Abstract
Transmembrane AMPA receptor regulatory proteins (TARPs) are AMPA receptor auxiliary subunits that influence diverse aspects of receptor function. However, the full complement of physiological roles for TARPs in vivo remains poorly understood. Here we find that double knock-out mice lacking TARPs γ-2 and γ-3 are profoundly ataxic and fail to thrive. We demonstrate that these TARPs are critical for the synaptic targeting and kinetics of AMPA receptors in cerebellar Golgi cells, but that either alone is sufficient to fully preserve function. By analyzing the few remaining synaptic AMPA receptors in the γ-2, γ-3 double knock-out mice, we unexpectedly find that these TARPs specify AMPA receptor subunit composition. This study establishes a new role for TARPs in regulating AMPA receptor assembly and suggests that TARPs are necessary for proper AMPA receptor localization and function in most, if not all, neurons of the CNS.
Keywords: glutamate receptor, auxiliary subunit, synaptic transmission, Golgi cell, stargazin, stargazer, interneuron
Introduction
Voltage- and ligand-gated ion channels form the foundation of neuronal signaling in the brain. Research has primarily focused on pore-forming principle subunits of ion channels; however, auxiliary subunits can also modify ion channel trafficking, localization, and gating (Isom et al., 1994; Yu et al., 2005; Levitan, 2006). Until recently, few ligand-gated ion channels were known to possess auxiliary subunits, but this changed with the identification of transmembrane AMPA receptor regulatory proteins (TARPs) as auxiliary subunits for AMPA receptors (Nicoll et al., 2006; Ziff, 2007). Because AMPA receptors underlie the majority of fast excitatory synaptic transmission in the brain and their mobility contributes to learning and memory, it is critical to understand how TARPs regulate AMPA receptor activity.
TARPs are a family of related four-pass transmembrane proteins (Letts et al., 1998), including γ-2, γ-3, γ-4, γ-7, and γ-8, which promote AMPA receptor function (Tomita et al., 2003; Kato et al., 2007). Like classical auxiliary subunits of voltage-gated channels, TARPs regulate many aspects of AMPA receptor activity. TARPs augment AMPA receptor plasma membrane trafficking, enhance synaptic clustering, increase glutamate affinity, increase kainate efficacy, determine antagonist pharmacology, and slow channel deactivation and desensitization (Yamazaki et al., 2004; Priel et al., 2005; Tomita et al., 2005; Turetsky et al., 2005; Zhang et al., 2006; Kott et al., 2007; Menuz et al., 2007).
Although loss of the prototypical TARP, stargazin or γ-2, results in the behavioral phenotypes of ataxia and epilepsy (Noebels et al., 1990), mice lacking either γ-4 or γ-8 appear behaviorally indistinguishable from littermates (Letts et al., 1998; Rouach et al., 2005; Milstein et al., 2007). Thus, it is unclear whether the requirement for TARPs in maintaining AMPA receptor function is limited to a few specific neuronal populations. Alternatively, functionally similar TARP family members may compensate for the loss of other TARPs given their overlapping expression profile. Such molecular redundancy appears to be common in synaptic protein families such as MAGUKs (membrane-associated guanylate kinases), neuroligins, and MALS (mammalian LIN-7) (Misawa et al., 2001; Olsen et al., 2005; Elias et al., 2006; Varoqueaux et al., 2006).
This study employs γ-2 and γ-3 knock-out (KO) mice to address key questions regarding the in vivo regulation of AMPA receptors by TARPs. We find that the functional redundancy of these TARPs in single knock-out mice avoids the neonatal lethality observed in double knock-outs. Importantly, we show that the presence of a single TARP family member in cerebellar Golgi cells can maintain synaptic AMPA receptor levels and kinetics. This redundancy likely explains the absence of behavioral phenotypes in most single TARP knock-out mice. Finally, we report an unexpected role for TARPs in regulating AMPA receptor subunit composition.
Materials and Methods
Knock-out mice.
All experiments followed animal welfare guidelines established by the University of California, San Francisco Institutional Animal Care and Use Committee. Stargazer mice (γ-2−/− mice) have been described previously (Letts et al., 1998). TARP γ-3−/− mice were generated by standard knock-out technology. Southern blot analysis was used to verify proper targeting. Genomic mouse tail DNA was digested with XbaI and blotted with a P32-labeled γ-3 probe that was located 5′ to exon 2. PCR primers for the γ-3 Southern probe were as follows: forward (F), TTCATAGATGGCCTTTCC; reverse (R), CCAACATTCCACTCTGGG. Mice carrying the γ-3 knock-out mutation were crossed to γ-2+/− mice to produce double knock-out γ-2+/−, γ-3−/− breeding pairs. PCR genotyping of tail DNA was performed with the following primers: for γ-3, F-wild type (wt), AACTAGGTTCCCAGATAGCC; R-wt, GCTTCTAATGGGTTGCGCCC; F-KO, GGCTGCTCTTTGGTTAATCGG; R-KO, TACCCGGTAGAATTGACCTGC; for γ-2, F-wt, CATTTGTTATACATGCTCTAG; R-wt, ACTGTCACTCTATCTGGAATC; F-KO, GAGCAAGCAGGTTTCAGGC; R-KO, ACTGTCACTCTATCTGGAATC.
Antibodies.
The rabbit polyclonal antibody to γ-3 has been characterized previously (Tomita et al., 2003). The following commercial antibodies were used: rabbit polyclonal antibodies to GluR1, GluR2/3, and GluR4 (Millipore Bioscience Research Reagents); monoclonal antibodies to NR1 (BD Pharmingen) and tubulin (Sigma).
Immunoblotting.
Brain regions from mice [postnatal day 25 (P25)–P45] were homogenized in 4.5 volumes of 320 mm sucrose buffer and then sonicated in 2% final SDS. Equal amounts (20 μg) of protein were separated on 8% polyacrylamide gels followed by transfer to polyvinylidene difluoride membranes. Proteins were detected by immunoblotting using the HRP-ECL kit from GE Healthcare. The densitometry function of ImageJ software, available from the NIH, was used to determine the relative amounts of AMPA and NMDA receptor protein. Statistical significance was determined by either a Student's paired t test or a one-way repeated-measures ANOVA, followed by the Tukey's post hoc test.
