Abstract
Nitric oxide (NO) produced by inducible NO synthase (iNOS) is responsible for endotoxin-induced vascular hyporeactivity and hypotension resulting in multiple organ failure. Endotoxic shock is also characterized by decreased expression of constitutive cyclooxygenase (COX-1), cytochrome P450 (CYP) 4A and endothelial NOS (eNOS). Our previous studies demonstrated that dual inhibition of iNOS and COX with a selective COX-2 inhibitor, NS-398, or a non-selective COX inhibitor, indomethacin, restores blood pressure presumably due to increased production of 20-hydroxyeicosatetraenoic acid (20-HETE) derived from arachidonic acid (AA) by CYP4A in endotoxaemic rats. The aim of this study was to investigate the effects of piroxicam, a preferential COX-1 inhibitor, on the endotoxin-induced changes in blood pressure, expression of COX-1, inducible COX (COX-2), CYP4A1, eNOS, iNOS and heat shock protein 90 (hsp90), and production of PGI2, PGE2, 20-HETE and NO. Injection of endotoxin (10 mg/kg, i.p.) to male Wistar rats caused a fall in blood pressure and an increase in heart rate associated with elevated renal 6-keto-PGF1α and PGE2 levels as well as an increase in COX-2 protein expression. Endotoxin also caused an elevation in systemic and renal nitrite levels associated with increased renal iNOS protein expression. In contrast, systemic and renal 20-HETE levels and renal expression of eNOS, COX-1 and CYP4A1 were decreased in endotoxaemic rats. The effects of endotoxin, except for renal COX-1 and eNOS protein expression, were prevented by piroxicam (10 mg/kg, i.p.), given 1 hr after injection of endotoxin. Endotoxin did not change renal hsp90 protein expression. These data suggest that a decrease in the expression and activity of COX-2 and iNOS associated with an increase in CYP4A1 expression and 20-HETE synthesis contributes to the effect of piroxicam to prevent the hypotension during rat endotoxaemia.
Enhanced expression of inducible nitric oxide (NO) synthase (iNOS) in many tissues in response to mediators released by endotoxin leads to increased generation of NO, which contributes to the fall in blood pressure, vascular hyporeactivity, multiple organ failure and the high mortality rate that are associated with septic shock [1–5]. Systemic blockade of iNOS opposes the fall in blood pressure in endotoxic shock [2,3,5]. This is not only due to withdrawal of the vasodilator effects of NO, but also is associated with increased activity of vasoconstrictor arachidonic acid (AA) products, such as 20-hydroxyeicosatetraenoic acid (20-HETE) [2,6]. Moreover, recent studies not only demonstrate the importance of the constitutive endothelial cell isoform of NOS (eNOS) for the up-regulation of pro-inflammatory protein expression, but also indicate the autoregulation of NOS expression by NO, since iNOS-derived NO is known to inhibit the expression and activity of eNOS [7–14]. Collectively, these data give rise to the hypothesis that eNOS plays a key role in the protein expression of iNOS and the pathogenesis of endotoxic shock.
20-HETE, an eicosanoid synthesized from AA primarily by cytochrome P450 (CYP) isoforms of the 4A and 4F classes in the vasculature, is one of the primary eicosanoids produced in the microcirculation [6,15]. 20-HETE participates in the regulation of vascular tone in several vascular beds, including kidney [6,15–19]. It has been reported that 20-HETE-induced constriction is abolished by inhibition of cyclooxygenase (COX) or with an endoperoxide/thromboxane receptor antagonist [16–19]. It has also been demonstrated that prostaglandin analogues of 20-HETE, 20-OH-PGG2 and 20-OH-PGH2, produced by COX in vascular endothelial cells mediate the vasoconstrictor effects of 20-HETE [18,19]. As opposed to its vasoconstrictor effect, 20-HETE also produces vasodilation in the renal, coronary, pulmonary and basilar arteries [20–23] through NO release [23] and conversion of 20-HETE to 20-OH-PGE2 and 20-OH-PGF2α by COX [18,21,24], and increased formation of PGE2 [21] and prostacyclin (PGI2) [20–22].
It has been reported that NO inhibits renal CYP4A1/A3 protein expression, CYP ω-hydroxylase activity and 20-HETE production [25–27]. We have also demonstrated that administration of a synthetic analogue of 20-HETE, N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine (5,14-HEDGE), prevents hypotension and vascular hyporeactivity associated with the changes in systemic and tissue NO production as well as iNOS protein expression in renal, cardiac and vascular tissues of rats treated with endotoxin [28–34]. These findings suggest that NO-induced inhibition of 20-HETE production and removal of its influence on vascular tone contributes to the endotoxin-induced cardiovascular changes.
COX-1 is constitutively expressed in several cell types and is responsible for prostaglandin release under physiological conditions, whereas COX-2 is expressed at high levels upon induction [35]. The down-regulation of COX-1 and up-regulation of inducible COX (COX-2) has also been reported to contribute to the systemic hypotension, multi-organ failure, and decreased survival in animals and humans with sepsis [36,37]. Non-selective COX inhibitors, such as indomethacin, prevent [38] or do not improve [39] the lethal effects of endotoxin in animal models of sepsis. The beneficial effects of indomethacin are correlated with decreased levels of nitrite and prostanoids in biological fluids from endotoxaemic rats [40]. Indomethacin also abolishes or significantly attenuates the decrease in MAP [41] or has no significant effect on blood pressure in endotoxaemic rats [42]. Our previous studies suggested that dual inhibition of iNOS and COX by indomethacin restores MAP presumably due to increased production of 20-HETE derived from AA by CYP4A in endotoxaemic rats [32]. More recently, we have demonstrated that dual inhibition of PGI2 and PGE2 synthesis and NO production by a selective COX-2 inhibitor, NS-398, restores blood pressure due to increased systemic and renal levels of 20-HETE [43]. Although COX-1 has also shown to be involved in the endotoxin-induced inflammatory response [44–49], there has been no previous attempt to examine the effects of COX-1 inhibitors on the hypotension and production of eicosanoids and NO during endotoxaemia in rodents. Therefore, we investigated the effects of piroxicam, a preferential COX-1 inhibitor, on the endotoxin-induced changes in blood pressure, renal COX-1, COX-2, CYP4A1, eNOS, iNOS and heat shock protein 90 (hsp90) protein expression as well as production of vasodilator prostanoids, PGI2 and PGE2 and a vasoconstrictor eicosanoid, 20-HETE, in rats. Preliminary results have been presented in abstract form [50,51].
Materials and Methods
Endotoxic shock model
Experiments were performed on male Wistar rats (n = 53) (Research Center of Experimental Animals, Mersin University, Mersin, Turkey) weighing 250 to 300 g that were fed a standard chow. The rats were housed in an animal facility with a 12-hr light:dark cycle. All experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Ethics Committee of Mersin University School of Medicine. Endotoxic shock was induced as previously described by Tunctan et al. [28]. Rats were randomly divided into saline (n= 21), endotoxin (n= 20), piroxicam (n= 6) and endotoxin+piroxicam (n= 6) groups. In the saline and piroxicam groups, rats received saline (4 ml/kg, i.p.) at time 0. Rats in the endotoxin and endotoxin+piroxicam groups were treated with endotoxin (Escherichia coli lipopolysaccharide, O111:B4; Sigma Chemical Co., St. Louis, MO, USA) (10 mg/kg, i.p.; sublethal dose) [28,52] at time 0. In the piroxicam and endotoxin+piroxicam groups, rats were treated with piroxicam (Sigma Chemical, St. Louis, MO, USA), at the dose of 10 mg/kg (i.p.) that inhibits prostanoid production in rat models of inflammation [53], 1 hr after injection of saline or endotoxin, respectively. MAP and heart rate (HR) were measured using a tail-cuff device (MAY 9610 Indirect Blood Pressure Recorder System, Commat Ltd., Ankara, Turkey) during a control period at time 0 and 1, 2, 3 and 4 hr. All animals were survived during the experiments. The rats were euthanized 4 hr after the administration of endotoxin, and blood samples and kidneys were collected from all animals. Sera were obtained from blood samples by centrifugation at 23,910 × g for 15 min. at 4°C and stored at −20°C until analysed for the measurement of eicosanoid and nitrite levels. The tissues were homogenized in 1 ml of an ice-cold 20 mM HEPES buffer (pH 7.5) containing 20 mM β-glycerophosphate, 20 mM sodium pyrophosphate, 0.2 mM sodium orthovanadate, 2 mM EDTA, 20 mM sodium fluoride, 10 mM benzamidine, 1 mM dithiothreitol, 20 mM leupeptin and 10 mM aprotinin [29]. The samples were centrifuged at 23,910 × g for 15 min. at 4°C and then supernatants were removed and stored at −20°C until analysed for the measurement of eicosanoids and nitrite levels, and α-smooth muscle actin, COX-1, COX-2, CYP4A1, eNOS, iNOS and hsp90 protein expression. The total protein amount was determined by Coomassie blue method using bovine serum albumin (BSA) as standard [28].
