Abstract
Background
Thrombotic thrombocytopenic purpura is a potentially fatal disease characterized by widespread platelet thrombi in the microcirculation. In the normal circulation, von Willebrand factor is cleaved by a plasma protease. We explored the hypothesis that a deficiency of this protease predisposes patients with thrombotic thrombocytopenic purpura to platelet thrombosis.
Methods
We studied the activity of von Willebrand factor–cleaving protease and sought inhibitors of this protease in plasma from patients with acute thrombotic thrombocytopenic purpura, patients with other diseases, and normal control subjects. We also investigated the effect of shear stress on the ristocetin cofactor activity of purified von Willebrand factor in the cryosupernatant fraction of the plasma samples.
Results
Thirty-nine samples of plasma from 37 patients with acute thrombotic thrombocytopenic purpura had severe deficiency of von Willebrand factor–cleaving protease. No deficiency was detected in 16 samples of plasma from patients with thrombotic thrombocytopenic purpura in remission or in 74 plasma samples from normal subjects, randomly selected hospitalized patients or outpatients, or patients with hemolysis, thrombocytopenia, or thrombosis from other causes. Inhibitory activity against the protease was detected in 26 of the 39 plasma samples (67 percent) obtained during the acute phase of the disease. The inhibitors were IgG antibodies. Shear stress increased the ristocetin cofactor activity of von Willebrand factor in the cryosupernatant of plasma samples obtained during the acute phase, but decreased the activity in cryosupernatant of plasma from normal subjects.
Conclusions
Inhibitory antibodies against von Willebrand factor–cleaving protease occur in patients with acute thrombotic thrombocytopenic purpura. A deficiency of this protease is likely to have a critical role in the pathogenesis of platelet thrombosis in this disease.
Thrombotic thrombocytopenic purpura is characterized by widespread platelet thrombi in arterioles and capillaries.1,2 Despite therapeutic advances,3 the age-adjusted mortality associated with the disease nearly tripled from 1971 to 1991.4 Among those who survive the acute phase, relapse is not uncommon.5 Infection with the human immunodeficiency virus (HIV) and other retroviruses may contribute to the increased frequency of the disease.6
Both endothelial-cell injury7,8 and intravascular platelet aggregation9 have been implicated in the pathogenesis of thrombotic thrombocytopenic purpura. Immunohistologic studies have demonstrated abundant von Willebrand factor in the thrombotic lesions.10 Abnormal multimers of von Willebrand factor, initially described in patients with chronic relapsing thrombotic thrombocytopenic purpura,11 are also common in the acute phase.12 However, the type of abnormality varies; many patients have fewer large multimers than normal, whereas others have normal levels of large multimers or even unusually large forms.
Von Willebrand factor is secreted from endothelial cells as an extra large polymer of a polypeptide joined by disulfide bonds13 and cleaved in the circulation at the peptide bond between tyrosine at position 842 and methionine at position 84314 by a 200-kd plasma metalloproteinase.15,16 Cleavage by the enzyme decreases the size of von Willebrand factor to dimers of 176-kd and 140-kd fragments.15,17 The enzyme, which is present in the cryosupernatant fraction of the plasma, requires a calcium or zinc cation for its activity.18 It is inhibited by tetracyclines but resistant to batimastat, a synthetic matrix metalloproteinase–specific inhibitor.18
In plasma the protease has little effect on von Willebrand factor unless the factor is unfolded by high levels of shear stress or other means.17 This suggests that in patients with thrombotic thrombocytopenic purpura, the multimers of von Willebrand factor ought to be relatively small, because the abnormal shear stress caused by platelet thrombi in the microcirculation should enhance proteolysis of von Willebrand factor. However, in some patients with acute thrombotic thrombocytopenic purpura, the size of the multimers is normal or very large. These findings point to a defect in the proteolysis of von Willebrand factor. Such a defect was suspected11 and recently described19 in patients with the chronic relapsing form of thrombotic thrombocytopenic purpura. In this study, we investigated the activity of von Willebrand factor–cleaving protease in patients with acute episodes of thrombotic thrombocytopenic purpura and the mechanisms by which a deficiency of the protease might lead to platelet thrombosis.
METHODS
Subjects
The diagnosis of acute thrombotic thrombocytopenic purpura was based on the standard criteria3 of thrombocytopenia (a platelet count of less than 100×103 per cubic millimeter), microangiopathic hemolytic anemia (as indicated by erythrocyte fragmentation on peripheral-blood smears) with a negative Coombs’ test, and the absence of identifiable causes of these abnormalities, such as disseminated intravascular coagulation, cancer, or preeclampsia. None of the patients had a serum creatinine concentration of more than 4 mg per deciliter (354 µmol per liter) or required renal dialysis. Thirty patients were treated at our institutions, and seven were referred from other hospitals. Six patients were known to have had prior episodes of the disease or additional episodes after the collection of plasma samples. Two of the patients had HIV infection, one had a history of systemic lupus erythematosus, and one had rheumatoid factor and antibodies to DNA.
Blood samples were collected in tubes containing citrate anticoagulant before or after the institution of plasma-exchange therapy or during remission when platelet counts were normal and stable. We also examined plasma samples from 35 normal subjects or outpatients or hospitalized patients without thrombotic thrombocytopenic purpura; 21 patients with miscellaneous autoimmune or blood disorders, including disseminated intravascular coagulopathy (1 patient), autoimmune thrombocytopenia (2 patients), drug-induced thrombocytopenia (2 patients), the HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count in association with preeclampsia, 1 patient), deep-vein thrombosis (1 patient), lupus anticoagulant with thrombosis (1 patient), myeloproliferative disorders (4 patients), sickle cell anemia (5 patients), hemophilia A (1 patient), and von Willebrand’s disease (3 patients); and 18 patients with heparin-induced thrombocytopenia. Sixteen of the 18 plasma samples from patients with heparin-induced thrombocytopenia were kindly provided by Dr. G.P. Visentin of the Blood Center of Southeastern Wisconsin in Milwaukee. The heparin-induced serotonin-release assay was positive in all 17 of the plasma samples that were tested. Eight of the patients with heparin-induced thrombocytopenia had thrombotic complications. The investigation protocol was approved by the institutional review boards of the participating centers.
Control plasma consisted of pooled normal plasma used in the clinical coagulation laboratory. Plasma was separated from whole blood by a two-stage centrifugation15 and stored at −70°C. Cryosupernatant fractions were obtained by centrifugation at 4°C from plasma samples thawed on ice.15 Aliquots of plasma from two patients were also studied before being frozen, after high-speed centrifugation at 30,000×g for 60 minutes and after passage through 0.2-µm filters.
