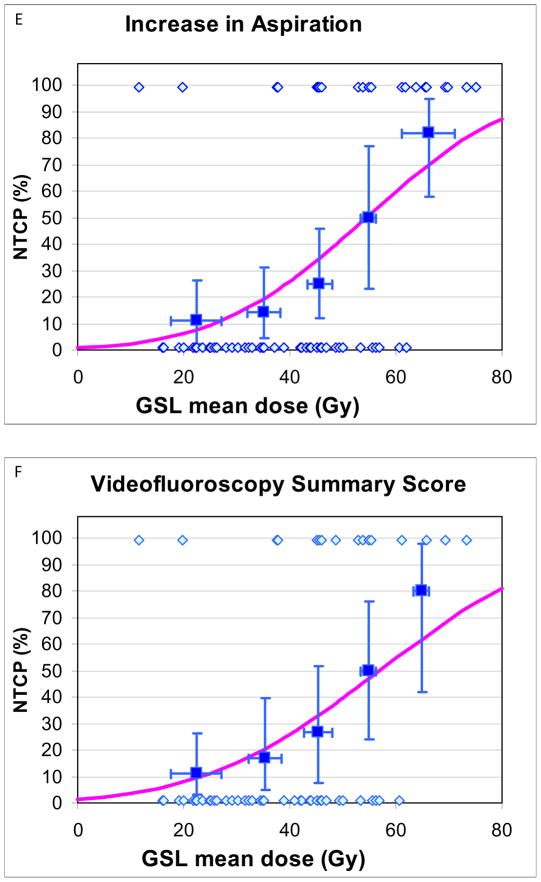
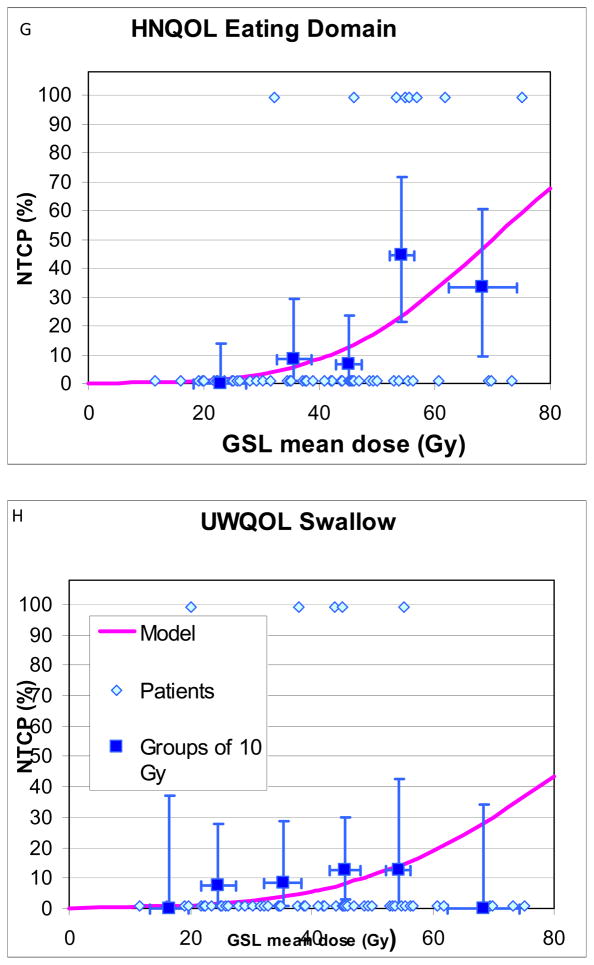
Details of patients with and without “complications”, and their mean PC and GSL doses, used for NTCP calculations and plotting, are provided in Table 4. NTCP curves are provided in Fig 1. NTCPs increased at moderate rates as mean doses increased, without any apparent threshold. For increased aspirations or worsening VF scores, PC TD50 and TD25 were 63Gy and 56Gy, respectively, (Fig 1A–B), and GSL TD50 and TD25 were 56Gy and 39Gy, respectively (Figs 1E–F). For both PC and GSL, TD 50 and TD25 for patient-reported worsened swallowing (Figs 1C–D, G–H) were substantially higher compared with the VF-based doses. Also, the 95% CIs in the derived TD50 values were smaller for the VF-based compared with the patient-reported-based NTCPs (Figs 1A–H)). The “m” parameters were small (0.16–0.23) for the PC fits but trended larger (0.31–0.46) for GSL, likely related to greater inter-patient heterogeneity among the GSL dose distributions, reflected in higher standard deviations around the mean doses (Table 2). No significant differences in the NTCP fits were observed when nominal mean doses or mean doses computed from EQD2 dose distributions (with either alpha/beta ratios of 10Gy or 3Gy) were used.
