Abstract
A preparative gas chromatography (pGC) method was developed for the separation of volatile components from the methanol extract of Curcuma rhizome. The compounds were separated on a stainless steel column packed with 10% OV-101 (3 m × 6 mm, i.d.), and then, the effluent was split into two gas flows. One percent of the effluent passed to the flame ionization detector (FID) for detection and the remaining 99% were directed to the fraction collector. Five volatile compounds were collected from the methanol extract of Curcuma rhizome (5 g/mL) after 83 single injections (20 uL) with the yield of 5.1–46.2 mg. Furthermore, the structures of the obtained compounds were identified as β-elemene, curzerene, curzerenone, curcumenol, and curcumenone by MS and NMR spectra, respectively.
1. Introduction
Essential oils are one of the most valuable natural products with multiple pharmacological activities. Among the Chinese medicines (CMs) recorded in Chinese Pharmacopoeia (2005 edition), there are about 20% herbs contain essential oils which are usually considered as bioactive fractions. However, reference compounds or chemical standards are the bottle neck for the quality control of CMs containing volatile compounds as their main active fractions. Therefore, it is necessary to separate and purify pure active chemicals, used as reference compounds, from CMs. However, the supply of reference compounds is far from the requirement for quality control of CMs. Especially, the pure volatile chemical compounds are even more difficult to be obtained because of their instability and low polarity, which hinders the development of quality control for TCMs. These problems, therefore, compromise the values of traditional Chinese medicine or even jeopardize the safety of the consumers.
Ezhu, one of the commonly used traditional Chinese medicines, is the dried rhizomes of three species of Curcuma, including Curcuma phaeocaulis, C. kwangsiensis, and C. wenyujin, according to the Chinese Pharmacopoeia [1]. At present, the essential oil of Ezhu is considered as its active fractions which possesses antitumour [2, 3] and antiviral activities [4, 5]. To date, β-elemene, curzerene, furanodienone, curcumol, isocurcumenol, germacrone, furanodiene, curdione, curcumenol, neocurdione, and curcumenone are considered as the main active volatile components in Ezhu [6–8]. Actually, the chemical compounds from Ezhu were prepared by silica gel column chromatography in most cases [6–8], but this classical isolation technique suffers from insufficient resolution for complex samples, requiring time-consuming fractionation in multiple steps with the risk of the compound being lost, altered or contaminated [9].
Preparative GC (pGC) is a powerful purification technique for the volatile and semivolatile compounds [10], which has been successfully used in a number of rather special applications such as the isolation of large quantities of the trace components of essential oils for organoleptic assessment [11], separation of isomers [12], isotopes [13], and enantiomers [14–17] from complex mixtures. Furthermore, the essential oil of many plants which is rich in volatile components is very propitious to be isolated by pGC [9, 18–25]. In present study, a pGC system was constructed and applied for the isolation of volatile constituents at milligram level from Ezhu, and the structures of the isolated compounds were determined by their MS and NMR spectra.
2. Materials and Methods
2.1. Materials
Rhizome of Curcuma was purchased from Wanhe pharmacy (Shapingba, Chongqing, China) in November 2009. Methanol, ethyl acetate, petroleum, and n-butanol were purchased from Chuandong Chemical Co., Ltd. (Chongqing, China). 60–100 mesh silica-gel for column chromatography was purchased from Branch of Qingdao Haiyang Chemical Plant (Shandong, China). The voucher specimens of Curcuma rhizomes were deposited at the Department of Pharmaceutics, College of Chemistry and Chemical Engineering, Chongqing University, Chongqing, China.
2.2. Sample Preparation
Ultrasonic extraction was performed on an AS3120A Ultrasonic Cleaner (Tianjin Automatic Science Instrument CO., Ltd, Tianjin, China). In brief, dried material of Ezhu was ground into powder of 0.2-0.3 mm diameter. Powder of Ezhu (100 g) was soaked in methanol (200 mL) for 24 h and then placed into ultrasonic tank for extraction of 15 minutes (120 W). The obtained methanol extract was added onto a silica gel column (3 × 45 cm) and washed by ethyl acetate and petroleum mixed solution (ratio 1 : 1), and the effluent was collected and condensed before injected into pGC system.
