Abstract
The first method for quantifying cannabinoids and cannabinoid glucuronides in whole blood by liquid chromatography-tandem mass spectrometry (LC-MS/MS) was developed and validated. Solid-phase extraction followed protein precipitation with acetonitrile. HPLC separation was achieved in 16 min via gradient elution. Electrospray ionization was utilized for cannabinoid detection; both positive (Δ9-tetrahydrocannabinol [THC], cannabinol [CBN]) and negative (11-hydroxy-THC [11-OH-THC], 11-nor-9-carboxy-THC [THCCOOH], cannabidiol [CBD], THC-glucuronide and THCCOOH glucuronide) polarity were employed with multiple reaction monitoring. Calibration by linear regression analysis utilized deuterium-labeled internal standards and a 1/x2 weighting factor, yielding R2 values > 0.997 for all analytes. Linearity ranged from 0.5–50 μg/L (THC-glucuronide), 1.0–100 μg/L (THC, 11-OH-THC, THCCOOH, CBD and CBN) and 5.0–250 μg/L (THCCOOH-glucuronide). Imprecision was < 10.5% CV, recovery was > 50.5% and bias within ± 13.1% of target for all analytes at three concentrations across the linear range. No carryover, endogenous or exogenous interferences were observed. This new analytical method should be useful for quantifying cannabinoids in whole blood and further investigating cannabinoid glucuronides as markers of recent cannabis intake.
Keywords: THC, cannabinoid glucuronides, LC-MS/MS, whole blood
Introduction
Cannabis use substantially impacts public safety, as many individuals drive or operate complex equipment soon after self-administration. The National Highway Traffic Safety Administration (NHTSA) reported that in 2007, 8.6% of nighttime drivers tested positive for cannabinoids in blood and/or oral fluid, a rate almost 4 times higher than the percentage of drunk drivers with a blood alcohol concentration ≥0.8 g/L [1]. While finding cannabinoids in blood or oral fluid does not necessarily imply impairment, windows of drug detection in these matrices are often short for occasional or moderate smokers [2–4], increasing impairment probability.
Δ9-tetrahydrocannabinol (THC) is the primary psychoactive component in cannabis and is metabolized via cytochrome P450 (CYP) 2C9 and 2C19 isozymes to several phase I metabolites, most prominently 11-hydroxy-THC (11-OH-THC) and 11-nor-9-carboxy-THC (THCCOOH) [5–6]. THC and its phase I metabolites also undergo UDP-glucuronosyltransferase-catalyzed phase II metabolism to form cannabinoid glucuronides in vivo [7–9], facilitating excretion. Currently, little is known about cannabinoid glucuronide pharmacological activity or detection windows following cannabis intake, although others hypothesized that these glucuronides could serve as markers of recent cannabis intake due to a shorter half-life in vivo [10–11]. Detection and quantification of these metabolites may provide scientific data permitting researchers, physicians and law enforcement personnel to document recent cannabis intake.
Analysis of glucuronides by gas chromatography-mass spectrometry (GC-MS) is difficult as chemical derivatization requirements and volatility issues preclude direct detection and quantification. Therefore, analytical procedures for cannabinoids in urine [12–14], blood [15], meconium [16–17] and oral fluid [18] typically include expensive and time-consuming alkaline and/or enzymatic glucuronide hydrolysis to liberate cannabinoids prior to extraction and GC-MS analysis. However, these hydrolyses introduce multiple confounding issues, including, but not limited to poor chromatography [15] and variable hydrolysis efficiencies of the ether- and ester-linked glucuronide species [15, 19–22].
To circumvent hydrolysis and facilitate direct quantification of phase II cannabinoid metabolites, sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods are required. Yet few LC-MS/MS methods are available for cannabinoids in whole blood [23–25], likely resulting from higher limits of quantification (LOQ) than typically achieved by GC-MS. Additionally, to date, these published methods do not included glucuronide metabolites. Furthermore, development of glucuronide analytical methods is hampered by a lack of commercially available native and isotopically-labeled cannabinoid glucuronide standards.
To this end, we developed and validated the first sensitive and specific LC-MS/MS method for simultaneous detection of free and glucuronidated cannabinoids in human whole blood. This method is unique in that THC, THCCOOH and their glucuronides, 11-OH-THC, cannabidiol (CBD) and cannabinol (CBN) are simultaneously extracted and quantified in 16 min. While whole blood is frequently the specimen collected in driving under the influence of drugs (DUID) cases and other investigations, to our knowledge no studies directly investigated whole blood pharmacokinetics following smoked cannabis. Therefore, we will utilize this method to investigate in vitro cannabinoid stability, evaluate cannabinoid glucuronides as markers of recent cannabis intake and determine whole blood cannabinoid pharmacokinetics in order to provide a scientific database for researchers, clinicians and forensic toxicologists interpreting whole blood cannabinoid concentrations.
Experimental
Clinical Samples
A healthy cannabis smoker provided written informed consent to participate in a study investigating cannabinoid pharmacokinetics, in vitro cannabinoid stability and novel markers of cannabis intake following a single smoked cannabis dose. The Institutional Review Board of the National Institute on Drug Abuse, National Institutes of Health approved this protocol. Cannabis cigarettes contained 6.8% THC (w/w) or approximately 56 mg THC and were smoked ad libitum over a 10 min period following an overnight stay on a secure residential unit. Whole blood was collected with sodium heparin 0.5 h prior to, 0.25 h after and 1.0 h after the start of cannabis smoking. Blood was transferred to polypropylene storage tubes and stored refrigerated until analysis within 24 h.
Instrumentation
All experiments were performed on an AB Sciex 3200 Qtrap triple quadrupole mass spectrometer with a TurboV ESI source (AB Sciex, Foster City, CA). The mass spectrometer was interfaced with a Shimadzu UFLCxr system consisting of two LC-20ADXR pumps, a SIL-20ACXR autosampler, and a CTO-20AC column oven (Shimadzu Corporation, Columbia, MD). Evaporation under nitrogen was completed using a TurboVap LV evaporator from Zymark (Hopkinton, MA).