Table 4.
Patients with or without “complications” at ≥12 months, and their PC and GSL doses. See definition of “complications” in Methods.
| Worsened VF scores | Increased VF-based aspirations | Worsened HNQOL Eating Domain | Worsened UWQOL swallow | ||
|---|---|---|---|---|---|
| No. patients with ‘complications”/total assessable patients | 16/58 | 21/67 | 8/59 | 5/60 | |
| Mean PC doses (Gy) | |||||
| Patients with “complications” | 62±8 | 64±8 | 62±8 | 61±10 | |
| Patients without “complications” | 52±8 | 53±8 | 54±9 | 55±9 | |
| p | <0.001 | <0.001 | 0.16 | 0.04 | |
| Mean GSL doses (Gy) | |||||
| Patients with “complications” | 48±16 | 53±17 | 54±12 | 40±13 | |
| Patients without “complications” | 36±12 | 36±12 | 38±14 | 40±15 | |
| p | 0.004 | 0.001 | 0.03 | 0.9 |
Abbreviations: NTCPs- normal tissue complications probabilities. PC- pharyngeal constrictors. GSL- glottis & supraglottic larynx. HNQOL- Head &Neck Quality of Life questionnaire. UWQOL-University of Washington Quality of Life questionnaire.
Fig 1.
Normal tissue complication probablility (NTCP) curves for long-term increase in various measures of videofluoroscopy-based and patient-reported dysphagia after therapy compared with pre-therapy, vs. doses to the pharyngeal constrictors (PC) (A–D) and glottic-supraglottic larynx (GSL) (E–H). UWQOL: University of Washington quality of life questionnaire. HNQOL: Head-neck cancer quality of life questionnaire.
In each figure the symbols at the bottom represent patients with no “complications” and the at the top patients with “complications”. The horizontal error bars are bins of 7 Gy for the PC plots and 10 Gy for the GSL plots. The y axis error bars represent 80% confidence intervals. The definitions of “complications” are detailed in Methods.
Discussion
This study demonstrates significant correlations of swallowing-related organ mean doses with various subjective and objective measures of worsened dysphagia after therapy compared to the baseline. An important aspect is the difference in the dose-effect relationships between the objective VF-based assessments of dysphagia and patient-reported worsening of dysphagia, the former having substantially lower TD50 compared with the latter. The rates of observer-rated (CTCAE-based) moderate/severe dysphagia (grade ≥2) at >6 months were very low in this study and precluded a meaningful statistical evaluation of this endpoint. This low rate is the likely outcome of the efforts to reduce dysphagia by sparing the non-involved parts of the swallowing-related organs.
What is the reason for the different dose-effect relationships of patient-reported and objective, VF-based dysphagia? One of the most important post-chemo-RT VF-based abnormalities is aspirations, which may be correlated with clinical aspiration pneumonia (2, 17). Some VF-based aspirations are ‘silent”, are not noticed by the patients and do not elicit a cough response, likely due to sensory decline after chemo-RT (1). Thus, functional swallow abnormalities after chemo-RT, which are clinically relevant, may not be appreciated by the patients, resulting in a shift of the patient-reported dose-response curve compared to the objective measurement-based curve. Another explanation is the shifting over time of patient-reported outcomes representing either a true improvement or an accommodation to their new functional status, termed “response shift” (25). These issues relate to the lack of a gold-standard for assessing dysphagia (26); In the absence of a single gold standard measure of dysphagia (and of other symptoms), reporting multiple measures using different and complementary well-developed methodologies is the current state of the art.
Which dosimetric measure should be used as a constraint or an objective for IMRT plans in efforts to reduce dysphagia? The mean doses as well as several VDs of the swallowing organs were highly statistically significant predictors of all endpoints of dysphagia. An additional important finding was very high correlations between the mean doses and the VDs, making the VDs redundant. Other studies found significant correlations between multiple VDs and various dysphagia measures, but did not test for inter-correlations or for correlations of the VDs with the mean doses (6,13,14). We have previously encountered similar findings in the parotid glands: a redundancy of the VDs when the mean dose is included in the dose-effect model (24). This phenomenon likely exists in all organs in any study in which the treatment techniques and dose distribution patterns are similar among the study patients. Thus, the mean dose is likely the only metric required for IMRT planning aiming to spare the swallowing organs.
