Abstract
Objective
The aim of the present study was to determine whether mitochondrial uncoupling protein (UCP)-2 is required for AMPK-dependent angiogenesis in ischemia in vivo.
Methods and Results
Angiogenesis was assayed by monitoring endothelial tube formation (a surrogate for angiogenesis) in human umbilical vein endothelial cells (HUVECs), isolated mouse aortic endothelial cells (MAECs), and pulmomary microvascular endothelial cells (PMECs), or in ischemic thigh adductor muscles from wild-type (WT) mice or mice deficient in either AMPKα1 or AMPKα2. AMPK inhibition with pharmacological inhibitor (compound C) or genetic means (transfection of AMPKα-specific siRNA) significantly lowered the tube formation in HUVECs. Consistently, compared with WT mice, tube formation in MAECs isolated from either AMPKα1−/− or AMPKα2−/− mice, which exhibited oxidative stress and reduced expression of UCP2, were significantly impaired. In addition, adenoviral overexpression of UCP2, but not adenoviruses encoding green florescence protein (GFP), normalized tube formation in MAECs from either AMPKα1−/− or AMPKα2−/− mice. Similarly, supplementation with sodium nitroprusside (SNP), a nitric oxide (NO) donor, restored tube formation. Furthermore, ischemia significantly increased angiogenesis, serine 1177 phosphorylation of endothelial NO synthase (eNOS), and UCP2 in ischemic thigh adductor muscles from WT mice, but not from either AMPKα1−/− or AMPKα2−/− mice.
Conclusion
We conclude that AMPK-dependent UCP2 expression in endothelial cells promotes angiogenesis in vivo.
Keywords: AMPK, uncoupling protein-2, nitric oxide, angiogenesis
Introduction
AMP-activated protein kinase (AMPK), an evolutionarily conserved serine/threonine protein kinase, is considered a major metabolic regulator at both the cellular and whole-body levels. AMPK is activated in response to physiological processes that deplete ATP (thereby increasing the AMP:ATP ratio), such as exercise and hypoxia. AMPK is ubiquitously expressed in every cell type in the vascular wall. In vascular endothelial cells, the AMPKα1β1γ1 heterotrimer is the dominant isoform expressed whereas AMPKα2 is a minor form.1 However, the AMPKα subunit is essential and a dual deficiency in AMPKα1 and AMPKα2 causes embryonic lethality in mice. 2
Angiogenesis is a central feature of normal embryonic and post-natal development, and this process plays a critical role in the neovascularization that is associated with tumor growth, wound healing, and occlusive vascular diseases.3–5 Angiogenesis is dependent on cell proliferation, migration, and capillary tube formation in endothelial cells (ECs).6–8 The most important pro-angiogenic factor is nitric oxide (NO) and in endothelial NO synthase (eNOS) knockout mice, the capacity for angiogenesis is significantly impaired.9 Increasing evidence suggests that NO is required for vascular endothelial growth factor (VEGF)-induced angiogenesis.10, 11 In addition, NO has been shown to assist in the proliferation and migration of endothelial cells during angiogenesis.12, 13
Mitochondrial uncoupling proteins (UCP) are mitochondrial transporters that are present in the inner mitochondrial membrane and belong to a family of mitochondrial anion carriers, which includes adenine nucleotide transporters.14 Mild uncoupling of respiration has been reported to diminish mitochondrial reactive oxygen species (ROS) formation.14 Recent evidence implies that the basic role of all UCPs is to prevent oxidative tissue injury by reducing oxidative stress.14 A role for UCP2 in the downregulation of mitochondrial ROS production is plausible, because available evidence suggests that this protein is expressed in numerous mammalian tissues.14 For example, we have recently demonstrated that upregulation of UCP2 by AMPK activation in ECs attenuates oxidative stress in diabetes.15
NO bioactivity is determined by both the synthesis of NO by nitric oxide synthases (NOS) and its inactivation by ROS.16 Specifically, superoxide anions (O2·−) not only reduce the bioavailability of NO, but also inhibit its main target, soluble guanylyl cyclase.17, 18 Increasing evidence suggests that AMPK is important in maintaining NO bioactivity. Both AMPKα1 and α2 have been reported to increase NO release by phosphorylating eNOS at Ser1177 and Ser635.1, 2, 19–21 In addition, recent data from our group indicate that AMPK functions as a sensor for redox status within a cell and AMPK activation suppresses oxidative stress in endothelial function. 22
AMPK has been demonstrated to be required for ischemic angiogenesis.23 However, the molecular mechanisms by which AMPK promotes angiogenesis remains poorly elucidated. Thus, the aim of the present study was to establish the molecular signaling pathways by which AMPK promotes vascular angiogenesis in response to hind limb hypoxia/ischemia in vivo.
Experimental Procedures
Animal experiments
Male AMPKα1−/− and AMPKα2−/− mice were generated, as previously described.24 Their genetic controls (C57BL/6 WT mice) were obtained from The Jackson Laboratory (Bar Harbor, ME) and were 10 weeks of age, with a weight of 20–25 g. Mice were housed in temperature-controlled cages under a 12-h light-dark cycle and given free access to water and normal chow. Unilateral hind limb ischemia was induced by resecting the left femoral arteries and veins of WT mice, AMPKα1−/−, and AMPKα2−/− mice under anesthesia with sodium pentobarbital (50 mg/kg, intraperitoneally), as described previously.25 Briefly, animals were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally), and unilateral hind limb ischemia was induced by resecting the left femoral arteries and veins of WT mice, AMPKα1−/−, and AMPKα2−/− mice. To ensure ischemia efficiency, the proximal portion of femoral artery and vein, and the distal portion of their major branches were ligated, followed by resection of the sections between ligations, and ischemia was confirmed by cyanosis in the distal hindlimb. When hindlimb ischemia was induced, new blood vessels grew into the ischemic limb. After recovery for two weeks, animals were euthanasia for excising muscle samples.
Capillary density assay
Arteriolar and capillary densities within the ischemic thigh adductor skeletal muscles were analyzed to obtain specific evidence of vascularity at the microcirculation level, similar to a previously described method.25 From each animal, three pieces of ischemic muscles were harvested, sliced, and then fixed in 4% formaldehyde. Next, tissues were embedded in paraffin, and multiple tissue slices of 5-mm thickness were prepared. Capillary ECs were identified by immunohistochemical staining with anti-mouse Von Willebrand Factor (VWF) antibody (Abcam, Boston, USA) and arteriolar smooth muscle cells were identified by an antibody against vascular smooth muscle α-actin. Fifteen random microscopic fields from three different sections in each tissue block were examined for the presence of capillary or arteriolar ECs and smooth muscle cells. Capillary density was expressed as the number of capillaries per high-power field (×400).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were grown in endothelial cell basal medium (EBM) supplemented with EGM™ SingleQuots from LONZA (Walkersville, MD), penicillin (100 U/ml), and streptomycin (100 µg/ml). In all experiments, cells were between passages 3 and 8. All cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Cells were grown to 70–80% confluence before being treated with different agents.
A primary mouse aortic endothelial cells (MAECs) culture was established according to previously published methods from our group.26
Pulmonary microvascular endothelial cells (PMECs) were isolated as described by Lips et al 27 with dynabeads coated with Bandeiraea simplicifolia lectin.
