Abstract
Background
Administration of cocaine during adolescence alters neurotransmission and behavioral sensitization in adulthood, but its effect on the acquisition of fear memories and the development of emotion-based neuronal circuits is unknown.
Methods
We examined fear learning and anxiety-related behaviors in adult male rats that were subjected to binge cocaine treatment during adolescence. We furthermore conducted gene expression analyses of the amygdala 22 hours after the last cocaine injection to identify molecular patterns that might lead to altered emotional processing.
Results
Rats injected with cocaine during adolescence displayed less anxiety in adulthood than their vehicle-injected counterparts. In addition, cocaine-exposed animals were deficient in their ability to develop contextual fear responses. Cocaine administration caused transient gene expression changes in the Wnt signaling pathway, of axon guidance molecules, and of synaptic proteins, suggesting that cocaine perturbs dendritic structures and synapses in the amygdala. Phosphorylation of glycogen synthase kinase 3 beta, a kinase in the Wnt signaling pathway, was altered immediately following the binge cocaine paradigm and returned to normal levels 22 hours after the last cocaine injection.
Conclusion
Cocaine exposure during adolescence leads to molecular changes in the amygdala and decreases fear learning and fear responses in adulthood.
Keywords: cocaine, adolescence, amygdala, anxiety, Wnt signaling, axon guidance
Introduction
Cocaine is a psychostimulant drug that has long lasting behavioral and neurobiological consequences (1-4). While cocaine usage in teenagers has shown a downward trend in the last decade, many young Americans still experiment with drugs and/or alcohol during their formative adolescent years (5). All drugs of abuse target subcortical dopaminergic reward pathways and it is important to fully understand how stimulation of these pathways can affect a developing brain with immature circuitry (6).
Chronic drug use impairs cortical inhibition of impulsive actions and subcortical dopamine release in reward pathways, and promotes risk-taking and drug-seeking behaviors (7). Administration of cocaine during adolescence and subsequent activation of dopaminergic pathways may restructure brain anatomy, physiology and function, and lead to various behavioral deficits in adulthood. We administered cocaine in a binge administration paradigm during adolescence (8) and studied the behavioral response of cocaine-exposed rats to anxiety-evoking situations and fear learning.
The amygdalar nuclei form a circuit with the prefrontal cortex (PFC) and hippocampus that is responsible for detecting contextual and spatial information during fear conditioning, and for discriminating dangerous from innocuous stimuli (9). We demonstrated previously that binge-cocaine exposure during adolescence alters normal PFC function in adult rats (8). The PFC processes information from external stimuli that encode cues for drug-seeking behaviors, anxiety and fear learning (10). Prelimbic FC afferent connections to the amygdala are required for the consolidation and expression of learned fear behaviors, whereas infralimbic FC-amygdala connections modulate extinction behaviors (11). Corticoafferent projections of the basolateral amygdala to the PFC modify glutamatergic tone after chronic cocaine exposure (12). Efferents from the hippocampus to the amygdala are critical for the extinction of fear (13).
Recent evidence suggests that drugs of abuse modulate axon guidance molecules in adult rodents and cause synaptic remodeling that may reinforce the cycle of addiction (14-16). Here we provide evidence that cocaine exposure during adolescence results in gene expression changes in axon guidance and Wnt signaling pathways in the amygdala, and disrupts performance in amygdala-mediated fear learning and anxiety tasks.
Material and Methods
Animals:
All animals were housed and maintained in accordance with the policies of the Vanderbilt Animal Care and Use Committee. Male Sprague-Dawley rats (n=111; Charles River, Wilmington, MA) weighing approximately 50g (postnatal day 28 – P28) on arrival were housed in pairs in clear plastic cages. Food and water was available ad libitum except where noted. The colony room was maintained on a 12h:12h light-dark cycle (lights on at 6:00 am). Animals were handled daily for at least a week before initiation of experiments. All behavioral testing took place during the light cycle and in independent groups of rats.
Drug Administration Protocol:
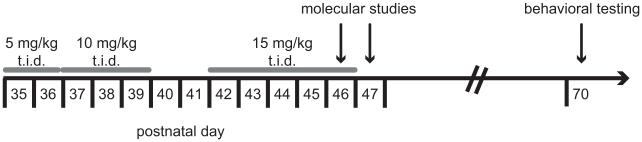
Cocaine hydrochloride (Sigma; St. Louis, MO) was dissolved in 0.9 % saline and administered intraperitoneally (i.p) at 5, 10, and 15 mg/kg, in a volume of 1μl/g body weight. 0.9% saline was used for all vehicle injections. Three injections were given per day, 1 hour apart, in accordance with the binge cocaine protocol previously developed by our group (8). Escalating doses of cocaine were administered within a 12-day period from P35 to P46, equivalent to the period from early adolescence to young adulthood in humans. From P35 to P36 rats received 5 mg/kg t.i.d. cocaine or vehicle. From P37 to P39, rats received 10 mg/kg t.i.d. cocaine or vehicle, and from P42-46, rats received 15 mg/kg t.i.d. cocaine or vehicle (figure 1).
Figure 1. Overview of experimental time courses.
Ascending doses of cocaine were administered to adolescent rats from P35 to P46. Rats received I.P. injections three times per day (t.i.d.), 1 hour apart, of 5 mg/kg (P35-P36), 10mg/kg (P37-P39), or 15 mg/kg (P42-P46) cocaine or saline vehicle. Subsets of rats were sacrificed at various timepoints after the last injection for molecular studies, or tested as adults in behavioral tasks that evaluated amygdalar and hippocampal functions.
Elevated Plus Maze:
All animals were habituated to the testing room for one hour. Adult male rats (P70) were placed on the elevated plus maze (Hamilton Kinder, Poway, CA) for 5 minutes and their movement was tracked using a ceiling-mounted video recording device and ANY-maze software (Stoelting, Wood Dale, IL). The maze was made of black plexiglass with 4-arms, 85 inches above the ground. The two closed arms had 40cm high walls while the open arms were without enclosing walls. All tests were carried out in red light. An animal was considered to be occupying a zone if 100% of its body was in that zone. Statistical significance was determined using the student’s t-test.
Contextual Fear Conditioning:
Video freeze software and operant chamber equipment (Med Associates, St. Albans, VT) were provided by the Vanderbilt Rat Neurobehavioral Core. On training day, adult male rats (P75) were habituated to the testing room for one hour prior to testing. Rats were placed in an operant chamber with natural scented oil as an odorant cue (The Body Shop; Littlehampton, UK) for a total of 7 minutes. After a two-minute acclimation period, animals were exposed to a 30 second, 5 kHz, 70 dB tone, the conditioned stimulus (CS), which coterminated with a 1 second, 0.5 mA foot-shock, the unconditioned stimulus (US). The tone and shock pairings were repeated three times and rats were removed 45 seconds after the last shock. 24 hours later, the animals were placed in the same chamber with scent, but without shock or auditory stimuli for 4 minutes. The animals’ fear response was recorded as the percentage of time the animal spent freezing. A freezing episode was defined as the absence of movement for at least three seconds. Repeated measures ANOVA for 1-minute bins, and post-hoc Tukey-Kramer HSD tests were used to determine statistical significance with JMP software (Cary, NC).