Preparation of von Willebrand Factor
Von Willebrand factor was purified from pooled normal plasma by precipitation with glycine salts and gel filtration.17 The fractions eluted from the chromatographic column that contained the largest multimers at a concentration of 150 to 250 µg per milliliter were exposed to guanidine (1.5 mol per liter) and used as the substrate for the protease assay.
Multimers of von Willebrand factor were analyzed by sodium dodecyl sulfate–agarose-gel electrophoresis as previously described.18 The size of the multimers was represented by the distance from the starting point of electrophoresis to the peak of multimer distribution, as determined by densitometry, divided by the peak distance of control von Willebrand factor (the normalized peak distance).18 An increase in the normalized peak distance indicated a decrease in the size of the multimers. Concentrations of von Willebrand factor were determined by an enzyme-linked immunosorbent assay as described previously.20
Assay of Protease Activity
The assay of von Willebrand factor–cleaving protease activity in plasma was based on the generation, from purified von Willebrand factor, of dimers of 176-kd and 140-kd fragments, which appeared as 350-kd and 200-kd bands, respectively, on sodium dodecyl sulfate–polyacrylamide-gel electrophoresis.15 Plasma samples were diluted 1:3 with a TRIS saline buffer containing 50 mmol of TRIS per liter and 50 mmol of sodium chloride per liter (pH 8.0) in the presence or absence of 5 mmol of EDTA per liter. Von Willebrand factor was added to the test samples at a dilution of 1:10. After a 60-minute incubation at 37°C, the reaction was terminated by the addition of a 1:5 dilution of a buffer containing 0.125 mmol of TRIS per liter (pH 6.8), 20 g of sodium dodecyl sulfate per liter, 5 percent glycerol (vol/vol), and 5 mmol of EDTA per liter.18 The optical intensity of von Willebrand factor fragments, after immunoblotting and autoradiography, was determined by scanning densitometry.18 The difference between the intensity of the 350-kd band in the reaction mixture without EDTA and that in the mixture with EDTA represented the amount generated by the protease in the test sample. The 350-kd band generated by serially diluted samples of control plasma, expressed as a percentage of that generated in an undiluted sample of control plasma, showed a linear correlation with the plasma concentration. Therefore, the optical intensity of the 350-kd band generated in the assay was used to represent the protease activity in the test sample.
Sixty-seven of the plasma samples from control subjects and patients with thrombotic thrombocytopenic purpura were obtained at the University of Miami and were randomly distributed in a blinded fashion to the laboratory where the assay was performed. Many of the plasma samples had been frozen at −70°C. Analysis of stored samples of control plasma showed that freezing for at least 20 years did not adversely affect the protease activity.
Preparation of IgG
IgG was isolated from plasma with staphylococcal protein A–agarose columns equilibrated in a buffer of 50 mmol of TRIS per liter (pH 8.0) and 0.5 mol of sodium chloride per liter. IgG was eluted with a buffer of 0.1 mol of glycine per liter (pH 2.8) and 0.5 mol of sodium chloride per liter (pH 2.8). The eluates, which were immediately neutralized by a 1:10 dilution of TRIS (1 mol per liter, pH 8.0), were dialyzed extensively against the TRIS saline buffer before being concentrated on polyethylene glycol 20,000. Protein concentrations were determined by a Coomassie brilliant blue dye–binding assay.15 Bovine immune globulin was used as the reference.
Detection of Inhibitor by Mixing Studies
For the mixing studies, plasma samples were incubated at 56°C for 60 minutes to inactivate the protease. Control plasma was then incubated at 37°C for 30 minutes with an equal volume of the test plasma or IgG. The protease activity in the mixture was expressed as a percentage of that in control plasma incubated with heated control plasma or control IgG.
In IgG-neutralization studies, IgG antibody was incubated with various concentrations of rabbit IgG antibodies against human IgG Fab region or Fc region or IgM mu chain (Sigma Chemical, St. Louis) before its inhibitory activity was determined by mixing studies.
Mixing studies were also performed with various dilutions of some plasma samples from patients with acute thrombotic thrombocytopenic purpura. Ten microliters of control plasma was mixed with 30 µl of undiluted or serially diluted plasma samples from the patients. The von Willebrand factor–cleaving protease activity in 10 µl of control plasma incubated with 30 µl of undiluted or serially diluted heated control plasma was used as the reference.
Effect of Shear Stress on von Willebrand Factor
Purified von Willebrand factor that contained large multimers was dissolved to a final concentration of 5 to 10 µg per milliliter in the cryosupernatant fraction of control plasma or plasma samples from patients with thrombotic thrombocytopenic purpura. Aliquots of von Willebrand factor were forced at controlled flow rates through stainless-steel tubing (length, 610 cm; internal radius, 0.0254 cm) at room temperature with a syringe pump.17 In this device, the aliquots were exposed to the highest rate of shear stress of 6404 per second for 15 seconds. The ristocetin cofactor activity in the aliquots was determined in triplicate within 60 minutes after the procedure as previously described.18 The values were compared with those of control aliquots that had not been exposed to shear stress with the use of Student’s t-test.