Tube formation assay
The formation of vessel-like structures by HUVECs on growth factor-reduced Matrigel (BD Biosciences) was performed as previously described.28 Briefly, 35-mm culture dishes were coated with Matrigel according to the manufacturer’s instructions. The indicated endothelial cells were seeded on coated dishes at 3×105 cells/dish in EBM containing VEGF (50 ng/ml) and incubated at 37°C for 8 h under normoxic or hypoxic conditions. In some dishes, sodium nitroprusside (SNP; 1 mM) was also added as indicated. Tube formation was observed using an inverted phase contrast microscope (Nikon, Tokyo, Japan). Images were captured with a videographic system (DEI-750 CE Digital Output camera; Optronics, Goleta, CA). The degree of tube formation was quantified by measuring the number of tubes in 30 randomly chosen low-power fields (×40) from each dish using the National Institutes of Health (NIH) Image Program. Each experiment was repeated three times.
Transfection of small interfering RNA (siRNA) into endothelial cells
Transient transfection of siRNA was performed according to the manufacturer’s protocol (Santa Cruz Biotechnology, Santa Cruz, CA). Briefly, siRNAs were dissolved in siRNA buffer (20 mM KCl, 6 mM HEPES [pH 7.5], 0.2 mM MgCl2) to prepare a 10-µM stock solution. HUVECs grown in 6-well plates were transfected with siRNA in transfection medium (Gibco/Invitrogen) containing liposomal transfection reagent (Lipofectamine RNAimax; Invitrogen). For each transfection, 100 µl transfection medium containing 4 µl siRNA stock solution was gently mixed with 100 µl transfection medium containing 4 µl transfection reagents. After 30-min incubation at room temperature, siRNA-lipid complexes were added to the cells in 1.0 ml transfection medium and then cells were incubated with this mixture for 6 h at 37°C. The final concentration of control or AMPK-specific siRNA is 40-nM. The transfection medium was then replaced with normal medium, and cells were cultured for 24 h.29
Adenoviral infection
Ad-GFP, a replication-defective adenoviral vector expressing GFP, served as control. The Ad-UCP2 adenoviral vector expresses the full-length UCP2 gene. MAECs were infected with Ad-GFP and Ad-UCP2 overnight in medium supplemented with 2% FBS. Cells were then washed and incubated in fresh medium for an additional 12 h before experimentation. These conditions typically produced an infection efficiency of >80%, as determined by GFP expression.
Western blot analysis
Cell lysates were subjected to western blot analysis. The protein content was assayed using the BCA protein assay reagent (Pierce, Rockford, IL). Proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to membranes. Membranes were incubated with a 1:1000 dilution of primary antibody, followed by a 1:2000 dilution of horseradish peroxidase-conjugated secondary antibody. Protein bands were visualized by enhanced chemiluminescence (ECL; GE Healthcare, Barrington, IN).
Assays of AMPK activity
AMPK activity was measured in immunoprecipitates from 200 µg cell lysate protein using antibodies bound to protein A/G-Sepharose, as described previously.30, 31 Briefly, immunocomplexes were collected by centrifugation at 8000 × g for 1 min. After being washed extensively with immunoprecipitation buffer, the immunoprecipitates were divided equally for further assays. AMPK activity assays were performed at 30°C in a 50-µl total volume containing Na-HEPES (40 mM, pH 7.4), NaCl (80 mM), dithiothreitol (1 mM), SAMs peptide (HMRSAMSGLHLVKRR, 200 µM).30 Variable concentrations of 5'-AMP (0 or 200 µM), 32P-ATP (200 µM), and magnesium acetate (5 mM) were used. Reactions were initiated by the addition of 32P-ATP. For each analysis, blanks were included without the immunoprecipitates and peptide. At the conclusion of the assay (10 min), an aliquot of the reaction mixture was spotted on a 1 × 1-cm square of P81 paper (Whatman), followed by immersion in a perforated plastic beaker inside a glass beaker containing ice-cold phosphoric acid (150 mM).
Immunohistochemistry
The thigh adductor skeletal muscles were fixed in 4% paraformaldehyde overnight, and then processed, embedded in paraffin, and sectioned as 5-µm slices. The deparaffinized, rehydrated sections were microwaved in citrate buffer for antigen retrieval. Sections were incubated in endogenous peroxidase (Dako) and protein block buffer, and then with primary antibodies overnight at 4°C. Slides were rinsed with washing buffer and incubated with labeled polymer-horseradish peroxidase-anti-mouse or rabbit antibodies followed by DAB+ chromogen detection (Dako). After final washes, sections were counterstained with hematoxylin. All positive staining was confirmed by ensuring that no staining occurred under the same conditions using non-immune rabbit or mouse control IgG. Semiquantitative analysis of tissue immunoreactivity was performed by four observers blinded to the identity of the samples using an arbitrary grading system to estimate the degree of positive staining for each individual marker.
Statistical analysis
Data are reported as the mean ± standard deviation of the mean (SEM). An unpaired Student t test was used to detect significant differences when two groups were compared. One-way or two-way ANOVA was used to compare the differences among three or more groups followed by Bonferroni multiple comparison tests as applicable; p<0.05 was considered statistically significant.
RESULTS
Functional AMPK is essential for tube formation in HUVECs in vitro
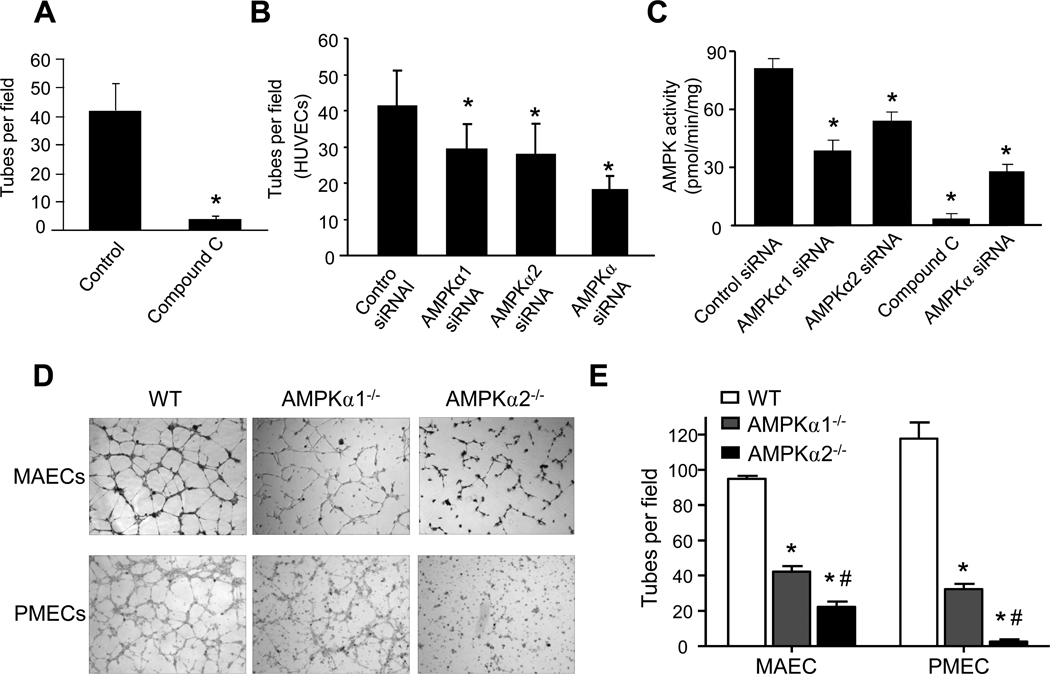
To demonstrate that vascular tube formation is dependent on a functional AMPK signaling system, we evaluated the contribution of AMPK in the formation of vessel-like tubes (EC tubes) in cultured HUVECs. HUVECs were cultured on Matrigel-coated plates with or without compound C (10 µM), a potent inhibitor of AMPK. As depicted in Fig. 1A, compound C significantly suppressed tube formation in HUVECs, suggesting that AMPK might be important for tube formation in HUVECs.