Reagents
Standards and deuterated internal standards were purchased from Cerilliant (Round Rock, TX) except for THC-glucuronide that was from ElSohly Laboratories, Inc (Oxford, MS). Ammonium acetate, formic acid and acetonitrile (ACN) were obtained from Sigma-Aldrich (St. Louis, MO). Methanol was from Fisher Scientific (Fair Lawn, NJ). Ammonium hydroxide and glacial acetic acid were from Mallinckrodt Baker (Phillipsburg, NJ). Water was purified in house by an ELGA Purelab Ultra Analytic purifier (Siemens Water Technologies, Lowell, MA). All solvents were HPLC grade or better. 200-mg, 6-mL Bond Elut Plexa solid-phase extraction (SPE) cartridges were utilized for preparing samples (Agilent Technologies, Culver City, CA). Blank human whole blood was evaluated for absence of cannabinoids prior to use.
Preparation of Standard Solutions
Individual stock solutions of 1.0 g/L THC, 11-OH-THC, THCCOOH, CBD and CBN, 100 mg/L THCCOOH-glucuronide and 10 mg/L THC-glucuronide were diluted with methanol to prepare calibration solutions. 11-OH-THC-glucuronide and di-glucuronide metabolites are not commercially available. A stock solution containing 10 mg/L of analytes other than THC-glucuronide was prepared in methanol and stored at −20°C. Dilutions of the stock solution (adding in THC-glucuronide) created calibrators at 0.5, 1.0, 2.0, 5.0, 10, 20, 50, 100, and 250μg/L when fortifying 25 μL of standard solution into 500 μL of blank human whole blood.
Quality control samples were prepared in methanol from different vials than utilized for preparing standards. Low-, medium-, and high-quality control samples were prepared across the linear dynamic range of the assay. Whole blood low-, medium-, and high-quality control target concentrations were: THC-glucuronide 1.5, 4.5, and 45 μg/L; 11-OH-THC, CBD, CBN, THC and THCCOOH 2.5, 7.5, and 75 μg/L; and THCCOOH-glucuronide 7.5, 22.5, and 225 μg/L, respectively. All quality control solutions were stored at −20°C.
Stock internal standard solution was prepared by diluting 100 mg/L solutions of THC-d3, 11-OH-THC-d3, THCCOOH-d9 and CBD-d3 1:10 with methanol and storing at −20°C. A 1:50 dilution of internal standard stock solution was prepared in methanol and 25 μL of the diluted solution was added to each 500 μL whole blood sample, providing a final internal standard concentration of 10 μg/L. Deuterated CBN, THCCOOH-glucuronide, and THC-glucuronide are not currently commercially available; THC-d3 was utilized for CBN quantification and THCCOOH-d9 for quantification of both glucuronides.
Procedures
Sample Preparation
Blank blood (0.5 mL) was pipetted into a 10-mL conical polypropylene tube (Sarstedt, Newton, NC). 25 μL of internal standard and either 25 μL of standard or quality control solution were added. 25 μL blank methanol was added to authentic specimens. Ice-cold ACN (1.5 mL) was added drop-wise while vortexing. Tubes were capped and centrifuged (4000g, 4°C) for 5 min. Supernatants were decanted into clean tubes, 4.5 mL 0.2% NH4OH in de-ionized water (v/v) was added, and samples mixed immediately prior to SPE loading.
Solid Phase Extraction
Extraction columns were conditioned with 2 mL methanol and 2 mL de-ionized water. Samples were decanted onto conditioned columns and loaded by gravity. Columns were washed with 2 mL 79:20:1 de-ionized water:acetonitrile:glacial acetic acid (v/v/v) and then dried under full vacuum (≥ 30 kPa) for 20 sec. Analytes were eluted with two separate 1.5 mL aliquots of 1% glacial acetic acid in ACN (v/v) under gravity. Vacuum was briefly applied after both aliquots were collected. Eluents were collected in a 10-mL conical polypropylene tube and dried under nitrogen at 42°C in a Zymark TurboVap evaporator. Samples were reconstituted in 150 μL of initial mobile phase (70:30 A:B), vortexed for 15 sec and transferred to 250 μL pulled-point glass inserts in autosampler vials.
Liquid Chromatography
Chromatographic separation was performed with an Ultra Biphenyl column (100 × 2.1 mm, 5μm) fitted with an Ultra II Biphenyl guard cartridge (10 × 2.0 mm) (Restek Corp, Malvern PA). The autosampler temperature was 4°C and column oven 40°C throughout analysis. The injection volume was 25 μL. Gradient elution was performed with (A) 10 mM ammonium acetate in water adjusted to pH 6.15 (± 0.05) with formic acid and (B) 15% methanol in acetonitrile (v/v) at a flow rate of 400 μL/min. The initial gradient conditions were 30% B, hold for 30 sec, then increase to 90% B at 6.0 min. 90% B was maintained for 7.5 min, at which time the column was re-equilibrated to 30% B over 0.75 min and held for 1.75 min. HPLC eluent was diverted to waste for the first 2.5 min and the final 9 min of analysis.
Mass Spectrometry
Mass spectrometric data were acquired with electrospray ionization (ESI). THC-glucuronide, THCCOOH-glucuronide, THCCOOH, 11-OH-THC and CBD were acquired in negative ionization mode while THC and CBN were acquired in positive ionization mode. MS/MS parameter settings (Table 1, compound-specific optimization) were optimized via direct infusion of individual analytes (500 μg/L in initial mobile phase) at 10 μL/min. Optimized source parameters were as follows: Gas (1) 0.31 MPa, Gas (2) 0.48 MPa, Curtain Gas 0.17 MPa, Source Temperature 650°C. Three acquisition periods were employed, with dwell times of 150 ms for each MS/MS transition in the first, 100 ms for the second and 150 ms for the final period. Unit resolution was used for all experiments.
Table 1.
