Abstract
A minimum in the biological response to materials that is observed to occur within a narrow surface energy range is related to the properties of water at these biology-contacting surfaces. Wetting energetics are calculated using a published theory from which it is further estimated that water molecules bind to these special surfaces through a single hydrogen bond, leaving three other hydrogen bonds to interact with proximal water molecules. It is concluded that, at this Goldilocks Surface, the local chemical environment of surface-bound water is nearly identical to that experienced in bulk water; neither deprived of hydrogen bond opportunities, as it is in contact with a more hydrophobic surface, nor excessively hydrogen bonded to a more hydrophilic surface. A minimum in the biological response occurs because water vicinal (near) to the Goldilocks Surface is not chemically different than bulk water. A more precise definition of the relative terms hydrophobic and hydrophilic for use in biomaterials becomes evident from calculations: > 1.3 kJ/mole-of-surface-sites is expended in wetting a hydrophilic surface whereas < 1.3 kJ/mole-of-surface-sites is expended in wetting hydrophobic surfaces; hydrophilic surfaces wet with > 1 hydrogen bond per water molecule whereas hydrophobic surfaces wet with < 1 hydrogen bond per water molecule.
Keywords: Biological response, biocompatible, surface energy, water wetting, hydrophilic, hydrophobic
1. Introduction
Robert Southey is credited with the modern version of the children’s story Goldilocks and the Three Bears in which the little blond-haired girl Goldilocks unlawfully enters the bears’ domicile and samples porridge left on a table to cool while the three bears were out on morning walk. Goldilocks found that one bowl of porridge was too hot, a second too cold, and the third just right to her felonious taste test. Henceforth, the adjective “Goldilocks” has been applied in popular and scientific literature in reference to anything that has a particularly balanced set of properties as in, for examples; the Goldilocks Principle that describes the perfect planetary conditions for life to arise, a Goldilocks Planet to which this principle refers, the Goldilocks Enigma that seeks a metaphysical reason why Earth happens to be a Goldilocks Planet, or the workings of a Goldilocks Economy for the planet.
Biomaterials generally seeks materials that meet different medical-device application needs and thereby exhibit the end-use criteria referred to as “biocompatible” [1–5]. No one material can satisfy very different performance requirements of diverse medical devices. A biomaterial that might be regarded as “too hot” (hydrophilic for example) for certain applications, such as blood contact [6], can be “just right” in a different application; adhesion of mammalian cells to cultureware surfaces for example [7–9]. Consequently, there is no single “Goldilocks Biomaterial” to be discovered. But if our core understanding of biocompatibility is correct [1], there should be a Goldilocks Principle appropriate to biomaterials that describes how to make a perfect biomaterial for a particular medical application. To suspect otherwise is to give up all hope of rational biomaterials engineering; a loss of faith few in the biomaterials community are willing to condone, even though this putative Goldilocks Principle has not been forthcoming from more than five decades of focused research.
Our core understanding of biocompatibility is built on the basic tenet that proteins adsorbed to biomaterial surfaces from solution catalyze, mediate, or moderate the biological response to materials in a manner that ultimately dictates biocompatibility. It is thus apparent that a full-and-quantitative understanding of how proteins arrive at, and adsorb to, biomaterial surfaces from complex biological milieu is essential to prospective biomaterials design for advanced medical devices. If the number and kind of proteins adsorbed to a surface is not clearly known, then evidence-based biochemical mechanisms of the biological response to materials cannot be responsibly proposed. And if mechanisms of the biological response to materials remain obscure, then structure-property relationships cannot be formulated, leaving biomaterials development dependent on design-directed or trial-and-error approaches [10, 11]. Thus, the entirety of biomaterials surface science seems critically dependent on a thorough understanding of protein adsorption.
Experimental evidence gathered in my laboratories over the last three decades strongly suggests that water controls energetics of protein adsorption and that the adsorption process has more to do with solvent properties than solute [7, 9, 12–23]. Combined with the above-stated theory of biocompatibility, it logically follows that water ultimately controls the biological response to materials, albeit through the convoluted agency of protein activity at hydrated surfaces. Indeed, it is observed that different biological responses to materials ranging from blood plasma coagulation [6] to mammalian cell adhesion [7] to zymogen activation [24–26] correlate with the structure and reactivity of water inferred from diverse studies of water properties drawn from the condensed-phase physics literature [15, 16] (note that refs. [6, 7] and [15, 16] are review articles summarizing and comparing work from a broad literature). All taken together, these experimental facts and correlations argue that, if a Goldilocks Principle exists for biomaterials, then it is reasonable to seek it in the way water reacts with different material surfaces.