What are the mean doses which should guide IMRT planning? Our previous study of MRI-based anatomical changes in the PCs three months after chemo-RT compared with pre-therapy showed clear dose-effect relationships for these changes, which were significantly more apparent at mean doses>50 Gy (27). The doses we have found in the current study regarding VF-assessed swallow dysfunction were TD50 and TD25 of 63Gy and 56Gy, respectively, for the PCs, and 56Gy And 39Gy, respectively, for the GSL, while the corresponding TDs for significant patient-reported worsening of swallow were substantially higher. The dosimetric measures in previous studies of dose-effect relationships for dysphagia have recently been summarized (28). The mean PC doses above which the risk of dysphagia increases significantly range in these studies between 45–60 Gy (28). Our study found TDs which are consistent with moderate slopes of the dose-effect relationships, rather than any threshold. In our view, using the VF-based TD25 as goals for optimization would be reasonable, and at the University of Michigan we now use the VF-based TD25 for the PC and GSL as dosimetric goals.
There are several reasons for the differences in the dose-effect relationships between the different published series. The first reason is differences in therapies, notably the inclusion in some series of many patients receiving RT alone without concurrent chemotherapy, which is expected to reduce the risk of dysphagia (14). Other reasons include different assessments of dysphagia in different series: aspiration and objective imaging (4,5,10,14), feeding tube dependency (12,13,15), patient reported dysphagia (4,6,9), strictures (5,13) or observer-reported like RTOG, CTCAE, or Performance Status Scale (6,9). The importance of the end-point used to measure dysphagia has been demonstrated in the current study, as different TD50 and TD25 were found for different endpoints. Additional reasons for the differences among the series relate to the nature of the study: while the current study was prospective and took into account the changes from pre- to post-therapy swallow measures, almost all other studies (except for 11, 14) were retrospective, assessing overt dysphagia at a single point in time after therapy. The problems inherent in the retrospective assessment of dose-response relationships for dysphagia are illustrated in the study by Langerman et al who compared the rates of aspiration before and after chemo-RT of HN cancer (29). While aspiration rates increased in oral cavity and oropharyngeal cancer, they actually decreased in patients with laryngeal cancer, from 37% pre-therapy to 28% post-therapy, likely due to reduced laryngeal dysfunction following tumor shrinkage. Had these authors performed only post-therapy tests, they might have concluded that the high RT doses delivered to the larynx in the patients with laryngeal cancer may have caused a 28% rate of aspirations. Similarly, both the functional results from the current series (17) as well as others (30) demonstrate that in some patients therapy achieves an improvement in dysphagia compared to the pre-therapy baseline. Thus, prospective studies that include the pre-therapy data are the most reliable means to evaluate dose-effect relationships.
Another potential cause of different reports of dose-effect relationships is different methods to delineate the organs. For example, drawing the PCs anatomically, as was done in this study, results in different mean doses compared to drawing only the posterior pharyngeal wall, without differences in the IMRT plans and dose distributions (31). This issue deserves further study. Also, we strived to spare the non-involved parts of the whole larynx, including both its glottis and supraglottic parts, as we hypothesized that the effect of RT on both may cause dysphagia (3), and therefore reported the doses to the GSL rather than the glottis alone. This reporting differed from some other series which reported the doses to the glottis alone (14). As other investigations reported significant correlations between the doses to the supraglottic larynx and dysphagia (6, 10), we feel that reporting GSL doses, and aiming to spare the GSL, rather than the glottic larynx alone, are justified. On the other hand, sparing of the glottic larynx in cases of oropharyngeal cancer is usually easier than sparing the supraglottic larynx, especially where the vallecula is included in the targets. Substantial sparing of the glottis can be equally achieved by split-field technique or, as done in the current study, by whole-neck IMRT, if the glottic larynx is assigned a high priority in the optimization cost function, and slight under-dosage of the lymphatic CTVs in the low neck is accepted (32).