LC-MS/MS Parameters for Cannabinoids and Cannabinoid Glucuronides in Whole Blood
| Analyte | Q1 | Q3 | DP (V) | CE (V) | RT (min) |
|---|---|---|---|---|---|
| Mass (m/z)b | Mass (m/z)b | ||||
| THCCOOH-glucuronide | 519.0 | 342.9 | −45 | −31 | 3.1 |
| 519.0 | 299.0 | −45 | −44 | 3.1 | |
| THC-glucuronide | 489.0 | 313.1 | −55 | −38 | 3.2 |
| 489.0 | 174.9 | −55 | −24 | 3.2 | |
| THCCOOH-d9 | 352.2 | 254.2 | −55 | −38 | 4.3 |
| 352.2 | 194.1 | −55 | −28 | 4.3 | |
| THCCOOH | 343.0 | 245.1 | −60 | −36 | 4.3 |
| 343.0 | 191.1 | −60 | −44 | 4.3 | |
| 11-OH-THC-d3 | 332.1 | 271.2 | −50 | −32 | 5.2 |
| 332.1 | 314.2 | −50 | −26 | 5.2 | |
| 11-OH-THC | 329.0 | 267.9 | −50 | −38 | 5.3 |
| 329.0 | 311.1 | −50 | −24 | 5.3 | |
| CBD-d3 | 316.1 | 248.1 | −50 | −32 | 5.7 |
| 316.1 | 182.2 | −50 | −26 | 5.7 | |
| CBD | 312.9 | 245.1 | −60 | −28 | 5.8 |
| 312.9 | 178.9 | −60 | −26 | 5.8 | |
| CBN | 311.2 | 223.1 | 61 | 27 | 6.2 |
| 311.2 | 178.3 | 61 | 81 | 6.2 | |
| THC-d3 | 318.3 | 196.2 | 70 | 29 | 6.2 |
| 318.3 | 123.1 | 70 | 43 | 6.2 | |
| THC | 315.2 | 193.2 | 70 | 29 | 6.3 |
| 315.2 | 123.1 | 70 | 43 | 6.3 |
Q1: Quadrupole 1; Q3: Quadrupole 3; DP: declustering potential; CE: collision energy; RT: retention time; THC: Δ9-tetrahydrocannabinol; THCCOOH: 11-nor-9-carboxy-THC; 11-OH-THC: 11-hydroxy-THC; CBD: cannabidiol; CBN: cannabinol.
Bold font denotes quantifier transition.
Data Analysis
Linear regression with 1/x2 weighting was employed for all analytes. Peak area ratios of target analytes and their respective internal standards were calculated for each concentration. Analyst Version 1.5 (AB Sciex, Foster City, CA) was utilized for all data collection and processing; statistical calculations were completed with GraphPad Prism 5 for Windows (GraphPad Software, La Jolla, CA).
Validation
Specificity, sensitivity, linearity, intra- and inter-batch imprecision, bias, extraction efficiency, matrix effect, carryover, dilution integrity, endogenous and exogenous interferences and analyte stability were investigated to evaluate method integrity. Specificity was based on relative retention time, precursor mass, and fragment ion. Retention times for QC and authentic specimens were required to be within ± 0.2 min of the mean calibrator retention time. Transition peak area ratios for QC and authentic specimens were required to be within ± 20% of the mean peak area ratios for calibrators of each respective analyte.
Sensitivity was evaluated by determining limits of detection (LOD) and (LOQ). A series of decreasing concentrations of drug-fortified whole blood was analyzed to empirically determine LOD and LOQ. LOD was determined as the concentration with a signal-to-noise ratio of at least 3, transition peak area ratios within 20% of the mean calibrator ratio and acceptable chromatographic retention time and peak shape. LOQ was the lowest concentration with a signal-to-noise ratio of at least 10, acceptable bias and imprecision (within at least 20% of target concentration and relative standard deviation within at least 20%, n = 6), transition peak area ratios within 20% of the mean calibrator ratio and acceptable chromatographic retention time and peak shape.
Linearity of the method was investigated by calculation of the regression line by the method of least squares and expressed by the squared correlation coefficient (R2). A 1/x2 weighting factor was applied to compensate for heteroscedasticity as evaluated through residuals analysis. Linearity of each analyte was determined with at least five concentration levels, not including the blank matrix, on 4 separate days.
Imprecision and bias were evaluated at three QC concentrations spanning the dynamic linear range. Intra-batch imprecision (% CV) was evaluated by six determinations per concentration in 1 day. Inter-batch imprecision (% CV) was evaluated for two replicates per concentration on 10 days (n total = 20). One-way ANOVA was employed to evaluate inter-batch repeatability as detailed by Peters and Maurer [26]; p< 0.05 indicated significance. Bias was determined comparing the mean measured concentration of six analyses to the target value and was expressed as the percent of target concentration.
Extraction efficiency (%) and matrix effect (%) for each analyte also were determined at low, medium, and high control concentrations according to the design proposed by Matuszewski et al [27]. For determination of extraction efficiency, quality control standard solution was added prior to or following SPE. Extraction efficiency, %, was expressed as the mean analyte area of samples with control solution added before SPE (n = 6) divided by the mean analyte area of samples with control solution added after SPE (n = 6). Matrix effect was investigated by comparing analyte peak areas of extracted blank samples that were fortified after SPE versus analyte peak areas of neat samples prepared in initial mobile phase (30:70 A:B) at equivalent concentrations. Matrix effect was computed by dividing the analyte areas of blank samples fortified after SPE by areas of neat samples, expressed as percent.
Carryover was determined by injecting a negative specimen containing internal standard after a specimen containing two times the upper LOQ. As high concentrations are sometimes observed in blood following cannabis smoking, dilution integrity (1:5 and 1:10) was assessed with three blank blood specimens fortified with high QC solution. Specimens were combined with additional blank whole blood at 1:5 and 1:10 ratios to yield a 500 μL sample. Internal standard was added and specimens were processed as normal.
Interference from endogenous whole blood compounds was assessed by fortifying aliquots from ten blank whole blood pools with low QC solution and evaluating calculated concentrations. Interferences from over 80 illicit and common therapeutic drugs, metabolites and related compounds were evaluated by adding potential interferents into whole blood aliquots fortified with low QC solution. A compound did not interfere if the low QC quantified within 20% of target and had stable retention times and correct transition ratios. All interferences (Table 2) were tested at 1000 μg/L except for the cannabinoids that were tested at 250 μg/L.
Table 2.