Figure 1. AMPKα subunit deletion suppresses endothelial tube formation in vitro.
A. HUVECs on Matrigel-coated plates were incubated with AMPK inhibitor compound C (10 µM) for 3 h. Tube number per visual field (×40) was counted as described in Methods and Materials. B. HUVECs were subjected to siRNA transfection targeting AMPKα, AMPKα1, or AMPKα2 for 24 h, respectively, and then were incubated on Matrigel-coated plates for 3 h to detect tube formation. C. Assays of AMPK activity in HUVECs treated with compound C (3 h) or transfection of siRNA specific for total AMPKα, AMPKα1, or AMPKα2 or control siRNA for 24 h. *p<0.05 vs. control siRNA; n=5. D & E. Assays of tube formation in mouse aortic endothelial cells (MAECs) and pulmomary microvascular endothelial cells (PMECs) from WT, AMPKα1−/−, and AMPKα2−/− mice. *p<0.05 vs. WT; #p<0.05 vs AMPKα1−/−, n=3 in each group.
To exclude potential off-target effects of compound C, HUVECs were transfected with control siRNA, AMPKα-specific siRNA, AMPKα1-, or AMPKα2-specific siRNA. As depicted in Fig. 1B, compared with cells transfected with control siRNA, transfection of non-selective AMPKα siRNA suppressed tube formation in HUVECs. Transfection of AMPKα1- or AMPKα2-specific siRNA consistently attenuated tube formation, but to a lesser degree than non-selective siRNA (Fig. 1B). Together, our results suggest that AMPKα is required for optimal tube formation.
We further evaluated the effects of compound C and AMPK siRNA on AMPK activity. As shown in Fig. 1C, transfection of AMPKα-specific siRNA in HUVECs caused an approximately 70% reduction of AMPK whereas compound C inhibited AMPK activity by over 90%. The greater reduction of AMPK activity by compound C compared with AMPKα-specific siRNA might help explain the greater effects of compound C on tube formation. Interestingly, silencing AMPKα1 caused a greater reduction of AMPK activity when compared to the transfection of AMPKα2 siRNA in HUVECs (Fig. 1C), indicating that AMPKα1 is a predominant isoform in HUVECs.
AMPKα1 or α2 deletion impairs tube formation in endothelial cells in vitro
To ensure that the effects of AMPKα deletion we observed in HUVECs were not limited to this cell type, we further tested the contribution of AMPKα1 and α2 in tube formation in cultured MAECs and pulmonary microvascular endothelial cells (PMECs) derived from AMPKα1−/−, AMPKα2−/−, and WT control animals. As depicted in Fig. 1D and 1E, tube formation was also dramatically reduced in MAECs and PMECs cultured from AMPKα1- and AMPKα2-deficient animals compared with WT controls.
AMPKα deletion in mice causes defective angiogenesis in vivo
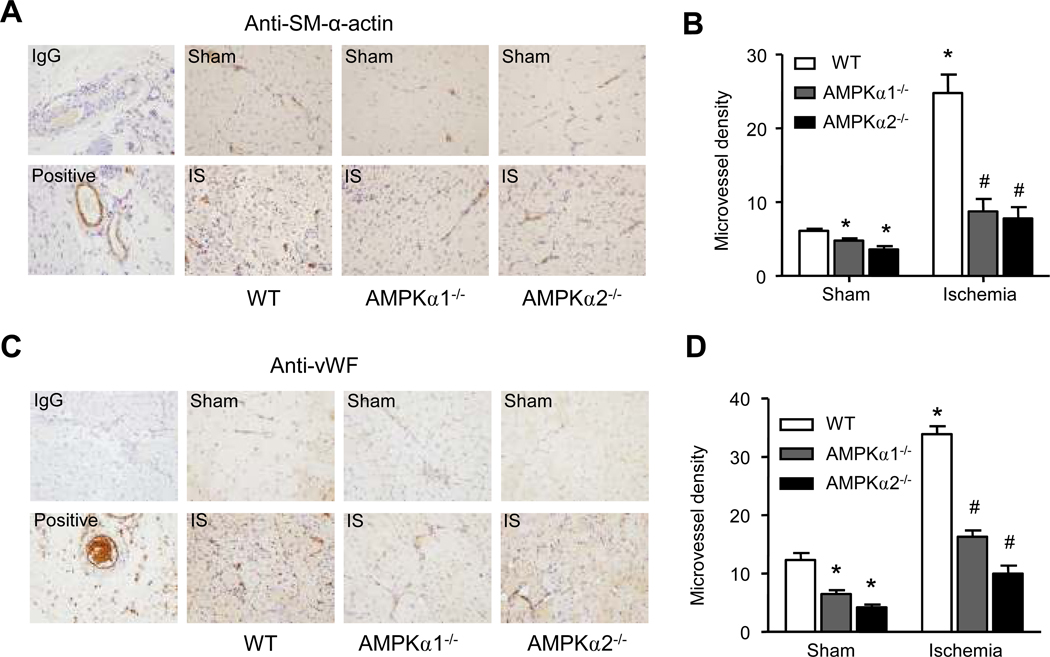
Next, we determined whether AMPKα was critically involved in the angiogenic response in vivo. To this end, WT mice and mice deficient in either AMPKα1 or AMPKα2 were subjected to hindlimb ischemia for 2 weeks. Ischemic muscle was harvested from the affected area and stained for various immunohistochemical markers on postoperative day 14. Arteriolar and capillary densities were assessed by staining with antibodies against vascular smooth muscle α-actin (SM-α-actin; Fig. 2A) or von Willebrand factor (vWF; Fig. 2C), respectively. Vessel density under basal conditions was decreased in tissues obtained from AMPKα1−/− and AMPKα2−/− animals when compared with WT controls (Fig. 2B and D). As expected, microvessel density exhibited a robust increase in ischemic WT muscles (Fig. 2B and D). By contrast, only a modest increase was found in AMPKα1−/− or AMPKα2−/− mice (Fig. 2B and D), implying that AMPKα was required for the angiogenic response in ischemia.
Figure 2. Impaired angiogenesis in AMPKα-deficient mice.