2.3. pGC System
The pGC system was modified based on an SC-2000 GC instrument (Chuanyi Analyzer Co., Ltd, Chongqing, China), the diagram is shown in Figure 1. It is equipped with a stainless steel column packed with 10% OV-101 (3 m × 6 mm, i.d.), a flame ionization detector (FID), a special effluent splitter with minimum dead volume, and a home-made preparative fraction collector. The data was collected and analyzed on a HW-2000 Chromatographic Workstation (Nanjing Qianpu Software Co. Ltd., China).
Figure 1.
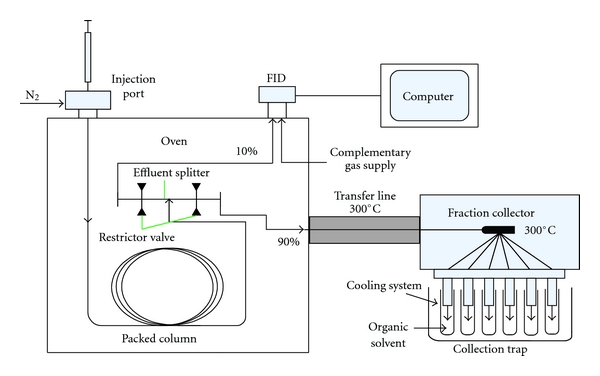
Preparative gas chromatography system equipped with packed column, flame ionization detector (FID), effluent splitter, and fraction collector.
High purity nitrogen (N2) was used as carrier gas at a flow rate of 30 mL/min. The inlet and FID temperature were 220°C, respectively. The column temperature was set at 180°C, then programmed at 3°C min−1 to 250°C, and held for 10 min. The effluent was splitted into two flows, one (1%) towards the FID and the other (99%) to the fraction collector using a special gas effluent splitter. Two restrictor valves were used to control the split flow. In order to supply sufficient gas flow for the FID detection, a supplementary gas (N2, 10 mL/min) was added before arrived at the detector. Volumes of 20 μL Ezhu essential oil were injected. After being separated by the column, the fractions were collected in a series of 2 mL traps filled with ethyl acetate. The trapping time and peak retention time were synchronized. The isolated fractions were analyzed under the same conditions of pGC and by following GC-MS.
2.4. GC-MS Analysis
GC-MS was performed on a Trace GC Ultra gas chromatography instrument coupled to a DSQ II mass spectrometer and an Xcalibur Version 2.0.7 software (Thermo Fisher Scientific, Boston, MA, USA). A capillary column (30 m × 0.25 mm i.d.) coated with 0.25 μm film 5% phenyl methyl siloxane was used for separation. High purity helium was used as carrier gas with flow-rate at 1.0 mL/min. The other GC conditions were as follows: inlet mode and temperature were pulsed splitless at 190°C; the column temperature was set at 60°C and held for 2 min for injection, then programmed at 5°C min−1 to 145°C and held for 25 min at the temperature of 145°C, then at 5°C min−1 to 200°C, and finally, at 20°C min−1 to 280°C, and held for 3 min at the temperature of 280°C.
The spectrometers were operated in electron-impact (EI) mode, the scan range was 40–550 amu, the ionization energy was 70 eV and the scan rate was 0.34 s per scan. The quadrupole, ionization source temperature were 150°C and 280°C, respectively.
3. Results and Discussion
3.1. Recovery of pGC
The recovery of pGC was tested by injection of 5 × 10 μL n-butanol, and methanol was used as trapping solvent. The yield amount n-butanol was calculated based on the calculation factor of n-butanol to methanol (f = 0.414) by injecting methanol and n-butanol mixed solvent (ratio 1 : 1) under the same conditions. Finally, a total of 40 μL n-butanol was recovered with the recovery percentage of 80%.