Exogenous Interferences Investigated by Fortification into a Low Quality Control Samplea
| 2-ethyl-5-methyl-3,3-diphenylpyrroline | clonazepam | norbenzoylecgonine |
| 3,4-(methylenedioxyphenyl)-2-butanamine | clonidine | norbuprenorphine |
| 3,4-methylenedioxyamphetamine | clonipramine | norcocaethylene |
| 3,4-methylenedioxyethylamphetamine | cocaethylene | norcocaine |
| 3,4-methylenedioxymethamphetamine | cocaine | norcodeine |
| 4-bromo-2,5-dimethoxyphenethylamine | codeine | norcotinine |
| 4-hydroxy-3-methoxyamphetamine | cotinine | nordiazepam |
| 4-hydroxy-3-methoxymethamphetamine | diazepam | norephedrine |
| 6-acetylcodeine | diphenhydramine | norfluxetine |
| 6-acetylmorphine | ethylamphetamine | normorphine |
| 7-aminoclonazapam | flunitrazapam | noroxycodone |
| 7-aminoflunitrazapam | fluoxetine | noroxymorphone |
| 7-aminonitrazapam | flurazepam | oxazepam |
| 8,11-dihydroxy-Δ9-tetrahydrocannabinol | hydrocodone | oxycodone |
| 8-hydroxy-Δ9-tetrahydrocannabinol | hydromorphone | oxymorphone |
| acetaminophen | ibuprofen | paroxetine |
| acetylsalicylic acid | imipramine | pentazocine |
| alprazolam | lorazepam | phenycyclidine |
| amphetamine | methadone | p-hydroxyamphetamine |
| benzoylecgonine | methamphetamine | p-hydroxybenzoylecgonine |
| bromazepam | m-hydroxybenzoylecgonine | p-hydroxycocaine |
| brompheniramine | m-hydroxycocaine | p-hydroxymethamphetamine |
| buprenorphine | morphine | p-hydroxynorephedrine |
| caffeine | morphine-3-glucuronide | propoxyphene |
| cannabigerol | morphine-6-glucuronide | temazepam |
| cathinone | nicotine | trans-3′-hydroxycotinine |
| chlorpheniramine | nitrazepam | |
| N-methyl-1-(3,4-methylenedioxyphenyl)-2-butanamine | 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine |
All compounds fortified at 1000 μg/L except cannabinoids fortified at 250 μg/L.
Hydrolysis of glucuronides during sample processing was evaluated with blank whole blood fortified to 50 μg/L THC-glucuronide and 250 μg/L THCCOOH-glucuronide. Quantifying THC and THCCOOH formed in these hydrolysis controls allowed the calculation of percent hydrolysis for glucuronide metabolites. THC-glucuronide and THCCOOH-glucuronide standards also were investigated individually for presence of THC and THCCOOH, respectively. Individual neat standards were evaporated, reconstituted in mobile phase and quantified against a neat calibration curve to quantify any free cannabinoids present.
Analyte stability in whole blood (n= 5) was evaluated at three QC concentrations under three conditions: 16 h at room temperature (RT), 72 h at 4 °C and three freeze-cycles at −20 °C (23 h freeze, 1 h thaw at RT). Stability of extracted whole blood samples while in the 4°C autosampler was evaluated over 24 h. Extracted low, medium, and high QC samples (n = 3 at each level) were analyzed immediately after extraction along with calibration standards. Another set of three low, medium, and high QC samples were analyzed 24 h after extraction and subsequent storage in autosampler vials at 4°C. All samples were quantified from the initial calibration curve.
Results and Discussion
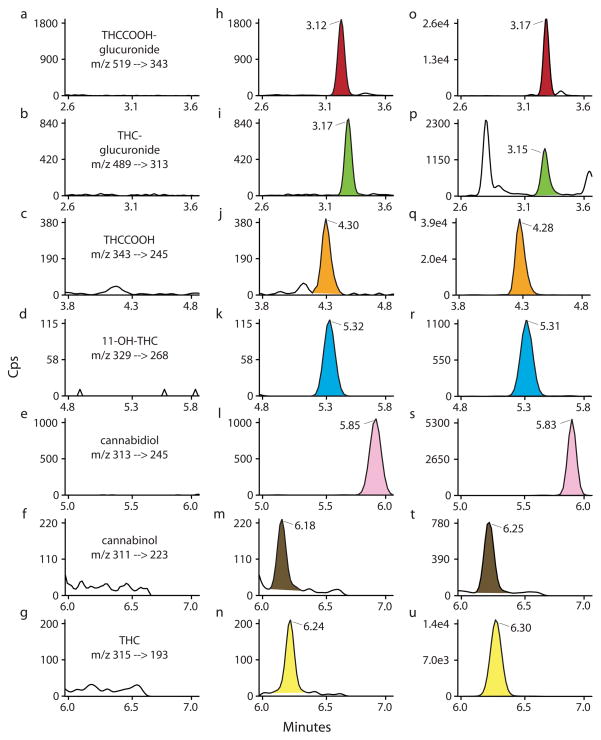
Cannabinoids are the most commonly abused illicit drugs, and cannabinoid medications are utilized for an increasing number of indications, documenting the need for accurate, sensitive and robust cannabinoid quantification. Numerous analytical methods are available to quantify cannabinoids in human whole blood, with and without conjugate hydrolysis [28–32]. However, these methods are limited to parent THC, phase I metabolites and other minor cannabinoids, and fail to consider implications of phase II metabolites. Specifically, factors such as poor hydrolysis efficiency [19,15] and glucuronide instability [33] can introduce unnecessary (and potentially substantial) error into quantitative determinations. Direct identification and quantification of glucuronides negates these issues and can yield novel insight into glucuronide pharmacokinetics and glucuronide in vitro stability while possibly providing an opportunity to utilize cannabinoid glucuronides as markers of recent cannabis intake. The present method sensitively and specifically quantifies these glucuronides directly in addition to typical cannabinoids of interest, including minor cannabinoids CBD and CBN (Figure 1). Thus, this first analytical method for directly analyzing free and glucuronidated cannabinoids in the same whole blood specimen is a significant advancement in the detection and quantification of this important class of compounds.
Fig. 1.
MRM ion chromatograms of (a–g) extracted blank whole blood, (h–n) analytes at limit of quantification, and (o–u) a whole blood specimen 0.25 h after smoking a 6.8% THC (w/w) cannabis cigarette. Limits of quantification (LOQ): Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC), 11-nor-9-carboxy-THC (THCCOOH), cannabidiol, cannabinol 1 μg/L; THC-glucuronide 0.5 μg/L and THCCOOH-glucuronide 5.0 μg/L. Authentic specimen concentrations are detailed in Table 7.