Immunoblotting
Immunoblotting for α-smooth muscle actin, COX-1, COX-2, CYP4A1, eNOS, iNOS and hsp90 proteins were performed according to the method as described by previously [30,34]. Briefly, tissue homogenates (100 μg of protein) were subjected to a 10% SDS-polyacrylamide gel electrophoresis and then proteins were transferred to a nitrocellulose membrane. The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline (mmol/L: Tris-HCl 25 [pH 7.4], NaCl 137, KCl 27 and 0.05% Tween 20) and incubated overnight with monoclonal antibodies (1:500 in 5% BSA) of anti-COX-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-COX-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-CYP4A1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-eNOS (Calbiochem, San Diego, CA, USA), anti-iNOS (BD Transduction Laboratories, San Jose, CA, USA) or anti-hsp90 (Calbiochem, San Diego, CA, USA) followed by incubation with a sheep anti-mouse immunoglobulin secondary antibody conjugated with horse radish peroxidase (Amersham Life Sciences, Cleveland, OH, USA) (1:1,000 in 0.1% BSA) for 1 hr. The blots were developed with enhanced chemiluminescence (ECL) (ECL Plus Western Blotting Detection Reagents) (Amersham Life Sciences, Cleveland, OH, USA) according to the manufacturer’s instructions. Immunoreactive proteins were visualized using a gel imaging system (EC3-CHEMI HR imaging system) (Ultra-Violet Products, UVP, UK). Densitometric analysis was performed with NIH image software (ImageJ 1.29). The same membrane was used to determine α-actin expression using a monoclonal antibody against α-smooth muscle actin (Sigma Chemical Co., St. Louis, USA) (1:1,000 in 5% BSA) and the content of the latter was used to normalize the expression of COX-1, COX-2, CYP4A1, eNOS, iNOS and hsp90 proteins in each sample.
Measurement of systemic and renal eicosanoid levels
Serum and tissue 6-keto-PGF1α, PGE2 and 20-HETE concentrations were measured as indexes for COX and CYP4A activity by ELISA according to the manufacturer’s instructions in the 6-keto-PGF1α and PGE2 (Cayman Chemical Co., Ann Arbor, MI, USA) and 20-HETE (Detroit R&D, Inc., Detroit, MI, USA) assay kits, respectively.
Measurement of systemic and renal nitrite levels
Nitrite (stable product of NO) levels in the sera and tissue homogenates were measured using the diazotization method based on the Griess reaction as an index for NOS activity [28,54]. Briefly, samples (50 μl) were pipetted into 96-well microtitre plates and an equal volume of Griess reagent (1% sulphanylamide (25 μl) and 0.1% N-1-naphtylethylenediamine dihydrochloride (25 μl) in 2.5% ortophosphoric acid) was added to each well. After incubation for 10 min. at room temperature, absorbance was measured at 550 nm with a microplate reader. Standard curves were also constructed using sodium nitrite concentrations ranging from 0.25–50 μM.
Statistical analysis
All data were expressed as means ± SEM. Data were analysed by one-way ANOVA followed by Student-Newman-Keuls test for multiple comparisons, Kruskal-Wallis test followed by Dunns test for multiple comparisons and Student’s t or Mann-Whitney U tests when appropriate. A P value < 0.05 was considered to be statistically significant.
Results
Effect of piroxicam on the cardiovascular response to endotoxin
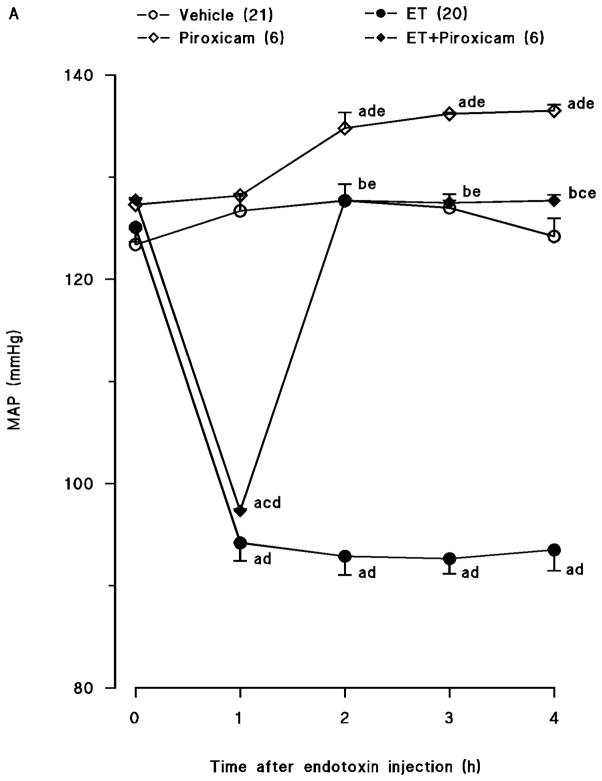
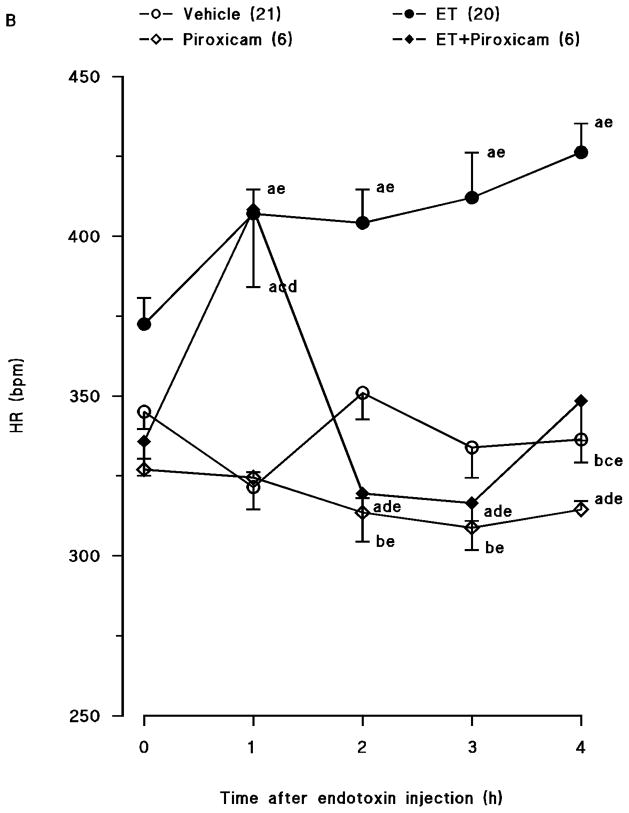
Endotoxin caused a gradual fall in MAP (fig. 1A) and an increase in HR (fig. 1B) over the 4-hr course of the experiment (p < 0.05). The changes in MAP and HR reached a maximum 4 hr after the administration of endotoxin. MAP fell by 31 mmHg and HR rose by 90 bpm in rats treated with endotoxin. Piroxicam prevented the fall in MAP and the increase in HR in rats given endotoxin (p < 0.05). An increase in MAP and a decrease in HR were also observed 1, 2 and 3 hr after injection of piroxicam in vehicle-treated rats (p < 0.05).
Fig. 1.
Time course of the effects of piroxicam on (A) mean arterial presure (MAP) and (B) heart rate (HR) following administration of saline (vehicle) (4 ml/kg, i.p.) or endotoxin (ET) (10 mg/kg, i.p.) to conscious rats. Piroxicam (10 mg/kg, i.p.) was given 1 hr after administration of endotoxin. Data are expressed as means ± SEM. Numbers in parentheses indicate the number of animals studied per group. aSignificant difference from the corresponding value seen in rats treated with saline (vehicle) (p < 0.05). bSignificant difference from the corresponding value seen in rats treated with endotoxin (p < 0.05). cSignificant difference from the corresponding value seen in rats treated with vehicle and piroxicam (p < 0.05). dSignificant difference from the time 0 hr value within a group (p < 0.05). eSignificant difference from the time 1 hr value within a group (p < 0.05).