Immunohistochemistry.
Anesthetized mice ages P18–P22 were transcardially perfused with PBS followed by 4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4, for 10 min. Brain tissues were postfixed for 4 h followed by a 2 h incubation in 10% sucrose/PBS and two 8 h incubations in 20% sucrose/PBS solutions. Sagittal sections (35 μm) were cut with a freezing microtome and were then blocked for 1 h in 3% normal goat serum and incubated overnight in GluR1, GluR2/3, and GluR4 antibodies at 4°C. The sections were processed with Vectastain ABC kit using 3,3′-diaminobenzidine as substrate.
Electrophysiology.
For Golgi cell recordings, parasagittal cerebellar slices (200 μm) from juvenile mice (2–3 weeks old) and young mice (P9–P10) mice were cut in cold (4–6°C) ACSF containing the following (in mm): 125 NaCl, 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 25 glucose, 4 MgCl2, and 1 CaCl2 saturated with 95% O2–5% CO2. Slices were incubated at 30–34°C for 1 h, then moved to room temperature for 30 min, and finally stored in the recording solution at room temperature. The recording solution was identical to the cutting solution, except that the concentration of MgCl2 and CaCl2 were 1 and 2 mm, respectively. Transverse hippocampal slices (300 μm) from 2- to 3-week-old mice for CA1 pyramidal cells recordings were prepared similarly but were incubated at 30–34°C for 30 min and then maintained at room temperature. The cutting ACSF for hippocampal slices contained the following (in mm): 119 NaCl, 2.5 KCl, 26.3 NaHCO3, 1 NaH2PO4, 11 glucose, 1.3 MgCl2, and 2.5 CaCl2. Hippocampal recording solution was similar but contained 4 mm MgCl2 and 4 mm CaCl2. All recording solutions contained 100 μm picrotoxin (Sigma). For Golgi cell recordings, 3 μm strychnine (Sigma) was added.
Whole-cell recordings were obtained using glass electrodes (2–6 MΩ). The internal pipette solution for recording hippocampal pyramidal cells consisted of the following (in mm): 110 Cs methanesulfonate, 10 CsCl, 10 HEPES, 2 MgCl2, 4 Na2-ATP, 0.4 Na-GTP, 10 Cs4-BAPTA, 5 N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium bromide (QX-314), and 0.1 spermine, pH 7.2–3, adjusted to 295–305 mOsm. Golgi cell recordings used the same internal solution as hippocampal cells, except that 0.1% Lucifer yellow was added, the osmolarity was adjusted to 305–315 mOsm, and QX-314 and spermine were omitted in miniature EPSC (mEPSC) recordings. Hippocampal pyramidal cells were visually identified. Cerebellar Golgi cells were distinguished from other neurons in the granule cell layer by their larger soma, slow capacitance kinetics, and characteristic dendritic arborization in both the molecular and granule cell layers visualized with Lucifer yellow (Dieudonne, 1998).
Hippocampal CA1 pyramidal cells were voltage clamped at +40 mV, and dual-component EPSCs were evoked by stimulating in the stratum radiatum. NMDA receptors were then blocked by adding (RS)-CPP [3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid] to obtain a pure AMPA EPSC, and the NMDA receptor EPSC was obtained by subtraction. Paired-pulse facilitation was measured by stimulating twice with a 40 ms interval at a holding potential of −60 mV. Rectification was measured by measuring the AMPA receptor EPSCs at +40 and −60 mV. The rectification index (RI) was defined as (I+40/I−60) × [(−60 − Erev)/(40 − Erev)], such that a linear response would have an RI of 1, and a fully rectifying response would have a value of 0.
Evoked currents were obtained in Golgi cells similarly. EPSCs were evoked by stimulating the molecular layer while holding the cell at −70 mV. Dual-component responses were obtained at +40 mV, and then the AMPA receptor current was isolated by adding 50 μm d-APV (Tocris Bioscience). The paired-pulse ratio and rectification index was measured in the same way as for pyramidal cells, with the exception that the holding current was −70 mV. For recording mEPSCs, 500 nm TTX (Tocris Bioscience) and 50 μm d-APV (Tocris Bioscience) were additionally added to the recording solution. Customized IgorPro (WaveMetrics) software was used to analyze mEPSCs off-line with a threshold of 10 pA.
Statistical significance was determined by either a Student's t test for comparisons between two groups or a one-way ANOVA for comparisons between multiple groups. If significant, the ANOVA was followed by either the Tukey's or Games–Howell post hoc tests depending on whether the data met the assumption of equal variance according to the Levene statistic. All data shown are the mean ± SEM.
Results
γ-2,3−/− mice fail to thrive
To investigate the essential in vivo role of TARPs, we first generated mice deficient in γ-3 (Fig. 1A–C), which is widely expressed in the brain (Lein et al., 2007). Because these knock-out mice were indistinguishable from wt littermates (Fig. 1D,E), either γ-3 is not required for normal brain function or another TARP compensates for its loss.
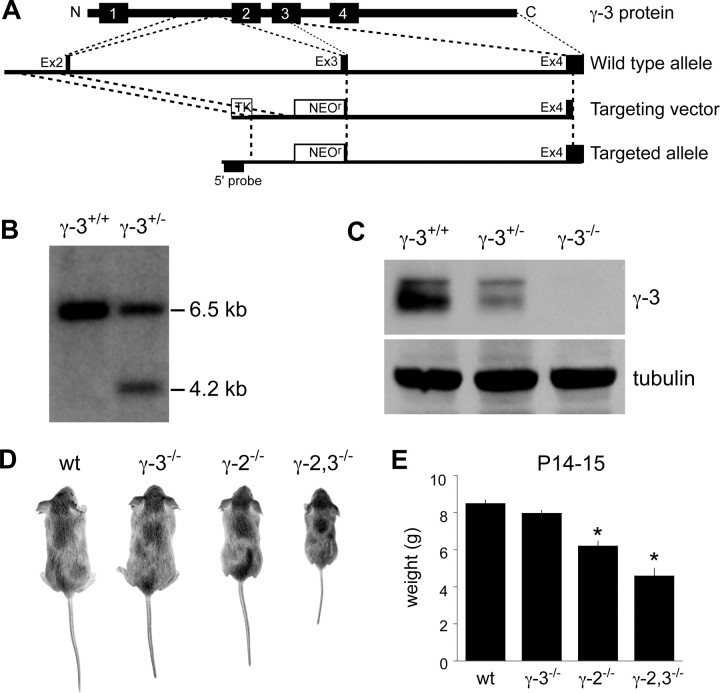
Figure 1.
