Summary
The aims of this study were to evaluate sensitivity, specificity, and accuracy with an epoch-by-epoch comparison of polysomnography (PSG) and actigraphy with activity counts scored at low, medium, and high thresholds, and to compare PSG derived total sleep time (TST), sleep efficiency (SE), and wake after sleep onset (WASO) to the same variables derived from actigraphy at low, medium, and high thresholds in 9-to-11 year-old children with Juvenile idiopathic arthritis (JIA), asthma, and healthy control children. One night of PSG and actigraphy were recorded. Pairwise group comparisons for sensitivity showed significant differences at the low (Tukey HSD p<.002), medium (p<.001) and high threshold (p<.001) between JIA and asthma groups, and at the high threshold between JIA and controls (p<.009). Significant differences were found for specificity at the low (p<.001), medium (p<.001) and high threshold (p<.001) between JIA and asthma groups, and between JIA and controls (low, p<.002: medium, p<.002: high, p<.008 threshold). PSG TST, WASO and SE were not significantly different among the groups, but significant group differences were found for actigraphy TST, WASO, and SE at all three thresholds. Actigraphy showed the least overestimation or underestimation of sleep or wakefulness at the medium threshold for TST and WASO for all three groups. Compared to PSG, actigraphy was most accurate in the identification of sleep from wakefulness in 9 to 11 year old healthy children, and less accurate in children with JIA and asthma.
Keywords: Polysomnography, actigraphy, concordance, school-age children
Introduction
Polysomnography (PSG) is the most accurate method to measure sleep stages and wakefulness. It requires that participants either come to a sleep laboratory or be connected to portable PSG equipment at home, creating considerable burden to participants and increasing study costs. Although the most accurate sleep measure, PSG data are usually obtained for 1 or 2 consecutive nights in a laboratory setting and thus, provides only a limited view of sleep, which may not represent a subject’s customary sleep at home. Nevertheless, PSG is the ‘gold standard’ in sleep research and in the clinical evaluation of suspected sleep disorders.
In contrast to PSG, actigraphs, worn at the wrist or ankle, are relatively unobtrusive, inexpensive and can record subjects’ sleep and wake patterns for multiple days and nights in the home environment. Wrist actigraphy has become a widely used technology for estimating sleep-wake patterns in community-based research (Sadeh and Acebo 2002; Berger, et al., 2008; Morgenthaler, et al., 2007; Littner, et al., 2003). The actigraph is a battery operated device worn on the non-dominant wrist and programmed for continuous limb movement data collection 24 hours a day for consecutive days or weeks at a time. Several types of actigraphs are commercially available and differ in the type of motion sensor used for the detection and recording of limb movements. Most modern actigraphs use accelerometers and have an event marker, which a participant presses when the actigraph is removed or replaced, and at bedtime and wake time. Limb movement (activity) counts can be recorded and stored in the actigraph in varying (e.g. 15 sec, 30 sec or 1 min) or fixed epoch lengths. Activity counts are downloaded from the actigraph to a computer for processing and scoring into epochs of sleep and wake using algorithms that are unique to the particular software associated with the device. The software and scoring algorithms for actigraphs vary in how the activity counts are summarized for each epoch (Sadeh and Acebo 2002; Jean-louis et al., 2001; Pollak et al., 1998; Sadeh et al., 1995). Some software programs provide operator determined activity count thresholds to specify for the discrimination of epochs of sleep and wake (Kushida et al., 2001; Cole et al., 1992; Jean-Louis et al., 1999). The differences in the devices and algorithms can pose significant challenges when comparing actigraph derived sleep and wake parameters across different study populations.
The validity of actigraphy to discriminate epochs of sleep and wake compared to PSG has been studied most extensively in adults (Van de Water et al., 2011; Kripke et al., 2010; Morgenthaler et al., 2007; Littner et al., 2003). Actigraphy agrees best with PSG in its detection of sleep duration in healthy subjects, but is less accurate in recognition of nocturnal wakefulness, especially in adults with various sleep disorders (Sadeh and Acebo 2002; Morgenthaler et al., 2007; Paquet et al., 2007), in infants (Insana et al., 2010), and in school age children (Spruyt et al., 2011). Actigraphy is best used to derive measures of sleep and wake patterns (e.g., total sleep and wake time, number of awakenings) over multiple sleep cycles (Morgenthaler et al., 2007), but not for estimates of time to fall asleep (sleep latency) or final awakening (Lichstein et al., 2006). Validity is increased when actigraphic data are supplemented with sleep diaries that can help the analyst control for artifacts in data collection, e.g. removal of the device (Kushida et al., 2001), or when subjects are contacted and reminded to press the event marker to denote wake and sleep times or times of actigraph removal (Tsai et al., 2008).