3.2. Isolation of Volatile Compounds from Ezhu by pGC
The GC chromatogram of Ezhu methanol extract recorded by pGC with FID detection is given in Figure 2. It was used as a basis for the collection of 5 fractions that were analyzed by the analytical GC and GC-MS system for an evaluation of resolution and yields of the preparative GC.
Figure 2.
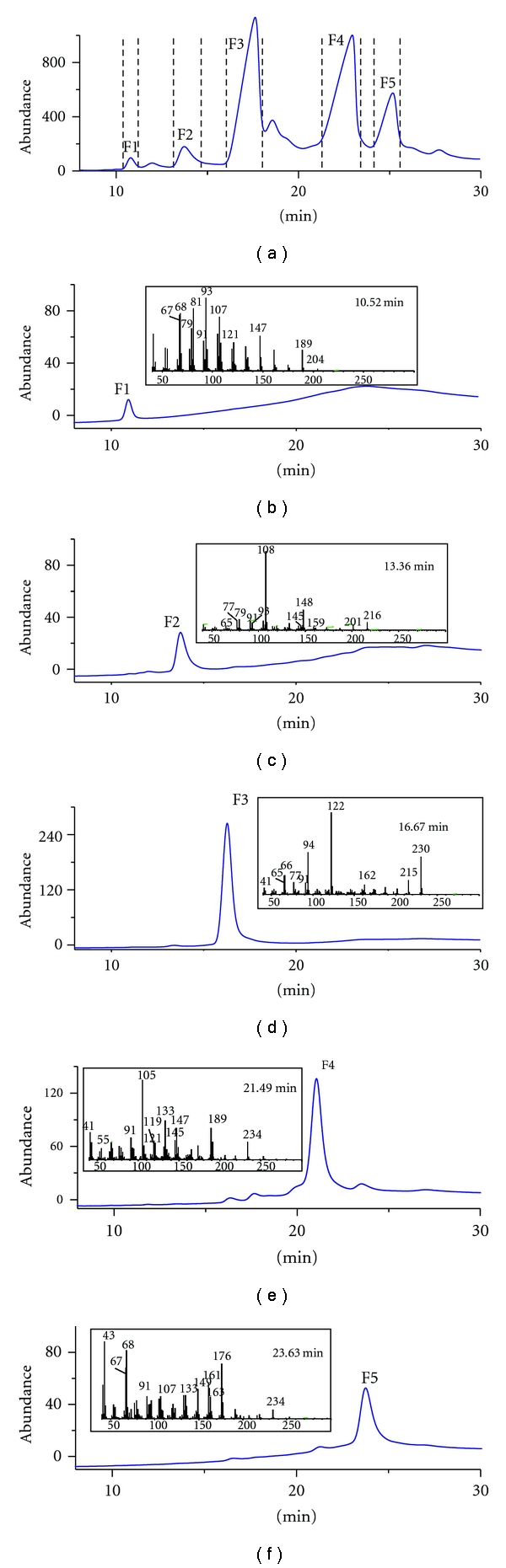
FID chromatogram for Ezhu extract (a), and FID chromatogram and MS spectra for the collected fractions (b–f).
Packed column analytical gas chromatograms of Ezhu essential oil and collected fractions, as well as mass spectra of peaks of every fraction collected, are given in Figure 2.
3.3. Identification and Yield of Collected Fractions
Five fractions of Ezhu essential oil were isolated and collected using preparative GC with 83 repeated injections, resulting in amounts of 5.1–46.2 mg for the compounds in the respective traps. The amounts of fractions F1–F5 were 5.1 mg, 6.6 mg, 41.6 mg, 46.2 mg, and 21.2 mg, respectively.
Five fractions were identified by MS (Table 1), 1H and 13C NMR spectra (shown in the appendix) of the individual peaks, fractions 1–5 were identified as β-elemene, curzerene, curzerenone, curcumenol, and curcumenone, respectively (Figure 3).
Table 1.