RESULTS
Protease Activity
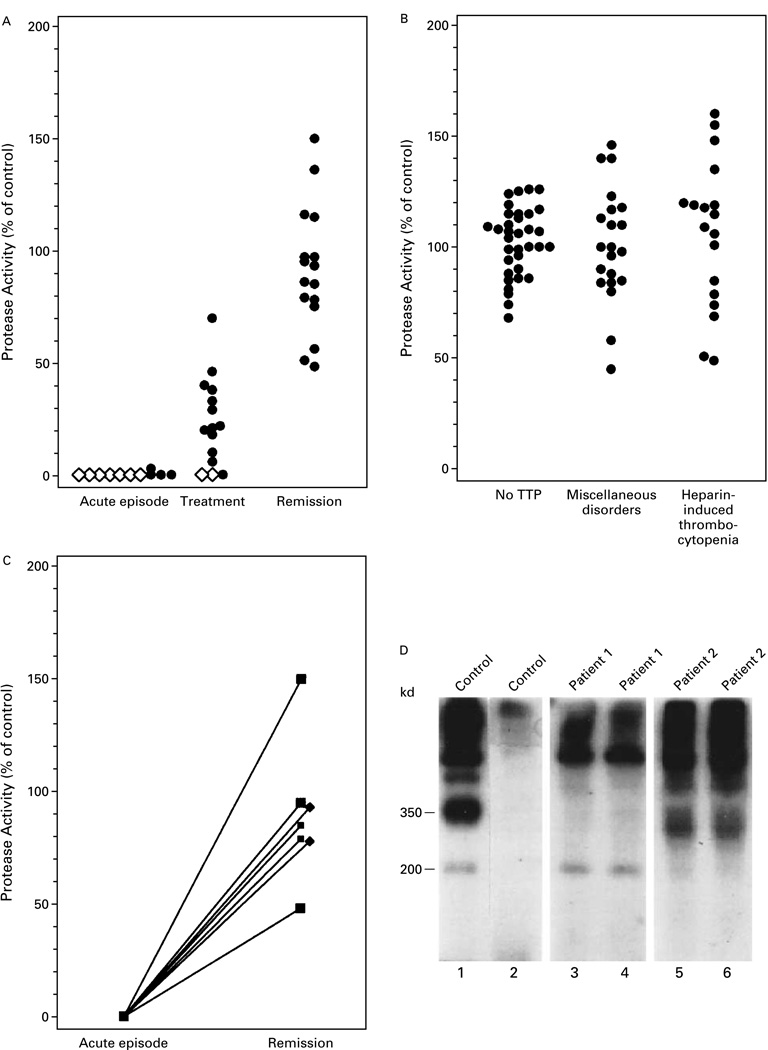
We studied 39 plasma samples obtained from 37 patients during acute episodes of thrombotic thrombocytopenic purpura (Fig. 1A). Two of the patients were studied during two acute episodes, at least two years apart. Thirty-eight samples had no detectable von Willebrand factor–cleaving protease activity, whereas one sample had a level that was 3 percent of the control value. The protease activity of 23 samples obtained after plasma-exchange therapy was instituted ranged from 0 to 70 percent (mean [±SD], 16±20 percent). As compared with the protease activity of plasma samples from subjects without thrombotic thrombocytopenic purpura (Fig. 1B), 22 of these samples had lower-than-normal activity. Samples with protease activity that was more than 57 percent of the value measured in control plasma samples were considered normal.
Figure 1.
Von Willebrand Factor–Cleaving Protease Activity in Plasma Samples from 37 Patients with Thrombotic Thrombocytopenic Purpura and 74 Control Subjects.
The protease activity in each sample was reflected by the intensity of the 350-kd band generated from purified von Willebrand factor and expressed as a percentage of the activity in control plasma. Panel A shows the protease activity of 39 plasma samples obtained during acute episodes of thrombotic thrombocytopenic purpura (TTP) before plasma-exchange therapy, 23 samples obtained after therapy, and 16 samples obtained during remission. Diamonds represent five samples, and circles one sample. Panel B shows the protease activity of plasma samples obtained from 35 randomly selected subjects without thrombotic thrombocytopenic purpura, 21 patients with miscellaneous autoimmune or blood disorders, and 18 patients with heparin-induced thrombocytopenia. Panel C shows the protease activity of plasma samples obtained from seven patients with thrombotic thrombocytopenic purpura during both an acute episode and remission. Panel D shows a composite immunoblot of 200-kd, 350-kd, and larger fragments generated from purified von Willebrand factor in control plasma (lanes 1 and 2) but not in plasma samples from two patients with acute thrombotic thrombocytopenic purpura (lanes 3, 4, 5, and 6). Each sample was assayed in duplicate. EDTA was absent from the buffer in lanes 1, 3, and 5 and present in lanes 2, 4, and 6.
Plasma samples from subjects without thrombotic thrombocytopenic purpura were also studied (Fig. 1B). Protease activity ranged from 68 to 126 percent of the value in control samples (mean, 102±15 percent) in 35 samples from normal subjects or outpatients or hospitalized patients without thrombotic thrombocytopenic purpura, from 45 to 145 percent (mean, 101±25 percent) in 21 samples from patients with miscellaneous autoimmune or blood disorders, and from 48 to 160 percent (mean, 106±33 percent) in 18 samples from patients with heparin-induced thrombocytopenia. These values did not differ significantly from those measured in 18 plasma samples obtained from patients with thrombotic thrombocytopenic purpura in remission (range, 48 to 150 percent; mean, 91±28 percent) (Fig. 1A).
Seven patients with thrombotic thrombocytopenic purpura were studied during both an acute episode and remission (Fig. 1C). In each case, the protease activity rose to a normal or a nearly normal level at remission. Figure 1D shows the absence of protease activity in plasma samples from two patients. Twenty-six of 39 plasma samples (67 percent) from patients with acute thrombotic thrombocytopenic purpura had poorly defined bands at 300 kd (as shown in Fig. 1D for Patient 2) that were not seen in subjects without thrombotic thrombocytopenic purpura. These bands were not derived from the added von Willebrand factor, since they were present in the unadulterated plasma.
Multimer analysis showed that after incubation with the cryosupernatant of control plasma, von Willebrand factor was cleaved to smaller forms, whereas it remained unchanged after incubation with plasma samples from patients with thrombotic thrombocytopenic purpura (data not shown).
In one patient, plasma samples were obtained from the time of an acute episode until remission and analyzed. Plasma samples obtained on days 1, 2, 5, and 22 had protease activity that was 0, 30, 65, and 95 percent of the control value, respectively, with corresponding platelet counts of 2×106, 2×106, 45×106, and 212×106 per cubic millimeter. The extraneous 300-kd bands were present on day 1 but disappeared in subsequent samples. High-speed centrifugation or filtration to remove platelet microparticles from the plasma samples did not affect the protease activity, nor did storage at −70°C.