Effect of piroxicam on the endotoxin-induced changes in COX-1 and COX-2 protein expression and prostanoid production
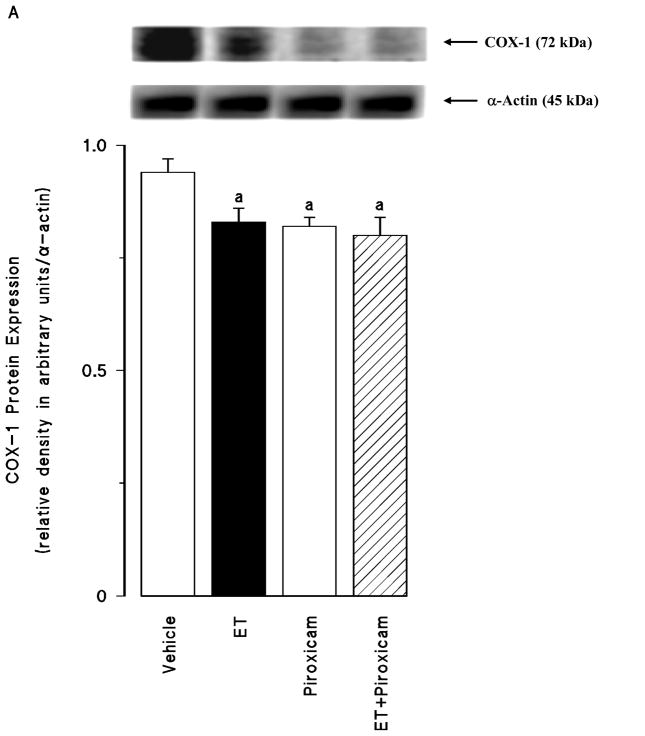
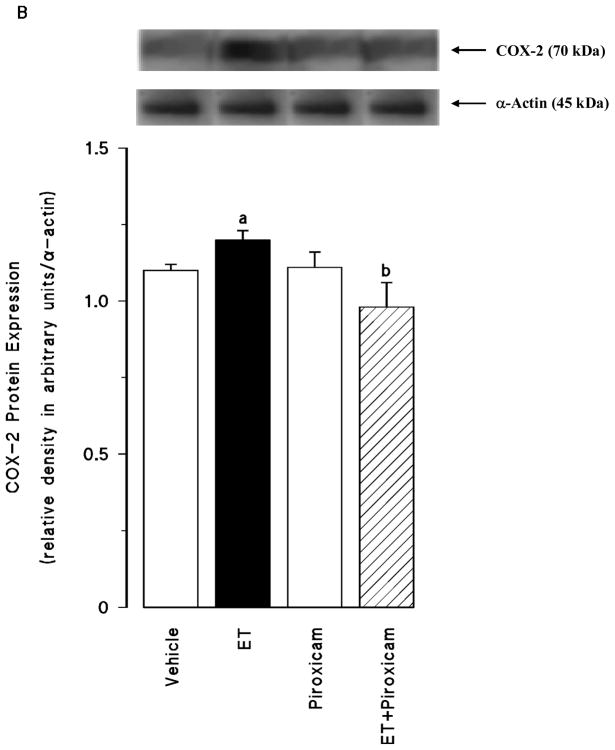
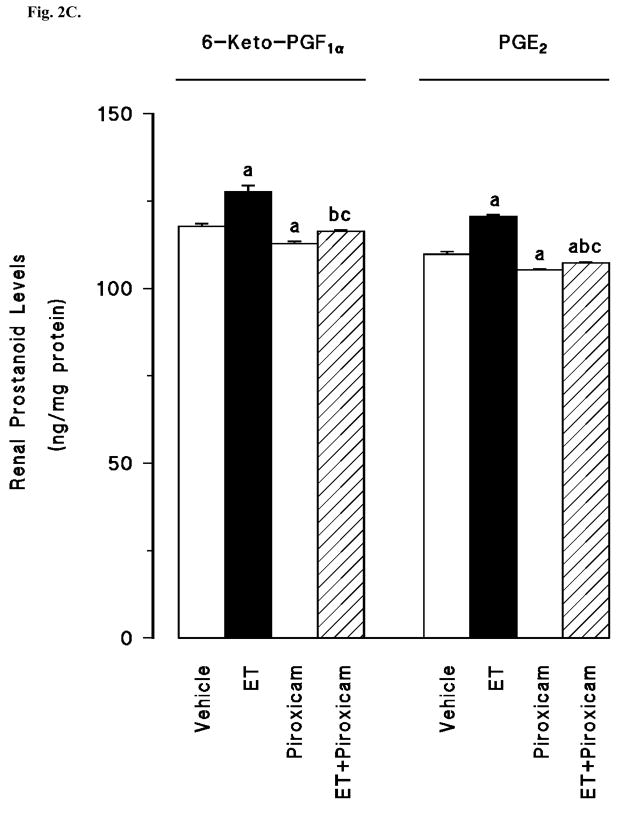
Renal COX-1 protein levels were decreased in the tissues of endotoxaemic rats (p < 0.05) (fig. 2A). Piroxicam had no effect on the decrease of COX-1 protein expression in the tissues of rats treated with endotoxin (p > 0.05). Piroxicam also caused a decrease in the basal levels of COX-1 protein when given to rats treated with vehicle (p < 0.05). In contrast to COX-1, COX-2 protein levels were increased in the tissues of endotoxaemic rats (p < 0.05) (fig. 2B). The increase in COX-2 protein expression was prevented in the tissues of rats treated with endotoxin and piroxicam. Piroxicam had no effect on the basal levels of COX-2 protein in vehicle-treated rats (p > 0.05). The endotoxin-induced increase in COX-2 protein expression was also associated with an increase in renal 6-keto-PGF1α and PGE2 levels (p < 0.05) (fig. 2C). Inhibition of COX-1 by piroxicam prevented the increase in tissue levels of 6-keto-PGF1α and PGE2 in rats given endotoxin (p < 0.05). Piroxicam caused a decrease in renal 6-keto-PGF1α and PGE2 levels when given to rats treated with vehicle (p < 0.05).
Fig. 2.
The effects of piroxicam on changes in (A) constitutive cyclooxygenase (COX-1) protein expression, (B) inducible cyclooxygenase (COX-2) protein expression, and (C) renal prostanoid levels measured 4 hr after saline (vehicle) (4 ml/kg, i.p.) or endotoxin (ET) (10 mg/kg, i.p.) injection to Wistar rats. Piroxicam (10 mg/kg, i.p.) given at 1 hr after saline or ET injection. Data are expressed as means ± SEM of 4–6 animals. aSignificant difference from the corresponding value seen in rats treated with saline (vehicle) (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with endotoxin (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with vehicle and piroxicam (p < 0.05).