Generation of γ-3−/− and γ-2,3−/− mice. A, A diagram depicts the γ-3 protein, wild-type genomic locus, targeting vector, and targeted allele. Exons (Ex) are marked as black boxes. B, Southern blot analysis using the 5′ probe shown in A detects both a 6.5 kb wild-type fragment and the 4.2 kb knock-out fragment in γ-3+/− mice. C, Immunoblot analysis of brain lysates from γ-3+/+, γ-3+/−, and γ-3−/− mice demonstrates the absence of γ-3 protein in γ-3−/− mice. Tubulin served as a loading control. D, E, P14–P15 γ-2−/− mice weigh only slightly less than wild-type littermates. In contrast, the few γ-2,3−/− mice that survive weigh ∼50% of their γ-3−/− littermates (wt, 8.49 ± 0.17 g; γ-3−/−, 7.97 ± 0.15 g; γ-2−/−, 6.20 ± 0.27 g; γ-2,3−/−, 4.59 ± 0.40 g; n = 86, 94, 14, and 15, respectively; p < 0.05 for γ-2,3−/− compared with γ-3−/−, γ-2−/−, or wt; p < 0.0001 for γ-2−/− vs wt littermates; p > 0.05 for γ-3−/− vs wt mice). Asterisks indicate statistical significance.
Given that γ-2 and γ-3 are closely related, we tested for molecular redundancy by generating γ-2−/−;γ-3−/− double knock-out mice (abbreviated γ-2,3−/−). We found that γ-2,3−/− mice are born at predicted Mendelian ratios, but that the majority die within the first 2 weeks after birth (Table 1). By culling littermates, some γ-2,3−/− mice lived into the third postnatal week, but few survived beyond the fourth week. At P14, surviving γ-2,3−/− mice were ∼55% the weight of γ-3−/− littermates, whereas γ-2−/− mice were ∼75% the weight of their wild-type littermates (Fig. 1D,E). No significant difference in weight was observed between γ-3−/− and wild-type mice. In addition to their smaller size, γ-2,3−/− mice displayed profound ataxia, which was considerably more severe than in γ-2−/− mice (supplemental Movies 1–4, available at www.jneurosci.org as supplemental material). That the loss of γ-3 exaggerates the γ-2 behavioral phenotype suggests that these two TARPs may serve redundant functions.
Table 1.
Survival of γ-2,3−/− mice in the absence of culling littermates
| γ-2+/+;γ-3−/− | γ-2+/−;γ-3−/− | γ-2−/−;γ-3−/− | Litters | χ2 | |
|---|---|---|---|---|---|
| At birth | 59 (28%) | 111 (53%) | 41 (19%) | 37 | p = 0.162 |
| At P14 (pups) | 62 (35%) | 106 (61%) | 7 (4%) | 46 | p < 0.0001 |
| Died <P14 | 5 (9%) | 23 (42%) | 27 (49%) | 30 | p < 0.0001 |
The number of progeny of each genotype in litters resulting from crosses of γ-2+/−;γ-3−/− mice is shown. The percentage of animals of each genotype is given in parentheses. The χ2 test was used to determine if the genotype distribution differed from Mendelian inheritance.
Loss of cerebellar AMPA receptor expression in γ-2,3−/− mice
TARPs chaperone AMPA receptors for proper protein folding and membrane trafficking (Vandenberghe et al., 2005). We therefore asked whether deletion of γ-2 and γ-3 yields a secondary loss of AMPA receptor protein levels, as occurs in the hippocampus of γ-8−/− mice (Rouach et al., 2005). Immunohistochemical staining of sagittal brain slices using antibodies raised against the AMPA receptor subunits GluR1, GluR2/3, and GluR4 did not reveal gross localization differences between wild-type and γ-2,3−/− mice (Fig. 2A). We then used immunoblot analysis to quantify changes in AMPA receptor protein levels. Although γ-2 and γ-3 both occur at high levels in cortical regions of forebrain (Tomita et al., 2003; Fukaya et al., 2005), no changes in AMPA receptor levels were found in either the cortex or the hippocampus of γ-2,3−/− mice (Fig. 2B). Accordingly, hippocampal synaptic AMPA receptor function, measured electrophysiologically as the ratio of AMPA to NMDA EPSC amplitudes, were unchanged in γ-2,3−/− mice compared with controls (Fig. 2C). There was also no change in the paired-pulse ratio (Fig. 2D) or the rectification index (Fig. 2E).
Figure 2.