Open field:
Animals were placed for 60 minutes in automated locomotor activity chambers (Med Associates, St.Albans, VT) measuring 43.2 × 43.2 × 30.5 cm (length × width × height). Movement and activity was monitored by photocell beam breaks and analyzed with the Activity Monitor Software (Med Associates). The perimeter along the walls of the chamber was designated as the “exterior” zone, while the space in the center of the arena 7.5 cm from the wall was designated as the “interior” zone. Resting time refers to episodes in which the animal did not ambulate for at least 2 seconds. Statistical analysis was carried out with repeated measures ANOVA for 5-minute bins.
Hole Board Food Search and Exploration Tasks:
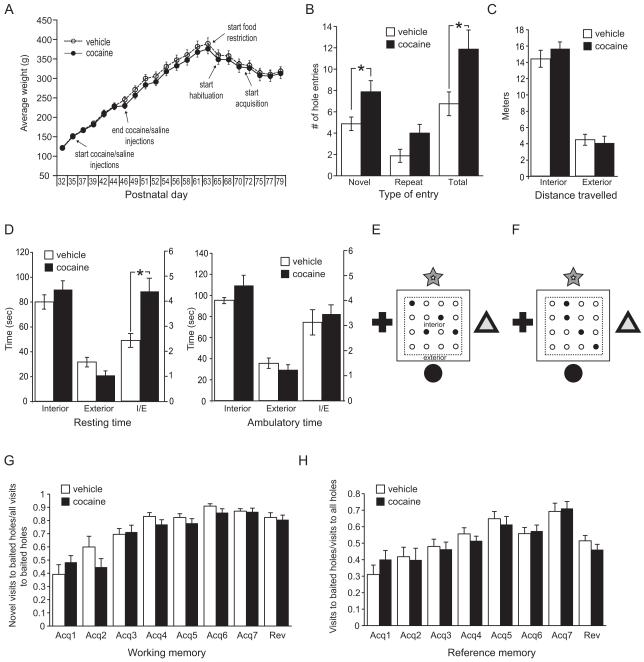
Experiments were carried out in sound-attenuated activity chambers (Med Associates) with pictures of easily identifiable geometrical shapes on each side (figure 3E,F). The chambers were fitted with floor inserts containing sixteen holes 1.25″ in diameter placed on 3″ centers (four rows of four equidistant holes) with an underlying food tray. The task was automated using infrared beams and software that logs hole entries. To increase the valence of food, rats were food restricted to 90% of their daily food intake measured over the previous five days, with their weights closely matched and monitored (figure 3A). Food restriction was initiated on P64 and maintained throughout the experiment. On P65, rats were placed in the chambers for 15 minutes with holes unbaited. Exploratory behavior was calculated by measuring the number of holes the animal entered (novel entries), the number of times the animal returned to the same hole (repeat entries), and the total number of entries into any hole during the first 5 minutes. On P66, P69 and P70 rats spent 15 minutes in the chambers and four holes were baited with sucrose pellets. Acquisition began on P71, with the same four holes baited. Acquisition consisted of blocks of 10 one-minute sessions per day. Rats were removed after one minute or when all four baited holes had been visited and all food was retrieved, whichever came first. Acquisition trials were carried out on P71, P72, P73, P76, P77, P78 and P79. A reversal trial was carried out on P80. For the reversal trial, four new holes were baited (figure 3F). The pertinent calculations of the software were working memory ratio (novel entries into baited holes / all entries into baited holes) and reference memory ratio (all entries into baited holes/total entries into all holes). Statistical significance was determined using a student’s t-test.
Figure 3. Exploration and novelty seeking is increased in adult rodents after binge cocaine administration in adolescence.
A: Weight curves and treatment schedules for animals undergoing the hole board tasks. B-D: Hole board exploration. E-H: Hole board food search task. B: Novel, repeat, and total entries into unbaited holes during a 5-minute novel exposure to the hole board chamber. C: Distance traveled in the interior and exterior part of the chamber during novel exposure. D: Resting (not ambulating for at least 2 seconds) and ambulatory time spent in the interior part of the chamber or the exterior part of the chamber during novel exposure. I/E is the ratio of interior time over exterior time. E, F: Hole board food search design with baited holes in black. High-contrast shapes were placed on the four walls of the chamber as reference points. Areas of internal and external measurements are shown. E: Holes baited during habituation and acquisition; F: Holes baited during the reversal trial. G: Working memory ratio (novel entries into baited holes / all entries into baited holes) in the hole board food search task. F: Reference memory ratio (all entries into baited holes/total entries into all holes) in the hole board food search task. All data average ± SEM; vehicle n=8; cocaine n=8.
Microarrays:
Rats were sacrificed 22 hours after the last cocaine injection on P47 by rapid decapitation. Brains were quickly removed and stored at −80°C until dissection on a freezing microtome. Amygdala was dissected in 2 mm round tissue punches at – 1.7 mm bregma and – 2.5 mm bregma (17), yielding two slices for the left and right side of brain. Each punch was 0.8 mm thick. The punches contained the central amygdaloid nucleus, basolateral amygdaloid nucleus, and basomedial amygdaloid nucleus with all their subdivisions. RNA was extracted with the RNagents kit (Promega, Madison, WI) according to company protocol. Double-stranded cDNA was synthesized with the help of an oligo-dT-T7 RNA polymerase primer and a cDNA synthesis kit (Invitrogen, Carlsbad, CA). Biotinylation was carried out with the Gene Chip Expression 3′ Amplification kit for IVT (Affymetrix,). Hybridization to the array and washing and staining were performed according to company protocol. Samples from individual subjects were hybridized to individual arrays. Only samples that reached commonly accepted quality control criteria defined by Affymetrix, dChip (18) and RMAExpress (19) were used in the analysis. Programs used for data collection included GeneChip Operating Software (GCOS, Expression Console; Affymetrix) for scanning and to obtain quality control data, and RMAExpress (19) for quantile normalization and background correction to compute expression values for all probe sets. The Database for Annotation, Visualization and Integrated Discovery (DAVID, v6.7), (20, 21) was used to group regulated genes into functional annotations provided by a number of databases.
QPCR:
Microarray findings were verified with quantitative PCR (QPCR) in technical as well as biological replicates. RNA was extracted using the PureLink Micro to Midi RNA extraction kit (Invitrogen). cDNA was synthesized from 0.3-1 μg of total RNA with the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA). A primer set for each gene was designed with the help of Primerblast (http://www.ncbi.nlm.nih.gov/tools/primer-blast) for amplicons between 150 and 250 base pairs. Melt curve analysis and polyacrylamide gel electrophoresis were used to confirm the specificity of each primer pair. QPCR reactions were carried out using a Stratagene ThermoCycler and iQ SYBR Green Supermix (Bio-Rad) or Brilliant II SYBR Green Supermix (Agilent Technologies, Santa Clara, CA). PCR cycling conditions were as follows: an initial step of 95°C for 10 min, followed by 40 cycles of 94°C for 15s, 55°C for 15s, 78°C for 15s. Data were collected at 78°C. Dilution curves were generated for each primer in every experiment and on every plate by diluting cDNA from a control sample 1:4 three times, yielding a dilution series of 1.00, 0.25, and 0.0625, and .015. All samples were examined in duplicate. Values were normalized to the internal controls ß-actin, alpha tubulin, 18s RNA, and general transcription factor IIB, which were not regulated by the drug paradigm. The list of primer pairs is shown in Table S1 in the Supplement.