Wild-type (WT), AMPKα1−/−, and AMPKα2−/− mice were subjected to hindlimb ischemia for 2 weeks as described in the methods. Three skeletal muscle samples were taken from the ischemic left hindlimb and the sham operated right hindlimb of each mouse. Three slices of each sample were used for IHC staining. Arteriole and capillary densities were assessed by staining with the antibody against vascular smooth muscle cell marker (SM-α-actin) or endothelial cell marker (Von Willebrand Factor, VWF) respectively. A. Representative immunohistochemical staining for SM-α-actin (×400) and (B) quantification for arteriole number per visual field were shown. C. Representative immunohistochemical staining for VWF antibody (×400) and (D) quantification for capillary number per visual field were shown. *p<0.05 vs. WT/sham group, # p<0.05 vs. WT/ischemia group, n=3 (WT) or n=5 (AMPKα1−/− and AMPKα2−/−).
AMPKα deletion in mice increases reactive nitrogen species (RNS) stress in endothelial cells post ischemia
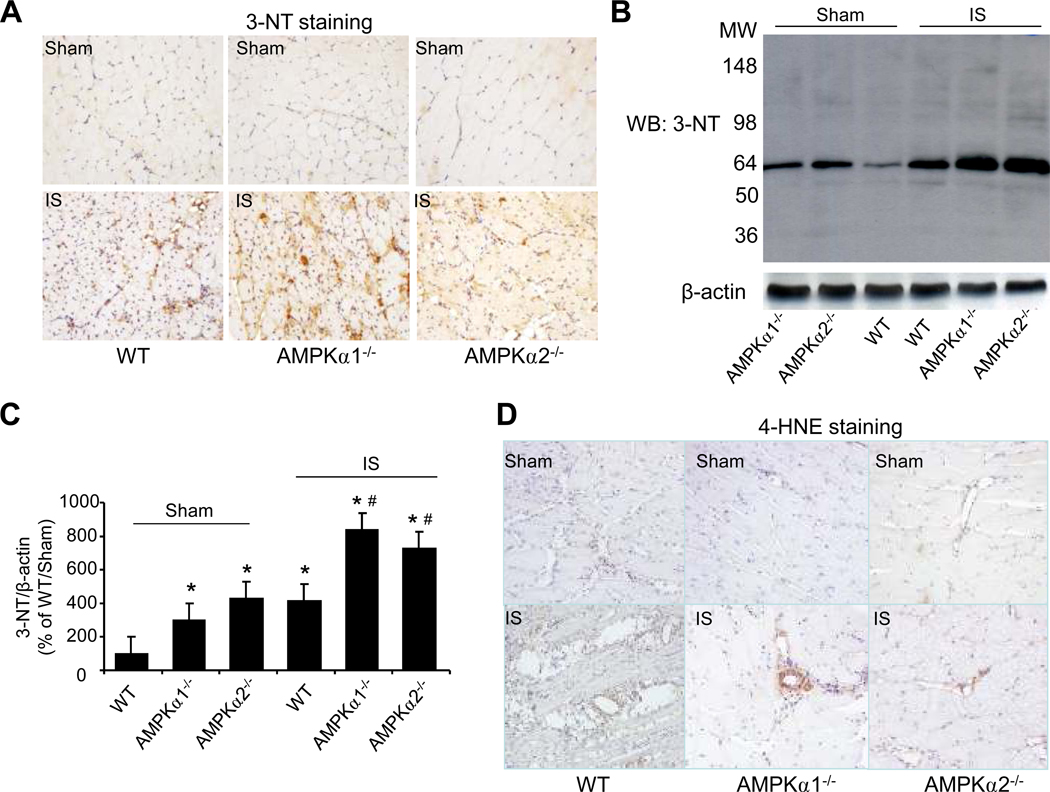
Increasing evidence suggests that hypoxia increases superoxide anions (O2·−) and peroxynitrite (ONOO−) upon its reaction with NO. 32 ONOO− can nitrate protein tyrosine residues forming 3-nitrotyrosine (3-NT).33 To assess RNS stress in endothelial cells following ischemia, we analyzed 3-NT immunostaining in ischemic- and sham-treated tissues in WT, AMPKα1−/−, and AMPKα2−/− mice. As expected, ischemia significantly increased 3-NT staining in WT mice. Compared with WT mice, the levels of 3-NT staining were significantly higher in ischemic tissues from both AMPKα1−/− and AMPKα2−/− mice (Fig. 3A).
Figure 3. Oxidative stress is increased in AMPKα-deficient mice post ischemia.
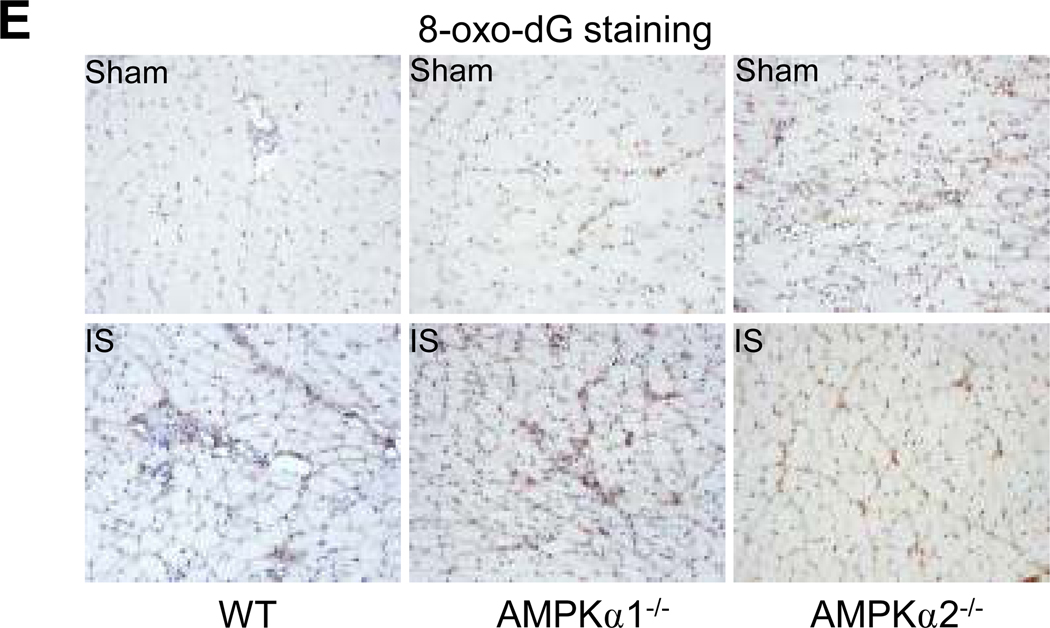
A. Representative immunohistochemical staining with 3-Nitrotyrosine (3-NT) antibody showed 3-NT levels in ischemia skeletal muscle (×400). B. Western blot detected 3-NT-positive proteins level in ischemic tissues with 3-NT-specific antibody. C. Quantification of 3-NT-positive proteins. *p<0.05 vs. WT/sham group; #p<0.05 vs. WT/ischemia (IS) group, n=3 (WT) or n=5 (AMPKα1−/− and AMPKα2−/−). D. Representative immunohistochemical staining with the antibody against 4-HNE, an end-product of lipid peroxidation (×400). The image is a representative of at least five images. E. Representative immunohistochemical staining (×400) with anti-8-hydroxy-2’-deoxyguanosine (8-oxo-dG), a sensitive indicator of DNA oxidation. The image is a representative of at least five images.