Calibration and Validation
The method was validated according to the criteria described in the Experimental Section. Table 3 details LOD, LOQ and calibration results for each analyte. LOQs were determined empirically through analysis of decreasing concentrations of drug-fortified whole blood and were 1 μg/L for THC, 11-OH-THC, THCCOOH, CBD and CBN with a 0.5 mL whole blood specimen, exceeding cutoff criteria proposed by Farrell et al [34] and meeting the 1 μg/L THC cutoff typically employed for DUID testing [35]. To extend the dynamic linear range for THCCOOH-glucuronide and minimize the number of re-extractions that might be required due to high THCCOOH-glucuronide concentrations, a 250 μg/L calibrator was included for this analyte. However, extending the linear range required increasing the LOQ from 2.0 to 5.0 μg/L to meet a priori specifications for calibration curve linearity. Linear ranges and R2 values (1/x2 weighting) were acceptable (R2 > 0.990) for all analytes. Linear ranges were THC-glucuronide 0.5–50 μg/L, THCCOOH-glucuronide 5.0–250 μg/L and THC, 11-OH-THC, THCCOOH, CBD and CBN 1.0–100 μg/L (Table 3); these ranges should prove useful for clinical and forensic casework. Calibrators for THC, 11-OH-THC, THCCOOH, CBD and CBN quantified within ± 15% (± 20% for LOQ and glucuronides) when quantified against the entire calibration curve. We expect that our ongoing clinical studies will help establish the utility of glucuronide metabolites for establishing recency of use, generating wider interest in cannabinoid glucuronide testing. Additional interest might prompt proper deuterated internal standards synthesis, allowing more stringent criteria (± 15%) to be applied to all analytes at concentrations > LOQ.
Table 3.
Limits of Detection (LOD), Limits of Quantification (LOQ), and Calibration Results for Cannabinoids and Cannabinoid Glucuronides in Whole Blood by LC-MS/MS
| Analyte | Internal standard | LOD (μg/L) | LOQ (μg/L) | Slope ± SD (n = 4) | y-int ± SD (n = 4)a | R2 ± SD (n = 4) | Linear range (μg/L) |
|---|---|---|---|---|---|---|---|
| THCb | THC-d3 | 0.5 | 1.0 | 0.115±0.013 | 0.015±0.022 | 0.999±0.001 | 1.0–100 |
| 11-OH-THCc | 11-OH-THC-d3 | 1.0 | 1.0 | 0.068±0.003 | 0.002±0.013 | 0.997±0.002 | 1.0–100 |
| THCCOOHd | THCCOOH-d9 | 1.0 | 1.0 | 0.114±0.006 | 0.019±0.015 | 0.999±0.001 | 1.0–100 |
| CBDe | CBD-d3 | 0.25 | 1.0 | 0.110±0.010 | 0.001±0.003 | 1.000±0.000 | 1.0–100 |
| CBNf | THC-d3 | 1.0 | 1.0 | 0.180±0.025 | −0.016±0.012 | 0.999±0.001 | 1.0–100 |
| THC-glucuronide | THCCOOH-d9 | 0.25 | 0.5 | 0.303±0.036 | 0.005±0.011 | 0.999±0.001 | 0.5–50 |
| THCCOOH-glucuronide | THCCOOH-d9 | 1.0 | 5.0 | 0.054±0.010 | −0.025±0.025 | 0.998±0.002 | 5.0–250 |
y-intercept.
Δ9-tetrahydrocannabinol.
11-hydroxy-THC.
11-nor-9-carboxy-THC.
cannabidiol.
cannabinol.
Deuterium-labeled analogues are not currently commercially available for THCCOOH-glucuronide, THC-glucuronide and CBN. The decision to implement THC-d3 and THCCOOH-d9 for CBN and glucuronides, respectively, was based on similarities in extraction efficiency and matrix effects. This choice was not ideal as differences in efficiencies were present and these can vary depending on the matrix pool; nevertheless, a priori specifications for sensitivity and linearity were met. Other glucuronide metabolites, including morphine-3-glucuronide-d3, buprenorphine-glucuronide and mefenamic acyl-β-D-glucuronide-d3 were investigated as potential internal standards. However, these were either not extracted efficiently (buprenorphine-glucuronide) or not well-retained on our chromatographic system (morphine-3-glucuronide-d3 and mefenamic acyl-β-D-glucuronide-d3). While we attempted to minimize matrix effects through sample preparation including solid phase extraction, some matrix effect remained. The matrix effects for glucuronides and their respective internal standards were not identical, but our approach is the best available at this time. Furthermore, we investigated matrix effect in 10 different whole blood pools demonstrating that low QC quantification remained within ± 20% in all 10 whole blood pools. Despite these efforts, differential matrix effect cannot be excluded, and glucuronide quantification could be affected. It should be noted that deuterated glucuronide analogues are recommended should they become available, as improvements in imprecision, bias and reliability could be realized.
Bias and imprecision were evaluated at three concentrations across the linear dynamic range of each analyte (Table 4). Intra-batch imprecision (% CV) was less than 7.9% for all analytes at all concentrations (n= 6); inter-batch imprecision (% CV) was less than 10.4% (n= 20). Bias, calculated as the percent of target concentrations at low, mid and high QC concentrations for each analyte, ranged from 93.8% to 113.1% of target concentrations (n= 6). One-way ANOVA yielded statistically significant differences in inter-batch repeatability for several analytes; however, differences were less than 10.4% CV and considered clinically insignificant.
Table 4.
Bias and Imprecision Data for Cannabinoids and Cannabinoid Glucuronides in Whole Blood by LC-MS/MS
| Analyte | Intra-batch imprecision (% CV, n = 6) | Inter-batch imprecision (% CV, n = 20) | Calculated Concentration (μg/L, n = 6) | Bias (% of target, n = 6) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Mid | High | Low | Mid | High | Low | Mid | High | Low | Mid | High | |
| THCa,b | 4.8 | 2.8 | 3.5 | 8.6 | 9.8 | 7.4 | 2.8 | 28.0 | 71.8 | 110.8 | 112.1 | 95.7 |
| 11-hydroxy-THC | 6.5 | 6.1 | 4.3 | 10.4 | 8.9 | 7.4 | 2.7 | 27.6 | 72.4 | 109.1 | 110.2 | 96.5 |
| 11-nor-9-carboxy-THC | 7.9 | 4.2 | 5.3 | 5.5 | 7.2 | 7.4 | 2.6 | 26.5 | 73.4 | 104.6 | 106.1 | 97.8 |
| cannabidiol | 5.9 | 3.0 | 3.5 | 4.9 | 6.4 | 6.1 | 2.8 | 27.2 | 74.2 | 111.1 | 108.7 | 99.0 |
| cannabinol | 3.3 | 2.5 | 3.2 | 9.9 | 10.1 | 8.6 | 2.8 | 26.6 | 70.3 | 111.1 | 106.5 | 93.8 |
| THC-glucuronidec | 5.7 | 4.4 | 5.7 | 5.7 | 7.6 | 8.2 | 1.7 | 15.8 | 43.0 | 110.1 | 105.3 | 95.6 |
| THCCOOH-glucuronided | 5.2 | 4.2 | 6.8 | 9.1 | 8.7 | 8.4 | 8.3 | 84.8 | 237.3 | 110.0 | 113.1 | 105.5 |
Δ9-tetrahydrocannabinol.