Western Blotting:
Groups of animals were sacrificed on P46 20 minutes after the first injection and 20 minutes after the final (third) injection, and on P47 22 hours after the final injection. Brains were removed and dissected as described above. Tissue was sonicated in Laemmli buffer, heated to 80°C for 10 minutes and proteins were electrophoresed on 10-20% gradient Tris-Glycine gels (Invitrogen). Proteins were transferred to PVDF membranes and blocked with animal-free blocking solution (Vector Laboratories, Burlingame, CA). Primary antibodies were diluted in blocking solution and incubated with membranes overnight at 4°C. The following antibodies were used: anti-actin (Sigma, St.Louis, MO), anti-phospho GSK3 beta (Serine 9), and anti-total GSK3 beta, (Cell Signaling, Danvers, MA). Membranes were washed in TBS-T and incubated for an hour at room temperature with HRP-conjugated secondary antibodies (Vector Laboratories) prepared in blocking solution. Blots were immersed in chemiluminescent reagents (Pierce, Rockford, IL) and exposed and analyzed on the KODAK Imaging Station IS440. Statistical significance was determined using a student’s t-test.
Results
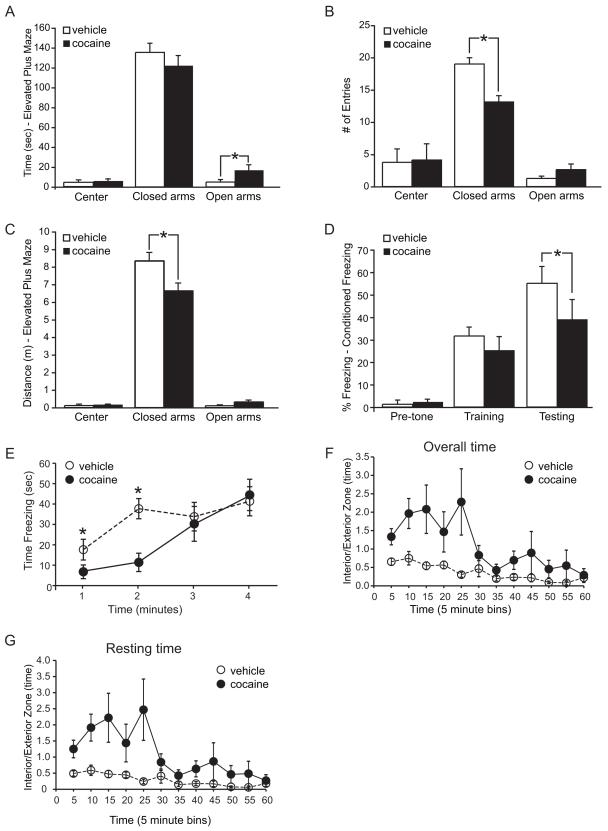
In the elevated plus maze, cocaine-exposed rats spent significantly more time in the open arm than vehicle–treated rats (figure 2A). Rats that received cocaine during adolescence had fewer entries into the closed arms and less distance traveled inside the closed arms than the vehicle-treated group (figure 2B,C). No difference was observed in the center and open arms in either distance traveled or number of entries.
Figure 2. Adolescent binge cocaine exposure disrupts fear learning and anxiety behaviors in adult rats.
A,B,C: Behavior in the elevated plus maze. A: Total time spent in center, closed, and open arms shows cocaine-exposed rats spent more time in the open arms than vehicle treated animals. B: Total number of entries into each zone of the maze showed cocaine-exposed rats entered the closed arms less frequently. C: Distance traveled in each zone showed cocaine-exposed animals traveled less in the closed arms. D,E: Results of the conditioned freezing paradigm. D: Percent freezing before and during training, and on testing day. ‘Pre-tone’ measures the first 190 seconds the animal was placed in the chamber prior to shock or tone. ‘Training’ refers to the pre- and post-tone, as well as the conditioning phase where animals received the conditioned (tone) and unconditioned (shock) stimuli. ‘Testing’ is the average measure of freezing over 4 minutes, 24 hours after conditioning. E: Freezing time during the testing period of the contextual fear conditioning paradigm. Post-hoc analysis revealed significant decreases in freezing behavior at 1 and 2 minutes. F,G: Behavior in the open field. Zone time was recorded for 60 minutes and separated into 5-minute bins. White circle, vehicle; black circle, cocaine. Shown is the ratio of time spent in the interior part of the chamber over time spent in the exterior part of the chamber (I/E) for all observed movements (F), and time spent resting, in which the animal did not ambulate for at least 2 seconds (G). All data average ± SEM; Elevated Plus Maze vehicle n= 8; cocaine n= 9; contextual fear conditioning vehicle n= 10; cocaine n= 9; open field vehicle n=6; cocaine n=8.
To examine learned fear we used the conditioned freezing paradigm. The amygdala, the brain area most closely associated with fear and anxiety, evolved with the olfactory system, and in the rat receives dense projections from the olfactory bulb to alert the animal to scents associated with danger (22, 23). Therefore, we used scented oils in the operant chambers during fear conditioning and on the testing day. While no difference in freezing was observed on the day of training, the cocaine-exposed group froze less on the testing day than the vehicle group (figure 2D). A time by group interaction was found (F(3,14)=4.89; p= 0.016), and post-hoc analysis confirmed significant decreases in freezing behavior at minute 1 (vehicle=29.4 ± 8.4%; cocaine=11.3 ± 5.6%) and minute 2 (vehicle=62.9 ± 8.2%; cocaine=19.0 ± 7.5%) (figure 2E). Thus, cocaine exposed animals did not develop the same contextual fear response as the vehicle - exposed rats.
Movement and behavior of rats in the open field area were monitored for a total of 60 minutes. The cocaine-exposed rats spent a greater proportion of time in the interior zone than vehicle - exposed rats (main effect of treatment, F(1,11)=8.8, p=0.01; figure 2F). This was not just restricted to a quick crossing of the interior, but was also observed in the resting time measures (F(1,11)=10.9, p=0.01; figure 2G). Overall distance traveled was not different between the groups (cocaine: 5901 ± 521; vehicle 6724 ± 659; cm average ± SEM).
In the hole board exploration task, exploration and anxiety were measured by the number of novel entries, repeat entries, and total entries with the snout into a hole during a 5-minute novel exposure to the 16-hole chamber. Cocaine-exposed rats entered the chamber’s holes at a higher frequency than the saline-exposed rats, although holes were not baited (figure 3B). Distance traveled and time spent ambulating or resting in the interior part of the chamber that included the holes, and the residual perimeter next to the walls of the chamber, were comparable for both groups (figure 3C, D). However, cocaine-exposed rats had a higher ratio of time spent resting in the interior part of the chamber/residual part of the chamber (figure 3D).
The hole board food search task and the Morris water maze task were used to assess spatial learning and memory function. The hole board food search task measures working memory and reference memory. The working memory ratios and reference memory ratios were between 30-50% on the first day of acquisition (figure 3G,H), after three days of habituation. Both groups of rats improved in their performance over time and no significant differences were seen in any aspect of the task, indicating that the cocaine-exposed rats learned the task as well as the vehicle-treated rats. Although upon novel exposure to the operant chamber (hole board exploration) differences in hole entries were observed between the groups, no differences were seen on the subsequent three days of habituation when holes were baited with sucrose pellets. Working and reference memory trials were therefore not influenced by different levels of anxiety to enter the holes. In a reversal test on the 8th day, no significant differences were observed between the groups. The reversal showed that rats had learned to not re-visit the baited holes, as their working memory ratio was similar to the last acquisition day (figure 3G). The decrease in reference memory ratio upon reversal shows that the rats first visited the previously baited, now unbaited holes, before checking the other holes (figure 3H). The decrease in reference memory ratio verified that the rats were using their memory to find the sucrose pellets.