This paper discusses theoretical and experimental evidence for a Goldilocks Surface that is “just right” from the perspective of bulk water properties. Water adjacent (vicinal) to a Goldilocks Surface is neither deprived of hydrogen bond opportunities, as it is in contact with a hydrophobic surface, nor excessively hydrogen bonded to a hydrophilic surface. In this sense, vicinal water interacts with a Goldilocks Surface in a way that is not different than the interaction with water molecules in bulk solution. A Goldilocks Surface is a water-contacting insoluble phase with water-like hydrogen-bonding properties. As a consequence of this unique chemistry, biological responses mentioned above are observed to “pivot” from low-to-high or high-to-low at the Goldilocks Surface Energy, depending on the specific case under consideration [7, 15, 16]. A refined definition of hydrophilic and hydrophobic in energetic terms emerges from this analysis.
2. Computational and Conceptual Methods
Work described herein was built upon wetting theory introduced by C. Extrand [27, 28] and expanded upon by linkage with the traditional Dupre’ work function and a lattice model of water (HOH) in a way that permitted calculation of the number of moles of water involved in wetting a mole of surface sites. Table 1 collects theoretical parameter definitions and potential relationships that could be drawn among these parameters. In particular, molar surface area Ā, molar HOH area H̄, Extrand’s wetting energy function ΔG, the Dupre’ work-of- adhesion W, and the combined term (W/ΔG)H̄ were used in this work. Outcome of theoretical calculations was compared to different measures of the biological response to material surface energy herein quantified by cosine of the advancing contact angle, cos θa. Three conceptual tools described below were essential to conclusions drawn from the calculations.
Table 1.
Definitions and Possible Relationships Among Parameters
| Row | Parameter | Symbol (units) | Relevant Relationships (units) | |
|---|---|---|---|---|
| Materials Constants |
1 | Molar Surface Area | Ā (cm2/mole surface sites) | |
| 2 | Molar HOH Area | H̄ (cm2/mole HOH) | H̄/Ā (mole-surface-sites/mole HOH) | |
| Functional Relationships |
3 | Wetting Energy Function |
ΔG (kJ/mole surface sites) | ΔG/Ā (kJ/cm2) |
| 4 | Wetting Work Function |
W (kJ/cm2) |
WĀ (kJ/mole-surface-sites) WH̄ (kJ/mole HOH) W/ΔG (mole-surface-sites/cm2) (W/ΔG)H̄ (mole-surface-sites/mole HOH) |
Notes: ΔG and (W/ΔG)H are the only combination of parameters considered in this work.
2.1 Molar Surface Area and Molar Wetting
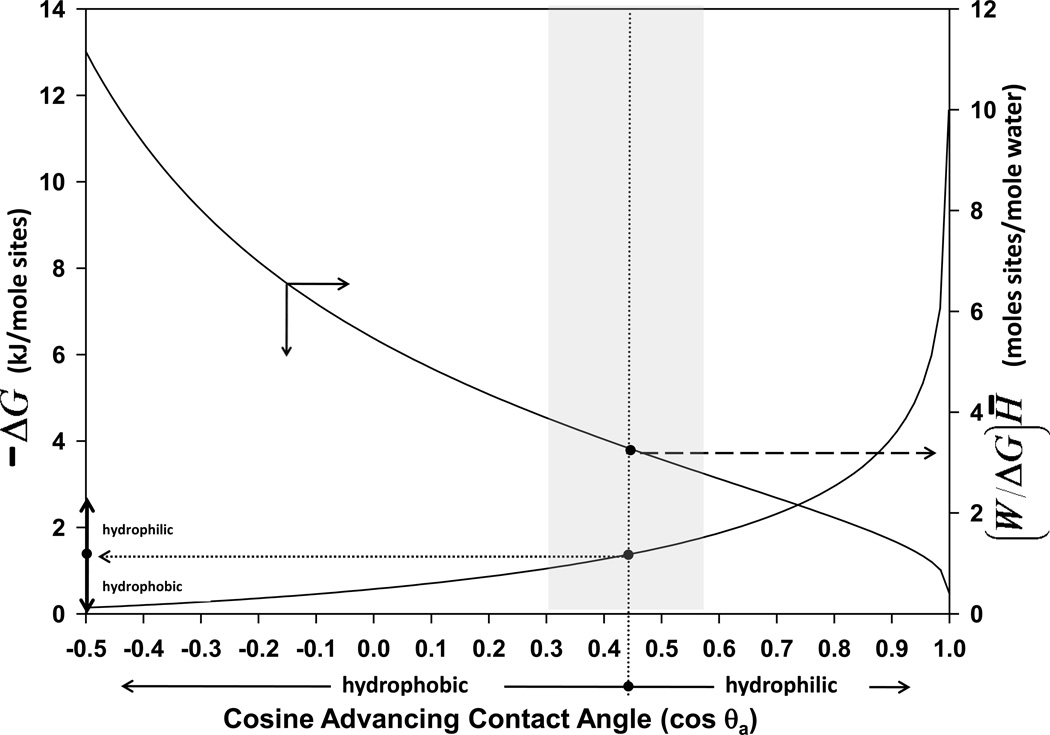
Extrand developed the concept of “molar surface area” [28] (row 1, Table 1) by envisioning a polymeric surface with smooth area A comprised of a repeat unit with molecular weight Mo. The surface area occupied by a repeat unit Asite was approximated as the 2/3 root of site volume (Vsite)2/3 which, in turn, was related to Mo and density ρ by Vsite = (Mo/ρNA), where NA is the Avogadro number. The molar surface area Ā was obtained by multiplication with NA, yielding Ā = [(Mo/ρ)2/3 NA1/3] with units of cm2/mole-of-surface-sites. Molar surface area was appropriate for any material that exhibited a discernable repeat unit, such as a polymer or SiO2 glass for example. Extrand applied this concept in deriving a relationship between the free energy ΔG of wetting a mole of surface sites and the advancing contact angle θa of a wetting liquid on that surface, concluding that in units of kJ/mole-of-surface-sites (row 3, Table 1) [27]; where RT the product of the gas constant and Kelvin temperature. It is thus evident that ΔG scales directly with temperature which was taken to be 298.15 °K in this work. This equation was used to calculate free energy as a function of cos θa shown on the left-hand ordinate of Fig. 1 and the number of moles of surface sites wetted by one mole of water (right-hand ordinate), as discussed further below. ΔG was interpreted as the strength of interaction of the wetting fluid with the surface and was equated with liquid-solid adhesion [27], or (negative of) the work required to remove a wetting fluid from the surface (dehydration energy).