THC, 11-hydroxy-THC, 11-nor-9-carboxy-THC, cannabidiol and cannabinol low-, mid- and high-quality control target concentrations were 2.5, 25 and 75 μg/L, respectively.
THC-glucuronide low-, mid- and high-quality control target concentrations were 1.5, 15 and 45 μg/L, respectively.
THCCOOH-glucuronide low-, mid- and high-quality control target concentrations were 7.5, 75 and 225 μg/L, respectively.
Extraction efficiency for native and deuterium-labeled analytes ranged from 50.5% to 93.9% (Table 5). Table 5 also displays ion suppression/enhancement produced by matrix effect; positive values indicate ion enhancement and negative values indicate ion suppression. While substantial matrix effects were observed for 11-OH-THC, similar results were obtained for the corresponding deuterated analogue and quantification was not adversely affected.
Table 5.
Extraction Efficiency, Matrix Effect and Process Efficiency for Cannabinoids and Cannabinoid Glucuronides in Whole Blood by LC-MS/MS
| Analyte | Extraction efficiency (%, n = 6)
|
Matrix effect (%, n = 6)
|
||||
|---|---|---|---|---|---|---|
| Low | Mid | High | Low | Mid | High | |
| THCa,b | 50.5 | 57.9 | 75.6 | −9.5 | −8.1 | 0.0 |
| 11-hydroxy-THC | 71.5 | 84.6 | 82.3 | −56.4 | −60.1 | −66.6 |
| 11-nor-9-carboxy-THC | 66.8 | 77.1 | 93.9 | −10.5 | −20.0 | −32.1 |
| cannabidiol | 63.2 | 68.5 | 83.8 | −16.8 | −16.1 | −30.1 |
| cannabinol | 56.4 | 63.2 | 76.5 | −19.7 | −12.8 | −3.7 |
| THC-glucuronidec | 73.2 | 75.6 | 87.6 | 48.1 | 41.3 | 1.7 |
| THCCOOH-glucuronided | 56.1 | 58.2 | 57.3 | 8.7 | 9.0 | −10.3 |
| THC-d3 | 50.6 | 57.3 | 71.8 | −11.3 | −8.2 | 6.0 |
| 11-hydroxy-THC-d3 | 79.9 | 84.2 | 79.3 | −57.6 | −59.3 | −65.3 |
| 11-nor-9-carboxy-THC-d9 | 70.9 | 77.6 | 87.3 | −14.1 | −17.8 | −26.5 |
| cannabidiol-d3 | 63.3 | 67.0 | 81.2 | −18.0 | −16.8 | −26.7 |
Δ9-tetrahydrocannabinol.
THC, 11-hydroxy-THC, 11-nor-9-carboxy-THC, cannabidiol and cannabinol low-, mid- and high-quality control target concentrations were 2.5, 25 and 75 μg/L, respectively.
THC-glucuronide low-, mid- and high-quality control target concentrations were 1.5, 15 and 45 μg/L, respectively.
THCCOOH-glucuronide low-, mid- and high-quality control target concentrations were 7.5, 75 and 225 μg/L, respectively.
Development of an effective sample cleanup that removed matrix interferences while maintaining high extraction efficiency proved to be the greatest challenge during method development. The extraction procedure (reversed-phase polymeric SPE), gentle wash step (20% acetonitrile in water) and polar elution solvent (acetonitrile) yielded high concentrations of phospholipids in extracts as evidenced through a positive precursor ion scan of m/z 184 as detailed by Xia and Jemal [36]. Extending the 90% acetonitrile hold to 7.5 min during the chromatographic gradient provided effective column washing and removal of phospholipids; forgoing this wash yielded substantial increases in ion suppression for subsequent injections. In addition to high phospholipid concentrations, rapid increases in system backpressure were observed during initial method development with a smaller HPLC column particle size (3 μm) and methanolic mobile phase. Backpressure increases were mitigated through replacement of methanol with acetonitrile, increasing column particle size to 5 μm and more frequent replacement of the guard column. Thus, slight decreases in resolution and cost-efficiency were offset by increased column life and a more reliable method.
Carryover in a negative specimen following a specimen containing twice the upper limit of quantification was assessed. No carryover was observed for any analyte; ion transition ratios were not within 20% of calibrators and any signal present was less than LODs. Common therapeutic and illicit drugs and metabolites at concentrations of 1000 μg/L (cannabinoids 250μg/L) did not interfere with analytes of interest at the low QC concentration. Additionally, ten pools of whole blood were tested for potential endogenous interferences; none were observed in any pool for any analyte. Dilution integrity was maintained up to 10 times dilution with blank whole blood and all analytes quantified within 20% of the theoretical high QC concentration.
Quantification of THCCOOH and THC formed in glucuronide control samples during extraction was conducted (n= 6 each). Mean (SD) percentages of THCCOOH-glucuronide and THC-glucuronide hydrolysis were 0.6 ± 0.05% and 3.7 ± 0.35%, respectively. However, these are both likely artifacts as neat THCCOOH-glucuronide and THC-glucuronide calibrators were determined to contain 0.5 ± 0.1% THCCOOH and 3.2 ± 0.2% THC, respectively (n = 5 each). While the ester-linked THCCOOH-glucuronide was reported as relatively labile [33], we observed minimal hydrolysis of THCCOOH-glucuronide during extraction. The THC impurity has a minor effect on THC quantification that is less than the analytical error for the method and a low LOQ of 1 μg/L was achieved. To confirm a lack of substantial effect on THC quantifications, samples fortified with only THC at the LOQ (1 μg/L) were quantified against the entire calibration curve containing all analytes. Acceptable quantifications were obtained (± 20%) for these samples, confirming minimal bias resulting from the THC impurity present in the THC-glucuronide standard.
Stability at 4 °C on the autosampler for 24 h was determined for extracted specimens. All analytes at all concentrations (low, mid and high QC) were stable under these conditions, with mean concentrations differing from samples injected immediately (n= 3) by less than −8.3% (Table 6). For fortified whole blood samples, THCCOOH, 11-OH-THC and both glucuronides were stable under all other conditions tested (three freeze-thaw cycles, 72 h at 4 °C and 16 h at RT). However, losses up to 35.7% were observed for THC after 72 h at 4 °C. Additionally, CBD, CBN and THC demonstrated relative instability under three freeze-thaw cycles, 72 h at 4 °C and 16 h at RT. It should be noted that these losses were observed in fortified samples; losses in authentic specimens may not reflect these findings due to differences in protein binding [15].