The Morris water maze task was carried out in morning and afternoon sessions on five consecutive days (Figure S1 in the Supplement). On the afternoon sessions of days 4 and 5, different reversals of the platform location were introduced (see Methods in the Supplement). No difference in performance was observed between the groups.
To determine if a molecular pattern was associated with impaired fear learning and anxiety, we conducted gene expression microarray assays on the amygdala from a subset of rats killed 22 hours after the last injection. Genes that were changed by less than 10% were excluded as well as those that were considered below detection level in 40% or more of all samples. Significance was determined with a student’s t-test and only genes that had a p value of less than 0.05 were considered for further analysis. We used the DAVID database to examine annotation clusters that identify common pathways in a list of regulated genes (20, 21). Because of redundancy in annotation records, we used annotation clustering to identify enriched gene groups in multiple categorical classifications.
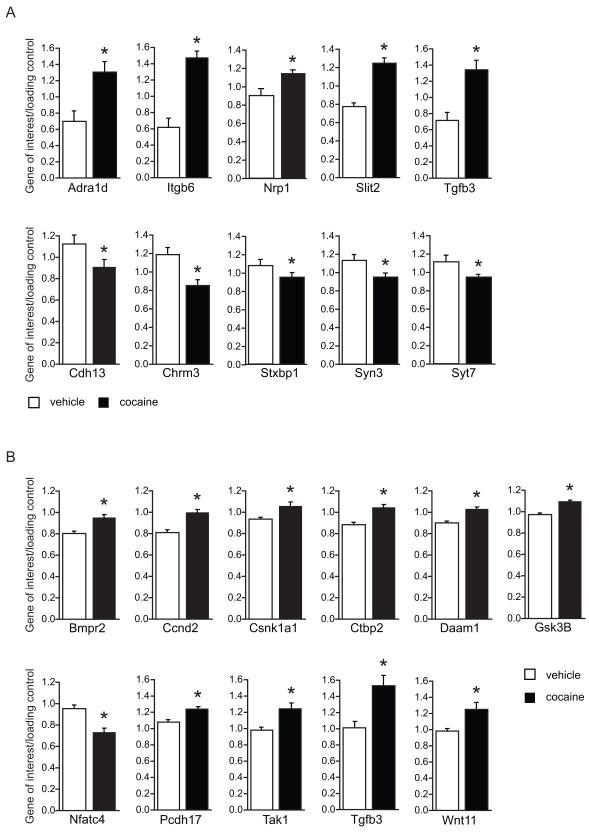
A group of downregulated genes was identified with an enrichment score of 3.37, which contained genes in pathways termed “synapse”, “synapse part”, “plasma membrane”, and “cell junction”. Members of this group are shown in table 1. No particular pathway was identified in the group of upregulated genes. However, in the entire group of regulated genes several pathways were significantly affected by cocaine exposure. These pathways were related to development of synapse structure and growth and included “axon guidance” (table 2) and “Wnt signaling” (table 3). Quantitative PCR (QPCR) on a subset of genes confirmed the gene array data (figure 4). The list of primer sequences is provided in Figure S1 in the Supplement. A technical replicate was performed for several genes in each pathway (figure 4A). Additional analysis in another cohort was performed to verify regulation of the Wnt signaling pathway (figure 4B). The altered expression of Wnt5a and Wnt7a could not be verified in the QPCR analysis and only showed trends for regulation (data not shown). However, Wnt11, a gene that had a low present call in the microarray analysis, was found to be significantly elevated in cocaine exposed animals (figure 4B).
Table 1. Adolescent cocaine exposure leads to downregulation of plasma membrane and synaptic genes in the amygdala.
Microarray analysis revealed a group of 36 genes classified as “synaptic” or “plasma membrane part” that were downregulated in animals that received cocaine during adolescence. Shown are the probe set IDs, gene name, accession number, fold changes, and p-value (based on log2-transformed data). Vehicle n=6; cocaine n=7.
| Probe ID | Gene name | Accession | Fold change |
P value |
|---|---|---|---|---|
| 1370121_at | Add1: adducin 1 (alpha) | NM_016990 | −1.19 | 4.27E-02 |
| 1368534_at | Adra1d: adrenergic receptor, alpha 1d | NM_024483 | 1.71 | 4.62E-02 |
| 1370621_at | Cd3z: CD3 antigen, zeta polypeptide | D13555 | −1.13 | 3.10E-02 |
| 1369559_a_at | Cd47: CD47 antigen (Rh-related antigen,
integrin-associated signal transducer) |
NM_019195 | −1.18 | 2.50E-02 |
| 1369025_at | Cd5: CD5 antigen | NM_019295 | −1.11 | 3.28E-02 |
| 1373102_at | Cdh13: cadherin 13 | BI282750 | −1.19 | 6.45E-03 |
| 1369112_at | Chrm3: cholinergic receptor, muscarinic 3 | M18088 | −1.17 | 4.30E-02 |
| 1373067_at | Ctnnb1: Catenin (cadherin associated protein), beta 1 | AI102738 | −1.18 | 4.84E-02 |
| 1370625_at | Faim2: Fas apoptotic inhibitory molecule 2 | AF044201 | −1.21 | 3.06E-02 |
| 1398246_s_at | Fcgr3: Fc receptor, IgG, low affinity III | NM_053843 | −1.15 | 4.41E-02 |
| 1369267_at | Gabrg3: gamma-aminobutyric acid A receptor, gamma 3 | NM_024370 | −1.25 | 1.85E-02 |
| 1370590_at | Gpsm1: G-protein signalling modulator 1 (AGS3-like) | AF107723 | −1.17 | 4.08E-02 |
| 1377546_at | Gria4: Glutamate receptor, ionotropic, 4 | BF397279 | −1.31 | 1.48E-02 |
| 1371180_a_at | Grm1: glutamate receptor, metabotropic 1 | Y18810 | −1.16 | 4.15E-02 |
| 1378625_at | Grm8: Glutamate receptor, metabotropic 8 | BF392502 | −1.19 | 2.01E-03 |
| 1368783_at | Icos: inducible T-cell co-stimulator | NM_022610 | −1.13 | 4.78E-02 |
| 1368979_at | Kalrn: kalirin, RhoGEF kinase | NM_032062 | −1.11 | 3.53E-02 |
| 1370078_at | Lin7b: lin-7 homolog b (C. elegans) | NM_021758 | −1.13 | 3.10E-03 |
| 1384190_at | Mapk8ip3: mitogen-activated protein kinase 8 interacting protein 3 | BF553848 | −1.17 | 2.53E-02 |
| 1395927_at | Pdzk1: PDZ domain containing 1 | BE116199 | −1.12 | 3.12E-02 |
| 1388966_at | P2ry1: purinergic receptor P2Y, G-protein coupled 2 | BM388250 | −1.13 | 4.61E-02 |
| 1393207_at | Rab35: RAB35, member RAS oncogene family | BF566116 | −1.12 | 4.31E-02 |
| 1369338_at | Robo1: roundabout homolog 1 (Drosophila) | NM_022188 | −1.17 | 4.98E-02 |
| 1369054_at | Rph3a: rabphilin 3A homolog (mouse) | NM_133518 | −1.27 | 2.29E-02 |
| 1368907_at | Scamp5: secretory carrier membrane protein 5 | NM_031726 | −1.14 | 4.45E-03 |
| 1368445_at | Shank1: SH3 and multiple ankyrin repeat domains 1 | BE105448 | −1.17 | 1.32E-02 |