To further confirm the increase in 3-NT-positive proteins in ischemic tissues, we first immunoprecipitated 3-NT-positive proteins from tissues of WT, AMPKα1−/−, and AMPKα2−/− mice, followed by western blot detection of 3-nitrotyrosine-positive protein. As shown in Fig. 3B and 3C, the levels of 3-NT proteins were markedly elevated in tissues from either AMPKα1−/− mice or AMPKα2−/− mice when compared with those from WT. As expected, ischemia significantly increased the levels of 3-NT-positive proteins in tissues from WT, AMPKα1−/−, and AMPKα2−/− mice (Fig. 3B and 3C). Interestingly, ischemia caused a greater increase in 3-NT-positive proteins in tissues from AMPKα1−/− and AMPK α2−/− mice than those from WT mice, suggesting that AMPKα deficiency might enhance the formation of reactive oxygen and nitrogen species in response to ischemic insults.
4-Hydroxynonenal (HNE), a lipid oxidation product, is a well characterized marker for oxidative stress in tissues.34 Ischemia increases the formation of 4 HNE-modified proteins in tissues.35 Therefore, we next determined 4-HNE-modified proteins in ischemia- and sham-treated tissues in WT, AMPKα1−/−, and AMPKα2−/− mice. As depicted in Fig. 3D, there was no marked difference among sham-treated tissues in WT, AMPKα1−/−, and AMPKα2−/− mice. As expected, ischemia markedly increased 4-HNE-modified protein staining in WT. A greater increase of 4-HNE-modified proteins were observed in tissues from ischemic-AMPKα1−/−, and AMPKα2−/− mice when compared to ischemic WT (Fig. 3D). Additionally, 8-hydroxy-2’-deoxyguanosine (8-oxo-dG), a marker of oxidative stress to DNA,36 was dramatically increased in the tissues from AMPKα1−/−, and AMPKα2−/− mice treated with either sham or ischemia when compared to WT mice (Fig. 3E).
UCP2 levels are lower in AMPKα1−/− or AMPKα2−/− mice post ischemia
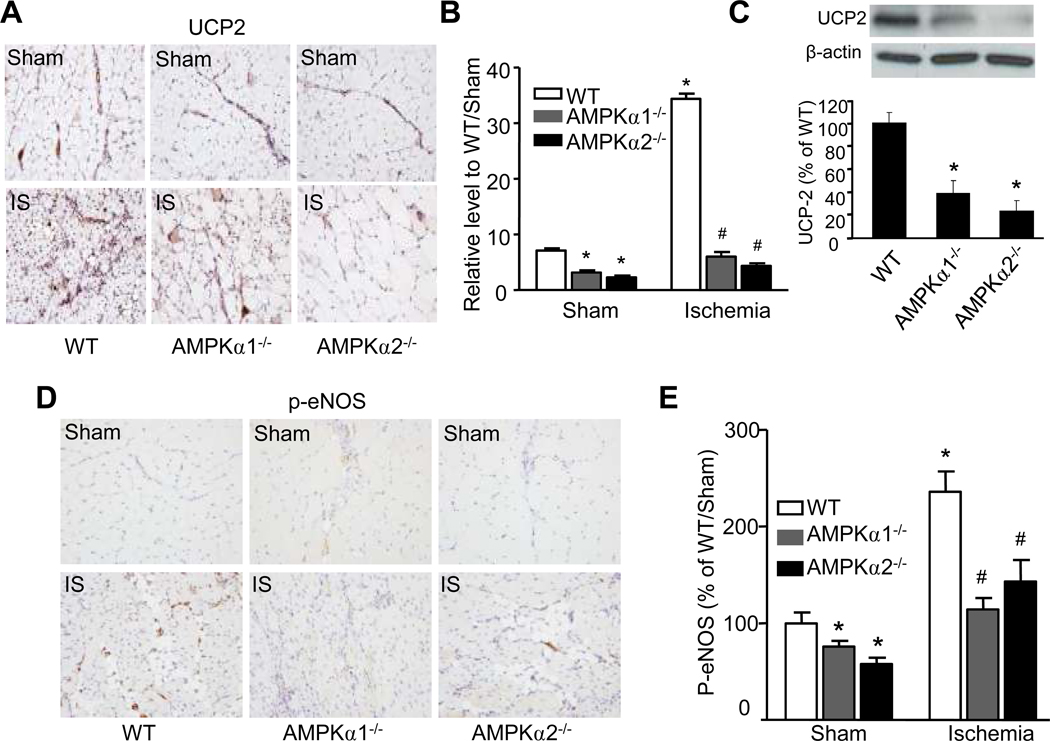
UCP2 has been reported to reduce mitochondrial ROS in several cell types, including endothelial cells, as well as in various organs.37–41 It was interesting to determine whether AMPK deletion altered UCP2 expression under ischemic conditions. Under normoxia, UCP2 expression was significantly lower in tissues from AMPKα1−/− and AMPKα2−/− mice compared to those from WT mice (Fig. 4A and 4B). As expected, ischemia markedly up-regulated the UCP2 levels in WT mice. However, ischemia did not significantly alter the UCP2 levels in tissues from either AMPKα1−/− or AMPKα2−/− mice (Fig. 4A and 4B).
Figure 4. AMPKα deficiency suppresses UCP2 expression and eNOS phosphorylation in vivo.
A. Immunohistochemical detection of UCP2 in hindlimb skeletal muscles from WT, AMPKα1−/−, and AMPKα2−/− mice under sham or ischemia condition; one representative image of each group was shown (×400). B. Quantification for anti-UCP2 staining intensity in vessels was calculated as arbitrary unit. *p<0.05 vs. WT/sham group; #p<0.05 vs. WT/ischemia (IS) group, n=3 (WT) or n=5 (AMPKα1−/− and AMPKα2−/−). C. western blot analyzed UCP2 level in cultured MAECs from WT, AMPKα1−/−, and AMPKα2−/− mice. *p<0.05 vs. WT; n=4. D & E. Immunohisto chemical detection (×400) of p-eNOS-Ser 1177 in WT, AMPKα1−/−, and AMPKα2−/− mice under sham or ischemia condition; *p<0.05 vs. WT/sham group; #p<0.05 vs. WT/ischemia (IS) group, n=3 (WT) or n=5 (AMPKα1−/− and AMPKα2−/−).
Because the amount of endothelial cells was not sufficient for western blot analysis of UCP2 protein levels in ischemic tissues, we also probed for UCP2 in sub-cultured MAECs from WT, AMPKα1−/−, and AMPKα2−/− mice by Western blot analysis and confirmed that it was significantly suppressed in both AMPKα1−/− and AMPKα2−/− mice compared with WT mice (Fig. 4C).
We next determined the phosphorylation of eNOS at Ser1177 in WT, AMPKα1−/−, and AMPKα2−/− mice. Compared with WT, the basal levels of p-eNOS in either AMPKα1−/− or AMPKα2−/− mice were significantly reduced (Fig. 4D and 4E). As expected, ischemia markedly increased the levels of p-eNOS in WT mice (Fig. 4D and 4E). Importantly, ischemia-enhanced p-eNOS in either AMPKα1−/− or AMPKα2−/− mice was markedly attenuated, compared with that in ischemic WT mice (Fig. 4D and 4E). Together, our results suggest an AMPK-dependent eNOS phosphorylation in vivo.