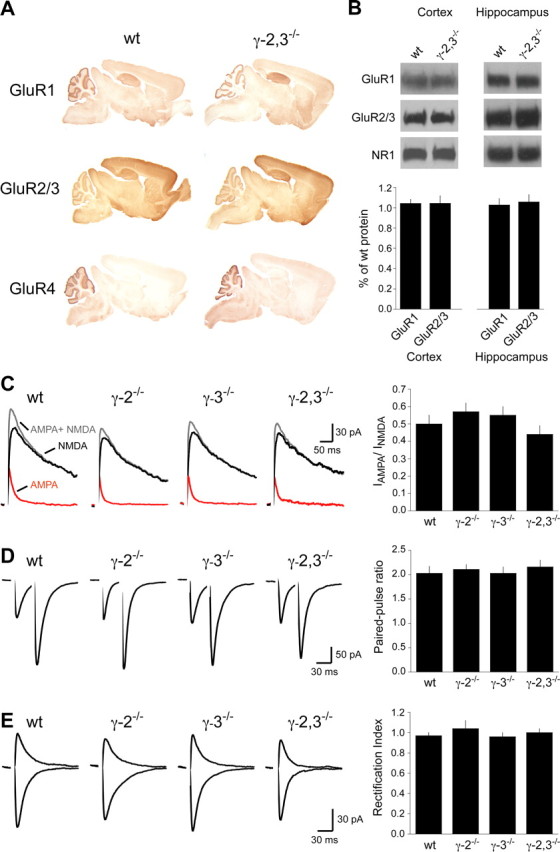
AMPA receptor expression and function in γ-2,3−/− mice forebrain. A, Immunohistochemical localizations of GluR1, GluR2/3, and GluR4 in wild-type and γ-2,3−/− mice. B, Immunoblot analysis of cortical and hippocampal extracts reveals no difference in AMPA receptor expression in γ-2,3−/− mice (hippocampus, n = 3 and 4 blots for GluR1 and GluR2/3, respectively; p > 0.5 for each subunit; cortex, n = 4 and 5 blots for GluR1 and GluR2/3, respectively; p > 0.3 for each). NMDA receptor subunit NR1 levels are also unchanged. C, Dual-component EPSCs (gray) in hippocampal CA1 pyramidal cells were pharmacologically separated into their AMPA- (red) and NMDA- (black) mediated components. No changes are seen in the IAMPA/INMDA ratio (wt, 0.50 ± 0.05; γ-2−/−, 0.57 ± 0.05; γ-3−/−, 0.55 ± 0.05; γ-2,3−/−, 0.44 ± 0.05; n = 12, 10, 9, and 9, respectively; p = 0.32). D, Paired-pulse facilitation is unchanged in all knock-out strains (wt, 2.03 ± 0.14; γ-2−/−, 2.11 ± 0.10; γ-3−/−, 2.03 ± 0.13; γ-2,3−/−, 2.16 ± 0.14; n = 12, 10, 9, and 9, respectively; p = 0.86). E, The rectification of AMPA receptor EPSCs in all mouse strains is linear (wt, 0.97 ± 0.03; γ-2−/−, 1.04 ± 0.08; γ-3−/−, 0.96 ± 0.04; γ-2,3−/−, 1.00 ± 0.04; n = 12, 9, 9, and 8, respectively; p = 0.68). Shown are EPSCs from cells held at −60 mV and +40 mV.
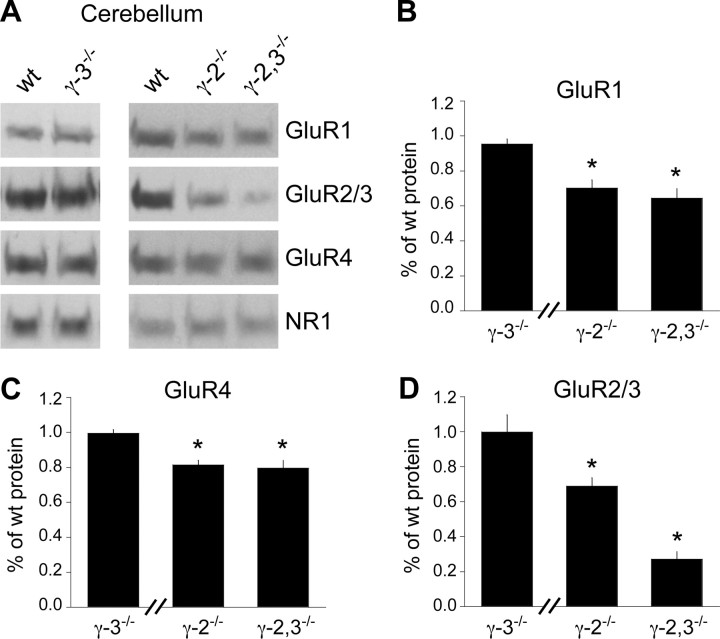
In contrast, loss of γ-2 and γ-3 reduced AMPA receptor subunit protein levels in the cerebellum (Fig. 3A). Both GluR1 and GluR4 were diminished by ∼25% in γ-2−/− mice (Fig. 3B,C), but no further reduction occurred in double knock-out mice. In contrast, GluR2/3 levels were unaltered in γ-3−/− mice, were reduced by ∼30% in γ-2−/− mice and were reduced by ∼70% in γ-2,3−/− mice (Fig. 3D). This synergistic reduction in GluR2/3 levels in the γ-2,3−/− mice implies a functional redundancy for these two closely related TARPs.
Figure 3.
Cerebellar AMPA receptor protein expression in γ-2 and γ-3 knock-outs. A, Immunoblotting of cerebellar extracts shows that AMPA receptor levels are unchanged in γ-3−/− mice (n = 3, 5, and 5 for GluR1, GluR2/3 and GluR4, respectively; p > 0.2 for each compared with wild type). B, C, GluR1 and GluR4 levels are significantly reduced in γ-2−/− mice without further loss in double knock-out mice (n = 5 and 4 blots for GluR1 and GluR4, respectively; p < 0.002 for either γ-2−/− or γ-2,3−/− vs wild type and p > 0.6 for γ-2−/− vs γ-2,3−/− for both GluR1 and GluR4). D, GluR2/3 protein levels are decreased by ∼30% in γ-2−/− mice and by ∼70% in γ-2,3−/− mice (n = 7 blots; p < 0.002 for either γ-2−/− or γ-2,3−/− vs wild type; p < 0.001 for γ-2−/− vs γ-2,3−/−). Hash marks on the bar graphs indicate that separate experiments were used to compare γ-3−/− mice versus wild types. Asterisks indicate statistical significance.
A single TARP family member is sufficient to preserve synaptic AMPA receptors
The loss of cerebellar AMPA receptors and severe ataxia in γ-2,3−/− mice suggested impaired synaptic transmission in a specific population of cerebellar neurons. To test this directly, we investigated AMPA receptor-mediated synaptic transmission in cerebellar Golgi cells (Palay and Chan-Palay, 1974), local interneurons that express high levels of γ-2 and γ-3 (Fukaya et al., 2005; Lein et al., 2007) and whose ablation causes ataxia (Watanabe et al., 1998). We quantified Golgi cell synaptic AMPA receptor-mediated transmission by stimulating parallel fibers to evoke a compound EPSC composed of NMDA and AMPA receptor-mediated currents. We then pharmacologically separated the currents by applying 50 μm d-APV and measured the ratio of AMPA to NMDA EPSC amplitudes. Double knock-out mice had a nearly 90% reduction of AMPA/NMDA, whereas no change was seen in either single knock-out (Fig. 4A). No difference was observed in the paired-pulse ratio, a measure of presynaptic function, consistent with the predicted postsynaptic loss of receptors (Fig. 4B).