| 1369715_at | Slc6a11: solute carrier family 6, member 11; GABA transporter | M95763 | −1.3 | 5.00E-02 |
| 1387693_a_at | Slc6a9: solute carrier family 6, member 9; glycine transporter | M95413 | −1.3 | 2.49E-02 |
| 1368896_at | Smad7: MAD homolog 7 (Drosophila) | NM_030858 | −1.14 | 1.47E-02 |
| 1381263_at | Slc27a4: solute carrier family 27 (fatty acid transporter), member 4 | H33747 | −1.15 | 4.24E-02 |
| 1370840_at | Stxbp1: syntaxin binding protein 1 | NM_013038 | −1.11 | 7.16E-03 |
| 1369423_at | Syn3: synapsin III | NM_017109 | −1.26 | 1.45E-02 |
| 1387527_at | Syngr1: synaptogyrin 1 | NM_019166 | −1.17 | 4.07E-02 |
| 1370514_a_at | Syt7: synaptotagmin VII | AI713274 | −1.13 | 1.48E-02 |
| 1378407_at | Trim9: tripartite motif-containing 9 | BF401415 | −1.23 | 2.07E-02 |
| 1369330_at | Unc13a: unc-13 homolog A (C. elegans) | NM_022861 | −1.12 | 1.74E-02 |
| 1384158_at | Unc13c: unc-13 homolog C (C. elegans) | AW522416 | −1.2 | 4.86E-02 |
| 1387716_at | Utrn: utrophin | NM_013070 | −1.32 | 2.47E-02 |
Table 2. Adolescent cocaine exposure alters the expression of axon guidance genes in the amygdala.
Regulated axon pathway genes are listed with their respective probe set IDs, gene name, and accession number. Vehicle n=6; cocaine n=7.
| Probe ID | Gene name | Accession | Fold change | P value |
|---|---|---|---|---|
| 1380330_at | Ablim2: actin binding LIM protein family, member 2 | BI288159 | −1.15 | 4.52E-02 |
| 1382389_at | Arhgef5: Rho guanine nucleotide exchange factor 5 | AI179755 | 1.21 | 2.93E-02 |
| 1389244_x_at | Cxcr4: chemokine (C-X-C motif) receptor 4 | AA945737 | 1.23 | 7.72E-03 |
| 1369476_at | Efnb1: ephrin B1 | NM_017089 | −1.13 | 3.11E-02 |
| 1378997_at | Ephb6: Eph receptor B6 | BM391684 | 1.19 | 3.31E-02 |
| 1370267_at | Gsk3b: glycogen synthase kinase 3 beta | BF287444 | 1.44 | 2.64E-02 |
| 1392582_at | Lrrc4c_predicted: leucine rich repeat containing 4C | AA819053 | 4.3 | 2.45E-03 |
| 1372032_at | neuroblastoma ras oncogene | AA851914 | 1.14 | 7.86E-03 |
| 1390573_a_at | Nfatc4: nuclear factor of activated T-cells, calcineurin-dependent 4 | BG377358 | −1.11 | 1.91E-02 |
| 1370570_at | Nrp1: neuropilin 1 | AF016296 | 1.29 | 2.59E-02 |
| 1396426_at | p21 protein (Cdc42/Rac)-activated kinase 4 | BF404920 | −1.14 | 3.02E-03 |
| 1369338_at | Robo: roundabout homolog 1 (Drosophila) | NM_022188 | −1.17 | 4.98E-02 |
| 1378389_at | similar to nuclear factor of activated T-cells, calcineurin-dependent 4 | BM385157 | 1.18 | 2.33E-02 |
| 1395986_at | Slit2: slit homolog 2 (Drosophila) | BF391439 | 1.33 | 5.19E-03 |
| 1377651_at | Trio: Triple functional domain (PTPRF interacting) | AI577848 | −1.37 | 1.70E-02 |
Table 3. Adolescent cocaine exposure alters the expression of Wnt signaling pathway genes in the amygdala.
Regulated Wnt signaling pathway genes are listed with their respective probe set IDs, gene name, and accession number. Vehicle n=6; cocaine n=7.
| Probe ID | Gene name | Accession | Fold change |
P value |
|---|---|---|---|---|
| 1376843_at | Bmpr2: bone morphogenic protein receptor, type II | BE118651 | 1.68 | 5.03E-03 |
| 1375353_at | Cables1: Cdk5 and Abl enzyme substrate 1 | BI296696 | 1.22 | 4.78E-02 |
| 1373089_at | Cdh3: cadherin 3, type 1, P-cadherin | AI010270 | 1.4 | 2.13E-02 |
| 1375619_at | Cdh8: Cadherin 8 | BF417982 | −1.18 | 3.60E-02 |
| 1375719_s_at | Cdh13: cadherin 13 | BI282750 | −1.32 | 1.68E-02 |
| 1372299_at | Cdkn1c: cyclin-dependent kinase inhibitor 1C (P57) | AI013919 | 1.53 | 7.78E-03 |
| 1372685_at | Cdkn3: cyclin-dependent kinase inhibitor 3 | BE113362 | 1.19 | 3.30E-03 |
| 1389759_at | Celsr1: cadherin EGF LAG seven-pass G-type receptor 1 | AW433901 | 1.25 | 3.47E-02 |
| 1383946_at | Cldn1: claudin 1 | AI137640 | 1.53 | 4.93E-02 |
| 1385852_at | Crebbp: CREB binding protein | BF566908 | 1.3 | 4.53E-02 |
| 1394689_at | Csnk1a1: Casein kinase 1, alpha 1 | BE117217 | 1.23 | 7.57E-03 |
| 1387113_at | Ctbp2: C-terminal binding protein 2 | NM_053335 | 1.25 | 3.88E-02 |
| 1373067_at | Ctnnb1: Catenin (cadherin associated protein), beta 1 | AI102738 | −1.18 | 4.84E-02 |
| 1375266_at | Ccnd2: cyclin D2 | BG380633 | 1.21 | 3.52E-02 |
| 1374480_at | Daam1: dishevelled associated activator of morphogenesis 1 | BE107255 | 1.12 | 2.74E-02 |
| 1374530_at | Fzd7: frizzled homolog 7 (Drosophila) | AI010048 | 1.35 | 3.95E-02 |
| 1368337_at | Glycam1: glycosylation dependent cell adhesion molecule 1 | NM_012794 | 1.72 | 1.74E-02 |
| 1370267_at | Gsk3b: glycogen synthase kinase 3 beta | BF287444 | 1.44 | 2.64E-02 |
| 1367571_a_at | Igf2: insulin-like growth factor 2 | NM_031511 | 1.47 | 4.38E-02 |
| 1367648_at | Igfbp2: insulin-like growth factor binding protein 2 | NM_013122 | 1.68 | 4.48E-02 |
| 1382439_at | Itgb6: integrin, beta 6 | AI070686 | 2.09 | 1.74E-02 |
| 1393138_at | Jund: Jun D proto-oncogene | BE329377 | −1.11 | 4.77E-02 |
| 1388155_at | Krt1-18: keratin complex 1, acidic, gene 18 | BI286012 | 1.49 | 4.43E-02 |
| 1371530_at | Krt2-8: keratin complex 2, basic, gene 8 | BF281337 | 1.81 | 2.16E-02 |
| 1399075_at | Map3k7: mitogen activated protein kinase
kinase kinase 7; Tak1: Tgf beta activated kinase 1 |