The American Academy of Sleep Medicine has issued practice parameters on the use of actigraphy, which includes use of them in children with sleep disorders and other pediatric populations, including children with chronic illnesses (Littner et al., 2003; Mogenthaler et al., 2007). Agreement between actigraphy and PSG has been reported in a study of infants (Insana et al., 2010), healthy school age children < 9 years old (Spruyt et al., 2011) and in children with sleep disordered breathing (Hyde et al., 2007), but to our knowledge studies have not reported similar comparisons in healthy children 9 to 11 years old, or in children with chronic illnesses such as arthritis or asthma. Chronic illnesses vary in underlying disease mechanisms and manifestation of symptoms that may have differential effects on sleep and wake patterns. For example, joint inflammation and pain in JIA represent different pathophysiological processes compared to bronchoconstriction and difficulty breathing in asthma. Varying disease-related symptoms could influence movement patterns of the children throughout the night. PSG studies in JIA have shown sleep fragmentation (e.g., sleep stage shifts, more arousals and awakenings, increased limb movements), snoring, mild sleep disordered breathing, and daytime sleepiness (Ward et al., 2010; Lopes et al., 2008; Ward et al., 2008; Passarelli et al., 2006; Zamir et al., 1998) but less is known about sleep as measured by actigraphy in JIA. Compared to healthy school-age children, PSG studies in asthma have shown decreased total sleep time, more arousals and awakenings (Ramagopal, et al., 2008; Sulit, et al., 2005), and actigraphy studies have shown decreased total night time sleep, more daytime napping, and higher activity counts during sleep (Kieckehfer, et al., 2008; Sadeh et al., 1995). Without studies of concordance between PSG and actigraphy in JIA or asthma, it is unclear for example, which threshold for scoring actigraphy would yield the most sensitive, specific and accurate measure of sleep and of nocturnal wakefulness. Further, given known developmental changes in sleep as children mature, concerns about the ability of actigraphy to accurately evaluate sleep quality in school age children (Spruyt et al., 2011), increasing use of actigraphy in community-based clinical studies, and a lack of reports in the literature comparing actigraphy to PSG in children with chronic illness, there is a need for further validation studies. In 9-to-11 year-old children with JIA, asthma, and healthy control children, the aims of this study were: 1) to evaluate sensitivity, specificity, and accuracy with an epoch-by-epoch comparison of (PSG and actigraphy with activity counts scored at low, medium, and high thresholds, and 2) to compare PSG derived total sleep time (TST), sleep efficiency (SE), and wake after sleep onset (WASO) to the same variables derived from actigraphy at low, medium, and high thresholds.
Methods
Participants
This study is a secondary analysis of data obtained from two research projects that examined sleep quality in children with JIA (Ward et al., 2010; Ward et al., 2008) and in children with and without asthma (Kieckhefer et al., 2009; Kieckhefer et al., 2008). Human subjects approval for the studies was obtained from the Institutional Review Boards at Seattle Children’s Hospital (JIA study) and the University of Washington (asthma study). From 2002 through 2007, children 9-to-11 years of age with JIA (n = 34), with asthma (n = 19) and controls (n=18) were studied. Children in either study were excluded if they had a diagnosis of a psychiatric condition, diabetes, cancer, cystic fibrosis; or were diagnosed with an upper respiratory infection or acute exacerbation of asthma within the past 2 weeks.
General Procedures
For both studies each child and a parent slept in the University of Washington School of Nursing sleep research laboratory. They were scheduled to arrive at the laboratory approximately 2 to 3 hours prior to the child’s usual bedtime. A schedule for bedtime and rise time was established based on a child’s usual school-night schedule, except during summer months when most children followed a similar schedule every night of the week. PSG and actigraphy were simultaneously recorded for each child using standard laboratory protocols. In the JIA study average bedtime was 9:25 PM and rise time was 7:13 AM, in the asthma study average bedtime was 9:15 PM and rise time was 7:30 AM.