Figure 1.
Free energy of dehydrating a surface (−ΔG, left-hand axis) compared to the number of moles of water wetting these sites ((W/ΔG)H̄, right-hand axis) as a function of site wettability measured by cosine of the advancing contact angle, cos θa. Wetting energy increases as surface sites become more hydrophilic (left-to-right on the abscissa), crossing a boundary near a 65° nominal contact angle (dotted vertical line within the gray band) that differentiates hydrophobic from hydrophilic. The wetting energy corresponding to this pivot point (dotted horizontal line) defines hydrophilicity on an energetic basis: hydrophilic wetting expends > 1.3 kJ/mole-of-surface-sites whereas hydrophobic wetting expends < 1.3 kJ/mole-of-surface-sites (see vertical arrow annotations, left-hand axis). The number of moles of water involved in wetting a mole of surface site increases nearly exponentially with site wettability over the hydrophilic range, causing the ratio (W/ΔG)H̄→0 as cos θa → 1 (right-hand axis). By contrast (W/ΔG)H̄→∞ as number of moles of water wetting increasingly hydrophobic sites decreases to zero. (W/ΔG)H̄→3.2 within the gray band pivot point range where the biological response to materials reaches a minimum as a function of surface energy. It is estimated that (W/ΔG)H̄ = 3.2 corresponds to one water molecule bonded with a single hydrogen bond, defining the Goldilocks Surface.
Extrand theory was not specific to a particular wetting fluid and did not explicitly depend on fluid liquid-vapor (lv) interfacial tension γlv because γlv is a measure of liquid-molecule cohesion, not intermolecular interactions with the wetted surface (assuming γlv does not change due to surface wetting) [27]. Furthermore, θa was an independent parameter that made no statement about the chemical nature of the surface supporting θa. In essence, Extrand’s theory computed the free energy of wetting for any wetting fluid on any surface from θa alone. In deriving this thermodynamics, Extrand neglected vapor spreading pressure, which becomes increasingly significant with increasing hydrophilicity, and equated θa with an equilibrium angle, but otherwise theory was without additional assumptions. The full range of applicability of Extrand theory was not determined but theory was shown to be in good agreement with experiment for polymeric materials spanning a broad range in water contact angle and wetting-fluid interfacial tensions. Simplifications and approximations utilized by Extrand were not considered to be crucial to the general conclusions of this work, especially in light of the approximate location of the pivot point in the biological response discussed in Section 2.3 (see gray band in Fig. 1). In other words, we assumed on the basis of evidence reported in ref. [27] that Extrand theory was applicable to ordinary polymeric materials of general interest to biomaterials in contact with high-ionic strength buffered aqueous solutions wherein charge interactions are screened to short Debye lengths. Fully-water-wettable surfaces or surfaces bearing strong Lewis acid/base functionalities with a strong surrounding electric field (such as ion-exchange functionalities [29, 30]) might fall outside this range. We considered the bonding of water to these ordinary surfaces and did not contemplate how this bonding might be subsequently perturbed by the adsorption of solutes, such as proteins, recognizing that hydration reactions almost assuredly precede such slower steps of the biological response to materials [15, 16, 27].