Figure 4.
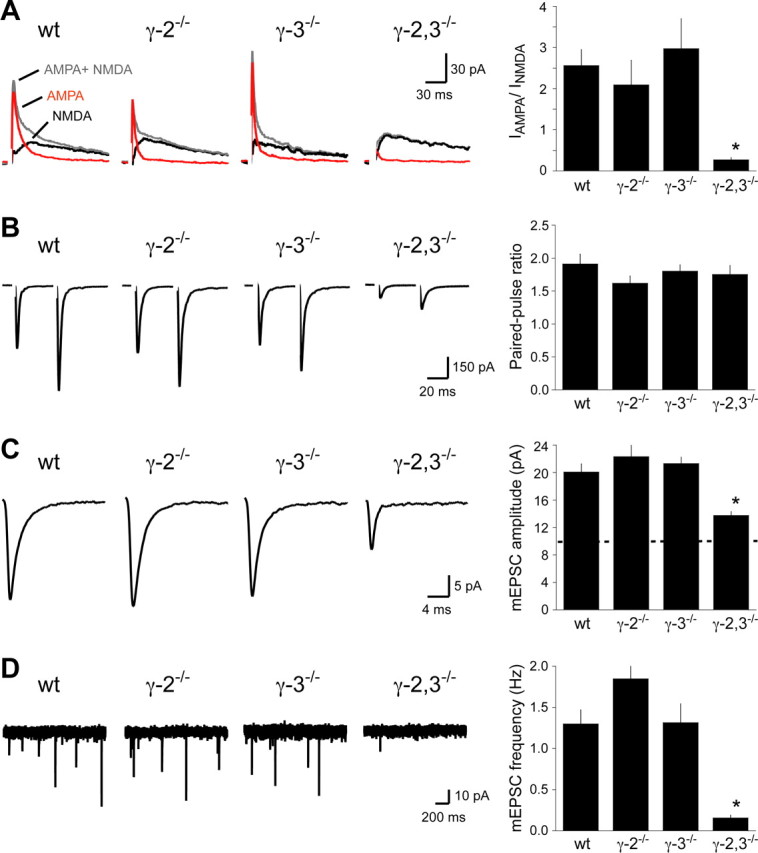
AMPA receptor EPSCs in cerebellar Golgi cells from γ-2 and γ-3 knock-out mice. A, Dual-component EPSCs (gray) composed of IAMPA (red) and INMDA (black) from cerebellar Golgi cells. Whereas the IAMPA/INMDA ratio was similar in wild-type, γ-2−/−, and γ-3−/− mice, it was severely compromised in γ-2,3−/− mice (wt, 2.56 ± 0.39; γ-2−/−, 2.09 ± 0.60; γ-3−/−, 2.97 ± 0.73; γ-2,3−/−, 0.27 ± 0.06; n = 9, 6, 7, and 7, respectively; p < 0.002 for wild type compared with γ-2,3−/−; p > 0.9 for each other genotype compared with each wild type). B, Paired-pulse facilitation of AMPA receptor EPSCs was similar in all genotypes (wt, 1.91 ± 0.15; γ-2−/−, 1.62 ± 0.11; γ-3−/−, 1.80 ± 0.10; γ-2,3−/−, 1.75 ± 0.14; n = 11, 8, 10, and 8, respectively; p > 0.4). C, The average mEPSC amplitude in γ-2,3−/− mice is significantly reduced compared with all other genotypes. The threshold for mEPSC detection is shown as a dotted line on the bar graph (wt, 20.1 ± 1.0 pA; γ-2−/−, 22.3 ± 1.6 pA; γ-3−/−, 21.3 ± 1.0 pA; γ-2,3−/−, 13.8 ± 0.6 pA; n = 14, 9, 10, and 8, respectively; p < 0.03 for each genotype compared with γ-2,3−/−; p > 0.4 for each other genotype compared with each other). D, The mEPSC frequency is significantly lower in γ-2,3−/− mice compared with controls (wt, 1.30 ± 0.15 Hz; γ-2−/−, 1.85 ± 0.40 Hz; γ-3−/−, 1.31 ± 0.23 Hz; γ-2,3−/−, 0.16 ± 0.04 Hz; n = 13, 9, 10, and 11, respectively; p < 0.02 for each genotype compared with γ-2,3−/−; p > 0.5 for each other genotype compared with each other). Sample traces consist of three overlaid consecutive sweeps. Asterisks indicate statistical significance.
The diminished synaptic AMPA receptor EPSCs in γ-2,3−/− mice may reflect fewer AMPA receptors in all Golgi synapses or selective loss from a subset of synapses. To distinguish between these possibilities, we measured Golgi cell mEPSCs. If the former were true, then both the amplitude and the frequency of mEPSCs would be reduced, because many events would fall below the threshold for detection. If the latter were true, only the mEPSC frequency would diminish. In contrast to wild-type or single knock-out mice, mEPSCs were extremely rare in γ-2,3−/− mice, and the few remaining had amplitudes just above the 10 pA detection threshold (Fig. 4C,D). This ∼90% decrease in frequency and large change in amplitude suggest an equal loss of AMPA receptors from all synapses in the double knock-out mice. Together, our evoked transmission and mEPSC data indicate that either γ-2 or γ-3 can support normal AMPA receptor-mediated synaptic transmission in Golgi cells.