Polysomnography
Both studies used the same laboratory PSG protocols. Details for the PSG recordings have been published previously (Ward et al., 2010; Ward et al., 2008). In brief, electrodes for electro-oculogram, electrocardiogram, electromyelogram, and leg movements were placed according to laboratory protocols based on published standards (Rechtschaffen and Kales. 1968). Electroencephalogram (EEG) electrodes were positioned at 2 frontal (F7, F8), 2 central (C3, C4), and 2 occipital (O1, O2 locations (International 10–20 system of measurement) and linked to reference electrodes placed over each mastoid bone (A1, A2). Nasal airflow was monitored with a pressure cannula placed in the nose (Pro-Tech Services, Inc., Mukilteo, WA) and respiratory effort was measured by piezo respiratory effort bands placed around the chest and abdomen (Pro-Tech Services Inc., Mukilteo, WA). Oxygen saturation was measured from the left or right index finger with a pulse oximeter (Nonin XPod, Nonin Med, Plymouth, MN). Electrophysiological signals were recorded and digitized by Somnologica data acquisition recording system (A10 recorders, Embla, Broomfield, CO) and displayed and stored on a desktop (Dell Pentium III) computer. All digitized data were acquired and stored unfiltered and continuously displayed in 30-sec intervals during each recording. PSG data from each laboratory night were scored (Somnological software version 3.1.2) manually into 30 sec epochs of wake and sleep stages by an experienced technologist according to standard criteria (Rechtschaffen and Kales. 1968).
PSG parameters of interest included: 1) total sleep time defined as the amount of time in NREM stages 1–4 and REM; 2) sleep efficiency expressed as a ratio of total sleep time/time in bed (e.g. from lights off to lights on); and 3) wake after sleep onset expressed as a percentage of wake during sleep period time (e.g. time from lights out until final awakening).
Actigraphy
Concurrent with PSG, each child wore an actigraph (Actiwatch 64 ™, Mini-Mitter Philips Respironics, Bend, OR) placed on the non-dominant wrist during each laboratory night. Actiwatch 64 ™ has an accelerometer that senses the occurrence and degree of motion in all directions. This motion is converted into an electric signal and digitally integrated to derive an activity count. Actigraphy data were recorded in 15 sec epochs (JIA) or 30 sec epochs (asthma and controls), visually inspected and screened of artifacts prior to running the scoring program. For the JIA data, prior to applying the scoring algorithm the raw activity counts in 15 sec epochs were combined to create 30 sec epochs.
Activity counts were scored using the Actiware software version 3.4 (Mini Mitter Inc.). The Actiware software scoring algorithm to discriminate activity counts into epochs of sleep and wake computes a weighted sum of activity in the current epoch, preceding 4 epochs, and the following 4 epochs (Mini-Mitter Philips Respironics, Inc. Bend, OR). This software has three different sensitivity thresholds. If the total activity count is above the defined threshold, the epoch is scored as wake, and if the total activity count is equal to or below the sensitivity threshold, the epoch is scored as sleep. The threshold sensitivity values include: 1) low, defined as 80 activity counts per epoch; 2) medium, defined as 40 activity counts per epoch; and 3) high, defined as 20 activity counts per epoch.
The actigraphy sleep interval was manually set for each record using PSG lights out and on for sleep onset and offset, respectively. Sleep onset was defined as the first 10 minute period in which no more than one epoch was scored as mobile, and sleep offset was defined as the last ten minute period in which no more than one epoch was scored as mobile. Actigraphy parameters of interest included 1) total sleep time, defined as the number of minutes scored as sleep between sleep onset and sleep offset during the sleep interval (similar to PSG sleep period time); 2) sleep efficiency, defined as the ratio of total sleep time/time in bed; and 3) wake after sleep onset, defined as the percentage of scored total wake time during the sleep interval multiplied by 100. We calculated the three actigraphy sleep parameters using each of the threshold sensitivities.