Inhibitors of the Protease
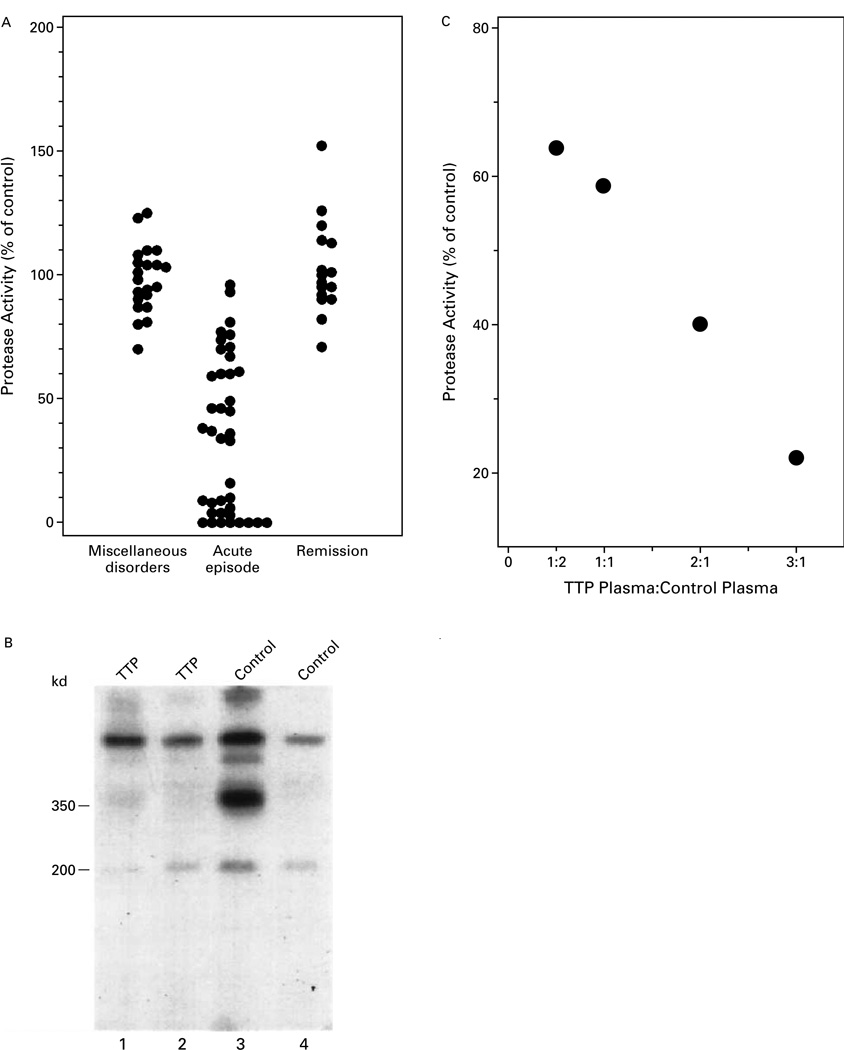
Mixing studies were performed to detect inhibitors of von Willebrand factor–cleaving protease (Fig. 2A). The protease activity in mixtures of control plasma and plasma samples from 21 subjects with miscellaneous autoimmune or blood disorders ranged from 70 to 125 percent of the value in control samples (mean, 98±14 percent). Of the 39 samples obtained from patients during acute episodes of thrombotic thrombocytopenic purpura, 26 (67 percent) reduced the level of protease activity in control plasma by more than 3 SD. No inhibitory activity was detected in 16 samples obtained during a remission of the disease; the protease activity ranged from 71 to 152 percent (mean, 103±19 percent). Proteolysis in control plasma was inhibited by incubation with a plasma sample from a patient with thrombotic thrombocytopenic purpura (lanes 1 and 2 in Fig. 2B) but not by incubation with heated control plasma (lanes 3 and 4 in Fig. 2B).
Figure 2.
Detection of an Inhibitor of von Willebrand Factor–Cleaving Protease.
In Panel A, von Willebrand factor–cleaving protease activity was measured in a mixture of control plasma and an equal volume of a plasma sample from 21 patients with miscellaneous autoimmune or blood disorders, 37 patients with acute thrombotic thrombocytopenic purpura (39 samples), and 16 patients with thrombotic thrombocytopenic purpura in remission. Panel B shows the results of autoradiography. Von Willebrand factor was cleaved in control plasma incubated with heated control plasma (lanes 3 and 4), whereas there was minimal cleavage in control plasma incubated with a plasma sample from a patient with thrombotic thrombocytopenic purpura (TTP) (lanes 1 and 2). EDTA was absent from the buffer in lanes 1 and 3 and present in lanes 2 and 4. In Panel C, protease activity was measured in 3:1 mixtures of various dilutions of a plasma sample from a patient with thrombotic thrombocytopenic purpura and control plasma.
The plasma samples from patients with thrombotic thrombocytopenic purpura that reduced the level of protease activity in control plasma by less than 3 SD but more than 2 SD were further investigated (Fig. 2C). When these plasma samples were present in an equal volume with control plasma, the protease activity was 59 percent of that in the control mixture. When the samples were present at a ratio of 3 to 1, the activity was 22 percent of that in the control mixture.
The inhibitory activity in plasma samples from patients with thrombotic thrombocytopenic purpura was not affected by dialysis, was stable at 56°C, and was resistant to serine or cysteine protease inhibitors such as isoflurophate, phenylmethylsulfonyl fluoride, aprotinin, leupeptin, iodoacetamide, and N-ethylmaleimide. However, it was abolished by passage through a protein A–agarose column, suggesting that the inhibition was mediated by IgG.
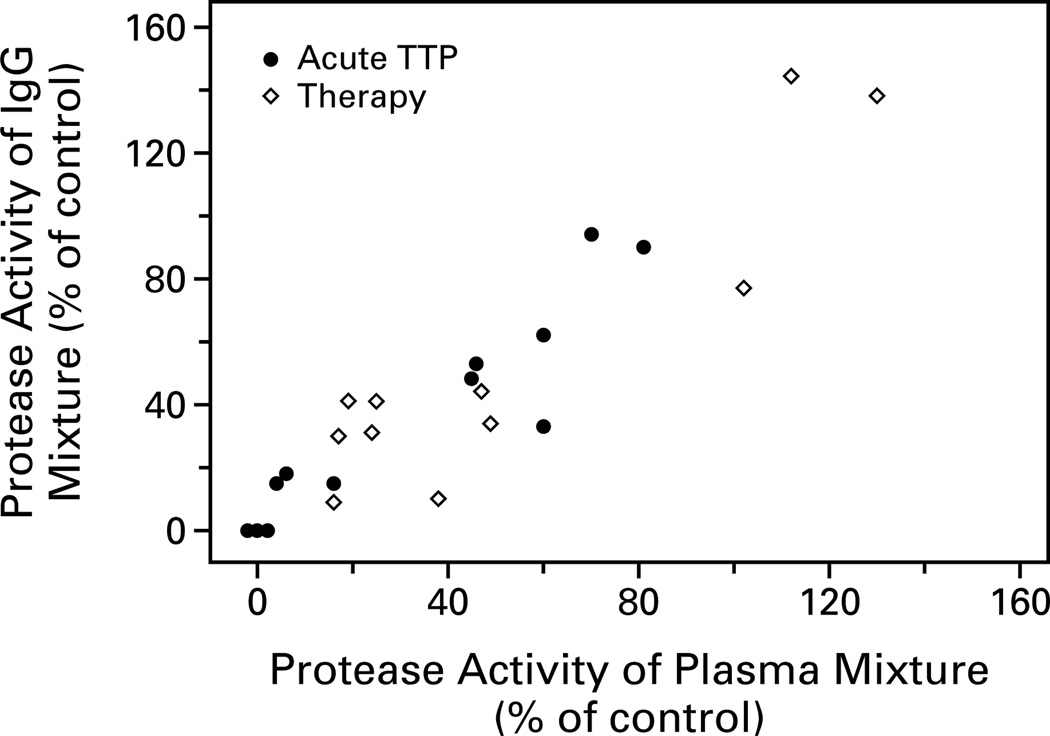
The IgG antibody isolated from 12 samples of plasma from patients with acute thrombotic thrombocytopenic purpura and 11 samples from patients after plasma-exchange therapy was instituted was compared with the inhibitory activity of the patients’ plasma. The IgG was isolated in a quantitative manner: 1 ml of plasma was loaded onto 1 ml of protein–A agarose beads, and IgG antibody was eluted with 4 ml of glycine buffer and concentrated to a final volume of 0.5 ml. The concentrations of the IgG antibody in the samples ranged from 8.6 to 39.0 mg per milliliter. The protease activity in the mixture of control plasma and the IgG from the patients was plotted against the protease activity in the mixture of control plasma and the plasma from which the IgG antibody had been isolated (Fig. 3). The positive correlation (correlation coefficient, 0.91) indicated that the inhibitory activity of plasma samples from the patients with thrombotic thrombocytopenic purpura was mediated by the IgG.