Loss of TARPs speeds AMPA receptor kinetics
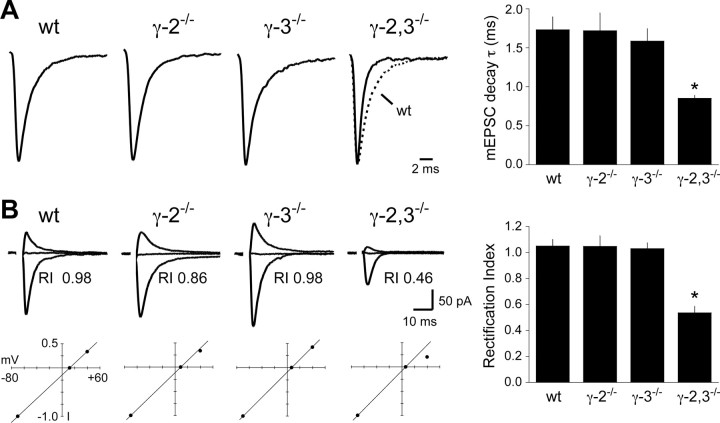
Previous work showed that TARPs slow the rates of AMPA receptor deactivation and desensitization both in heterologous systems and in some excitatory neurons (Priel et al., 2005; Tomita et al., 2005; Milstein et al., 2007). However, it is unclear whether TARPs can also slow AMPA receptor kinetics in interneurons given that their decay is often markedly faster than those of excitatory neurons. Although some factors underlying the faster kinetics in interneurons have been identified, including expression of specific AMPA receptor subunits and highly synchronized glutamate release (Jonas, 2000; Jonas et al., 2004), a role for TARPs has not been examined. Like many interneurons, Golgi cell mEPSCs in wild-type mice have extremely rapid kinetics (τ ≈ 1.7 ms). However, we found that the decay of mEPSCs in γ-2,3−/− mice was nearly twice as fast as those in single knock-out and wild-type mice (Fig. 5A). Thus, TARPs can contribute significantly to AMPA receptor kinetics at fast interneuron synapses.
Figure 5.
TARPs modulate synaptic EPSC kinetics and subunit composition. A, The decay kinetics of mEPSC in Golgi cells from γ-2,3−/− mice are nearly twice as fast as in controls (wt, 1.73 ± 0.14 ms; γ-2−/−, 1.72 ± 0.23 ms; γ-3−/−, 1.59 ± 0.16 ms; γ-2,3−/−, 0.85 ± 0.04 ms; n = 14, 9, 10, and 8, respectively; p < 0.03 for each genotype compared with γ-2,3−/−; p > 0.9 for each other genotype compared with each other). Sample traces show the peak-normalized average mEPSC from a representative cell. A wild-type trace (dotted line) is superimposed over the γ-2,3−/− trace to illustrate the change in kinetics. B, The RI of evoked AMPA receptor EPSCs is linear in wild-type and single knock-out mice, but partially rectifying in double knock-out mice (wt, 1.05 ± 0.05; γ-2−/−, 1.05 ± 0.08; γ-3−/−, 1.03 ± 0.05; γ-2,3−/−, 0.54 ± 0.05; n = 9, 7, 8, and 7, respectively; p < 0.0001 for each genotype compared with γ-2,3−/−; p > 0.9 for each other genotype compared with each other). Traces from a sample cell held at −70 mV, Erev, and +40 mV are shown and labeled with the RI. The corresponding I–V relationship is also plotted. Asterisks indicate statistical significance.
Loss of TARPs affects AMPA receptor subunit composition
We were surprised to find that the remaining synaptic AMPA receptors in γ-2,3−/− mice have a different subunit composition than in wild-type mice. Whereas the I–V relationships of synaptic AMPA receptor-mediated currents in wild-type and single knock-out mice were linear, the I–V curve in γ-2,3−/− mice inwardly rectified (Fig. 5B). Given that native AMPA receptors require GluR2 subunits to generate a linear I–V relationship, our data suggest that AMPA receptors in control animals contain GluR2, whereas synapses in γ-2,3−/− mice contain a mixed population of GluR2-containing and GluR2-lacking AMPA receptors. The AMPA receptor I–V relationship was linear even in young (P9–P10) wild-type Golgi cells (rectification index in P9–P10 mice, 0.96 ± 0.07, n = 5), indicating that the AMPA receptor rectification in γ-2,3−/− mice did not result from impaired developmental maturation of AMPA receptor composition. Thus, our data suggest a novel additional mechanism involving subunit assembly by which TARPs modulate AMPA receptor function.
Discussion
Our results demonstrate that TARPs are essential genes that control multiple aspects of AMPA receptor function in vivo. We show that TARPs γ-2 and γ-3 are molecularly redundant. Furthermore, we show that TARPs control AMPA receptor synaptic levels and EPSC decay kinetics in a cerebellar interneuron. Finally, our data reveal a surprising role for TARPs in regulating AMPA receptor subunit composition.
Molecular redundancy of TARP family members
Although γ-2−/− mice (stargazer) have a dramatic behavioral phenotype, other single TARP knock-out mice, including the γ-3−/− mice reported here, do not show obvious behavioral phenotypes (Letts et al., 2005; Rouach et al., 2005; Milstein et al., 2007). Given the demonstrated importance of TARPs in vitro in regulating AMPA receptor maturation, trafficking, and gating, one might have expected greater behavioral abnormalities. However, our study of γ-2,3−/− mice supports the possibility that loss of individual TARPs can be functionally compensated by other TARP family members with overlapping expression patterns (Fukaya et al., 2005; Lein et al., 2007). The early postnatal lethality and decreased AMPA receptor function in γ-2,3−/− mice support this model of functional redundancy.
In hippocampal pyramidal neurons, γ-8 appears to be the primary TARP regulating AMPA receptor activity, because γ-8−/− mice display a profound reduction of extrasynaptic receptors and an ∼40% reduction of synaptic receptors (Rouach et al., 2005). In wild-type hippocampal neurons, γ-8 occurs at considerably higher levels than γ-2 and γ-3 (Tomita et al., 2003; Fukaya et al., 2005), indicating that γ-8 constitutes the majority of TARP expression in these neurons. Thus the loss of γ-8 leads to a severe reduction in overall TARP expression and the loss of synaptic AMPA receptors in a single TARP knock-out mouse. Expression of the other TARPs, γ-2 and γ-3, is only sufficient to partially maintain synaptic AMPA receptors in γ-8−/− mice. We report here that the loss of both γ-2 and γ-3 does not affect synaptic AMPA receptors in hippocampal pyramidal cells, consistent with γ-8 being the primary TARP expressed. In contrast, Golgi cells appear to be equally dependent on γ-2 and γ-3, because either is sufficient to maintain synaptic AMPA receptor levels. In both types of neurons, TARP levels appear to be saturating in wild-type mice, because the loss of either γ-2 or γ-3 in Golgi cells or the loss of γ-2 and γ-3 in hippocampal neurons does not lead to a loss of synaptic AMPA receptors.
