Figure 4. Molecular basis of rotational switching.
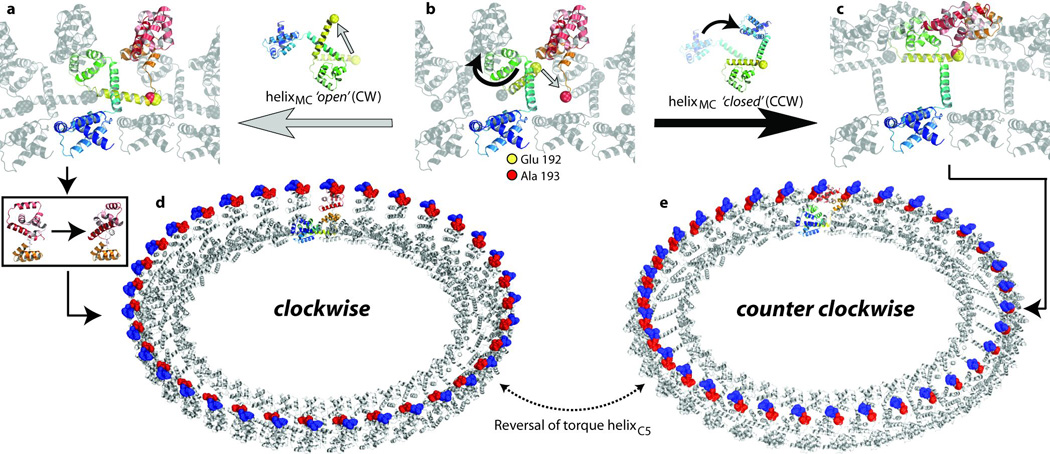
a–c, An expanded view of the encircled region in Fig. 3e is shown with one coloured FliG polypeptide chain. E192 and A193 are shown as yellow and red spheres respectively. a, b, Transition from the closed to the open conformation of helixMC. b, c, An alternative conformational change when helixMC remains in the closed conformation. d, e, The FliG ring with the monomers in the FliGMC and the FliGFL crystal structures, respectively. Charged residues on torque helixC5 are shown in blue (positive) and red (negative).