AI146037 | 1.12 | 8.56E-03 |
| 1390573_a_at | Nfatc4: nuclear factor of activated
T-cells, cytoplasmic, calcineurin- dependent 4 |
BG377358 | −1.11 | 1.91E-02 |
| 1393144_at | Nmi: N-myc (and STAT) interactor | BM388202 | 1.14 | 2.44E-02 |
| 1395408_at | Nostrin: nitric oxide synthase trafficker | AI058709 | 1.13 | 3.63E-02 |
| 1377676_at | Nucks: nuclear ubiquitous casein kinase and
cyclin-dependent kinase substrate |
AI599187 | 1.13 | 3.37E-02 |
| 1384509_s_at | Pcdh17: protocadherin 17 | BF558981 | 1.49 | 4.84E-02 |
| 1370490_at | Pcdh3: protocadherin 3 | L43592 | −1.35 | 7.82E-03 |
| 1370950_at | Ppap2b: phosphatidic acid phosphatase type 2B subunit B delta | AW253995 | 1.29 | 2.70E-02 |
| 1395502_at | Ppp2r5d: protein phosphatase 2, regulatory | BF557865 | −1.11 | 3.60E-02 |
| 1382274_at | Rarres1: retinoic acid receptor responder 1 | AA819288 | −1.26 | 1.08E-02 |
| 1378389_at | similar to nuclear factor of activated T-cells, calcineurin-dependent 1 | BM385157 | 1.18 | 2.33E-02 |
| 1391557_at | Sox15: SRY (sex determining region Y)-box 15 | AI009685 | −1.14 | 2.96E-02 |
| 1367859_at | Tgfb3: transforming growth factor, beta 3 | NM_013174 | 1.33 | 4.50E-02 |
| 1368359_a_at | Vgf: VGF nerve growth factor inducible | NM_030997 | −1.21 | 3.29E-02 |
| 1382375_at | Wnt5a: Wingless-type MMTV integration site 5A | AI639128 | −1.27 | 4.87E-02 |
| 1380958_at | Wnt7a: wingless-related MMTV integration site 7A | BG671935 | −1.18 | 2.61E-03 |
Figure 4. Adolescent cocaine exposure affects the expression of synaptic and developmental genes in the amygdala.
Quantitative PCR verification of gene expression changes observed in the microarray analyses. A: Technical replicate performed on the original cohort of animals from microarray studies confirms the microarray results. B: Biological replicate performed on an additional cohort of animals confirms regulation of genes of the Wnt signaling pathway. Slit2 (p<=0.01; not shown) and Tgfb3 were also examined to verify findings in the original cohort. Values were normalized to the control genes beta actin, alpha tubulin, 18s RNA, and GtfIIB. For a complete list of gene names, please refer to tables 1-3. Statistical significance was determined with a student’s t-test, * p<=0.05. Average + SEM; technical replicate vehicle n=6; cocaine n=6; biological replicate vehicle n=10; cocaine n=8.
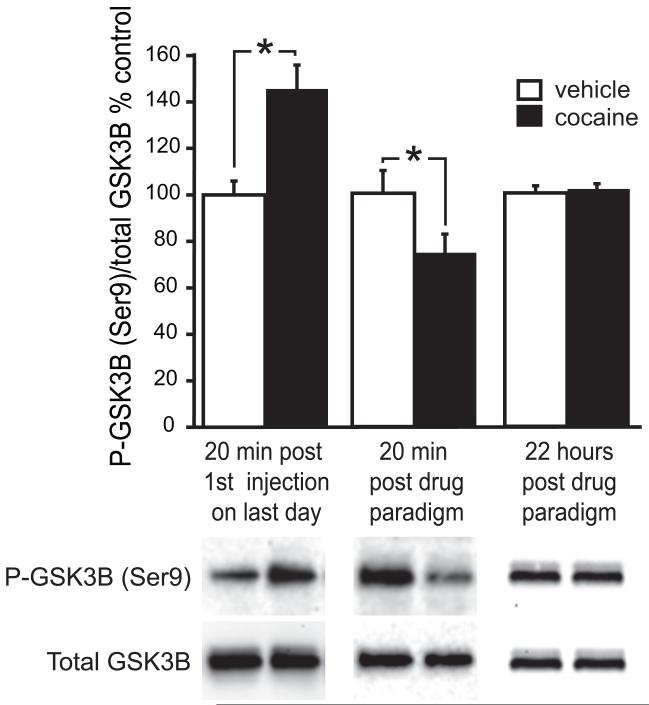
The microarray analyses revealed an increase in glycogen synthase kinase 3 beta (GSK3B) mRNA levels in the amygdala 22 hours after the last injection (table 3). To determine if cocaine regulates GSK3B activity, we measured total GSK3B protein as well as levels of GSK3B phosphorylated at serine residue 9, in the amygdala of rats that were sacrificed 20 minutes after the first or third injection on the last day of the dosing paradigm, or 22 hours after the last injection. Phosphorylation of GSK3B at serine residue 9 was increased after the first injection but decreased after the third injection. No changes were seen in the total amount of GSK3B protein (figure 5). No changes in phosphorylated or total GSK3B were seen 22 hours after the last injection, indicating that GSK3B phosphorylation had returned to normal levels.
Figure 5. Cocaine administration during adolescence regulates GSK3B phosphorylation patterns in the amygdala.
Representative western blots of total GSK3B protein and phosphorylated GSK3B in amygdala tissue punches from animals sacrificed 20 minutes after the first injection on the last day of the paradigm (left bar graphs), 20 minutes after the last injection (center bar graphs), or 22 hours after the last injection (right bar graphs). Phosphorylated GSK3B protein was normalized to total GSK3B protein. Percent change in intensity relative to vehicle samples is graphed. Average + SEM; for blots of rats sacrificed 20 minutes after the first or last injection, vehicle n=7; cocaine n=7; for blots of rats sacrificed 22 hours after the injection paradigm, vehicle n=6; cocaine n=6. * p<=0.05.
Discussion:
Exposure to drugs of abuse during adolescence could affect the developmental trajectory of the brain with lasting consequences for structure, function and behavior. Here we provide evidence that adolescent cocaine abuse has deleterious behavioral effects in adulthood, well after cessation of drug use. During cocaine exposure, protein phosphorylation and gene expression patterns were altered, as measured on the last day of cocaine treatment. This interference with normal molecular processes led to altered behavior in adulthood. A previous study in the PFC showed that the changes in gene expression patterns are mostly transient, while the behavioral consequences are long-lasting (8). Since we used the same experimental paradigm, it is reasonable to assume that the molecular patterns in the amygdala normalize as well, an assumption supported by the fact that in the present study GSK3B phosphorylation was normalized 22 hours after the last cocaine injection. However, transient changes in gene and protein expression can interfere with the normal program of brain development and have permanent consequences beyond that age period.