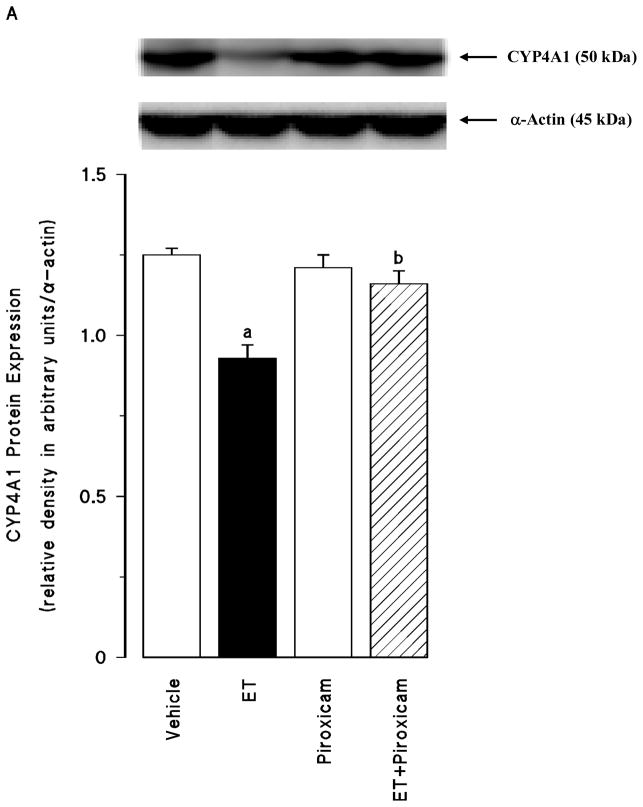
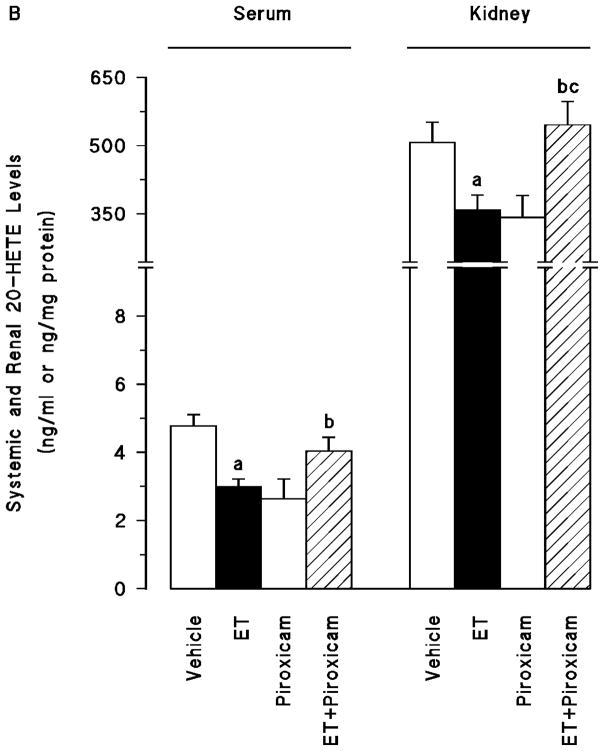
Effect of piroxicam on the endotoxin-induced decrease in CYP4A1 protein expression and 20-HETE production
Endotoxin caused a decrease in renal CYP4A1 protein expression (p < 0.05) (fig. 3A) associated with decreased systemic and renal levels of 20-HETE (p < 0.05) (fig. 3B). Piroxicam prevented the decrease in the CYP4A1 protein expression and 20-HETE synthesis in the tissues of rats treated with endotoxin (p > 0.05). Piroxicam had no effect on the basal levels of CYP4A1 protein and 20-HETE levels when given to rats treated with vehicle (p > 0.05).
Fig. 3.
The effects of piroxicam on changes in (A) cytochrome P450 (CYP) 4A1 protein expression and (B) systemic and renal 20-hydroxyeicosatetraenoic acid (20-HETE) levels measured 4 hr after saline (vehicle) (4 ml/kg, i.p.) or endotoxin (ET) (10 mg/kg, i.p.) injection to Wistar rats. Piroxicam (10 mg/kg, i.p.) given at 1 hr after or saline ET injection. Data are expressed as means ± SEM of 4–8 animals. aSignificant difference from the corresponding value seen in rats treated with saline (vehicle) (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with endotoxin (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with vehicle and piroxicam (p < 0.05).
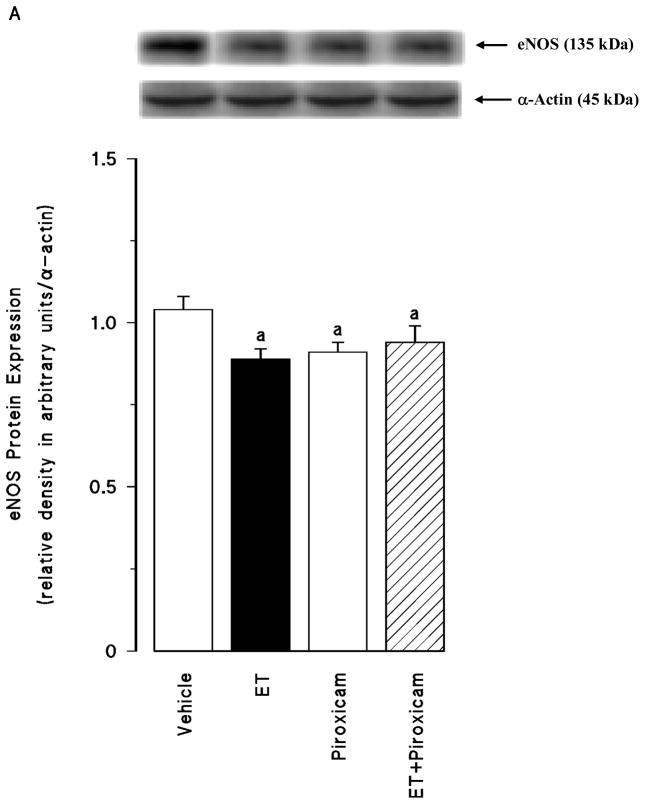
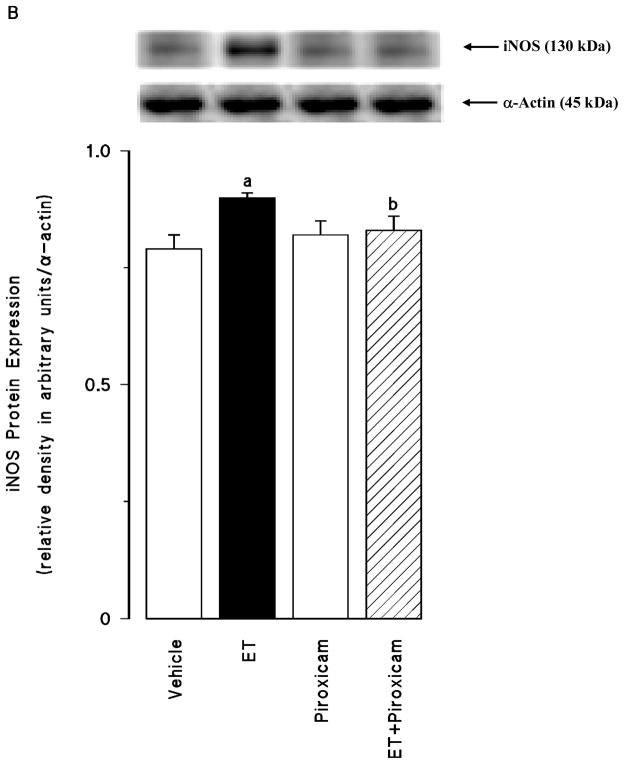
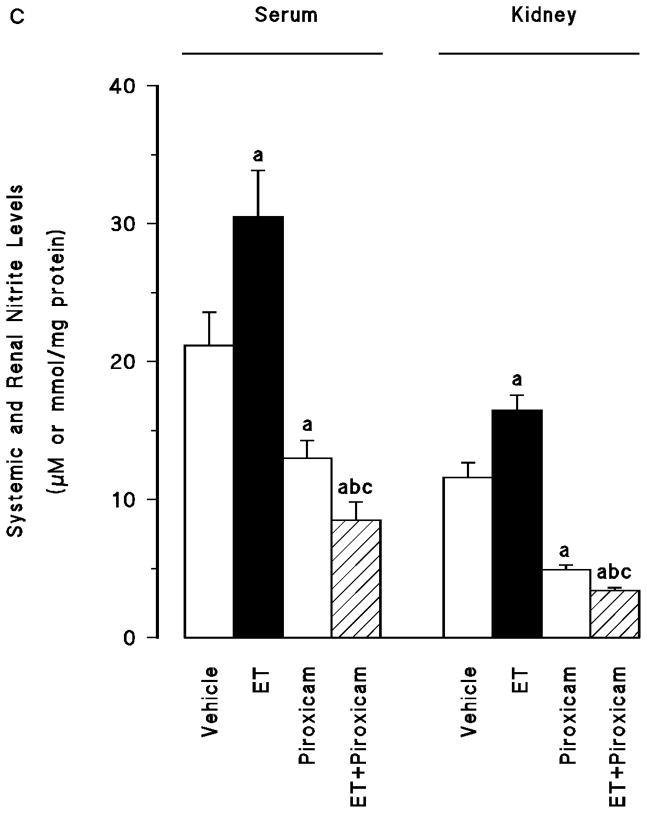
Effect of piroxicam on the endotoxin-induced changes in eNOS and iNOS protein expression and NO production
Renal eNOS protein levels were decreased in the tissues of endotoxaemic rats (p < 0.05) (fig. 4A). Piroxicam had no effect on the decrease in the eNOS protein expression in the tissues of rats treated with endotoxin (p > 0.05). Piroxicam also caused a decrease in the basal levels of eNOS protein when given to rats treated with vehicle (p < 0.05). In contrast to eNOS, renal iNOS protein levels were increased in the tissues of endotoxaemic rats (p < 0.05) (fig. 4B). The increase in iNOS protein expression was prevented in the tissues of rats treated with endotoxin and piroxicam. Piroxicam had no effect on the basal levels of iNOS protein in vehicle-treated rats (p > 0.05). Endotoxin-induced increase in iNOS protein expression was also associated with an increase in systemic and renal nitrite levels (p < 0.05) (fig. 4C). Inhibition of COX-1 by piroxicam prevented the increase in serum and tissue levels of nitrite in rats given endotoxin (p < 0.05). Piroxicam also caused a decrease in nitrite levels when given to rats treated with vehicle (p < 0.05).