Figure 3.
Inhibitory Activity of IgG from 12 Patients with Acute Thrombotic Thrombocytopenic Purpura (TTP) and from 11 Patients after Plasma-Exchange Therapy Was Instituted as a Function of the Inhibitory Activity of Plasma Samples from Which IgG Was Isolated.
IgG antibody was isolated from plasma samples from the patients and mixed with control plasma, and protease activity was plotted against the protease activity measured in the mixture of control plasma and the patients’ plasma samples.
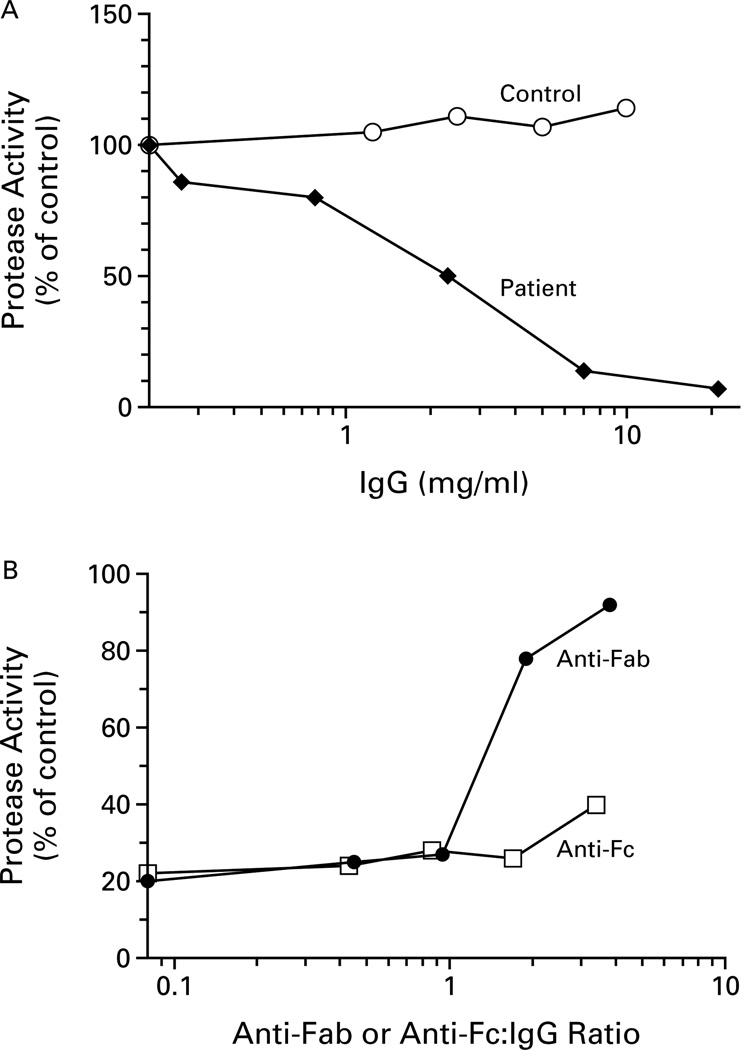
Figure 4A depicts the concentration-dependent inhibition of protease activity by IgG antibody isolated from one of these plasma samples. At a concentration of 2 mg per milliliter, the IgG antibody inhibited the protease activity in control plasma by 50 percent. Control IgG exhibited no inhibitory activity.
Figure 4.
Inhibition of von Willebrand Factor–Cleaving Protease Activity by an IgG Antibody Isolated from Plasma from a Patient with Thrombotic Thrombocytopenic Purpura (TTP) (Panel A) and Neutralization of the Inhibitory Activity by the Antibody against Human Fab Region (Panel B).
In Panel A, the protease activity of control plasma and IgG antibody isolated from the patient was plotted against the final IgG concentration in the mixture (log scale). The protease activity of the mixture of control plasma and control IgG is also shown. In Panel B, the protease activity of a mixture of control plasma, IgG antibody isolated from the patient, and IgG antibodies to the Fab region (anti-Fab) or the Fc region (anti-Fc) was plotted against the ratio of anti-Fab or anti-Fc to IgG in the mixture (log scale).
To confirm that the inhibition involved an antigen–antibody interaction, we treated the patient’s IgG with rabbit IgG antibodies against human IgG Fab region or Fc region before the assay (Fig. 4B). Only the antibody against the Fab region abolished the inhibitory activity of the patient’s IgG antibody, and this was true in all six IgG preparations that were tested. In separate experiments, the inhibitory activity of the IgG antibody from the patient’s plasma was not affected by the rabbit antibody against human IgM mu chain.