Table 6.
Stability Data for Cannabinoids and Cannabinoid Glucuronides in Whole Blood by LC-MS/MSa
| Analyte | 24 h autosampler (% difference, n = 3) | 72 h 4 °C (% difference, n = 3) | 16 h RTb (% difference, n = 3) | 3 Freeze/thaw cycles (% difference, n = 3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Mid | High | Low | Mid | High | Low | Mid | High | Low | Mid | High | |
| THCc,d | 2.1 | 0.9 | 3.6 | −32.1 | −35.7 | −33.0 | −32.9 | −21.9 | −21.9 | −30.4 | −20.9 | −17.8 |
| 11-hydroxy-THC | −2.9 | −4.0 | −3.7 | 1.5 | −4.9 | −5.2 | −19.5 | 1.6 | 0.0 | −5.6 | 1.5 | −0.9 |
| 11-nor-9-carboxy-THC | −6.9 | 1.8 | 3.7 | 2.1 | 1.2 | 1.5 | 4.7 | 7.6 | 10.4 | 9.2 | 6.8 | 7.6 |
| cannabidiol | 1.3 | 0.4 | −0.3 | −27.6 | −29.5 | −28.6 | −13.5 | −14.3 | −15.7 | −12.1 | −17.9 | −13.3 |
| cannabinol | 4.9 | −4.6 | −2.0 | −5.9 | −12.8 | −13.2 | −14.7 | −21.2 | −20.6 | −8.0 | −21.2 | −20.0 |
| THC-glucuronidee | −8.3 | −1.5 | 4.7 | −11.3 | −6.2 | −9.2 | 5.3 | 8.0 | 5.9 | −2.9 | −2.2 | −2.4 |
| THCCOOH-glucuronidef | −6.6 | −2.2 | 3.8 | −4.7 | 5.2 | 5.0 | 13.1 | 14.6 | 7.3 | 7.6 | 10.5 | 5.3 |
Positive values indicate % increase from theoretical, negative values indicate % loss from theoretical.
Room temperature
Δ9-tetrahydrocannabinol.
THC, 11-hydroxy-THC, 11-nor-9-carboxy-THC, cannabidiol and cannabinol low-, mid- and high-quality control target concentrations were 2.5, 25 and 75 μg/L, respectively.
THC-glucuronide low-, mid- and high-quality control target concentrations were 1.5, 15 and 45 μg/L, respectively.
THCCOOH-glucuronide low, mid- and high-quality control target concentrations were 7.5, 75 and 225 μg/L, respectively.
Application of Method
Whole blood was collected from a clinical research participant prior to and after smoking a single cannabis cigarette ad libitum. Baseline concentrations were less than LOQ for all cannabinoids except THCCOOH and THCCOOH-glucuronide. 15 and 60 min after the start of smoking, blood was collected and concentrations determined by this new analytical method (Table 7). THC-glucuronide quantified at 0.6 μg/L in the first specimen, demonstrating the necessity for the low LOQ that this method achieved. It should be noted that specimens were analyzed within 24 h of collection, minimizing any potential losses due to analyte degradation. Concentrations suggest THC-glucuronide may serve as possible marker of recent cannabis intake, given that it is detectable following cannabis smoking, albeit at a low concentration. Further research is required to assess detection windows for THC-glucuronide or other minor cannabinoids, such as CBD or CBN, following smoked cannabis.
Table 7.
Cannabinoids and Cannabinoid Glucuronides Quantified in Whole Blood Collected from a Volunteer during a Controlled Smoked Cannabis Administration Study
| Analyte | Baselinea (μg/L) | 0.25 hb (μg/L) | 1.0 hb (μg/L) |
|---|---|---|---|
| THCc | < LODd | 54.8 | 13.4 |
| 11-hydroxy-THC | < LOD | 7.2 | 4.1 |
| 11-nor-9-carboxy-THC | 12.5 | 59.2 | 51.9 |
| cannabidiol | < LOD | 2.1 | < LOQe |
| cannabinol | < LOD | 2.9 | < LOD |
| THC-glucuronide | < LOD | 0.6 | < LOQ |
| THCCOOH-glucuronide | 33.0 | 38.1 | 96.0 |
Baseline samples collected 0.5 h prior to the start of ad libitum smoking of a single 6.8% THC (w/w) cannabis cigarette.
Time from the start of smoking.
Δ9-tetrahydrocannabinol.
Concentration below method limit of detection.
Concentration below method limit of quantification.
Conclusions
This method is the first robust, sensitive and specific LC-MS/MS technique for direct detection and quantification of several cannabinoids and two cannabinoid glucuronides in human whole blood, yielding a comprehensive cannabinoid whole blood profile following cannabis intake. The rapid and simple extraction and 16 min analysis are beneficial; however, care should be taken to prevent buildup of phospholipids and other matrix components, leading to increased HPLC backpressure and loss of resolution. This method is utilized for several controlled cannabinoid administration studies and will provide whole blood pharmacokinetic and cannabinoid stability data useful to clinicians and forensic toxicologists interpreting whole blood cannabinoid concentrations often obtained during DUID cases and other investigations. This new analytical method for cannabinoids in whole blood offers advantages in sensitivity and spectrum of cannabinoid analytes included over existing LC-MS/MS and GC-MS assays, and when applied to controlled cannabinoid administration studies, may improve our ability to interpret cannabinoid whole blood results.