Mass data of 5 collected fractions.
| Fraction | Mass data | Rt (min) | Compound |
|---|---|---|---|
| F1 | 204(M+, 4), 189(45), 147(48), 121(39), 107(75), 93(100), 91(41), 81(86), 79(59), 68(79), 67(77) | 10.9 | β-elemene |
| F2 | 216(M+, 9), 201(6), 159(4), 148(24), 145(5), 108(100), 93(9), 91(11), 79(13), 77(11), 65(5) | 13.7 | Curzerene |
| F3 | 230(M+, 46), 215(17), 162(11), 122(100), 94(51), 91(14), 77(15), 66(23), 65(23), 41(8) | 16.3 | Curzerenone |
| F4 | 234(M+, 22), 189(45), 147(42), 145(26), 133(54), 121(20), 119(24), 105(100), 91(27), 55(16), 41(34) | 21.1 | Curcumenol |
| F5 | 234(M+, 26), 176(78), 163(29), 161(48), 149(43), 133(37), 107(32), 91(29), 68(91), 67(75), 43(100) | 23.8 | Curcumenone |
Figure 3.
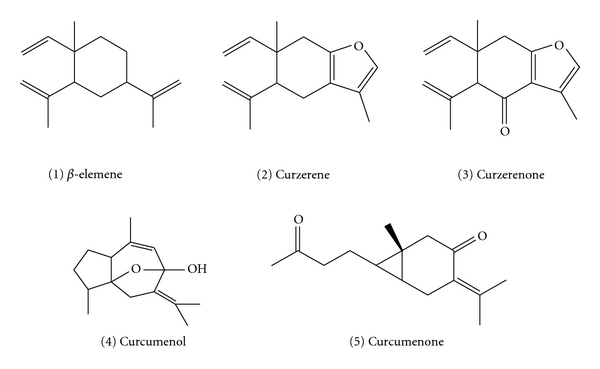
Chemical structures of 5 collected chemicals.
4. Conclusions
Preparative GC on a 3 m × 6 mm peaked column using an FID, an effluent splitter, and a fraction collector was shown an appropriate resolution, yield, and recovery rate of Ezhu essential oil to obtain pure volatile constituents at milligram level. The combination of preparative GC with analytical GC using the same column and GC conditions allows a direct transfer of retention times and facilitates fractions identification. Altogether, these results show that preparative GC is very fit to obtain small amount of pure compounds from volatile oil. Therefore, preparative GC should be developed on resolution of volatile oil and yield of target compounds.
Acknowledgment
This work was supported by Natural Science Foundation Project of CQ CSTC (no. 2010BB5070).
Appendix
NMR data of β-elemene, curzerene, curzerenone, curcumenol, and curcumenone, Analyzed by AV400 NMR (Bruker, Switzerland), solvent: CDCl3, internal standard: TMS.
(1) β-elemene [26] —
1H-NMR (400 MHz, CDCl3) δ : 5.80 (1H, dd, J = 10.5, 17.9 Hz, H-13), 4.87–4.91 (2H, m, H-14Z and H-8Z), 4.82 (1H, t, J = 1.6 Hz, H-8E), 4.58 (1H, s, H-11Z), 4.56 (1H, s, H-11E), 3.57 (1H, d, J = 10.9 Hz, H-14E), 1.95 (1H, dd, J = 3.7, 12.3 Hz, H-5), 1.68 (3H, s, H-12), 16.7–1.70 (1H, m, H-3), 1.32–1.60 (6H, m, H-1, -4 and -6), 1.13 (3H, s, H-9), 0.98 (3H, s, H-15).
13C-NMR (100 MHz, CDCl3) δ : 39.9 (C-1), 39.8 (C-2), 52.7 (C-3), 32.9 (C-4), 45.7 (C-5), 26.8 (C-6), 150.2 (C-7), 108.1 (C-8), 24.7 (C-9), 147.5 (C-10), 109.7 (C-11), 21.0 (C-12), 150.1 (C-13), 112.0 (C-14), 16.7 (C-15).