Cocaine exposure during adolescence decreased guarded behaviors and fear learning in adult rats. Although fear learning was abnormal, learning and memory paradigms not related to fear were normal. Rats exposed to cocaine during adolescence were more likely to enter into the open arm of an elevated plus maze, or the less protected areas of the open field, and to inspect the holes in the hole board without hesitation. These behavioral changes indicated that cocaine exposure in adolescence reduces cautious behavior in adulthood.
Increased impulsivity and risk-taking in human cocaine users are well known (24, 25), though it is not known if this is a pre-existing trait leading to drug use, or a consequence of drug use. Here we used a rat model with no pre-existing traits and show that drug exposure during adolescence decreases cautious behavior in adulthood. Thus, drug use during adolescence can lead to long-term adverse behaviors. Although it is presently not known if similar adaptations can occur during adult cocaine use, it should not detract from the importance of the long-lasting effects of adolescent cocaine use. Onset of drug use during adolescence is a significant predictor of the subsequent development of addiction (26), and as we show here, as well as in our previous study (8), alters behavior in adulthood.
The behavioral changes observed indicate that cocaine affects amygdalar physiology. Therefore we conducted gene expression analyses to identify groups of genes or pathways in the amygdala that are altered immediately after cocaine exposure. Groups of genes involved in synaptic function, axon guidance and Wnt signaling, were significantly changed in the amygdala of cocaine-exposed rats. Changes of axon guidance molecules by psychostimulants have also been reported in other brain areas but this study is the first to report cocaine-induced alterations in axon guidance molecules in the amygdala (14-16, 27-29). These systems are dynamically regulated by cocaine and a given gene or protein may be initially increased and subsequently repressed, or vice versa. Thus, although the direction of regulation might be dependent on the timing of tissue harvest after the final cocaine injection, the fact that cocaine affects these transcripts is a crucial observation.
Axon guidance and Wnt signaling are important developmental processes that modulate the correct target selection of synapses and dendritic structures, as well as the patterning of neurotransmission and overall neuronal circuit formation (30, 31). In the adult striatum, GSK3B regulates the heightened locomotor activity and sensitivity after cocaine administration (32). Decreased phosphorylation of GSK3B at serine residue 9 in the amygdala was seen previously in the adult rodent after cocaine exposure (33). After the first injection on the last day of our paradigm, GSK3B was hyperphosphorylated but after the third injection phosphorylation was decreased, presumably through the activation of a feed-back mechanism. The molecular data indicate that cocaine dysregulates the signaling pathways associated with GSK3B in the amygdala. GSK3B regulates the activity of beta catenin, a transcription factor that promotes the expression of many target genes, including receptors, cell adhesion molecules, cell cycle regulators, growth factors, and cytoskeletal proteins (figure 6). The downregulation of synaptic proteins, together with the alterations in axon guidance and Wnt pathway genes, points to a reorganization of synapses and dendritic structures in the amygdala by cocaine, which might be the reason for the long-term behavioral changes we observed.
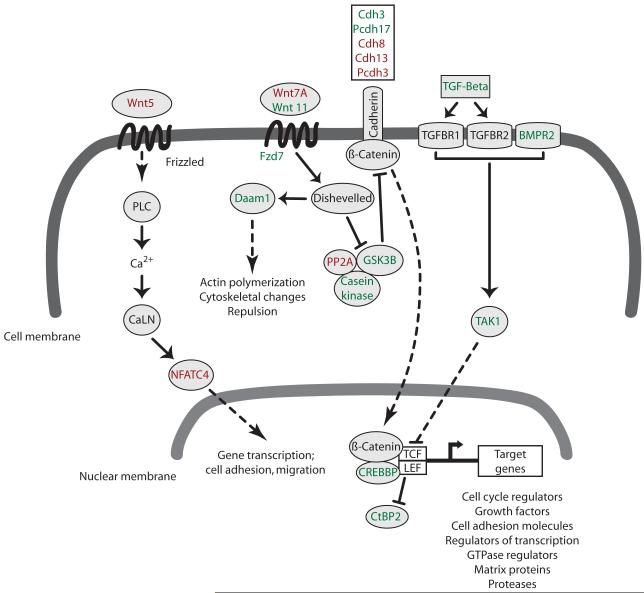
Figure 6. Schematic representation of cocaine-induced amygdalar gene changes in the Wnt pathway.
Adolescent cocaine exposure regulated the mRNA expression of many genes involved in Wnt signaling pathways. These signaling pathways can alter the morphology of the actin cytoskeleton and participate in the remodeling of synaptic and dendritic structures following exposure to drugs of abuse. Signaling by Wnt molecules leads to the activation of transcription factors and target genes. In the canonical Wnt pathway, dishevelled inhibits a kinase-associated scaffolding complex (GSK3B, casein kinase, PP2A) that normally facilitates the degradation of beta catenin. Free beta catenin translocates to the nucleus where it activates the transcription of Wnt target genes. Dishevelled, as well as axon guidance molecules, also induce changes in actin polymerization and cytoskeletal proteins via the activation of Rho GTPases. The calcium-mediated Wnt signaling pathway is controlled by the Wnt5 molecules and activates transcription of cell surface proteins and cell adhesion molecules. Shown in green are upregulated genes and shown in red are downregulated genes. Solid arrows show direct interactions and dashed arrows denote signaling processes with intermediates not shown. Abbreviations: CaLN, calmodulin; LEF, lymphoid enhanced binding factor; PLC, phospholipase C; TAK1, Tgf beta activated kinase 1; TCF, t-cell transcription factor; TGFBR1/2, transforming growth factor beta receptor 1/2. For a detailed list of all other genes names see table 3.
Psychostimulants like amphetamine and cocaine prevent the clearance of dopamine and other biogenic amines from the synapse and potentiate their signaling (34). Dopamine as well as serotonin modulate developmental processes such as proliferation, cell migration, and differentiation (35-40). From the present study we conclude that during adolescence aberrant monoamine signaling can affect developmental processes that pattern connectivity in the amygdala, with lasting effects on fear, anxiety, and emotion. The transient exposure to cocaine during adolescence could result in “mis-wiring” of emotional circuitry and fear recognition systems, leading to detrimental behaviors later in life.
Decreased anxiety could be perceived as a favorable characteristic, but recognition of danger and judicious behavior is imperative for the survival of a species (41). Since healthy levels of anxiety mediate cautious behavior in novel or dangerous situations, we conclude that the decreased caution after adolescent cocaine exposure could lead to increased “risk-taking” in adulthood.
Supplementary Material
Acknowledgements:
The work was supported by DA19152 and T32MH064913. Graham Goenne, Nicole Herring, Ph.D. and Andrew Luksik assisted in the drug injection paradigms and Randy Barrett, Ph.D., assisted with the behavioral experiments. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding institutes or the National Institutes of Health.