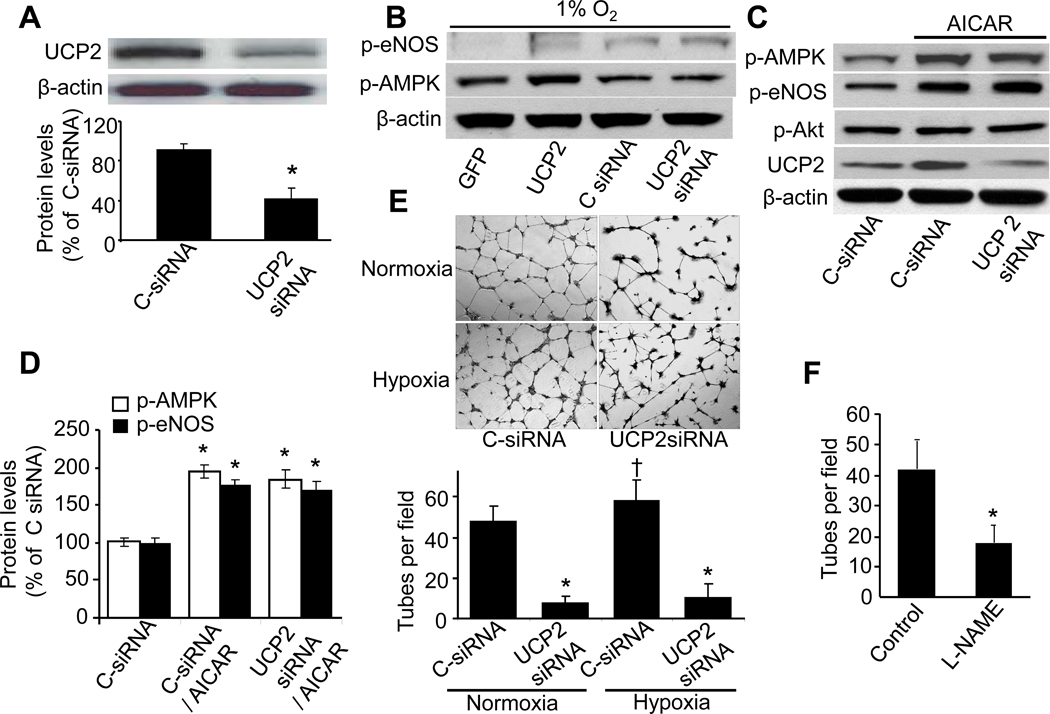
Effects of UCP2 gene silencing on the phosphorylation of both AMPK and eNOS in HUVECs
We next detected if endogenous UCP2 altered the phosphorylation of both AMPK and eNOS. As depicted in Fig. 5A, the transfection of UCP2-specific siRNA but not control siRNA reduced the levels of UCP2 by ~60% in HUVECs. In line with an earlier report,42 gene silencing of UCP2 had no effect on either p-AMPK or p-eNOS (Fig. 5B). As expected, adenoviral overexpression of UCP2 but not Ad-GFP slightly increased the phosphorylation of both p-AMPK and p-eNOS (Fig. 5B).
Figure 5. NO- and UCP2- dependent EC tube formation in cultured HUVECs.
A. UCP2 protein level in HUVECs transfected with control siRNA (C-siRNA) or UCP2 siRNA for 24 h. B. HUVECs infected with either GFP or UCP2, or transfected with either control siRNA or UCP2 siRNA for 72 h were treated by 1% O2 for 1.5 h. The levels of p-AMPKα-Thr172, p-eNOS-Ser1177, and β-actin (loading control) were detected by western blot. The blot is a representative of four blots from four individual experiments. C & D. HUVECs transfected with either control siRNA or UCP2 siRNA were treated with AICAR (2 mM) for 16 h. The levels of p-AMPKα-Thr172, p-eNOS-Ser1177, p-Akt-Thr308, UCP2, and β-actin (loading control) were detected by western blot. *p<0.05 vs. control siRNA; n=4. E. Tube formation was impaired in HUVECs with UCP2 knocked-down. HUVECs transfected with either control siRNA or UCP2 siRNA for 24h were treated with either normoxia or hypoxia (1% O2) for 16h, then were subcultured on Matrigel-coated plates with the treatment of normoxia or hypoxia for 8h. †p<0.05 vs. C-siRNA/normoxia; *p<0.05 vs. C-siRNA/normoxia or hypoxia, respectively, n=5. F. Tube formation was monitored in HUVECs with or without L-NAME, as described in Methods; tube number per visual field (×40) was counted. *p<0.05 vs. control; n=5.
It was interesting to determine if UCP2 knockdown altered AICAR-induced phosphorylation of eNOS and Akt. As expected, AICAR markedly increased the levels of p-AMPK, p-eNOS, and UCP2 in HUVECs (Fig. 5C and 5D). Interestingly, neither UCP2 siRNA nor control siRNA altered AICAR-enhanced phosphorylation of AMPK, eNOS, and Akt (Fig. 5C and 5D). Taken together, these results suggest that AMPK is an upstream kinase for eNOS and UCP2.
Inhibition of UCP2 or eNOS attenuates the tube formation in HUVECs
We reasoned that UCP2 would be critically involved in tube formation under ischemic conditions by increasing NO bioactivity in endothelial cells. To this end, we examined tube formation in HUVECs transfected with UCP2 siRNA or with the NOS inhibitor L-NAME. As shown in Figure 5E, UCP2 siRNA inhibited HUVECs tube formation under either normoxia or hypoxia condition. Inhibition of NOS also dramatically abrogated tube formation in HUVECs (Fig. 5F).
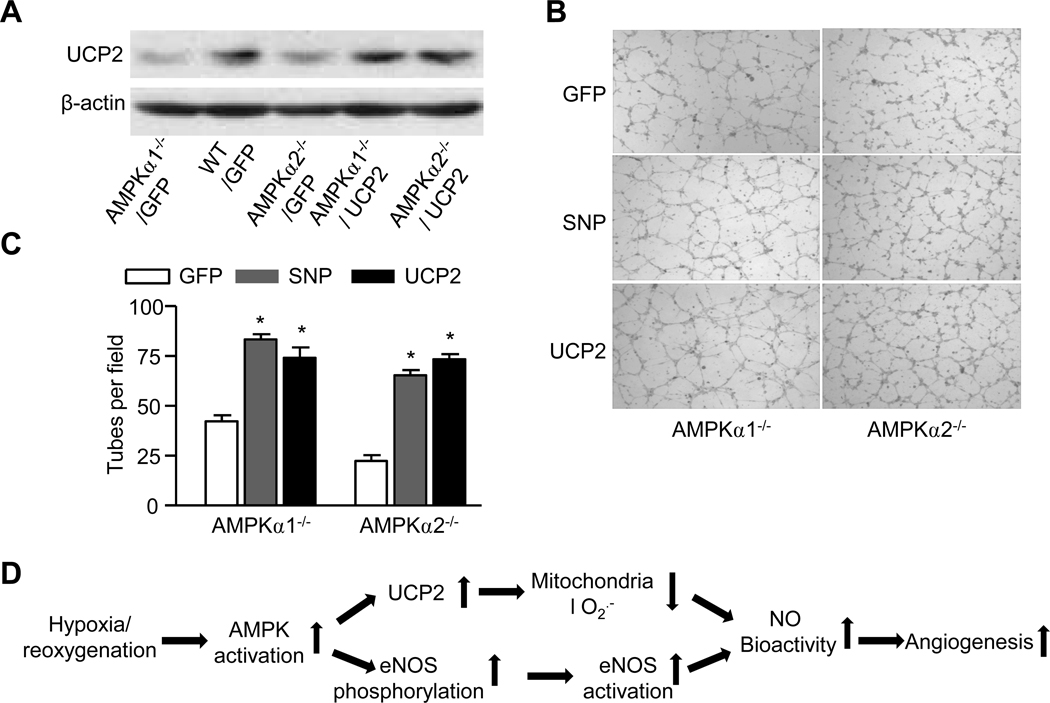
Supplementation of UCP2 or exogenous NO normalizes the tube formation in mouse aortic endothelial cells from mice deficient of AMPKα1 or AMPKα2
We determined if supplementation of UCP2 or exogenous NO restored defective tube formation in MAECs from AMPKα1−/− and AMPKα2−/−. Consistent with our earlier report, UCP2 was markedly reduced in MAECs from AMPKα1−/− and AMPKα2−/− when compared to those from WT (Fig. 6A). As expected, adenoviral overexpression of UCP2 markedly increased the levels of UCP2 in MAECs from AMPKα1−/− and AMPKα2−/− (Fig. 6A). More compellingly, when MAECs derived from AMPKα1−/− and AMPKα2−/− mice were infected with either adenoviral overexpression of UCP2 or treated with the NO donor, SNP, robust recovery of the tube forming response was observed (Fig. 6B and 6C). These findings strongly suggest that NO and UCP2 are both critically required by endothelial cells to mount a normal angiogenic response.