Which are most important: the dose to the SPC, IPC, or larynx, in determining dysphagia? The answer: it depends on where the primary tumor is. This study has confirmed our previous results: in OPC, all of the above are statistically significant, with the SPC having the highest p value. Similar results were reached by others who assessed dose-response relationships in patients with OPC (9, 14, 15). In comparison, series which included patients with all cancer sites, including larynx cancer, found that the doses to the IPC or the larynx are most significantly correlated with dysphagia (5,10, 13). Thus, in series of OPC, where the larynx and IPC receive low or moderate doses while the SPC receives the highest doses, the doses to the latter likely determine the risk of dysphagia. In comparison, patients with laryngeal cancers receive high IPC and laryngeal doses, and these determine in these patients the risk and rate of dysphagia. An example was provided by Caglar et al (5): in the whole patient population, which included laryngeal and oropharyngeal cancers, only the laryngeal doses were statistically significant predictors of dysphagia, however, when only the OPC patients in that series were analyzed, the SPC became statistically significant. The bottom-line: An effort should be made to spare all the swallowing structures when possible.
In conclusion, this study has demonstrated significant relationships between long-term swallowing dysfunction and the mean doses delivered to the swallowing organs. These data supply dosimetric guidelines for efforts to reduce late dysphagia by reducing the doses to the swallowing structures. The dependence of the NTCPs on the specific methods used to assess dysphagia, found in this study, highlights the need to establish a “gold tandard” for dysphagia evaluation.
Acknowledgments
Supported by NIH Grants PO1 CA59827 and the Newman Family Foundation.
Eric Chanowski and Assuntina Sacco for data management.
Footnotes
Presented in part at the 52nd Annual Meeting of the American Society of Therapeutic Radiology and Oncology (ASTRO), San Diego, CA, October 31–Nov 4, 2010.
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation. J Clin Oncol. 2006;24:2636–43. doi: 10.1200/JCO.2006.06.0079. [DOI] [PubMed] [Google Scholar]
- 2.Eisbruch A, Lyden T, Bradford CR, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:23–8. doi: 10.1016/s0360-3016(02)02712-8. [DOI] [PubMed] [Google Scholar]
- 3.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425–39. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 4.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68:1289–98. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 5.Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts for swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:1110–8. doi: 10.1016/j.ijrobp.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 6.Dirix P, Abbeel S, Vanstraelen B, et al. Dysphagia after chemoradiotherapy for head and neck cancer: dose-effect relationships for the swallowing structures. Int J Rad Onc Biol Phys. 2009;75:385–392. doi: 10.1016/j.ijrobp.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 7.Dornfeld K, Simmons JR, Karnell L, et al. Radiation doses to structures within and adjacent to the larynx are correlated with long-term diet- and speech-related quality of life. Int J Radiat Oncol Biol Phys. 2007;68:750–7. doi: 10.1016/j.ijrobp.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 8.Werbrouck J, De Ruyck K, Duprez F, et al. Acute normal tissue reactions in head-and-neck cancer patients treated with IMRT: influence of dose and association with genetic polymorphisms in DNA DSB repair genes. Int J Radiat Oncol Biol Phys. 2009;73:1187–95. doi: 10.1016/j.ijrobp.2008.08.073. [DOI] [PubMed] [Google Scholar]
- 9.Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx. Radiother Oncol. 2007;85:64–73. doi: 10.1016/j.radonc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Jensen K, Lambertsen K, Grau C. Late swallowing dysfunction and dysphagia after radiotherapy for pharynx cancer: correlation with dose and volume parameters. Radiother Oncol. 2007;85:74–82. doi: 10.1016/j.radonc.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Langendijk JA, Doornaert P, Rietveld DH, et al. A predictive model for swallowing dysfunction after curative radiotherapy in head and neck cancer. Radiother Oncol. 2009;90:189–95. doi: 10.1016/j.radonc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Li B, Li D, Lau DH, et al. Clinical-dosimetric analysis of measures of dysphagia in head and neck cancer. Radiation Oncology. 