Effects of Shear Stress on von Willebrand Factor
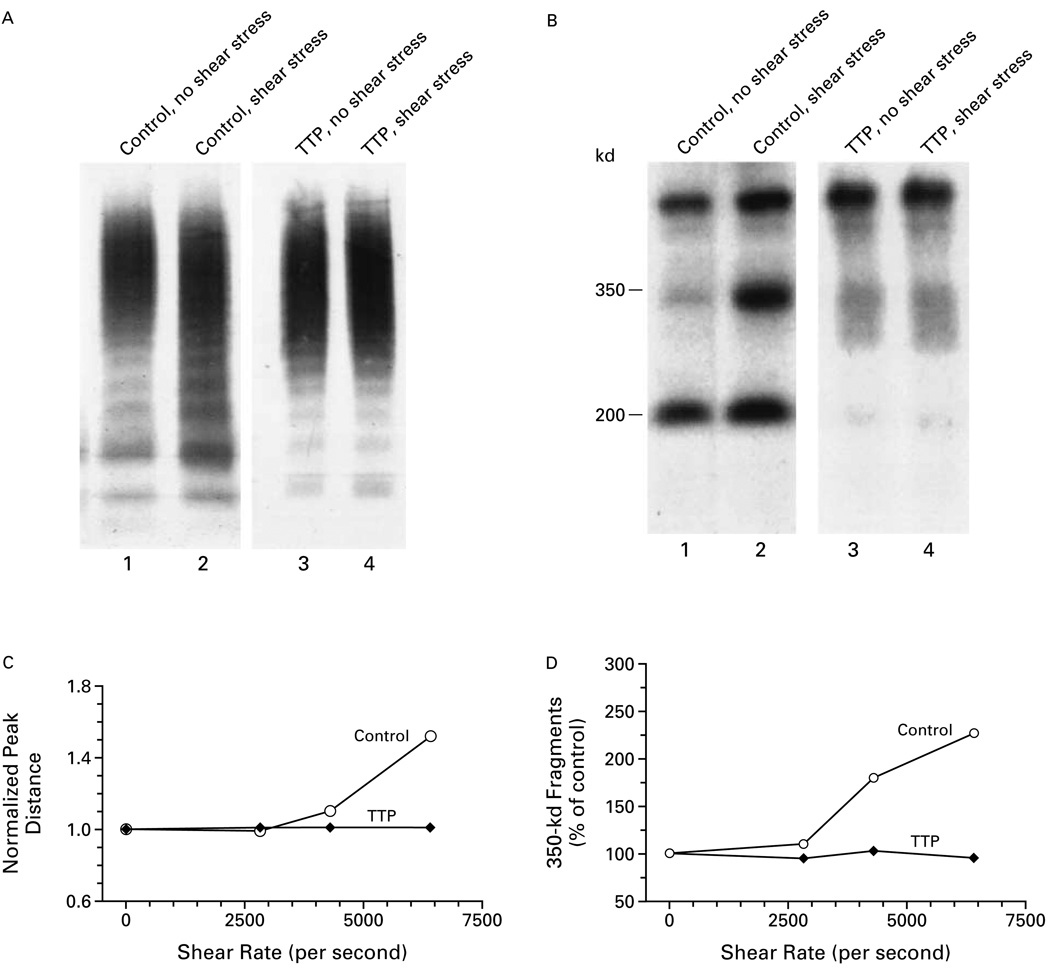
Von Willebrand factor was dissolved in the cryosupernatant of control plasma or a plasma sample from a patient with thrombotic thrombocytopenic purpura, and ristocetin cofactor activity was studied before and after shear stress (Fig. 5). The size and proteolysis of multimers of von Willebrand factor were also investigated. Cryosupernatants that were depleted of large and intermediate-sized multimers had no ristocetin cofactor activity, and exposure to shear stress did not change the concentration of von Willebrand factor (data not shown). Shear stress at a rate of 6404 per second decreased the size of the multimers and increased the number of cleaved fragments of von Willebrand factor only in control plasma (Fig. 5A and 5B). Only the von Willebrand factor in the cryosupernatant of control plasma had an increase in both the normalized peak distance (Fig. 5C) and von Willebrand factor proteolytic fragments (Fig. 5D) that was dependent on the level of shear stress.
Figure 5.
Effects of Shear Stress on the Proteolysis of Purified von Willebrand Factor Multimers.
Purified von Willebrand factor was dissolved in the cryosupernatant of control plasma or a plasma sample from a patient with thrombotic thrombocytopenic purpura (TTP). Panel A shows the sizes of the von Willebrand factor multimers on agarose-gel electrophoresis in the cryosupernatants in the presence and absence of shear stress. Panel B shows the frequency of the cleaved 350-kd and 200-kd fragments on polyacrylamide-gel electrophoresis and immunoblotting in the presence and absence of shear stress. Shear stress at a rate of 6404 per second decreased the size of the multimers and increased the numbers of cleaved fragments only in control cryosupernatant. The von Willebrand factor in the cryosupernatant of control plasma had an increase in the normalized peak distance (Panel C) and the amount of 350-kd fragments (Panel D) that was dependent on the rate of shear stress. A normalized peak distance of more than 1 indicates that the size of the multimers was decreased as compared with that measured in the absence of shear stress. The amount of 350-kd fragments was expressed as a percentage of the amount in the absence of shear stress.
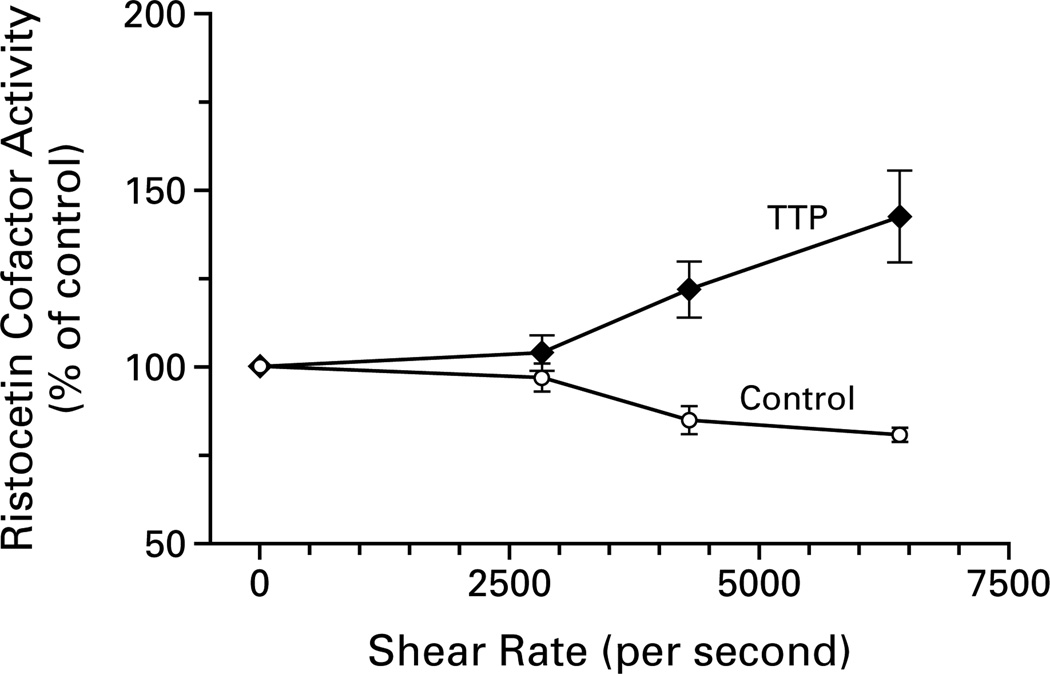
Figure 6 demonstrates that high levels of shear stress increased the ristocetin cofactor activity of the von Willebrand factor in the cryosupernatant of plasma from a patient with thrombotic thrombocytopenic purpura and decreased the activity in the cryosupernatant of control plasma. For example, a shear rate of 4307 per second decreased the ristocetin cofactor activity of the von Willebrand factor in the control cryosupernatant by 15 percentage points (P<0.03) and increased the activity in the cryosupernatant from the patient by 22 percentage points (P<0.05).