Figure 6. Adenoviral over-expression of UCP2 normalizes tube formation in MAECs isolated from AMPKα1−/− and AMPKα2−/−.
A. Adenoviral over-expression of UCP2 in MAECs isolated from WT or AMPKα1−/− and AMPKα2−/−. The blot is a representative from three blots from three individual experiments. B. Representative images for the tube formation in MAECs isolated from WT, AMPKα1−/− and AMPKα2−/− with SNP or adenoviral overexpression of GFP or UCP2; C. Effects of SNP, a NO donor, and overexpression of UCP2 on the tuber formation in MAECs isolated from AMPKα1−/− and AMPKα2−/−; tube number per visual field (×40) was counted. *p<0.05 vs. corresponding MAECs infected with GFP; n=3 to 5. D. Proposed scheme showing that AMPK activation leads to increased angiogenesis.
Discussion
The present study has demonstrated that intact AMPK signaling is critical in the angiogenic response to ischemia in vitro as well as in vivo. Most importantly, we have provided convincing evidence that implicates UCP2 in mitochondrial uncoupling under ischemic conditions resulting in the suppression of ROS. Mitigation of oxidative stress by UCP2 in turn increases NO bioavailability and this ultimately integrates endothelial tube formation and microvascular angiogenesis in ischemia. Our novel observations might help explain how hypoxia/ischemia, via ROS suppression by AMPK and UCP2, underlies the angiogenic response in vivo (Fig. 6D).
The most important finding of the present study is AMPK-regulated UCP2 expression in hypoxic angiogenesis. In our experiments, we found that in AMPKα1−/− and AMPKα2−/− mice, UCP2 expression was markedly reduced compared with that in WT control mice. In addition, when HUVECs were transfected with siRNA specific to UCP2, they exhibited significant abrogation of endothelial tube formation. When these cells were treated with an NOS inhibitor, tube formation was also suppressed significantly. These findings were validated in MAECs derived from AMPKα1−/− and AMPKα2−/− mice that were treated with SNP or adenoviral overexpression of UCP2. Both interventions partly but significantly mitigated the tube formation defect. Our findings likely represent an early report of a possible physiological role of UCP2, which is usually classified as an atypical or novel UCP, in part because its exact function is uncertain. Our findings also appear to be consistent with previous reports suggesting a critical role for AMPK in compensatory new vessel formation when tissues become ischemic/hypoxic.23
Another important finding of this study is that UCP2 increases angiogenesis by reducing reactive oxygen and nitrogen species in vivo. Hypothetically, UCP2 can lead to more rapid electron flux through the respiratory chain, thereby dampening mitochondrial ROS synthesis.43 Downregulation of mitochondrial ROS production may be the most plausible role for UCP2, because its expression has been confirmed in numerous mammalian tissues. The ability of UCP2 to reduce ROS not only in mitochondria, but within the cytosol or even in the extracellular space, has also been documented. Duval et al. have recently shown that UCP2-mediated uncoupling in endothelial cells may decrease extracellular ROS in co-incubated low-density lipoproteins (LDLs).40 Furthermore, mice with deleted LDL receptors exhibit extensive diet-induced atherosclerotic plaques when they received bone marrow transplants from UCP2−/− mice, and appearance of these plaques was prevented when they received bone marrow transplants from UCP2+/+ mice, suggesting that the basic role of all UCP2 is to reduce oxidant stress to avoid oxidative tissue injury.39 In a previously published report from our laboratory, we have laid a foundation for the mechanism we are proposing in the present study. In that paper, our group demonstrated that when HUVECs or mice were treated with AICAR (a potent activator of AMPK), UCP2 expression was upregulated and oxidative stress was attenuated.15 Overall, our results, together with previous reports,10, 11 suggest that hypoxia/ischemia can activate AMPK. In turn, AMPK can suppress ROS synthesis via mitochondrial uncoupling mediated through UCP2. The significant attenuation of ROS that ensues can then enhance the bioavailability of NO. NO, through its stimulatory impact in VEGF as well as its role in promoting endothelial proliferation and migration, can then promote new vessel formation.10, 11
In summary, the present study proposes a role for UCP2 in the tissue response of angiogenesis to ischemic stress. Specifically, when ischemia is induced in tissues, AMPK is activated via multiple stimuli such as increased AMP/ATP ratios, VEGF, NO, and the formation of ROS or RNS such as H2O2 and ONOO−. AMPK activation appears to enhance the expression of UCP2, which then blocks ROS synthesis by the mitochondrial respiratory chain. ROS suppression serves to maintain high levels of NO bioavailability ultimately necessary for a normal angiogenic response to ischemia and/or hypoxia (Fig. 6D). Further delineation of these proposed mechanisms will be necessary before a complete understanding of this process is achieved.