2.2 Dupre’ Work-of-Adhesion and Relationship to ΔG
The traditional thermodynamic work of adhesion of a wetting fluid to a surface was taken to be W = γlv (1 + cos θa) in kJ/cm2 (row 4, Table 1). W measured the work-per-unit-interfacial-area required to remove a drop of fluid with interfacial tension γlv from a surface exhibiting an advancing contact angle θa [9, 31, 32]. There a number of critical thermodynamic criteria built into this Dupre’ relationship, especially including those related to attainment of thermodynamic equilibrium in an isolated system. It was assumed herein that these criteria applied equally to ΔG and were approximately applicable to experimentally-measured biological responses considered in this work, though no proof of this latter assumption was pursued herein. Rather, thermodynamic arguments were applied as a modeling tool that tested the feasibility of interpretative concepts in a highly-structured yet semi-quantitative manner.
The ratio −(W/ΔG) was particular to a particular wetting fluid (e.g. water) with surface tension γiv with units of moles-surface-sites/cm2, where the negative sign converts ΔG to work (row 4 column 5, Table 1). It was of interest to convert this ratio into (W/ΔG)H̄ with units of moles-of-surface-sites/mole-of-water by using the molar HOH area H̄ (row 2, Table 1). In this work, a value of H̄ = 4.35×108 cm2/mole HOH (2.3 nmole/cm2) derived from a lattice model of water [33–37] was used as an estimate of the area occupied by adsorbed water.
2.3 A Pivot Point in Vicinal Water Properties and the Biological Response to Materials
An extensive survey of the structure and reactivity of water at surfaces taken from the condensed-matter physics literature revealed a sharp change in water properties near a nominal water contact angle θ = 65° [15, 16] (water adhesion tension dyne/cm, where the superscript distinguishes pure water from solutions of proteins or surfactants). Physicochemical interpretation of some of this experimental evidence has proven controversial and an exact value for this “pivot point” has yet to be determined, but a change in vicinal water properties as a function of surface energy seems both experimentally and theoretically apparent, quite independent of the exact physical cause. A correlation of this θ = 65° pivot point was made with a sharp change in the biological response to material surface energy observed in diverse circumstances including blood plasma coagulation [38–40], blood factor XII activation [24–26], cell adhesion [7], and protein adsorption [14, 22, 23] (refs. [6, 7] and [15, 16] are review articles summarizing and comparing work from a broad literature). In fact, the hydrophobic/hydrophilic contrast in the biological response centered around θ = 65° has been used as a quantitative definition of hydrophilic and hydrophobic for biomaterials purposes (see refs. [6, 18, 41] and citations therein). I have hypothesized that there is a direct connection between the properties of water at a surface and the nature of the biological response to materials [15, 16, 26].
3. Results
3.1 Molar Surface Area
The concept of molar surface area discussed in Section 2.1 effectively permitted conversion of wetting measurements typically in units of kJ/cm2 into units of kJ/mole-surface-sites (row 1, Table 1). This is especially useful for comparing wetting energetics to adsorption energetics. Taking SiO2 glass as an example using ρ = 2.2 g/cm3 and Mo = 60.1 g/mole monomer unit, it works out that Ā = 7.7×108 cm2/mole-SiO2-sites (1.3 nmole-SiO2-sites/cm2). This can be compared to H̄ = 4.35×108 cm2/mole HOH (2.3 nmole/cm2) introduced in Section 2.2, revealing that 1.8 water molecules can geometrically fit onto one SiO2 repeat unit. The Ā value calculated for 1 g/cm3 water ice of 5.80×108 cm2/mole HOH (1.7 nmole/cm2, 1.3 water molecules per SiO2 repeat unit) is an alternative approach to a molar surface area for water, but this was not considered to be as realistic as Besseling’s lattice model [33–37] that is presumably a more faithful rendition of liquid water-molecule orientation. General conclusions of this work were not dependent on which water footprint was chosen but of course exact numbers vary with the parameter value estimates applied.
3.2 Site Wetting Energetics and Moles Water Involved
Extrand wetting theory outlined in Section 2 was used to calculate wetting energetics (row 3, Table 1) as a function of wetting site contact angle and was subsequently used to calculate moles of water involved in the wetting process. In agreement with chemical intuition, the energetic cost of displacing water from a surface (dehydration, −ΔG) rose sharply with hydrophilicity of the surface sites, as shown in Fig. 1 (left-hand ordinate is the same as Fig. 3 of Extrand’s ref. [27] on a cos θa rather than θa axis). This dehydration energy passed through approximately 1.3 kJ/mole-of-surface-sites (0.3 kCal/mole) near the 65° (cos θ = 0.4) dividing line discussed in Section 2.3 that distinguishes hydrophobic from hydrophilic and where protein adsorption decreases to immeasurably small values (see vertical bar annotation and double arrow annotation on the left-hand ordinate of Fig. 1).