Acknowledgments
The authors thank Allan Barnes, Erin Karschner, Teresa Gray, Amanda Rigdon and the clinical staff of the NIDA Intramural Research Program for technical assistance. This work was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
References
- 1.Compton R, Berning A. DOT HS. Vol. 811. NHTSA; Washington, DC: 2009. Results of the 2007 National Roadside Survey of Alcohol and Drug Use by Drivers; p. 175. [Google Scholar]
- 2.Huestis MA, Henningfield JE, Cone EJ. J Anal Toxicol. 1992;16:276–282. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- 3.Kauert GF, Ramaekers JG, Schneider E, Moeller MR, Toennes SW. J Anal Toxicol. 2007;31:288–293. doi: 10.1093/jat/31.5.288. [DOI] [PubMed] [Google Scholar]
- 4.Niedbala RS, Kardos KW, Fritch DF, Kardos S, Fries T, Waga J, Robb J, Cone EJ. J Anal Toxicol. 2001;25 (5):289–303. doi: 10.1093/jat/25.5.289. [DOI] [PubMed] [Google Scholar]
- 5.Matsunaga T, Iwawaki Y, Watanabe K, Yamamoto I, Kageyama T, Yoshimura H. Life Sci. 1995;56:2089–2095. doi: 10.1016/0024-3205(95)00193-a. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I. Life Sci. 2007;80:1415–1419. doi: 10.1016/j.lfs.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Halldin MM, Carlsson S, Kanter SL, Widman M, Agurell S. Arzneim Forsch. 1982;32:764–768. [PubMed] [Google Scholar]
- 8.Williams PL, Moffat AC. J Pharm Pharmacol. 1980;32:445–448. doi: 10.1111/j.2042-7158.1980.tb12966.x. [DOI] [PubMed] [Google Scholar]
- 9.Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M. Clin Pharmacol Ther. 1983;34:352–363. doi: 10.1038/clpt.1983.179. [DOI] [PubMed] [Google Scholar]
- 10.Mareck U, Haenelt N, Geyer H, Guddat S, Kamber M, Brenneisen R, Thevis M, Schänzer W. Drug Test Anal. 2009;1:505–510. doi: 10.1002/dta.106. [DOI] [PubMed] [Google Scholar]
- 11.Skopp G, Pötsch L, Ganßmann B, Mauden M, Richter B, Aderjan R, Mattern R. Z Rechtsmed. 1999;10:21–28. [Google Scholar]
- 12.Abraham TT, Lowe RH, Pirnay SO, Darwin WD, Huestis MA. J Anal Toxicol. 2007;31:477–485. doi: 10.1093/jat/31.8.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stout PR, Horn CK, Klette KL. J Anal Toxicol. 2001;25:550–554. doi: 10.1093/jat/25.7.550. [DOI] [PubMed] [Google Scholar]
- 14.Dietz L, Glaz-Sandberg A, Nguyen H, Skopp G, Mikus G, Aderjan R. Ther Drug Monit. 2007;29:368–372. doi: 10.1097/FTD.0b013e31805ba6fd. [DOI] [PubMed] [Google Scholar]
- 15.Schwilke EW, Schwope DM, Karschner EL, Lowe RH, Darwin WD, Kelly DL, Goodwin RS, Gorelick DA, Huestis MA. Clin Chem. 2009;55:2180–2189. doi: 10.1373/clinchem.2008.122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray TR, Barnes AB, Huestis MA. Anal Bioanal Chem. 2010;397:2335–2347. doi: 10.1007/s00216-010-3772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ElSohly M, Feng S. J Anal Toxicol. 1998;22:329–335. doi: 10.1093/jat/22.4.329. [DOI] [PubMed] [Google Scholar]
- 18.Moore C, Rana S, Coulter C, Day D, Vincent M, Soares J. J Anal Toxicol. 2007;31:187–194. doi: 10.1093/jat/31.4.187. [DOI] [PubMed] [Google Scholar]
- 19.Kemp PM, Abukhalaf IK, Manno JE, Manno BR, Alford DD, McWilliams ME, Nixon FE, Fitzgerald MJ, Reeves RR, Wood MJ. J Anal Toxicol. 1995;19:292–298. doi: 10.1093/jat/19.5.292. [DOI] [PubMed] [Google Scholar]
- 20.Kemp PM, Abukhalaf IK, Manno JE, Manno BR, Alford DD, Abusada GA. J Anal Toxicol. 1995;19:285–291. doi: 10.1093/jat/19.5.285. [DOI] [PubMed] [Google Scholar]
- 21.Stephanson N, Josefsson M, Kronstrand R, Beck O. J Chromatogr B: Anal Technol Biomed Life Sci. 2008;871:101–108. doi: 10.1016/j.jchromb.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 22.Huestis MA. Chem Biodivers. 2007;4:1770–1804. doi: 10.1002/cbdv.200790152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jagerdeo E, Schaff JE, Montgomery MA, LeBeau MA. Rapid Commun Mass Spectrom. 2009;23:2697–2705. doi: 10.1002/rcm.4174. [DOI] [PubMed] [Google Scholar]
- 24.Teixeira H, Verstraete A, Proenca P, Corte-Real F, Monsanto P, Vieira DN. Forensic Sci Int. 2007;170:148–155. doi: 10.1016/j.forsciint.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Coulter C, Miller E, Crompton K, Moore C. J Anal Toxicol. 2008;32:653–658. doi: 10.1093/jat/32.8.653. [DOI] [PubMed] [Google Scholar]
- 26.Peters FT, Maurer HH. Accredit Qual Assur. 2002;7:441–449. [Google Scholar]
- 27.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Anal Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 28.Scurlock RD, Ohlson GB, Worthen DK. J Anal Toxicol. 2006;30:262–266. doi: 10.1093/jat/30.4.262. [DOI] [PubMed] [Google Scholar]
- 29.Chu MH, Drummer OH. J Anal Toxicol. 2002;26:575–581. doi: 10.1093/jat/26.8.575. [DOI] [PubMed] [Google Scholar]
- 30.Kintz P, Cirimele V. Biomed Chromatogr. 1997;11:371–373. doi: 10.1002/(SICI)1099-0801(199711)11:6<371::AID-BMC685>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 31.König S, Aebi B, Lanz S, Gasser M, Weinmann W. Anal Bioanal Chem. 2011;400:9–16. doi: 10.1007/s00216-011-4708-x. [DOI] [PubMed] [Google Scholar]
- 32.Schwilke EW, Karschner EL, Lowe RH, Gordon AM, Cadet JL, Herning R, Huestis MA. Clin Chem. 2009;55:1188–1195. doi: 10.1373/clinchem.2008.114405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skopp G, Potsch L. Clin Chem. 2002;48:301–306. [PubMed] [Google Scholar]
- 34.Farrell LJ, Kerrigan S, Logan BK. J Forensic Sci. 2007;52:1214–1218. doi: 10.1111/j.1556-4029.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- 35.Pil K, Raes E, Van den Neste T, Verstraete A. Working paper “Uniform design and protocols for carrying out case-control studies” DRiving Under the Influence of Drugs, alcohol and medicines (DRUID); Bergisch Gladbach (Germany). 2007. p. 10. [Google Scholar]
- 36.Xia YQ, Jemal M. Rapid Commun Mass Spectrom. 2009;23:2125–2138. doi: 10.1002/rcm.4121. [DOI] [PubMed] [Google Scholar]