Footnotes
Disclosure/conflicts of interest: The authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 2.Nestler EJ. The neurobiology of cocaine addiction. Sci Pract Perspect. 2005;3:4–10. doi: 10.1151/spp05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lidow MS. Consequences of prenatal cocaine exposure in nonhuman primates. Brain Res Dev Brain Res. 2003;147:23–36. doi: 10.1016/j.devbrainres.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Marin MT, Cruz FC, Planeta CS. Cocaine-induced behavioral sensitization in adolescent rats endures until adulthood: lack of association with GluR1 and NR1 glutamate receptor subunits and tyrosine hydroxylase. Pharmacol Biochem Behav. 2008;91:109–114. doi: 10.1016/j.pbb.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 5.NIDA InfoFacts . NIDA InfoFacts: High School and Youth Trends. National Institute of Drug Abuse; 2010. Monitoring the Future Study: Trends in Prevalence of Various Drugs for 8th-Graders, 10th-Graders, and 12th-Graders. [Google Scholar]
- 6.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 8.Black YD, Maclaren FR, Naydenov AV, Carlezon WA, Jr., Baxter MG, Konradi C. Altered attention and prefrontal cortex gene expression in rats after binge-like exposure to cocaine during adolescence. J Neurosci. 2006;26:9656–9665. doi: 10.1523/JNEUROSCI.2391-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs RA, Bell GH, Ramirez DR, Eaddy JL, Su ZI. Basolateral amygdala involvement in memory reconsolidation processes that facilitate drug context-induced cocaine seeking. Eur J Neurosci. 2009;30:889–900. doi: 10.1111/j.1460-9568.2009.06888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biological Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 11.Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orozco-Cabal L, Liu J, Pollandt S, Schmidt K, Shinnick-Gallagher P, Gallagher JP. Dopamine and corticotropin-releasing factor synergistically alter basolateral amygdala-to-medial prefrontal cortex synaptic transmission: functional switch after chronic cocaine administration. J Neurosci. 2008;28:529–542. doi: 10.1523/JNEUROSCI.2666-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yetnikoff L, Labelle-Dumais C, Flores C. Regulation of netrin-1 receptors by amphetamine in the adult brain. Neuroscience. 2007;150:764–773. doi: 10.1016/j.neuroscience.2007.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halladay AK, Yue Y, Michna L, Widmer DA, Wagner GC, Zhou R. Regulation of EphB1 expression by dopamine signaling. Brain Res Mol Brain Res. 2000;85:171–178. doi: 10.1016/s0169-328x(00)00249-7. [DOI] [PubMed] [Google Scholar]
- 16.Bahi A, Dreyer JL. Cocaine-induced expression changes of axon guidance molecules in the adult rat brain. Mol Cell Neurosci. 2005;28:275–291. doi: 10.1016/j.mcn.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Paxinos G, Watson C. stereotaxic coordinates. 4th ed. Academic Press; San Diego: 1998. The rat brain. [Google Scholar]
- 18.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 20.Dennis G, Jr., Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 21.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 22.Moreno N, Gonzalez A. Evolution of the amygdaloid complex in vertebrates, with special reference to the anamnio-amniotic transition. J Anat. 2007;211:151–163. doi: 10.1111/j.1469-7580.2007.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 24.Bornovalova MA, Daughters SB, Hernandez GD, Richards JB, Lejuez CW. Differences in impulsivity and risk-taking propensity between primary users of crack cocaine and primary users of heroin in a residential substance-use program. Exp Clin Psychopharmacol. 2005;13:311–318. doi: 10.1037/1064-1297.13.4.311. [DOI] [PubMed] [Google Scholar]
- 25.Marzuk PM, Tardiff K, Smyth D, Stajic M, Leon AC. Cocaine use, risk taking, and fatal Russian roulette. JAMA. 1992;267:2635–2637. [PubMed] [Google Scholar]
- 26.Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10:163–173. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- 27.Grant A, Hoops D, Labelle-Dumais C, Prevost M, Rajabi H, Kolb B, et al. Netrin-1 receptor-deficient mice show enhanced mesocortical dopamine transmission and blunted behavioural responses to amphetamine. Eur J Neurosci. 2007;26:3215–3228. doi: 10.1111/j.1460-9568.2007.05888.x. [DOI] [PubMed] [Google Scholar]
- 28.Jassen AK, Yang H, Miller GM, Calder E, Madras BK. Receptor regulation of gene expression of axon guidance molecules: implications for adaptation. Mol Pharmacol. 2006;70:71–77. doi: 10.1124/mol.105.021998. [DOI] [PubMed] [Google Scholar]
- 29.Xiao D, Miller GM, Jassen A, Westmoreland SV, Pauley D, Madras BK. Ephrin/Eph receptor expression in brain of adult nonhuman primates: implications for neuroadaptation. Brain Research. 2006;1067:67–77. doi: 10.1016/j.brainres.2005.10.073. [DOI] [PubMed] [Google Scholar]
- 30.Bashaw GJ, Klein R. Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol. 2010;2:a001941. doi: 10.1101/cshperspect.a001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen SY, Cheng HJ. Functions of axon guidance molecules in synapse formation. Curr Opin Neurobiol. 2009;19:471–478. doi: 10.1016/j.conb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller JS, Tallarida RJ, Unterwald EM. Cocaine-induced hyperactivity and sensitization are dependent on GSK3. Neuropharmacology. 2009;56:1116–1123. doi: 10.1016/j.neuropharm.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrine SA, Miller JS, Unterwald EM. Cocaine regulates protein kinase B and glycogen synthase kinase-3 activity in selective regions of rat brain. J Neurochem. 2008;107:570–577. doi: 10.1111/j.1471-4159.2008.05632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, et al. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc Natl Acad Sci U S A. 2005;102:3495–3500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Popolo M, McCarthy DM, Bhide PG. Influence of dopamine on precursor cell proliferation and differentiation in the embryonic mouse telencephalon. Dev Neurosci. 2004;26:229–244. doi: 10.1159/000082140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10:588–597. doi: 10.1038/nn1896. [DOI] [PubMed] [Google Scholar]
- 37.Crandall JE, Hackett HE, Tobet SA, Kosofsky BE, Bhide PG. Cocaine exposure decreases GABA neuron migration from the ganglionic eminence to the cerebral cortex in embryonic mice. Cereb Cortex. 2004;14:665–675. doi: 10.1093/cercor/bhh027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crandall JE, McCarthy DM, Araki KY, Sims JR, Ren JQ, Bhide PG. Dopamine receptor activation modulates GABA neuron migration from the basal forebrain to the cerebral cortex. J Neurosci. 2007;27:3813–3822. doi: 10.1523/JNEUROSCI.5124-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones LB, Stanwood GD, Reinoso BS, Washington RA, Wang HY, Friedman E, et al. In utero cocaine-induced dysfunction of dopamine D1 receptor signaling and abnormal differentiation of cerebral cortical neurons. J Neurosci. 2000;20:4606–4614. doi: 10.1523/JNEUROSCI.20-12-04606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohtani N, Goto T, Waeber C, Bhide PG. Dopamine modulates cell cycle in the lateral ganglionic eminence. J Neurosci. 2003;23:2840–2850. doi: 10.1523/JNEUROSCI.23-07-02840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griskevicius V, Goldstein NJ, Mortensen CR, Sundie JM, Cialdini RB, Kenrick DT. Fear and Loving in Las Vegas: Evolution, Emotion, and Persuasion. J Mark Res. 2009;46:384–395. doi: 10.1509/jmkr.46.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.