The right-hand ordinate of Fig. 1 plots the moles of surface sites interacting with one mole of water (conversely, moles of water interacting with one mole of surface sites). The number of moles of water interacting with one mole of surface sites increased monotonically with surface energy, causing (W/ΔG)H̄→0 as cos θa → 1. By contrast, the number of moles of water interacting with one mole of surface sites decreased with increasing hydrophobicity, causing (W/ΔG)H̄→∞ as cos θa → −1 (hypothetical 180° contact angle case not shown in Fig. 1). It is noteworthy from Fig. 1 that (W/ΔG)H̄ = 3.2 moles-surface-sites-per-mole-of-water at the 65° (cos θ = 0.4) pivot point (~ 0.3 moles of water per mole of surface sites). This value was compared to the estimated 3.6 moles of hydrogen bonds per mole of liquid water at room temperature [42], as discussed in the following section.
4. Discussion
4.1 Energetics of Surface Dehydration
It is apparent from the left-hand ordinate of Fig. 1 that the energetics of wetting increases with surface site hydrophilicity as measured by decreasing site contact angle θa. Wetting energetics rise in a linear-like way with cos θa through the range considered to be hydrophobic (cos θa < 0.4, θa > 65°; see Section 2.3). Wetting energetics rise much more steeply within the hydrophilic range (cos θa > 0.4, θa < 65°). Extrand’s wetting theory thus affords an additional specification on hydrophilicity; hydrophilic wetting expends > 1.3 kJ/mole-of-surface-sites whereas hydrophobic wetting expends < 1.3 kJ/mole-of-surface-sites (see arrow annotations).
4.2 Hydrogen Bonding in Wetting
Inspection of the right-hand ordinate of Fig. 1 reveals that the number of moles of water involved in wetting a mole of surface sites increases monotonically with hydrophilicity, causing (W/ΔG)H̄ → 0 as cos θa → 1. As complete wettability is approached, the cohesive energy of a water droplet in contact with the surface is nearly entirely consumed by adhesion to the surface. Water spreads evanescently on the wetting surface, requiring a large number of water molecules to react with a mole of surface sites. By contrast, the number of moles of water interacting with a mole of surface sites decreases with increasing hydrophobicity, falling to zero moles of water molecules per mole of surface sites for the purely hypothetical case of 180° contact angle (not shown in Fig. 1). At this extreme, no cohesive energy is consumed by adhesion to the perfectly non-wetting surface and no water molecules interact with the surface.
It is particularly noteworthy that (W/ΔG)H̄ = 3.2 at the 65° (cos θ = 0.4) pivot point discussed in Section 2.3, meaning that 3.2 moles of surface sites interact with one mole of water (or ~ 0.3 moles of water interacts with 1 mole of surface sites). This should not be interpreted to mean that 1 molecule of water occupies ~ 3 surface sites because the geometric area of a surface site is a material property determined by Ā, not by wetting energetics. Rather, (W/ΔG)H̄ = 3.2 means that ~ 3 surface sites interact with 1 molecule of water at the pivot-point wettability. If surface sites were more hydrophobic than the pivot point (left side of the vertical dividing line of Fig. 1), then these same sites with size dictated by the material constant Ā would interact with fewer water molecules. And if these same sites were more hydrophilic (left side of the vertical dividing line of Fig. 1), then more water molecules would interact per surface site. In this way, Fig. 1 compares the number of water molecules interacting with sites of any particular dimension but with varying hydrophilicity.
Computation of ΔG and (W/ΔG)H̄ is purely a matter of thermodynamics and, as such, does not provide direct chemical insights into how the wetting fluid interacts with surface sites. However, traditional interfacial tension component theory has it that interactions across an interface between two mutually-insoluble phases (1, 2) are partly due to Lifshitz-van der Waals (dispersion, LW) forces and partly due to Lewis acid/base (AB) chemical reactions; , where γ terms are interfacial tensions between phases 1 and 2 in mJ/m2 and the superscripted tensions represent the LW and AB interfacial components. On this basis, it must be presumed that, at the pivot point wettability, 3.2 moles of surface sites interacts with 1 mole of water partly through LW interactions and partly through AB interactions. LW interactions are physical in nature and ubiquitous in material interactions but, in the case of water wetting ordinary materials such as glass and polymers, the AB interaction is dominated by hydrogen bonding (see ref. [15] and citations therein). On this basis, it is reasonable to conclude that 3.2 moles of surface sites interacts with 1 mole of water partly through LW interactions and partly by hydrogen bonding.