Fig. 4.
The effects of piroxicam on changes in (A) endothelial nitric oxide synthase (eNOS) protein expression, (B) inducible nitric oxide synthase (iNOS) protein expression and (C) systemic and renal nitrite levels measured 4 hr after saline (vehicle) (4 ml/kg, i.p.) or endotoxin (ET) (10 mg/kg, i.p.) injection to Wistar rats. Piroxicam (10 mg/kg, i.p.) were given at 1 hr after or saline ET injection. Data are expressed as means ± SEM of 6–19 animals. aSignificant difference from the corresponding value seen in rats treated with saline (vehicle) (p < 0.05). bSignificant difference from the corresponding value seen in rats treated with endotoxin (p < 0.05). cSignificant difference from the corresponding value seen in rats treated with vehicle and piroxicam (p < 0.05).
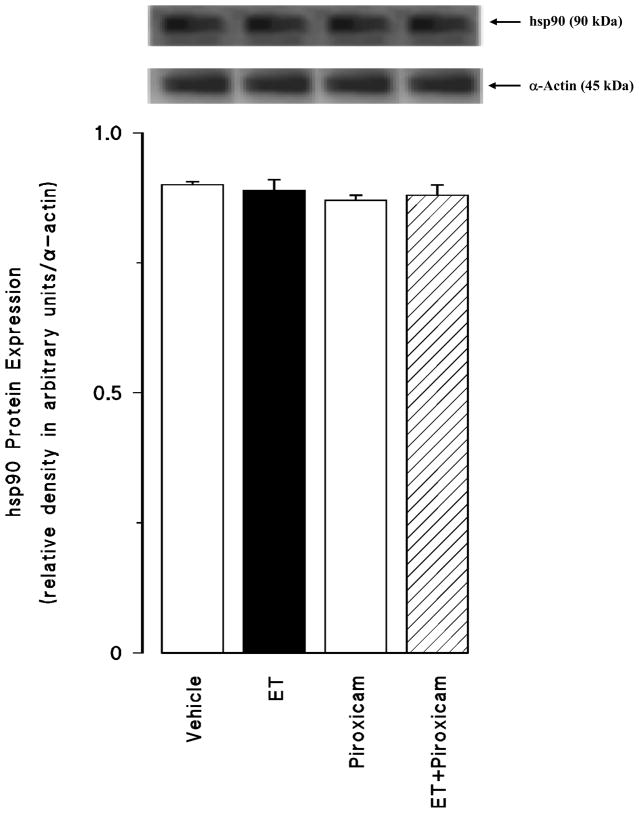
Effect of piroxicam on the hsp90 protein expression
Endotoxin did not alter the expression level of hsp90 in the renal tissue (p > 0.05) (fig. 5). Piroxicam had also no effect on the basal hsp90 protein expression in the tissues of rats treated with vehicle (p > 0.05).
Fig. 5.
The effects of piroxicam on changes in heat shock protein 90 (hsp90) protein expression measured 4 hr after saline (vehicle) (4 ml/kg, i.p.) or endotoxin (ET) (10 mg/kg, i.p.) injection to Wistar rats. Piroxicam (10 mg/kg, i.p.) were given at 1 hr after or saline ET injection. Data are expressed as means ± SEM of 6 animals.
Discussion
The results of the present study indicate that an increase in the expression and activity of COX-2 and iNOS associated with decreased CYP4A1 protein levels and 20-HETE synthesis contributes to the fall in blood pressure in rats treated with endotoxin. These data also demonstrate that decreased production of vasodilator prostanoids, PGI2 and PGE2, and NO formation by piroxicam, a preferential COX-1 inhibitor, restores MAP and HR due to increased systemic and renal levels of 20-HETE in endotoxaemic rats.
There are several reports suggesting a direct link between AA metabolites and NO under physiological and pathophysiological conditions [6,15,55,56]. The constitutive isoforms of COX and NOS enzymes play an important role in the regulation of several physiological states. On the other hand, under inflammatory conditions such as endotoxic shock, the inducible isoforms of these enzymes are induced in a variety of cells resulting in the production of large amounts of prostanoids and NO. Increasing evidence suggests that there is a considerable cross-talk between COX, NOS and CYP4A enzymes. Indeed, AA and its metabolites generated by COX isoforms have been shown to interfere with NO biosynthesis [55,56]. NO has also been demonstrated to activate COX enzymes, an event leading to overt production of prostanoids [55,56]. Moreover, it has been reported that NO inhibits renal CYP ω-hydroxylase activity and the production of 20-HETE [6,15], suggesting contribution of increased production of COX-2-derived prostanoids and NO to the endotoxin-induced decrease in CYP4A expression and activity during rat endotoxaemia. In the present study, the endotoxin-induced fall in MAP and rise in HR were associated with an increase in renal COX-2 expression and production of PGI2 and PGE2. In endotoxin-treated rats, increased renal iNOS protein levels were also associated with an increase in the systemic and renalP nitrite levels. In contrast to COX-2 and iNOS, COX-1 and eNOS protein levels were decreased in the kidneys of endotoxaemic rats. Endotoxin also caused a decrease in renal CYP4A1 protein expression associated with a decrease in systemic and renal 20-HETE levels. These effects of endotoxin, except for COX-1 and eNOS protein expression, were prevented by piroxicam. Our previous findings with a selective iNOS inhibitor, 1,3-PBIT, demonstrate that activation of mitogen-activated protein kinase kinase-1/extracellular signal-regulated kinase-1/2/iNOS/soluble guanylyl cyclase/protein kinase G pathway contributes to the fall in MAP and vascular reactivity in endotoxaemic rats, and the endotoxin-induced increase in iNOS-derived NO production suppresses renal CYP4A protein expression and activity [30,57]. We have also previously demonstrated that the fall in MAP in endotoxaemic rats is associated with a decrease in the expression of CYP4A1/A3 protein in the kidney and increased levels of nitrite in the serum, kidney, heart, thoracic aorta and superior mesenteric artery [28–32]. Furthermore, administration of a synthetic analogue of 20-HETE, 5,14-HEDGE, prevented the hypotension and vascular hyporeactivity associated with the changes in systemic and tissue NO production as well as endotoxin-induced cardiac, renal and vascular iNOS protein expression and endotoxin-induced decrease in eNOS protein levels in rats treated with endotoxin [33,34]. These findings suggest that iNOS-derived NO-induced inhibition of the formation of an AA metabolite, most likely 20-HETE, and removal of its influence on vascular tone contributes to the fall in blood pressure and vascular hyporeactivity in endotoxic shock. We have recently demonstrated that prostanoids produced during endotoxaemia increase iNOS protein expression and NO synthesis, and decrease CYP4A1 protein expression and CYP4A activity [32]. Moreover, dual inhibition of iNOS or COX by indomethacin restored renal CYP4A protein level and CYP4A activity and MAP in endotoxin-treated rats, suggesting that the effects of non-selective inhibition of COX might be due to increased production of AA metabolites derived via CYP4A. More recently, we have demonstrated that dual inhibition of PGI2 and PGE2 synthesis, and NO production by a selective COX-2 inhibitor restores blood pressure due to increased systemic and renal levels of 20-HETE [43]. Based on the results from previous studies [28–34,43,58] and our present findings, these results suggest that a decrease in the expression and activity of COX-2 and iNOS associated with increased expression of CYP4A1 and 20-HETE levels contributes to the effect of piroxicam to prevent the endotoxin-induced decrease in MAP and increase in HR in rats. In the present study, while piroxicam had no effect on the MAP and HR in vehicle-treated rats, it caused a decrease in renal COX-1 and eNOS protein expression as well as systemic and/or renal levels of 6-keto-PGF1α, PGE2 and nitrite. A possible mechanism for the effect of piroxicam might be through inhibition of endogenously produced NO on COX-1 expression and prostanoid formation. It is also possible that piroxicam might inhibit COX-1 activity leading to a decrease in eNOS expression and NO synthesis. However, additional experiments need to be conducted to demonstrate the validity of the proposed hypothesis. Further characterization of the molecular mechanisms of interactions between COX, NOS and CYP4A enzymes will provide the framework for extension of this work into understanding the role of arachidonic acid products and NO on the decrease in blood pressure during endotoxaemia.