Remaining AMPA receptors in γ-2,3−/− Golgi cells may associate with γ-7
Golgi cells from γ-2,3−/− mice express very low levels of functional AMPA receptors. Whether these residual receptors associate with another TARP or alternatively traffic without TARPs remains uncertain. Cerebellar Golgi cells do not express γ-4 or γ-8, but do express γ-7, a recently identified member of the TARP family (Kato et al., 2007; Lein et al., 2007). Thus, the relatively few remaining AMPA receptors in Golgi cells from γ-2,3−/− mice are likely to associate with γ-7. It is noteworthy that γ-7 does not maintain full synaptic AMPA receptor levels in the absence of γ-2 and γ-3, consistent with the finding that γ-7 does not increase membrane trafficking of AMPA receptors to the same extent as γ-2 in dissociated cerebellar granule cells (Kato et al., 2007). It is possible that whereas γ-2 and γ-3 have similar functions, γ-7 serves other aspects of AMPA receptor regulation.
Alternatively, the remaining Golgi cell AMPA receptors may not be associated with TARPs, because γ-7 slows heterologously expressed receptors (Kato et al., 2007). Analysis of the remaining synaptic receptors in Golgi cells found that the decay kinetics in γ-2,3−/− mice (τ ≈ 0.85 ms) were approximately twice as fast as in wild types (τ ≈ 1.6–1.7 ms). These decay kinetics resemble the values previously published for deactivation of receptors in the absence and presence of TARPs. That is, in heterologous expression systems, AMPA receptor subunits and splice variants have deactivation time constants of ∼0.6–1.2 ms (Dingledine et al., 1999; Sekiguchi et al., 2002), and receptors with TARPs decay in the range of ∼1.4–2.4 ms (Tomita et al., 2005; Zhang et al., 2006; Kato et al., 2007). Thus, the 10% remaining AMPA receptors in Golgi cells of γ-2/3−/− mice may not be associated with TARPs.
Although our data indicate that TARPs are a primary regulator of AMPA receptor trafficking and function in vivo, other mechanisms have been proposed to influence the membrane trafficking and synaptic targeting of AMPA receptors, including their C-terminal tail interactions with various cytoplasmic proteins (Barry and Ziff, 2002; Ziff, 2007). These alternative processes may support a limited level of AMPA receptor function in the absence of TARPs.
TARPs influence AMPA receptor subunit composition
The change from linear to rectifying synaptic responses in Golgi cells from γ-2,3−/− mice indicates that TARPs can specify AMPA receptor subunit composition. This change in subunit composition was surprising, because TARPs functionally interact with and regulate all four AMPA receptor subunits (Turetsky et al., 2005), and no change in rectification was found at hippocampal synapses in γ-8−/− mice (Rouach et al., 2005). Based on previous studies (Verdoorn et al., 1991; Mansour et al., 2001), the Golgi cell I–V relationships imply that wild-type cells exclusively express GluR2-containing receptors, whereas the remaining receptors in γ-2,3−/− cells are composed of a mixture of GluR2-containing and -lacking receptors. Although TARPs have been shown recently to decrease the extent of rectification of GluR2-lacking receptors (Soto et al., 2007), this effect is unlikely to account for the changes we observed in cerebellar Golgi cells. TARPs are unable to linearize the I–V relationship of GluR2-lacking receptors, indicating that the linear synaptic EPSCs we detected in wild-type Golgi cells are attributable to GluR2-containing receptors, whose I–V relationship remains linear in the presence of TARPs (Soto et al., 2007). The selective decrease of cerebellar GluR2/3 protein in γ-2,3−/− mice (Fig. 3D) provides further evidence that the change in AMPA receptor rectification reflects the loss of GluR2 receptors. However, the effects of TARPs on GluR2-lacking receptors makes it difficult to determine their precise contribution to the γ-2,3−/− Golgi cell EPSCs.
Already early in cerebellar development (P9–P10), AMPA receptors in wild-type Golgi cells have a linear I–V relationship, suggesting that the rectification in γ-2,3−/− Golgi cells is a direct result of the lack of TARP association rather than arrested development. There are at least three mechanisms by which TARPs might specify subunit composition. TARPs may preferentially associate with GluR2-containing receptors early in the biosynthetic pathway, enhance the trafficking of GluR2-containing receptors to the cell surface, or increase the insertion of GluR2-containing receptors into synapses. The profound loss of GluR2 protein from cerebellar extracts in γ-2,3−/− mice may indicate that the GluR2 subunits themselves or GluR2-containing receptors are preferentially stabilized by TARPs and are degraded in their absence. On the other hand, previous surface biotinylation assays have indicated that γ-2 enhances the plasma membrane expression of both GluR1 and GluR2 homomeric receptors in heterologous systems (Yamazaki et al., 2004; Turetsky et al., 2005), making the first and second possibilities unlikely. This may support a role for TARPs in specifically targeting GluR2-containing receptors to synapses, but further studies will be needed to distinguish between these and other mechanisms.
Although AMPA receptor subunits have been studied in isolation for nearly two decades, coexpression with TARPs endows receptors with features seen in neurons. Our work on native AMPA receptors in TARP double knock-out mice supports and expands the roles of TARPs seen in heterologous systems, and indicates that TARPs mediate the proper function of AMPA receptors in a wide variety of neurons in vivo.
Footnotes
This work was supported by National Institutes of Health grants (R.A.N., D.S.B) and L'Oreal United Nations Educational, Scientific, and Cultural Organization and Epilepsy Foundation predoctoral fellowships (K.M.).