Molecular simulations suggest that the nominal hydrogen bond capacity of room temperature water is 3.6 mole of hydrogen bonds per mole of water (~ 0.3 mole HOH/mole-hydrogen-bonds) [42]. Thus, if the hydrogen bonding capacity expressed in liquid water is the same as at pivot-point surfaces, it is possible that 3.2 mole of pivot-point surface sites interact with 1 mole of water molecules through 1.1 moles of hydrogen bonds (3.2 mole sites/mole HOH × 0.3 mole HOH/mole-hydrogen-bonds ~ 1.1 mole sites/mole-hydrogen-bonds). In other words, approximately 1 hydrogen bond per water molecule interacts with pivot point surfaces. It is to be emphasized that this chemical accounting of thermodynamic results is highly speculative with neither computational nor experimental support. But, to the extent this speculation is a reasonable interpretation of how LW and AB interactions occur between water and a pivot-point surface, it can be further inferred that surfaces within the gray-band range of Fig. 1 exhibit a unique balance of properties that binds a water molecule through a single hydrogen bond, leaving the remaining 2.6 hydrogen bonds to associate with water proximal to the pivot-point surface. The implication is that water vicinal to pivot-point surfaces (gray band in Fig. 1) is neither deficient in hydrogen-bond opportunities nor excessively hydrogen bonded to the surface relative to bulk water. By contrast, water vicinal to more hydrophobic or hydrophilic surfaces is unbalanced in hydrogen bonding compared to bulk water. This perspective on the hydrogen bond environment surrounding water involved in surface wetting thus affords an additional specification on the relative terms hydrophilic/hydrophobic: hydrophilic surfaces wet with > 1 hydrogen bond per water molecule whereas hydrophobic surfaces wet with < 1 hydrogen bond per water molecule.
4.3 A Pivot Point in the Biological Response to Materials
The gray band imposed on Fig. 1 marks the range wherein different biological responses are observed to pivot from low-to-high or high-to-low (see further Section 2.3). In particular, among these biological responses, protein adsorption capacity is largest for the most hydrophobic surfaces and falls monotonically through detection limits near the pivot point (with the exception of surfaces bearing ion-exchange functionalities [29]). Apparently, at the hydrophilic/hydrophobic dividing line, the energetic cost of displacing interphase water by an adsorbing protein nearly equals the net energy gained by partitioning protein from bulk solution into the interphase, and the adsorption process consequently becomes energetically unfavorable [14, 22, 23].
Certain biological responses such as mammalian cell adhesion are unfavorable at hydrophobic surfaces with a high protein adsorbent capacity but favorable at hydrophilic surfaces that adsorb little or no protein (see ref. [7] and citations therein). Other biological responses such as contact activation of blood plasma coagulation are low at hydrophobic surfaces but high at hydrophilic surfaces (see ref. [6] and citations therein). Still other responses such as contact activation of blood factor XII (Hageman factor) in neat buffer solution exhibit a parabolic response with surface energy, high a both extremes of water wetting but falling to a minimum within the gray band region of Fig. 1 [26]. Some of these latter biological responses are apparently not mediated by adsorbed protein (e.g. cell adhesion, activation of FXII by hydrophilic surfaces) but rather by lack thereof.
These correlations motivate the proposition that water controls protein adsorption which, in turn, mediates the biological response to materials. The reason water is influential in the biological response to materials is that solvent properties correlate with the extent of self association by hydrogen bonding [15, 16]. Water is a relatively poor solvent at low temperatures near the density maximum (3.98 °C) because nearly all hydrogen bonds are involved in self association. On the other hand, water steam (100 °C) is quite corrosive because all hydrogen bonds are available to do chemical work. Thus it may be anticipated that changes in hydrogen bonding induced by contact with surfaces at ambient temperatures will have a significant effect on vicinal-water solvent properties [18] which, in turn, will influence the distribution of ions near the water-contacting surface [15, 16, 43], and possibly affect pH within the vicinal-water region. A biological entity such as a protein or a cell entering the vicinal-water region can encounter significantly different chemistry than experienced in bulk solution depending on the extent to which self association of vicinal water has been affected by the presence of the surface. Water vicinal to pivot-point surfaces (gray band in Fig. 1) is chemically similar to bulk water, unperturbed by the presence of the imposed surface [15].
Metaphorically stated, the pivot point represents a “Goldilocks Surface” that is neither too cold (hydrophobic) nor too hot (hydrophilic) for contacting biology, inducing a muted response compared to biology in contact with surfaces falling at more polar extremes of hydrophilicity. The Goldilocks Surface does not correspond to a zero degree water contact angle, the contact angle of water on water, because perfect wetting of an insoluble water-contacting surface phase requires complete destruction of the bulk water hydrogen-bonded network by adhesion to the surface. Rather, a Goldilocks Surface participates in hydrogen bonding with vicinal water molecules in a way that does not significantly perturb the hydrogen-bond network of water relative to that of the bulk-solution phase.