A chaperone molecule, hsp90, has been identified as a signalling molecule in the activation of all the isoforms of NOS [58]. Hsp90 associates with NOS and facilitates its phosphorylation, and thereby increases NO production. Vo et al. [13] demonstrated that maximal vascular iNOS expression and its function are achieved via the up-regulation and increased association of eNOS with hsp90, and that in the absence of functional eNOS, the vascular effects of iNOS are delayed during rodent endotoxaemia. Yoshida and Xia [58] also reported that hsp90 is an important post-translational modulator of iNOS in iNOS-transfected cells. On the other hand, there are several studies reporting that endotoxin up-regulates iNOS protein, but does not alter basal hsp90 expression in in vitro [60] and in vivo studies [61]. Recently, Cheng et al. [62] have shown that 20-HETE impairs NO production in vitro and its function in vivo by inhibiting association of eNOS with hsp90. We have previously demonstrated that 5,14-HEDGE did not prevent the endotoxin-induced decrease in endothelium-dependent relaxations induced by acetylcholine in thoracic aorta and superior mesenteric artery, although it reversed the effects of endotoxin on vascular hyporeactivity to norepinephrine and overproduction of NO in the tissues [33]. More recently, we have demonstrated that 5,14-HEDGE decreases iNOS-hsp90 association (unpublished data). In the present study, the endotoxin-induced fall in systemic and renal 20-HETE levels was accompanied by increased iNOS protein levels and decreased eNOS protein expression without alterations in hsp90 expression in the kidneys of endotoxaemic rats. These effects of endotoxin, except for eNOS protein expression, were not prevented by piroxicam. Based on these data and our previous findings [28–31,33], it is likely that iNOS-derived NO inhibits renal eNOS protein expression in endotoxaemic rats. On the other hand, the ineffectiveness of piroxicam in preventing the decrease in eNOS protein expression does not completely support this hypothesis. However, the negative feedback of iNOS on eNOS expression could be explained by several mechanisms. For example, down-regulation of eNOS at the posttranscriptional level associated with up-regulation of iNOS mRNA by endotoxin [63] may contribute to the decrease in eNOS protein levels. Several kinases such as Rho-kinase, p38 mitogen-activated protein kinase, 5′-adenosine monophosphate-activated protein kinase, protein kinase A and protein kinase G as well as impaired phosphotidylinositol 3-kinase/Akt pathway have been shown to modulate eNOS expression and activity [64,65]. Therefore, further studies are required to clarify mechanisms of the endotoxin-induced decrease in eNOS protein expression. More importantly, these results indicate that an increase in 20-HETE production contributes to the effect of piroxicam to prevent the increase in iNOS protein expression during rat endotoxaemia. Although we did not investigate the effect of piroxicam on iNOS at the transcriptional level in the present study, it is possible that piroxicam might directly or indirectly, via 20-HETE, PGI2, PGE2 and/or transcription factors (such as NF-κB) [55,56], inhibits iNOS mRNA expression leading to a decrease in iNOS protein expression and NO production in endotoxaemic rats. Further characterization of the molecular mechanisms of piroxicam on eNOS and iNOS protein expression and association of these enzymes with hsp90 will provide the framework for extension of this work in understanding the role of 20-HETE and NO on the decrease in blood pressure during endotoxaemia.
In conclusion, the present study indicates that a decrease in the eNOS, COX-1 and CYP4A1 protein levels and the fall in the synthesis of 20-HETE, a vasoconstrictor arachidonic acid metabolite, and an increase in the formation of vasodilator prostanoids, PGI2 and PGE2, and NO is associated with increased COX-2 and iNOS protein expression in rats treated with endotoxin. Our findings also demonstrate that tissue hsp90 protein expression level does not change in endotoxaemic rats. Overall, these findings provide cogent evidence that an increase in renal CYP4A1 protein expression and 20-HETE levels associated with a decrease in protein expression and activity of COX-2 and iNOS contributes to the effect of piroxicam to prevent the hypotension during rat endotoxaemia. Our results suggest that an increase in 20-HETE levels by piroxicam causes dual inhibition of iNOS and COX-2 protein expression and activity in endotoxaemic rats. Although COX-1 has shown to be involved in the endotoxin-induced inflammatory response [44–50], our results suggest that COX-1 is not responsible for the hypotension and production of eicosanoids and NO during endotoxaemia in rodents. Impairment of renal and cardiovascular function is critically involved in the pathophysiological sequelae in septic shock finally resulting in multiorgan failure and death; restoration of these impaired functions should improve therapeutic benefit. In light of the important role of prostanoids, 20-HETE, and NO in endotoxin-induced hypotension, vascular hyporeactivity, multiple organ failure, and mortality, the interaction of CYP4A, COX and NOS pathways should be considered when developing new strategies for drug development in the treatment of septic shock.
Acknowledgments
This work was supported by the Research Foundation of Mersin University (Project Code No: BAP ECZF EMB (BT) 2006-3 and BAP SBE EMB (TC) 2008-6 DR), Novartis Turkey, USPHS NIH Grant HLBI-19134-34, and USPHS NIH Grant HLBI-19134-33A1. We greatly acknowledge Dr. John R. Falck for all of his helpful advice.