(2) Curzerene [27] —
1H-NMR (400 MHz, CDCl3) δ : 7.07 (1H, brs, H-8), 5.89 (1H, dd, J = 10.8, 17.0 Hz, H-12), 5.02 (1H, dd, J = 1.0, 17.0 Hz, H-13Z), 4.98 (1H, dd, J = 10.8, 17.0 Hz, H-13E), 4.88(1H, d, J = 1.2 Hz, H-10E), 4.77 (1H, d, J = 1.2 Hz, H-10Z), 2.69 (1H, d, J = 1.5 Hz, H-1β), 2.43 (2H, dd, J = 1.1, 1.5 Hz, H-4α and H-4β), 2.31 (1H, t, J = 1.5 Hz, H-3), 1.94 (3H, s, H-14), 1.76 (3H, s, H-11), 1.08 (3H, s, H-15).
13C-NMR (100 MHz, CDCl3) δ : 36.1 (C-1), 40.1 (C-2), 50.0 (C-3), 24.2 (C-4), 116.5 (C-5), 149.5 (C-6), 119.3 (C-7), 137.2 (C-8), 147.2 (C-9), 112.7 (C-10), 24.4 (C-11), 147.1 (C-12), 110.9 (C-13), 8.1 (C-14), 19.5 (C-15).
(3) Curzerenone [28] —
1H-NMR (400 MHz, CDCl3) δ : 7.07 (1H, brs, H-11), 5.81 (1H, brs, H-5), 5.18 (1H, t, J = 7.5 Hz, H-1), 3.72 (2H, AB-system, J = 15 Hz, H-9a, H-9b), 2.20 (3H, d, J = 1.5 Hz, H-13), 1.76 (3H, d, J = 1.5 Hz, H-14), 1.31 (3H, s, H-15), 1.60–2.48 (4H, m, H-2 and H-3).
13C-NMR (100 MHz, CDCl3) δ : 130.5 (C-1), 26.4 (C-2), 41.6 (C-3), 145.7 (C-4), 132.4 (C-5), 189.7 (C-6), 122.2 (C-7), 156.5 (C-8), 40.6 (C-9), 135.4 (C-10), 138.1 (C-11), 123.7 (C-12), 9.5 (C-13), 18.9 (C-14), 15.7 (C-15).
(4) Curcumenol [29] —
1H-NMR (400 MHz, CDCl3) δ : 5.75 (1H, s, H-9), 3.05 (1H, dd, J = 1.2, 2.1 Hz, H-1), 2.65 (1H, d, J = 15.6 Hz, H-6β), 2.10 (1H, d, J = 15.6 Hz, H-6α),1.66–1.97 (6H, m, H-1, H-2, H-3 and H-4) 1.81 (3H, s, H-12), 1.66 (3H, s, H-13), 1.66 (3H, s, H-14), 1.03 (3H, d, J = 6.2 Hz, H-15).
13C-NMR (100 MHz, CDCl3) δ : 51.3 (C-1), 27.6 (C-2), 31.2 (C-3), 40.3 (C-4), 85.4 (C-5), 37.2 (C-6), 137.3 (C-7), 101.5 (C-8), 125.8 (C-9), 137.3 (C-10), 122.1 (C-11), 18.9 (C-12), 22.9 (C-13), 21.4 (C-14), 11.8 (C-15).
(5) Curcumenone [30] —
1H-NMR (400 MHz, CDCl3) δ : 2.81 (2H, m, H-7), 2.55 (1H, d, J = 15.6 Hz, H-10β or H-10α), 2.51 (1H, d, J = 15.6 Hz, H-10β or H-10α), 2.47 (2H, t, J = 7.3 Hz, H-4), 2.13 (3H, s, H-15), 2.09 (3H, s, H-12), 1.79 (3H, s, H-13), 1.60 (2H, t, J = 7.3 Hz, H-3), 1.12 (3H, s, H-14), 0.67 (1H, q, J = 4.4 Hz, H-6), 0.45 (1H, dt, J = 7.3, 4.4 Hz, H-2).
13C-NMR (100 MHz, CDCl3) δ : 20.1 (C-1), 30.0 (C-2), 24.1 (C-3), 43.9 (C-4), 208.7 (C-5), 24.3 (C-6), 28.0 (C-7), 128.0 (C-8), 201.6 (C-9), 48.9 (C-10), 147.4 (C-11), 23.4 (C-12), 23.4 (C-13), 19.0 (C-14), 23.2 (C-15).