5. Conclusions
Water in contact with surfaces exhibiting a contact angle near 65° exhibits bulk-water like properties and is neither deficient in hydrogen-bond opportunities compared to bulk water molecules nor excessively hydrogen bonded relative to bulk water. This Goldilocks Surface represents a unique surface chemistry that does not perturb the structure and reactivity (solvent properties) of vicinal water. As a consequence, a minimum in the biological response to the Goldilocks Surface occurs because vicinal water is not different than bulk water. According to this interpretation, water controls the biological response to artificial materials. A full appreciation of role of water in this regard may lead to a Goldilocks Principle that explains the basic mechanisms underlying biocompatibility.
Acknowledgments
This work was supported the National Institute of Health grant PHS 2R01HL069965. The author is grateful to Drs. Avantika Golas and Waseem Haider for careful reading and discussion of the manuscript. Author further appreciates support from the Materials Research Institute and Department of Materials Science and Engineering, The Pennsylvania State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- 1.Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29:2941–2953. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Williams DF. General concepts of biocompatibility. In: Black J, Hastings G, editors. Handbook of Biomaterial Properties. London: Chapman and Hall; 1998. pp. 481–488. [Google Scholar]
- 3.Williams DF. The blood-device interface. Medical Device Technol. 1993:8–12. [Google Scholar]
- 4.Williams DF. proceedings of a consensus conference of the european society for biomaterials. New York: Elsevier; 1987. Definitions in biomaterials. [Google Scholar]
- 5.Williams DF. Review: Tissue-biomaterial interactions. J Mat Sci. 1987;22:3421–3445. [Google Scholar]
- 6.Vogler EA, Siedlecki CA. Contact activation of blood plasma coagulation. Biomaterials. 2009;30:1857–1869. doi: 10.1016/j.biomaterials.2008.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parhi P, Golas A, Vogler EA. Role of water and proteins in the attachment of mammalian cells to surfaces: a review. J Adhesion Sci and Tech. 2010;24:853–888. [Google Scholar]
- 8.Parhi P, Golas A, Vogler EA. Role of proteins and water in the initial attachment of mammalian cells to biomedical surfaces: a review. In: Carré A, Mittal KL, editors. Surface and Interfacial Aspects of Cell Adhesion. Koninklijke Brill NV: Leiden; 2010. pp. 103–138. [Google Scholar]
- 9.Vogler EA. Interfacial chemistry in biomaterials science. In: Berg J, editor. Wettability. New York: Marcel Dekker; 1993. pp. 184–250. [Google Scholar]
- 10.Brien CP, Stuart SJ, Bruce DA, Latour RA. Modeling of peptide adsorption interactions with a poly(lactic acid) surface. Langmuir. 2008:14115–14124. doi: 10.1021/la802588n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnthip N, Parhi P, Golas A, Vogler EA. Volumetric interpretation of protein adsorption: kinetics of protein-adsorption competition from binary solution. Biomaterials. 2009;30:6495–6513. doi: 10.1016/j.biomaterials.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogler EA. Practical use of concentration-dependent contact angles as a measure of solid-liquid adsorption i: theoretical aspects. Langmuir. 1992;8:2005–2012. [Google Scholar]
- 13.Vogler EA. Practical use of concentration-dependent contact angles as a measure of solid-liquid adsorption ii: experimental aspects. Langmuir. 1992;8:2013–2020. [Google Scholar]
- 14.Vogler EA, Martin DA, Montgomery DB, Graper JC, Sugg HW. A graphical method for predicting protein and surfactant adsorption properties. Langmuir. 1993;9:497–507. [Google Scholar]
- 15.Vogler EA. Structure and reactivity of water at biomaterial surfaces. Adv Colloid and Interface Sci. 1998;74:69–117. doi: 10.1016/s0001-8686(97)00040-7. [DOI] [PubMed] [Google Scholar]
- 16.Vogler EA. Water and the acute biological response to surfaces. J Biomat Sci Polym Edn. 1999;10:1015–1045. doi: 10.1163/156856299x00667. [DOI] [PubMed] [Google Scholar]
- 17.Vogler EA. Role of water in biomaterials. In: Ratner B, Hoffman A, editors. Biomaterials Science: An Introduction to Materials in Medicine. 2 ed. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- 18.Vogler EA. How water wets biomaterials. In: Morra M, editor. Water in Biomaterials Surface Science. New York: John Wiley and Sons; 2001. pp. 269–290. [Google Scholar]
- 19.Krishnan A, Siedlecki C, Vogler EA. Traube-rule interpretation of protein adsorption to the liquid-vapor interface. Langmuir. 2003;19:10342–10352. [Google Scholar]