Acknowledgments
This work was supported by NIH grants (HL074399, HL079584, HL080499, HL089920, HL096032, HL105157, and HL110448), a research award from the American Diabetes Association, and Warren Chair in Diabetes Research from the University of Oklahoma Health Sciences Center. Dr. Zou is a recipient of the National Established Investigator Award of American Heart Association. This work is partly supported by a grant from Chinese National Science Foundation (81028002).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schulz E, Anter E, Zou MH, Keaney JF., Jr Estradiol-mediated endothelial nitric oxide synthase association with heat shock protein 90 requires adenosine monophosphate-dependent protein kinase. Circulation. 2005;111:3473–3480. doi: 10.1161/CIRCULATIONAHA.105.546812. [DOI] [PubMed] [Google Scholar]
- 2.Viollet B, Athea Y, Mounier R, Guigas B, Zarrinpashneh E, Horman S, Lantier L, Hebrard S, Devin-Leclerc J, Beauloye C, Foretz M, Andreelli F, Ventura-Clapier R, Bertrand L. AMPK: Lessons from transgenic and knockout animals. Front Biosci. 2009;14:19–44. doi: 10.2741/3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breier G, Damert A, Plate KH, Risau W. Angiogenesis in embryos and ischemic diseases. Thromb Haemost. 1997;78:678–683. [PubMed] [Google Scholar]
- 4.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J. Angiogenesis inhibitors generated by tumors. Mol Med. 1995;1:120–122. [PMC free article] [PubMed] [Google Scholar]
- 7.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 8.Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res. 2006;71:226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest. 1997;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziche M, Morbidelli L, Masini E, Granger H, Geppetti P, Ledda F. Nitric oxide promotes DNA synthesis and cyclic GMP formation in endothelial cells from postcapillary venules. Biochem Biophys Res Commun. 1993;192:1198–1203. doi: 10.1006/bbrc.1993.1543. [DOI] [PubMed] [Google Scholar]
- 13.Murohara T, Witzenbichler B, Spyridopoulos I, Asahara T, Ding B, Sullivan A, Losordo DW, Isner JM. Role of endothelial nitric oxide synthase in endothelial cell migration. Arterioscler Thromb Vasc Biol. 1999;19:1156–1161. doi: 10.1161/01.atv.19.5.1156. [DOI] [PubMed] [Google Scholar]
- 14.Pecqueur C, Couplan E, Bouillaud F, Ricquier D. Genetic and physiological analysis of the role of uncoupling proteins in human energy homeostasis. J Mol Med. 2001;79:48–56. doi: 10.1007/s001090000150. [DOI] [PubMed] [Google Scholar]
- 15.Xie Z, Zhang J, Wu J, Viollet B, Zou MH. Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes. 2008;57:3222–3230. doi: 10.2337/db08-0610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 17.Munzel T, Daiber A, Ullrich V, Mulsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol. 2005;25:1551–1557. doi: 10.1161/01.ATV.0000168896.64927.bb. [DOI] [PubMed] [Google Scholar]
- 18.Price DT, Vita JA, Keaney JF., Jr Redox control of vascular nitric oxide bioavailability. Antioxid Redox Signal. 2000;2:919–935. doi: 10.1089/ars.2000.2.4-919. [DOI] [PubMed] [Google Scholar]
- 19.Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 20.Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA. Modulation by peroxynitrite of Akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J Biol Chem. 2002;277:32552–32557. doi: 10.1074/jbc.M204512200. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, Zhu Y, DeFea K, Pan S, Tsai MD, Shyy JY. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res. 2009;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou MH, Wu Y. AMP-activated protein kinase activation as a strategy for protecting vascular endothelial function. Clin Exp Pharmacol Physiol. 2008;35:535–545. doi: 10.1111/j.1440-1681.2007.04851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278:31000–31006. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- 24.Viollet B, Andreelli F, Jorgensen SB, Perrin C, Flamez D, Mu J, Wojtaszewski JF, Schuit FC, Birnbaum M, Richter E, Burcelin R, Vaulont S. Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem Soc Trans. 2003;31:216–219. doi: 10.1042/bst0310216. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki K, Murohara T, Ikeda H, Sugaya T, Shimada T, Shintani S, Imaizumi T. Evidence for the importance of angiotensin II type 1 receptor in ischemia-induced angiogenesis. J Clin Invest. 2002;109:603–611. doi: 10.1172/JCI13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Y, Zhang M, Liang B, Xie Z, Zhao Z, Asfa S, Choi HC, Zou MH. Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation. 121:792–803. doi: 10.1161/CIRCULATIONAHA.109.900928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lips KS, Pfeil U, Reiners K, Rimasch C, Kuchelmeister K, Braun-Dullaeus RC, Haberberger RV, Schmidt R, Kummer W. Expression of the high-affinity choline transporter CHT1 in rat and human arteries. J Histochem Cytochem. 2003;51:1645–1654. doi: 10.1177/002215540305101208. [DOI] [PubMed] [Google Scholar]
- 28.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song P, Zhang M, Wang S, Xu J, Choi HC, Zou MH. Thromboxane A2 receptor activates a Rho-associated kinase/LKB1/PTEN pathway to attenuate endothelium insulin signaling. J Biol Chem. 2009;284:17120–17128. doi: 10.1074/jbc.M109.012583. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5'-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528(Pt 1):221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou MH, Hou XY, Shi CM, Kirkpatick S, Liu F, Goldman MH, Cohen RA. Activation of 5'-AMP-activated kinase is mediated through c-Src and phosphoinositide 3-kinase activity during hypoxia-reoxygenation of bovine aortic endothelial cells. Role of peroxynitrite. J Biol Chem. 2003;278:34003–34010. doi: 10.1074/jbc.M300215200. [DOI] [PubMed] [Google Scholar]
- 32.Zou MH, Bachschmid M. Hypoxia-reoxygenation triggers coronary vasospasm in isolated bovine coronary arteries via tyrosine nitration of prostacyclin synthase. J Exp Med. 1999;190:135–139. doi: 10.1084/jem.190.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou MH, Shi C, Cohen RA. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H(2) receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes. 2002;51:198–203. doi: 10.2337/diabetes.51.1.198. [DOI] [PubMed] [Google Scholar]
- 34.Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 35.McCracken E, Valeriani V, Simpson C, Jover T, McCulloch J, Dewar D. The lipid peroxidation by-product 4-hydroxynonenal is toxic to axons and oligodendrocytes. J Cereb Blood Flow Metab. 2000;20:1529–1536. doi: 10.1097/00004647-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339:1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- 38.Negre-Salvayre A, Hirtz C, Carrera G, Cazenave R, Troly M, Salvayre R, Penicaud L, Casteilla L. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 1997;11:809–815. [PubMed] [Google Scholar]
- 39.Blanc J, Alves-Guerra MC, Esposito B, Rousset S, Gourdy P, Ricquier D, Tedgui A, Miroux B, Mallat Z. Protective role of uncoupling protein 2 in atherosclerosis. Circulation. 2003;107:388–390. doi: 10.1161/01.cir.0000051722.66074.60. [DOI] [PubMed] [Google Scholar]
- 40.Duval C, Negre-Salvayre A, Dogilo A, Salvayre R, Penicaud L, Casteilla L. Increased reactive oxygen species production with antisense oligonucleotides directed against uncoupling protein 2 in murine endothelial cells. Biochem Cell Biol. 2002;80:757–764. doi: 10.1139/o02-158. [DOI] [PubMed] [Google Scholar]
- 41.Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T, Gonzalez-Zulueta M, Nikolich K, Wieloch T. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med. 2003;9:1062–1068. doi: 10.1038/nm903. [DOI] [PubMed] [Google Scholar]
- 42.Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschop MH, Shanabrough M, Cline G, Shulman GI, Coppola A, Gao XB, Horvath TL, Diano S. UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan SH, Wu CA, Wu KL, Ho YH, Chang AY, Chan JY. Transcriptional upregulation of mitochondrial uncoupling protein 2 protects against oxidative stress-associated neurogenic hypertension. Circ Res. 2009;105:886–896. doi: 10.1161/CIRCRESAHA.109.199018. [DOI] [PubMed] [Google Scholar]