References
- 1.Pharmacopoeia Commission of PRC. Pharmacopoeia of the People’s Republic of China. 1st edition. Beijing, China: Chemical Industry Press; 2000. [Google Scholar]
- 2.Jiang JP. Anti-tumor activities of Ezhu. Jilin Journal of Traditional Chinese Medicine. 2000;33(2):62–64. [Google Scholar]
- 3.Nie XH, Ao ZH, Yin GY, Tao WY. Effect of extraction techniques on the components and antitumor activity in vitro of volatile oil from Curcuma wenyujin. Pharmaceutical Biotechnology. 2003;10(3):152–154. [Google Scholar]
- 4.Xia Q, Huang ZG, Li SP, Zhang P, Wang J, He LN. The experiment study of the anti-virus effects of zedoary oil on influenza virus and respiratory syncytial virus. Chinese Pharmacological Bulletin. 2004;20(3):357–358. [Google Scholar]
- 5.Ming Q, Sun F, Liu JW, et al. The effective concentration of zedoary oil on the inhibition of respiratory virus infections. Chinese Journal of Gerontology. 2004;24:267–268. [Google Scholar]
- 6.Yang FQ, Li SP, Zhao J, Lao SC, Wang YT. Optimization of GC-MS conditions based on resolution and stability of analytes for simultaneous determination of nine sesquiterpenoids in three species of Curcuma rhizomes. Journal of Pharmaceutical and Biomedical Analysis. 2007;43(1):73–82. doi: 10.1016/j.jpba.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Yang FQ, Li SP, Chen Y, et al. Identification and quantitation of eleven sesquiterpenes in three species of Curcuma rhizomes by pressurized liquid extraction and gas chromatography-mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2005;39(3-4):552–558. doi: 10.1016/j.jpba.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Yang FQ, Wang YT, Li SP. Simultaneous determination of 11 characteristic components in three species of Curcuma rhizomes using pressurized liquid extraction and high-performance liquid chromatography. Journal of Chromatography A. 2006;1134(1-2):226–231. doi: 10.1016/j.chroma.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 9.Eyres GT, Urban S, Morrison PD, Marriott PJ. Application of microscale-preparative multidimensional gas chromatography with nuclear magnetic resonance spectroscopy for identification of pure methylnaphthalenes from crude oils. Journal of Chromatography A. 2008;1215(1-2):168–176. doi: 10.1016/j.chroma.2008.10.102. [DOI] [PubMed] [Google Scholar]
- 10.Nojima S, Kiemle DJ, Webster FX, Roelofs WL. Submicro scale NMR sample preparation for volatile chemicals. Journal of Chemical Ecology. 2004;30(11):2153–2161. doi: 10.1023/b:joec.0000048780.26118.c0. [DOI] [PubMed] [Google Scholar]
- 11.Scott RPW. Gas Chromatography. (Chrom-Ed Book Series). 2011, http://www.library4science.com. [Google Scholar]
- 12.Meinert C, Moeder M, Brack W. Fractionation of technical p-nonylphenol with preparative capillary gas chromatography. Chemosphere. 2007;70(2):215–223. doi: 10.1016/j.chemosphere.2007.06.055. [DOI] [PubMed] [Google Scholar]
- 13.Holmstrand H, Mandalakis M, Zencak Z, Gustafsson O, Andersson P. Chlorine isotope fractionation of a semi-volatile organochlorine compound during preparative megabore-column capillary gas chromatography. Journal of Chromatography A. 2006;1103(1):133–138. doi: 10.1016/j.chroma.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Staerk DU, Shitangkoon A, Vigh G. Preparative gas chromatographic separation of the enantiomers of methyl 2-chloropropionate using a cyclodextrin-based stationary phase. Journal of Chromatography A. 1995;702(1-2):251–257. [Google Scholar]