- 20.Noh H, Vogler EA. Volumetric interpretation of protein adsorption: partition coefficients, interphase volumes, and free energies of adsorption to hydrophobic surfaces. Biomaterials. 2006;27:5780–5793. doi: 10.1016/j.biomaterials.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 21.Noh H, Vogler EA. Volumetric interpretation of protein adsorption: mass and energy balance for albumin adsorption to particulate adsorbents with incrementally-increasing hydrophilicity. Biomaterials. 2006;27:5801–5812. doi: 10.1016/j.biomaterials.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Cha P, Krishnan A, Fiore VF, Vogler EA. Interfacial energetics of protein adsorption from aqueous buffer to surfaces with varying hydrophilicity. Langmuir. 2008;24:2553–2563. doi: 10.1021/la703310k. [DOI] [PubMed] [Google Scholar]
- 23.Parhi P, Golas A, Barnthip N, Vogler EA. Volumetric interpretation of protein adsorption: capacity scaling with adsorbate molecular weight and adsorbent surface energy. Biomaterials. 2009;30:6814–6824. doi: 10.1016/j.biomaterials.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhuo R, Siedlecki CA, Vogler EA. Competitive-protein adsorption in contact activation of blood factor XII. Biomaterials. 2007;28:4355–4369. doi: 10.1016/j.biomaterials.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuo R, Siedlecki CA, Vogler EA. Autoactivation of blood factor XII at hydrophilic and hydrophobic surfaces. Biomaterials. 2006;27:4325–4332. doi: 10.1016/j.biomaterials.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Golas A, Parhi P, Dimachkie ZO, Siedlecki CA, Vogler EA. Surface-energy dependent contact activation of blood factor XII. Biomaterials. 2010;31:1068–1079. doi: 10.1016/j.biomaterials.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Extrand CW. A thermodynamic model for wetting free energies from contact angles. Langmuir. 2003;19:646–649. [Google Scholar]
- 28.Extrand CW. A thermodynamic model for contact angle hysteresis. J Colloid and Interface Sci. 1998;207:11–19. doi: 10.1006/jcis.1998.5743. [DOI] [PubMed] [Google Scholar]
- 29.Noh H, Vogler EA. Volumetric interpretation of protein adsorption: ion-exchange adsorbent capacity, protein pi, and interaction energetics. Biomaterials. 2008;29:2033–2048. doi: 10.1016/j.biomaterials.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, Lenhoff AM. A predictive approach to correlating protein adsorption isotherms on ion-exchange media. J Phys Chem B. 2008 doi: 10.1021/jp0754233. [DOI] [PubMed] [Google Scholar]
- 31.Hiemenz PC. Principles of colloid and surface chemistry. New York: M. Dekker; 1977. [Google Scholar]
- 32.Birdi KS. Handbook of surface and colloid chemistry. Boca Raton, FL: CRC Press; 1997. p. 763. [Google Scholar]
- 33.Besseling NAM. Theory of hydration forces between surfaces. Langmuir. 1997;13:2113–2122. [Google Scholar]
- 34.Besseling NAM, Lyklema J. Hydrophobic hydration of small apolar molecules and extended surfaces: a molecular model. Pure & Appl Chem. 1995;67:881–888. [Google Scholar]
- 35.Besseling NAM, Scheutjens JMH. Statistical thermodynamics of molecules with orientation-dependent interactions in homogeneous and inhomogeneous systems. J Phys Chem. 1994;98:11597–11609. [Google Scholar]
- 36.Besseling NAM, Lyklema J. Equilibrium properties of water and its liquid-vapor Interface. J Phys Chem. 1994;98:11610–11622. [Google Scholar]
- 37.Besseling NAM, Lyklema J. Molecular thermodynamics of hydrophobic hydration. J Phys Chem B. 1997;101:7604–7611. [Google Scholar]
- 38.Vogler EA, Graper JC, Harper GR, Lander LM, Brittain WJ. Contact activation of the plasma coagulation cascade. 1. procoagulant surface energy and chemistry. J Biomed Mat Res. 1995;29:1005–1016. doi: 10.1002/jbm.820290813. [DOI] [PubMed] [Google Scholar]
- 39.Zhuo R, Colombo P, Pantano C, Vogler EA. Silicon oxycarbide glasses for blood-contact applications. acta biomaterialia. 2005;1:583–589. doi: 10.1016/j.actbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Zhuo R, Miller R, Bussard KM, Siedlecki CA, Vogler EA. Procoagulant stimulus processing by the intrinsic pathway of blood plasma coagulation. Biomaterials. 2005;26:2965–2973. doi: 10.1016/j.biomaterials.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Vogler EA. On the origins of water wetting terminology. In: Morra M, editor. Water in Biomaterials Surface Science. New York: John Wiley and Sons; 2001. pp. 150–182. [Google Scholar]
- 42.Markovitch O, Agmon N. Structure and energetics of the hydronium hydration shells. The Journal of Physical Chemistry A. 2007;111:2253–2256. doi: 10.1021/jp068960g. [DOI] [PubMed] [Google Scholar]
- 43.Collins KD. Sticky ions in biological systems. Proc Natl Acad Sci USA. 1995;92:5553–5557. doi: 10.1073/pnas.92.12.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]