References
- 1.Kleinert H, Schwarz PM, Förstermann U. Regulation of the expression of inducible nitric oxide synthase. Biol Chem. 2003;384:1343–64. doi: 10.1515/BC.2003.152. [DOI] [PubMed] [Google Scholar]
- 2.Tunctan B, Altug S. The use of nitric oxide synthase inhibitors in inflammatory diseases: a novel class of anti-inflammatory agents. Cur Med Chem Anti-Inflammatory & Anti-Allergy Agents. 2004;3:271–301. [Google Scholar]
- 3.Cauwels A. Nitric oxide in shock. Kidney Int. 2007;72:557–65. doi: 10.1038/sj.ki.5002340. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes D, Assreuy J. Nitric oxide and vascular reactivity in sepsis. Shock. 2008;30:10–3. doi: 10.1097/SHK.0b013e3181818518. [DOI] [PubMed] [Google Scholar]
- 5.Hauser B, Bracht H, Matejovic M, Radermacher P, Venkatesh B. Nitric oxide synthase inhibition in sepsis? Lessons learned from large-animal studies. Anesth Analg. 2005;101:488–98. doi: 10.1213/01.ANE.0000177117.80058.4D. [DOI] [PubMed] [Google Scholar]
- 6.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res. 2005;41:175–93. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee A, Catravas JD. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vascul Pharmacol. 2008;49:134–40. doi: 10.1016/j.vph.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buga GM, Griscavage JM, Rogers NE, Ignarro LJ. Negative feedback regulation of endothelial cell function by nitric oxide. Circ Res. 1993;73:808–12. doi: 10.1161/01.res.73.5.808. [DOI] [PubMed] [Google Scholar]
- 9.Chauhan SD, Seggara G, Vo PA, Macallister RJ, Hobbs AJ, Ahluwalia A. Protection against lipopolysaccharide-induced endothelial dysfunction in resistance and conduit vasculature of iNOS knockout mice. FASEB J. 2003;17:773–5. doi: 10.1096/fj.02-0668fje. [DOI] [PubMed] [Google Scholar]
- 10.Connelly L, Madhani M, Hobbs AJ. Resistance to endotoxic shock in endothelial nitric-oxide synthase (eNOS) knock-out mice: a pro-inflammatory role for eNOS-derived no in vivo. J Biol Chem. 2005;280:10040–6. doi: 10.1074/jbc.M411991200. [DOI] [PubMed] [Google Scholar]
- 11.Cuzzocrea S, Mazzon E, Di Paola R, Esposito E, Macarthur H, Matuschak GM, et al. A role for nitric oxide-mediated peroxynitrite formation in a model of endotoxin-induced shock. J Pharmacol Exp Ther. 2006;319:73–81. doi: 10.1124/jpet.106.108100. [DOI] [PubMed] [Google Scholar]
- 12.Lu JL, Schmiege LM, 3rd, Kuo L, Liao JC. Downregulation of endothelial constitutive nitric oxide synthase expression by lipopolysaccharide. Biochem Biophys Res Commun. 1996;225:1–5. doi: 10.1006/bbrc.1996.1121. [DOI] [PubMed] [Google Scholar]
- 13.Vo PA, Lad B, Tomlinson JA, Francis S, Ahluwalia A. Autoregulatory role of endothelium-derived nitric oxide (NO) on Lipopolysaccharide-induced vascular inducible NO synthase expression and function. J Biol Chem. 2005;280:7236–43. doi: 10.1074/jbc.M411317200. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita T, Kawashima S, Ohashi Y, Ozaki M, Ueyama T, Ishida T, et al. Resistance to endotoxin shock in transgenic mice overexpressing endothelial nitric oxide synthase. Circulation. 2000;101:931–7. doi: 10.1161/01.cir.101.8.931. [DOI] [PubMed] [Google Scholar]
- 15.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–85. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 16.Escalante B, Omata K, Sessa W, Lee SG, Falck JR, Schwartzman ML. 20-hydroxyeicosatetraenoic acid is an endothelium-dependent vasoconstrictor in rabbit arteries. Eur J Pharmacol. 1993;235:1–7. doi: 10.1016/0014-2999(93)90812-v. [DOI] [PubMed] [Google Scholar]
- 17.Randriamboavonjy V, Busse R, Fleming I. 20-HETE-induced contraction of small coronary arteries depends on the activation of Rho-kinase. Hypertension. 2003;41:801–6. doi: 10.1161/01.HYP.0000047240.33861.6B. [DOI] [PubMed] [Google Scholar]
- 18.Schwartzman ML, Falck JR, Yadagiri P, Escalante B. Metabolism of 20-hydroxyeicosatetraenoic acid by cyclooxygeanse: formation and identification of novel endothelium-dependent vasoconstrictor metabolites. J Biol Chem. 1989;264:11658–62. [PubMed] [Google Scholar]
- 19.Escalante B, Sessa WC, Falck JR, Yadagiri P, Schwartzman ML. Vasoactivity of 20-hydroxyeicosatetraenoic acid is dependent on metabolism by cyclooxygenase. J Pharmacol Exp Ther. 1989;248:229–32. [PubMed] [Google Scholar]
- 20.Carroll MA, Garcia MP, Falck JR, McGiff JC. Cyclooxygenase dependency of the renovascular actions of cytochrome P450-derived arachidonate metabolites. J Pharmacol Exp Ther. 1992;260:104–9. [PubMed] [Google Scholar]
- 21.Fang X, Faraci FM, Kaduce TL, Harmon S, Modrick ML, Hu S, et al. 20-Hydroxyeicosatetraenoic acid is a potent dilator of mouse basilar artery: role of cyclooxygenase. Am J Physiol Heart Circ Physiol. 2006;291:H2301–7. doi: 10.1152/ajpheart.00349.2006. [DOI] [PubMed] [Google Scholar]
- 22.Pratt PF, Falck JR, Reddy KM, Kurian JB, Campbell WB. 20-HETE relaxes bovine coronary arteries through the release of prostacyclin. Hypertension. 1998;31:237–41. doi: 10.1161/01.hyp.31.1.237. [DOI] [PubMed] [Google Scholar]
- 23.Yu M, McAndrew RP, Al-Saghir R, Maier KG, Medhora M, Roman RJ, et al. Nitric oxide contributes to 20-HETE-induced relaxation of pulmonary arteries. J Appl Physiol. 2002;93:1391–9. doi: 10.1152/japplphysiol.00247.2002. [DOI] [PubMed] [Google Scholar]
- 24.Carroll MA, Capparelli MF, Doumand AB, Cheng MK, Jiang H, McGiff JC. Renal vasoactive eicosanoids: interactions between cytochrome P450 and cyclooxygenase metabolites during salt depletion. Am J Hypertens. 2001;14:159A. [Google Scholar]
- 25.Alonso-Galicia M, Sun CW, Falck JR, Harder DR, Roman RJ. Contribution of 20-HETE to the vasodilator actions of nitric oxide in renal arteries. Am J Physiol. 1998;275:F370–8. doi: 10.1152/ajprenal.1998.275.3.F370. [DOI] [PubMed] [Google Scholar]
- 26.Wang MH, Wang J, Chang HH, Zand BA, Jiang M, Nasjletti A, et al. Regulation of renal CYP4A expression and 20-HETE synthesis by nitric oxide in pregnant rats. Am J Physiol. 2003;285:F295–302. doi: 10.1152/ajprenal.00065.2003. [DOI] [PubMed] [Google Scholar]
- 27.Alonso-Galicia M, Drummond HA, Reddy KK, Falck JR, Roman RJ. Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension. 1997;29:320–5. doi: 10.1161/01.hyp.29.1.320. [DOI] [PubMed] [Google Scholar]
- 28.Tunctan B, Korkmaz B, Yildirim H, Tamer L, Atik U, Buharalioglu CK. Increased production of nitric oxide contributes to renal oxidative stress in endotoxemic rat. Am J Infect Dis. 2005;1:111–5. [Google Scholar]
- 29.Tunctan B, Korkmaz B, Yildirim H, Tamer L, Atik U, Buharalioglu CK. Reversal of endotoxin-induced hypotension by inhibition of inducible nitric oxide synthase activity is associated with improved oxidative status in rat heart, aorta and mesenteric artery. Turkish J Med Sci. 2006;36:71–80. [Google Scholar]
- 30.Tunctan B, Yaghini FA, Estes A, Malik KU. Inhibition by nitric oxide and cyclooxygenase of cytochrome P450 4A expression and activity contributes to endotoxin-induced hypotension in rats. Nitric Oxide: Biol Chem. 2006;14:51–7. doi: 10.1016/j.niox.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Tunctan B, Korkmaz B, Dogruer ZN, Tamer L, Atik U, Buharalioglu CK. Inhibition of extracellular signal-regulated kinase (ERK1/2) activity reverses endotoxin-induced hypotension via decreased nitric oxide production in rats. Pharmacol Res. 2007;56:56–64. doi: 10.1016/j.phrs.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Tunctan B, Yaghini FA, Estes A, Malik KU. Prostaglandins inhibit cytochrome P450 4A activity and contribute to endotoxin-induced hypotension in rats via nitric oxide production. Arch Pharm Res. 2008;31:856–65. doi: 10.1007/s12272-001-1238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tunctan B, Korkmaz B, Buharalioglu CK, Sahan Firat S, Anjaiah S, Falck J, et al. A 20-HETE agonist, N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine, opposes the fall in blood pressure and vascular reactivity in endotoxin-treated rats. Shock. 2008;30:329–35. doi: 10.1097/SHK.0b013e31816471c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuez T, Korkmaz B, Buharalioglu CK, Sahan-Firat S, Falck J, Tunctan B, et al. A synthetic analogue of 20-HETE, 5,14-HEDGE, reverses endotoxin-induced hypotension via increased 20-HETE levels associated with decreased iNOS protein expression and vasodilator prostanoid production in rats. Basic Clin Pharmacol. 2009;106:378–88. doi: 10.1111/j.1742-7843.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res. 2009;50:S129–34. doi: 10.1194/jlr.R800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu SF, Newton R, Evans TW, Barnes PJ. Differential regulation of cyclo-oxygenase-1 and cyclo-oxygenase-2 gene expression by lipopolysaccharide treatment in vivo in the rat. Clin Sci. 1996;90:301–6. doi: 10.1042/cs0900301. [DOI] [PubMed] [Google Scholar]