- 15.Shitangkoon A, Staerk DU, Vigh G. Gas chromatographic separation of the enantiomers of volatile fluoroether anesthetics using derivatized cyclodextrin stationary phases. I. Journal of Chromatography A. 1993;657(2):387–394. [Google Scholar]
- 16.Staerk DU, Shitangkoon A, Vigh G. Gas chromatographic separation of the enantiomers of volatile fluoroether anesthetics by derivatized cyclodextrins. II. Preparative-scale separations for isoflurane. Journal of Chromatography A. 1994;663(1):79–85. [Google Scholar]
- 17.Staerk DU, Shitangkoon A, Vigh G. Gas chromatographic separation of the enantiomers of volatile fluoroether anesthetics by derivatized cyclodextrins. III. Preparative-scale separations for enflurane. Journal of Chromatography A. 1994;677(1):133–140. [Google Scholar]
- 18.Saritas Y, Sonwa MM, Iznaguen H, König WA, Muhle H, Mues R. Volatile constituents in mosses (Musci) Phytochemistry. 2001;57(3):443–457. doi: 10.1016/s0031-9422(01)00069-3. [DOI] [PubMed] [Google Scholar]
- 19.Adio AM, Paul C, König WA, Muhle H. Volatile components from European liverworts Marsupella emarginata, M. aquatica and M. alpina. Phytochemistry. 2002;61(1):79–91. doi: 10.1016/s0031-9422(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 20.Lu R, Paul C, Basar S, König WA, Hashimoto T, Asakawa Y. Sesquiterpene constituents from the liverwort Bazzania japonica. Phytochemistry. 2003;63(5):581–587. doi: 10.1016/s0031-9422(03)00188-2. [DOI] [PubMed] [Google Scholar]
- 21.Adio AM, Paul C, König WA, Muhle H. Volatile constituents in the liverwort Tritomaria polita. Phytochemistry. 2003;64(2):637–644. doi: 10.1016/s0031-9422(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 22.Kreipl AT, König WA. Sesquiterpenes from the east African sandalwood Osyris tenuifolia. Phytochemistry. 2004;65(14):2045–2049. doi: 10.1016/j.phytochem.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Tesso H, König WA. Terpenes from Otostegia integrifolia. Phytochemistry. 2004;65(14):2057–2062. doi: 10.1016/j.phytochem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Eyres GT, Urban S, Morrison PD, Dufour JP, Marriott PJ. Method for small-molecule discovery based on microscale-preparative multidimensional gas chromatography isolation with nuclear magnetic resonance spectroscopy. Analytical Chemistry. 2008;80(16):6293–6299. doi: 10.1021/ac8007847. [DOI] [PubMed] [Google Scholar]
- 25.Rühle C, Eyres GT, Urban S, Dufour JP, Morrison PD, Marriott PJ. Multiple component isolation in preparative multidimensional gas chromatography with characterisation by mass spectrometry and nuclear magnetic resonance spectroscopy. Journal of Chromatography A. 2009;1216(30):5740–5747. doi: 10.1016/j.chroma.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Dunlop RW, Wells RJ. Isolation of some novel diterpenes from a soft coral of the genus Lobophytum. Australian Journal of Chemistry. 1979;32(6):1345–1351. [Google Scholar]
- 27.Hikino H, Agatsuma K, Konno C, Takemoto T. Structure of furanodiene and isofuranogermacrene (curzerene) Chemical and Pharmaceutical Bulletin. 1970;18(4):752–755. [Google Scholar]
- 28.Dekebo A, Dagne E, Hansen LK, Gautun OR, Aasen AJ. Crystal structures of two furanosesquiterpenes from Commiphora sphaerocarpa. Tetrahedron Letters. 2000;41(50):9875–9878. [Google Scholar]
- 29.Firman K, Kinoshita T, Itai A, Sankawa U. Terpenoids from Curcuma heyneana. Phytochemistry. 1988;27(12):3887–3891. [Google Scholar]
- 30.Kuroyanagi M, Ueno A, Ujiie K, Sato S. Structures of sesquiterpenes from Curcuma aromatica SALISB. Chemical and Pharmaceutical Bulletin. 1987;35(1):53–59. [Google Scholar]