References
- Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Dieudonne S. Submillisecond kinetics and low efficacy of parallel fibre-Golgi cell synaptic currents in the rat cerebellum. J Physiol. 1998;510:845–866. doi: 10.1111/j.1469-7793.1998.845bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Fukaya M, Yamazaki M, Sakimura K, Watanabe M. Spatial diversity in gene expression for VDCCgamma subunit family in developing and adult mouse brains. Neurosci Res. 2005;53:376–383. doi: 10.1016/j.neures.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Isom LL, De Jongh KS, Catterall WA. Auxiliary subunits of voltage-gated ion channels. Neuron. 1994;12:1183–1194. doi: 10.1016/0896-6273(94)90436-7. [DOI] [PubMed] [Google Scholar]
- Jonas P. The time course of signaling at central glutamatergic synapses. News Physiol Sci. 2000;15:83–89. doi: 10.1152/physiologyonline.2000.15.2.83. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Fricker D, Miles R. Interneuron diversity series: fast in, fast out—temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci. 2004;27:30–40. doi: 10.1016/j.tins.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Kato AS, Zhou W, Milstein AD, Knierman MD, Siuda ER, Dotzlaf JE, Yu H, Hale JE, Nisenbaum ES, Nicoll RA, Bredt DS. New transmembrane AMPA receptor regulatory protein isoform, gamma-7, differentially regulates AMPA receptors. J Neurosci. 2007;27:4969–4977. doi: 10.1523/JNEUROSCI.5561-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kott S, Werner M, Körber C, Hollmann M. Electrophysiological properties of AMPA receptors are differentially modulated depending on the associated member of the TARP family. J Neurosci. 2007;27:3780–3789. doi: 10.1523/JNEUROSCI.4185-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett FS, 2nd, Mori Y, Campbell KP, Frankel WN. The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat Genet. 1998;19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- Letts VA, Mahaffey CL, Beyer B, Frankel WN. A targeted mutation in Cacng4 exacerbates spike-wave seizures in stargazer (Cacng2) mice. Proc Natl Acad Sci U S A. 2005;102:2123–2128. doi: 10.1073/pnas.0409527102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan IB. Signaling protein complexes associated with neuronal ion channels. Nat Neurosci. 2006;9:305–310. doi: 10.1038/nn1647. [DOI] [PubMed] [Google Scholar]
- Mansour M, Nagarajan N, Nehring RB, Clements JD, Rosenmund C. Heteromeric AMPA receptors assemble with a preferred subunit stoichiometry and spatial arrangement. Neuron. 2001;32:841–853. doi: 10.1016/s0896-6273(01)00520-7. [DOI] [PubMed] [Google Scholar]
- Menuz K, Stroud RM, Nicoll RA, Hays FA. TARP auxiliary subunits switch AMPA receptor antagonists into partial agonists. Science. 2007;318:815–817. doi: 10.1126/science.1146317. [DOI] [PubMed] [Google Scholar]
- Milstein AD, Zhou W, Karimzadegan S, Bredt DS, Nicoll RA. TARP subtypes differentially and dose-dependently control synaptic AMPA receptor gating. Neuron. 2007;55:905–918. doi: 10.1016/j.neuron.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misawa H, Kawasaki Y, Mellor J, Sweeney N, Jo K, Nicoll RA, Bredt DS. Contrasting localizations of MALS/LIN-7 PDZ proteins in brain and molecular compensation in knockout mice. J Biol Chem. 2001;276:9264–9272. doi: 10.1074/jbc.M009334200. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- Noebels JL, Qiao X, Bronson RT, Spencer C, Davisson MT. Stargazer: a new neurological mutant on chromosome 15 in the mouse with prolonged cortical seizures. Epilepsy Res. 1990;7:129–135. doi: 10.1016/0920-1211(90)90098-g. [DOI] [PubMed] [Google Scholar]
- Olsen O, Moore KA, Fukata M, Kazuta T, Trinidad JC, Kauer FW, Streuli M, Misawa H, Burlingame AL, Nicoll RA, Bredt DS. Neurotransmitter release regulated by a MALS-liprin-alpha presynaptic complex. J Cell Biol. 2005;170:1127–1134. doi: 10.1083/jcb.200503011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar cortex: cytology and organization. Berlin: Springer; 1974. [Google Scholar]
- Priel A, Kolleker A, Ayalon G, Gillor M, Osten P, Stern-Bach Y. Stargazin reduces desensitization and slows deactivation of the AMPA-type glutamate receptors. J Neurosci. 2005;25:2682–2686. doi: 10.1523/JNEUROSCI.4834-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Byrd K, Petralia RS, Elias GM, Adesnik H, Tomita S, Karimzadegan S, Kealey C, Bredt DS, Nicoll RA. TARP gamma-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat Neurosci. 2005;8:1525–1533. doi: 10.1038/nn1551. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M, Nishikawa K, Aoki S, Wada K. A desensitization-selective potentiator of AMPA-type glutamate receptors. Br J Pharmacol. 2002;136:1033–1041. doi: 10.1038/sj.bjp.0704804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto D, Coombs ID, Kelly L, Farrant M, Cull-Candy SG. Stargazin attenuates intracellular polyamine block of calcium-permeable AMPA receptors. Nat Neurosci. 2007;10:1260–1267. doi: 10.1038/nn1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR, Nicoll RA, Bredt DS. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435:1052–1058. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- Turetsky D, Garringer E, Patneau DK. Stargazin modulates native AMPA receptor functional properties by two distinct mechanisms. J Neurosci. 2005;25:7438–7448. doi: 10.1523/JNEUROSCI.1108-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe W, Nicoll RA, Bredt DS. Interaction with the unfolded protein response reveals a role for stargazin in biosynthetic AMPA receptor transport. J Neurosci. 2005;25:1095–1102. doi: 10.1523/JNEUROSCI.3568-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Südhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Verdoorn TA, Burnashev N, Monyer H, Seeburg PH, Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991;252:1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Inokawa H, Hashimoto K, Suzuki N, Kano M, Shigemoto R, Hirano T, Toyama K, Kaneko S, Yokoi M, Moriyoshi K, Suzuki M, Kobayashi K, Nagatsu T, Kreitman RJ, Pastan I, Nakanishi S. Ablation of cerebellar Golgi cells disrupts synaptic integration involving GABA inhibition and NMDA receptor activation in motor coordination. Cell. 1998;95:17–27. doi: 10.1016/s0092-8674(00)81779-1. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Ohno-Shosaku T, Fukaya M, Kano M, Watanabe M, Sakimura K. A novel action of stargazin as an enhancer of AMPA receptor activity. Neurosci Res. 2004;50:369–374. doi: 10.1016/j.neures.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev. 2005;57:387–395. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- Zhang W, Robert A, Vogensen SB, Howe JR. The relationship between agonist potency and AMPA receptor kinetics. Biophys J. 2006;91:1336–1346. doi: 10.1529/biophysj.106.084426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff EB. TARPs and the AMPA receptor trafficking paradox. Neuron. 2007;53:627–633. doi: 10.1016/j.neuron.2007.02.006. [DOI] [PubMed] [Google Scholar]