2009;4:52. doi: 10.1186/1748-717X-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caudell JJ, Schaner PE, Desmond RA, et al. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy. Int J Rad Oncol Biol Phys. 2010;76:403–409. doi: 10.1016/j.ijrobp.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Scwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term dysphagia following oropharyngeal IMRT. Int J Rad Onc Biol Phys. doi: 10.1016/j.ijrobp.2009.10.002. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanguineti G, Gunn GB, Parker BC, et al. Weekly dose-volume parameters of mucosa and constrictor muscles predict the need for feeding tubes. Int J Rad Onc Biol Phys. 2010 doi: 10.1016/j.ijrobp.2009.10.057. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 16.Caudell JJ, Schaner PE, Meredith RF, et al. Factors associated with long-term dysphagia after definitive radiotherapy. Int J Rad Onc Biol Phys. 2009;73:410–415. doi: 10.1016/j.ijrobp.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 17.Feng FY, Kim HM, Lyden TH, et al. Intensity modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol. 2010;28:2732–8. doi: 10.1200/JCO.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Logemann JA. Evaluation and treatment of swallowing disorders. 2. Austin, TX: PRO-ED; 1998. [Google Scholar]
- 19.Karnell MP, MacCracken E. A database information storage and reporting system for videofluorographic oropharyngeal motility (OPM) swallowing evaluations. Am J Speech Language Pathol. 1994;3:54–60. [Google Scholar]
- 20.Terrell JE, Nanavati KA, Esclamado RM, et al. Head and neck cancer-specific quality of life: instrument validation. Arch Otolaryngol Head Neck Surg. 1997;123:1125–32. doi: 10.1001/archotol.1997.01900100101014. [DOI] [PubMed] [Google Scholar]
- 21.Hassan SJ, Weymuller EA., Jr Assessment of quality of life in head and neck cancer patients. Head Neck. 1993;15:485–96. doi: 10.1002/hed.2880150603. [DOI] [PubMed] [Google Scholar]
- 22.Thomas L, Jones TM, Tandon S, et al. University of Washington Quality of Life swallowing domain following oropharyngeal cancer. Eur Arch Otorhinolaryngol. 2008;265(Suppl 1):S29–37. doi: 10.1007/s00405-007-0470-2. [DOI] [PubMed] [Google Scholar]
- 23.Bentzen SM, Overgaard J. Clinical normal-tissue radiobiology. In: Tobias JS, Thomas PRM, editors. Current Radiation Oncology. Vol. 2. London; Arnold: 1995. pp. 37–67. [Google Scholar]
- 24.Eisbruch A, Ten Haken RK, Kim HM, et al. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity modulated irradiation of head and neck cancer. Int J Rad Onc Biol Phys. 1999;45:577–587. doi: 10.1016/s0360-3016(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 25.Jansen SJ, Stiggelbout AM, Nooij MA, et al. Response shift in quality of life measurement in breast cancer patients undergoing radiotherapy. Qual Life Res. 2000;9:603–15. doi: 10.1023/a:1008928617014. [DOI] [PubMed] [Google Scholar]
- 26.Gluck I, Feng FY, Lyden TH, et al. Evaluating and reporting dysphagia in trials of chemoirradiation for head and neck cancer. Int J Radiat Oncol Biol Phys. 2010;77:727–33. doi: 10.1016/j.ijrobp.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popovtzer A, Cao Y, Feng FY, Eisbruch A. Anatomical changes in the pharyngeal constrictors after chmo-irradiation of head and neck cancer and their dose-effect relationships : MRI-based study. Radiother Oncol. 2009;93 :510–10. doi: 10.1016/j.radonc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rancati T, Schwartz M, Allen A, et al. Radiation dose-volume effects in the larynx and pharynx. Int J Rad Onc Biol Phys. 2010;76 (3 Suppl):S63–S68. doi: 10.1016/j.ijrobp.2009.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langerman A, MacCracken E, Kasza K, et al. Aspiration in chemoradiated patients with head and neck cancer. Arch Otolaryngol Head neck Surg. 2007;133:1289–95. doi: 10.1001/archotol.133.12.1289. [DOI] [PubMed] [Google Scholar]
- 30.Salama JK, Stenson KM, List MA, et al. Characteristics associated with swallowing changes after concurrent chemo-radiotherapy. Arch otolaryngol Head neck Surg. 2008;134:1060–5. doi: 10.1001/archotol.134.10.1060. [DOI] [PubMed] [Google Scholar]
- 31.Vineberg KA, Eisbruch A, Feng M. Consequences of pharyngeal constrictor contouring method on dose calculation and optimization. Abstract, 52nd Annual meeting of the American Society of Therapeutic Radiation Oncology (ASTRO); San Diego, CA. Oct 31–Nov 4 2010. [Google Scholar]
- 32.Webster G, Rowbottom CG, Ho KF, et al. Evaluation of larynx sparing techniques with IMRT when treating the head and neck. Int J Rad Onc Biol Phys. 2008;72:617–622. doi: 10.1016/j.ijrobp.2008.06.1495. [DOI] [PubMed] [Google Scholar]