Figure 6.
Effect of Shear Stress on Ristocetin Cofactor Activity of Purified von Willebrand Factor.
Shear stress decreased the ristocetin cofactor activity of von Willebrand factor in the cryosupernatant of control plasma, but increased it in the cryosupernatant of plasma from a patient with thrombotic thrombocytopenic purpura (TTP). The ristocetin cofactor activity is expressed as a percentage of the activity in the absence of shear stress.
DISCUSSION
We found a severe deficiency of von Willebrand factor–cleaving protease activity in plasma samples from 37 patients during 39 episodes of acute thrombotic thrombocytopenic purpura. By contrast, samples obtained during remission had normal protease activity. In one patient who was studied serially from the time of an acute episode to remission, protease activity gradually became normal.
The protease deficiency was specific for thrombotic thrombocytopenic purpura. It was not detected in patients with thrombocytopenia, thrombosis, or hemolysis from other causes, suggesting that the deficiency did not result from hemolysis, thrombocytopenia, platelet activation, or thrombosis. Nonetheless, we have not ruled out the possibility that other diseases may be associated with a deficiency of this protease.
Plasma from normal subjects contained trace amounts of 350-kd and 200-kd fragments of von Willebrand factor, whereas plasma from patients with thrombotic thrombocytopenic purpura did not always have these fragments. When present, such fragments may have been produced before the protease activity was suppressed by inhibitors. Many plasma samples from patients with acute thrombotic thrombocytopenic purpura also had 300-kd bands. These bands differed from the 350-kd band produced by the protease or that produced by plasmin.17 Whether cysteine proteases or platelet microparticles, which have been detected in plasma from patients with thrombotic thrombocytopenic purpura,21,22 generated these bands in vivo and what role, if any, they have in the disease remain to be determined.
We found that plasma from most of our patients contained inhibitors of von Willebrand factor–cleaving protease. In each of the samples that we studied, the inhibition was mediated by IgG antibodies. These antibodies were probably directed against the protease, but they may also have been directed against unknown protease cofactors. Since the incubation of plasma from patients with thrombotic thrombocytopenic purpura with purified von Willebrand factor before the addition of control plasma resulted in less inhibition than did incubation of the plasma with control plasma followed by the addition of von Willebrand factor (data not shown), it is unlikely that the inhibitors were directed against the cleavage sites of the von Willebrand factor substrate.
Four patients with the chronic relapsing form of thrombotic thrombocytopenic purpura were reported to have reduced levels of von Willebrand factor–cleaving protease activity.19 In none of these patients was an inhibitor of the protease detected. However, as reported by Furlan et al.23 elsewhere in this issue of the Journal, inhibitors of this protease activity have been detected in patients with acute thrombotic thrombocytopenic purpura.
A link between thrombotic thrombocytopenic purpura and abnormal immune reactions has been suggested.24–26 Treatment with glucocorticoids or immunoadsorption with staphylococcal protein A may induce a remission of the disease,27 but even in the absence of immunosuppression thrombotic thrombocytopenic purpura does not recur in most patients who are successfully treated with plasmapheresis. The transient nature of acute thrombotic thrombocytopenic purpura raises the possibility that the antibodies to the protease represent a deranged response to certain triggering events.
How does a deficiency of von Willebrand factor–cleaving protease lead to platelet thrombosis in thrombotic thrombocytopenic purpura? Shear stress unfolds von Willebrand factor28 and enhances its proteolysis by protease in normal plasma.15,17 Shear stress may also expose the platelet-binding sites of von Willebrand factor. Our finding that the increase in ristocetin cofactor activity was dependent on the level of shear stress supports this possibility. Thus, in the absence of the protease, large, unfolded multimers may be more likely to bind platelets. This may explain why in patients with thrombotic thrombocytopenic purpura the numbers of large multimers are frequently decreased12 and the binding of von Willebrand factor to platelets is increased.29 In our capillary-tube device, von Willebrand factor was sheared at a rate of 4307 per second for 22 seconds. This brief exposure, and perhaps differences in temperature and other unknown factors, may explain why in a recent study the shear stress required to increase ristocetin cofactor activity in vitro (77 dyn per square centimeter) was higher than that encountered in normal circulation (60 dyn per square centimeter).30
The presence of inhibitory antibodies to von Willebrand factor–cleaving protease may explain why in our study thrombotic thrombocytopenic purpura responded to plasma infusion or plasma exchange. Presumably, patients with low titers of inhibitor would have a response to simple plasma infusion, whereas patients with high titers would require plasmapheresis to remove the inhibitory antibodies and supply normal protease. Plasmapheresis without plasma infusion is relatively ineffective in patients with thrombotic thrombocytopenic purpura,31 perhaps because it does not increase the protease activity quickly.
Acknowledgments
We are indebted to Drs. G.P. Visentin, H. Billett, and V. Chandrasyekaran for supplying plasma samples and to Drs. Ronald L. Nagel and Ira I. Sussman for their generous support of the study and critical comments.