- 37.Tsiotou AG, Sakorafas GH, Anagnostopoulos G, Bramis J. Septic shock; current pathogenetic concepts from a clinical perspective. Med Sci Monit. 2005;11:RA76–85. [PubMed] [Google Scholar]
- 38.Ashorobi RB, Williams PA. Indomethacin and alpha-tocopherol enhanced survival in endotoxic rats. Cent Afr J Med. 1995;41:216–19. [PubMed] [Google Scholar]
- 39.Tunctan B, Altug S, Uludag O, Abacioglu N. Time-dependent variations in serum nitrite, 6-keto-prostaglandin F1α and thromboxane B2 levels induced by lipopolysaccharide in mice. Biol Rhythm Res. 2000;1:499–514. [Google Scholar]
- 40.Futaki N, Takahashi S, Kitagawa T, Yamakawa Y, Tanaka M, Higuchi S. Selective inhibition of cyclooxygenase-2 by NS-398 in endotoxin shock rats in vivo. Inflam Res. 1997;46:496–502. doi: 10.1007/s000110050232. [DOI] [PubMed] [Google Scholar]
- 41.Fatehi-Hassanabad Z, Muller M, Andriantsitohaina R, Furman BL, Parratt JR, Stoclet JC. Influence of indomethacin on the haemodynamic effects of lipopolysaccharide in rats. Fundam Clin Pharmacol. 1996;10:258–63. doi: 10.1111/j.1472-8206.1996.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 42.Vayssettes-Courchay C, Bouysset F, Verbeuren TJ. Involvement of COX and NOS induction in the sympatho-activation during sepsis. Auton Neurosci. 2002;98:33–6. doi: 10.1016/s1566-0702(02)00027-9. [DOI] [PubMed] [Google Scholar]
- 43.Tunctan B, Korkmaz B, Cuez T, Buharalioglu CK, Sahan-Firat S, Falck J, et al. Contribution of vasoactive eicosanoids and nitric oxide production to the effect of selective cyclooxygenase-2 inhibitor, NS-398, on endotoxin-induced hypotension in rats. Basic Clin Pharmacol Toxicol. 2010;107:877–82. doi: 10.1111/j.1742-7843.2010.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adibkia K, Siahi Shadbad MR, Nokhodchi A, Javadzedeh A, Barzegar-Jalali M, Barar J, et al. Piroxicam nanoparticles for ocular delivery: physicochemical characterization and implementation in endotoxin-induced uveitis. J Drug Target. 2007;15:407–16. doi: 10.1080/10611860701453125. [DOI] [PubMed] [Google Scholar]
- 45.Cho JY. Immunomodulatory effect of nonsteroidal anti-inflammatory drugs (NSAIDs) at the clinically available doses. Arch Pharm Res. 2007;30:64–74. doi: 10.1007/BF02977780. [DOI] [PubMed] [Google Scholar]
- 46.Choi SH, Langenbach R, Bosetti F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J. 2008;22:1491–501. doi: 10.1096/fj.07-9411com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dunn AJ, Swiergiel AH. The role of cyclooxygenases in endotoxin- and interleukin-1-induced hypophagia. Brain Behav Immun. 2000;14:141–52. doi: 10.1006/brbi.1999.0580. [DOI] [PubMed] [Google Scholar]
- 48.Gadek-Michalska A, Bugajski J. Role of prostaglandins and nitric oxide in the lipopolysaccharide-induced ACTH and corticosterone response. J Physiol Pharmacol. 2004;55:663–75. [PubMed] [Google Scholar]
- 49.Teeling JL, Cunningham C, Newman TA, Perry VH. The effect of non-steroidal anti-inflammatory agents on behavioural changes and cytokine production following systemic inflammation: implications for a role of COX-1. Brain, Behaviour, and Immunity. 2010;24:409–19. doi: 10.1016/j.bbi.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tunctan B, Korkmaz B, Cuez T, Buharalioglu CK, Sahan-Firat S, Falck J, et al. Interactions between cytochrome P4504A, cyclooxygenase and nitric oxide synthase during endotoxemia: therapeutic implications for inflammatory diseases. EHRLICH II - 2nd World Conference on Magic Bullets, Celebrating the 100th Anniversary of the Nobel Prize Award to Paul Ehrlich; Nurnberg, Germany. October 3–5, (2008); p. A-330. Abstract Book. [Google Scholar]
- 51.Tunctan B, Korkmaz B, Cuez T, Sahan-Firat S, Yildirim H, Tamer L, et al. Increased production of 20-HETE contributes to the effects of COX inhibitors to prevent the decrease in lipid peroxidation and increase in catalase activity during endotoxemia. FASEB J. 2009;23:937.13. [Google Scholar]
- 52.Luss H, Watkins SC, Freeswick PD, Imro AK, Nussler AK, Billiar TR, et al. Characterization of inducible nitric oxide synthase expression in endotoxemic rat cardiac myocytes in vivo and following cytokine exposure in vitro. J Mol Cell Cardiol. 1995;27:2015–29. doi: 10.1016/0022-2828(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 53.Gilroy DW, Tomlinson A, Willoughby DA. Differential effects of inhibitors of cyclooxygenase (cyclooxygenase 1 and cyclooxygenase 2) in acute inflammation. Eur J Pharmacol. 1998;355:211–7. doi: 10.1016/s0014-2999(98)00508-1. [DOI] [PubMed] [Google Scholar]
- 54.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 55.Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev. 2005;57:217–52. doi: 10.1124/pr.57.2.1. [DOI] [PubMed] [Google Scholar]
- 56.Cuzzocrea S, Salvemini D. Molecular mechanisms involved in the reciprocal regulation of cyclooxygenase and nitric oxide synthase enzymes. Kidney Int. 2007;71:290–7. doi: 10.1038/sj.ki.5002058. [DOI] [PubMed] [Google Scholar]
- 57.Korkmaz B, Buharalioglu K, Demiryürek TA, Sahan-Firat S, Cuez T, Tunctan B. Activation of MEK1/ERK1/2/iNOS/sGC/PKG pathway associated with peroxynitrite formation contributes to hypotension and vascular hyporeactivity in endotoxemic rats. Nitric Oxide: Biol Chem. doi: 10.1016/j.niox.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Antonova G, Lichtenbeld H, Xia T, Chatterjee A, Dimitropoulou C, Catravas JD. Functional significance of hsp90 complexes with NOS and sGC in endothelial cells. Clin Hemorheol Microcirc. 2007;37:19–35. [PubMed] [Google Scholar]
- 59.Yoshida M, Xia Y. Heat shock protein 90 as an endogenous protein enhancer of inducible nitric-oxide synthase. J Biol Chem. 2003;278:36953–8. doi: 10.1074/jbc.M305214200. [DOI] [PubMed] [Google Scholar]
- 60.Chakravortty D, Kato Y, Sugiyama T, Koide N, Mu MM, Yoshida T, et al. The inhibitory action of sodium arsenite on lipopolysaccharide-induced nitric oxide production in RAW 267.4 macrophage cells: a role of Raf-1 in lipopolysaccharide signaling. J Immunol. 2001;166:2011–7. doi: 10.4049/jimmunol.166.3.2011. [DOI] [PubMed] [Google Scholar]
- 61.Li FC, Chan JY, Chan SH, Chang AY. In the rostral ventrolateral medulla, the 70-kDa heat shock protein (HSP70), but not HSP90, confers neuroprotection against fatal endotoxemia via augmentation of nitric-oxide synthase I (NOS I)/protein kinase G signaling pathway and inhibition of NOS II/peroxynitrite cascade. Mol Pharmacol. 2005;68:179–92. doi: 10.1124/mol.105.011684. [DOI] [PubMed] [Google Scholar]
- 62.Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA, Jr, et al. 20-hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2008;294:H1018–26. doi: 10.1152/ajpheart.01172.2007. [DOI] [PubMed] [Google Scholar]
- 63.Liu SF, Adcock IM, Old RW, Barnes PJ, Evans TW. Differential regulation of the constitutive and inducible nitric oxide synthase mRNA by lipopolysaccharide treatment in vivo in the rat. Crit Care Med. 1996;24:1219–25. doi: 10.1097/00003246-199607000-00026. [DOI] [PubMed] [Google Scholar]
- 64.Searles CD. Transcriptional and posttranscriptional regulation of endothelial nitric oxide transcription. Am J Physiol. 2006;291:C803–16. doi: 10.1152/ajpcell.00457.2005. [DOI] [PubMed] [Google Scholar]
- 65.Tai SC, Robb GB, Marsden PA. Endothelial nitric oxide synthase: a new paradigm for gene regulation in the injured blood vessel. Arterioscler Thromb Vasc Biol. 2004;24:405–12. doi: 10.1161/01.ATV.0000109171.50229.33. [DOI] [PubMed] [Google Scholar]