REFERENCES
- 1.Moschcowitz E. Hyaline thrombosis of the terminal arterioles and capillaries: a hitherto undescribed disease. Proc N Y Pathol Soc. 1924;24:21–24. [Google Scholar]
- 2.Baehr G, Klemperer P, Schifrin A. Acute febrile anemia and thrombocytopenic purpura with diffuse platelet thromboses of capillaries and arterioles. Trans Am Physicians. 1936;51:43–58. [Google Scholar]
- 3.Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med. 1991;325:393–397. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 4.Török TJ, Holman RC, Chorba TL. Increasing mortality from thrombotic thrombocytopenic purpura in the United States — analysis of national mortality data, 1968–1991. Am J Hematol. 1995;50:84–90. doi: 10.1002/ajh.2830500203. [DOI] [PubMed] [Google Scholar]
- 5.Bell WR. Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome relapse: frequency, pathogenesis, and meaning. Semin Hematol. 1997;34:134–139. [PubMed] [Google Scholar]
- 6.Hymes KB, Karpatkin S. Human immunodeficiency virus infection and thrombotic microangiopathy. Semin Hematol. 1997;34:117–125. [PubMed] [Google Scholar]
- 7.Burns ER, Zucker-Franklin D. Pathologic effects of plasma from patients with thrombotic thrombocytopenic purpura on platelets and cultured vascular endothelial cells. Blood. 1982;60:1030–1037. [PubMed] [Google Scholar]
- 8.Mitra D, Jaffe EA, Weksler B, Hajjar KA, Soderland C, Laurence J. Thrombotic thrombocytopenic purpura and sporadic hemolytic-uremic syndrome plasmas induce apoptosis in restricted lineages of human microvascular endothelial cells. Blood. 1997;89:1224–1234. [PubMed] [Google Scholar]
- 9.Siddiqui FA, Lian EC-Y. Novel platelet-agglutinating protein from a thrombotic thrombocytopenic purpura plasma. J Clin Invest. 1985;76:1330–1337. doi: 10.1172/JCI112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asada Y, Sumiyoshi A, Hayashi T, Suzumiya J, Kaketani K. Immunohistochemistry of vascular lesion in thrombotic thrombocytopenic purpura, with special reference to factor VIII antigen. Thromb Res. 1985;38:469–479. doi: 10.1016/0049-3848(85)90180-x. [DOI] [PubMed] [Google Scholar]
- 11.Moake JL, Rudy CK, Troll JH, et al. Unusually large plasma factor VIII:von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med. 1982;307:1432–1435. doi: 10.1056/NEJM198212023072306. [DOI] [PubMed] [Google Scholar]
- 12.Moake JL, McPherson PD. Abnormalities of von Willebrand factor multimers in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. Am J Med. 1989;87:9N–15N. [PubMed] [Google Scholar]
- 13.Tsai HM, Nagel RL, Hatcher VB, Sussman II. Multimeric composition of endothelial cell-derived von Willebrand factor. Blood. 1989;73:2074–2076. [PubMed] [Google Scholar]
- 14.Dent JA, Berkowitz SD, Ware J, Kasper CK, Ruggeri ZM. Identification of a cleavage site directing the immunochemical detection of molecular abnormalities in type IIA von Willebrand factor. Proc Natl Acad Sci U S A. 1990;87:6306–6310. doi: 10.1073/pnas.87.16.6306. [Erratum, Proc Natl Acad Sci U S A 1990;87: 9508.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai HM, Sussman II, Nagel RL. Shear stress enhances the proteolysis of von Willebrand factor in normal plasma. Blood. 1994;83:2171–2179. [PubMed] [Google Scholar]
- 16.Furlan M, Robles R, Lämmle B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87:4223–4234. [PubMed] [Google Scholar]
- 17.Tsai HM. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 1996;87:4235–4244. [PubMed] [Google Scholar]
- 18.Tsai HM, Sussman II, Ginsburg D, Lankhof H, Sixma JJ, Nagel RL. Proteolytic cleavage of recombinant type 2A von Willebrand factor mutants R834W and R834Q: inhibition by doxycycline and by monoclonal antibody VP-1. Blood. 1997;89:1954–1962. [PubMed] [Google Scholar]
- 19.Furlan M, Robles R, Solenthaler M, Wassmer M, Sandoz P, Lämmle B. Deficient activity of von Willebrand factor-cleaving protease in chronic relapsing thrombotic thrombocytopenic purpura. Blood. 1997;89:3097–3103. [PubMed] [Google Scholar]
- 20.Nichols WC, Cooney KA, Mohlke KL, et al. Von Willebrand disease in the RIIIS/J mouse is caused by a defect outside of the von Willebrand factor gene. Blood. 1994;83:3225–3231. [Erratum, Blood 1995;86:2461.] [PubMed] [Google Scholar]
- 21.Kelton JG, Warkentin TE, Hayward CP, Murphy WG, Moore JC. Calpain activity in patients with thrombotic thrombocytopenic purpura is associated with platelet microparticles. Blood. 1992;80:2246–2251. [PubMed] [Google Scholar]
- 22.Consonni R, Falanga A, Barbui T. Further characterization of platelet-aggregating cysteine proteinase activity in thrombotic thrombocytopenic purpura. Br J Haematol. 1994;87:321–324. doi: 10.1111/j.1365-2141.1994.tb04916.x. [DOI] [PubMed] [Google Scholar]
- 23.Furlan M, Robles R, Galbusera M, et al. Von Willebrand factor–cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic–uremic syndrome. N Engl J Med. 1998;339:1578–1584. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 24.Neame PB. Immunologic and other factors in thrombotic thrombocytopenic purpura (TTP) Semin Thromb Hemost. 1980;6:416–429. doi: 10.1055/s-2007-1005113. [DOI] [PubMed] [Google Scholar]
- 25.Joseph G, Smith KJ, Hadley TJ, et al. HLA-DR53 protects against thrombotic thrombocytopenic purpura/adult hemolytic uremic syndrome. Am J Hematol. 1994;47:189–193. doi: 10.1002/ajh.2830470308. [DOI] [PubMed] [Google Scholar]
- 26.Tandon NN, Rock G, Jamieson GA. Anti-CD36 antibodies in thrombotic thrombocytopenic purpura. Br J Haematol. 1994;88:816–825. doi: 10.1111/j.1365-2141.1994.tb05122.x. [DOI] [PubMed] [Google Scholar]
- 27.Kwaan HC, Soff GA. Management of thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Semin Hematol. 1997;34:159–166. [PubMed] [Google Scholar]
- 28.Siediecki CA, Lestini BJ, Kottke-Marchant KK, Eppel SJ, Wilson DL, Marchant RE. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood. 1996;88:2939–2950. [PubMed] [Google Scholar]
- 29.Chow TW, Turner NA, Chintagumpala M, et al. Increased von Willebrand factor binding to platelets in single episode and recurrent types of thrombotic thrombocytopenic purpura. Am J Hematol. 1998;57:293–302. doi: 10.1002/(sici)1096-8652(199804)57:4<293::aid-ajh5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 30.Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood. 1996;88:1525–1541. [PubMed] [Google Scholar]
- 31.Miura M, Koizumi S, Nakamura K, et al. Efficacy of several plasma components in a young boy with chronic thrombocytopenia and hemolytic anemia who responds repeatedly to normal plasma infusions. Am J Hematol. 1984;17:307–319. doi: 10.1002/ajh.2830170311. [DOI] [PubMed] [Google Scholar]