PSG and Actigraphy Data Processing for Analysis
PSG and actigraphy data were synchronized to National Institute of Standards and Technology (NIST) using the same computerized system for each recording session so that each 30-second epoch of PSG could be matched epoch by epoch with actigraphy for comparison. Each scored PSG record was exported from Somnologica as a text file and read into SPSS (version 15.0) and wake and sleep stage data were collapsed into binary variables (e.g. wake = 0; REM and sleep stages 1, 2, 3, 4= 1. Each scored actigraphy record was exported as a text file from Actiware and subsequently read into an Excel (Microsoft Office, version 2007) worksheet and also read into SPSS as binary sleep or wake variables. Agreement and disagreement between actigraphy and PSG was assessed for each matched 30-second epoch. When the actigraph data agreed with PSG data, the epoch was coded as true sleep (TS) or true wake (TW). When the actigraph data did not agree with PSG data it was scored as false sleep (FS) or false wake (FW).
Two sets of analytic methods were used to calculate PSG and actigraphy agreement using SPSS. An epoch-by-epoch agreement analysis provided estimates of sensitivity, specificity, and accuracy parameters. Sensitivity was defined as the proportion of all epochs scored as sleep by PSG that were also scored as sleep by actigraphy (True sleep). Specificity was defined as the proportion of all epochs scored as wake by PSG that were also scored as wake by actigraphy (True wake). Accuracy was the proportion of all PSG scored epochs correctly identified by actigraphy. False positive fraction (FPF) was defined as the fraction or probability of actigraphy reporting sleep when PSG detects wakefulness (Pepe 2003). The second set of analytic methods involved group comparisons of sleep parameters (e.g. TST, SE, and WASO) estimated with PSG and with actigraphy.
Statistical Analyses
Data were analyzed using SPSS for Windows version 15.0 (SPSS Inc, Chicago, IL). The first set of analysis was conducted to address group differences in clinical characteristics (e.g. age and sex) using a separate Chi-square test for each variable. Statistical significance was set at p <.05 (2-sided).
For the first aim, we examined sensitivity, specificity, and accuracy with an epoch-by-epoch comparison between PSG and actigraphy scored at low, medium, high thresholds among the 3 groups. Contingency tables were used to count the number of occurrences of true sleep, true wake, false sleep, and false wake. Estimates of sensitivity, specificity, and accuracy were derived from these occurrences. General linear model (GLM) analyses (i.e., repeated/related measures analyses) for the epoch-by-epoch agreement measures (sensitivity, specificity, and accuracy) were used to evaluate the main effects and interactions with threshold as within subject factor and group the between subject factor. To aid interpretation, GLM analyses (repeated/related measures) were followed by a series of univariate one-way ANOVAs, with pair-wise group comparisons executed using Tukey’s Honest Significant Difference (HSD).
For the second aim, we examined whether PSG derived TST, SE, WASO differed from the same variables derived by actigraphy at the low, medium, and high threshold among the 3 groups. A series of univariate one-way ANOVAs, with pair-wise group comparisons executed using Tukey’s (HSD), were used to evaluate the differences in TST, SE, WASO between PSG and actigraphy at the low, medium, and high threshold.
Finally, the Bland-Altman concordance technique was used to determine if a meaningful agreement could be found between concurrent PSG and each of actigraphy threshold for TST and WASO. Bland-Altman plots represent graphically the difference between two measurement techniques (on Y axes) against the average of the two techniques (on the X axis). Mean difference represents the bias between the 2 measures. Standard deviation of the bias (SD) provides an estimate of the scale of the variation of the difference between the two measures. For each of the sleep parameter comparisons, the average of actigraphy at a specific threshold and PSG was plotted on the X-axis, and the difference between actigraphy at a specific threshold and PSG was plotted on the Y-axis for each group. A positive bias thus indicates an overestimation of the variable with actigraphy; a negative bias indicates an underestimation of the variable with actigraphy.
Results
Sample Characteristics
No group differences were found for age, but sex differences were found (X2 = 18.7, p <.001) (See Table 2). As is characteristic of JIA, there were more girls in the JIA group (85%) compared to the asthma (31.6%) and control (38.9%) groups. Of the 34 children with JIA, 27% had pauciarticular disease and 71% had polyarticular disease. Children diagnosed with asthma were well or partially controlled based on frequency of symptoms and pulmonary function testing (http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf).
Table 2.
Demographic and Clinical Characteristics
| JIA (n=34) | Asthma (n=19) | Controls (n=18) | |
|---|---|---|---|
|
| |||
| Age, years | 10.2 ± .82 | 9.8 ± .86 | 9.8 ± .71 |
|
| |||
| Sex, n (%)a | |||
| Girls | 29 (85.3) | 6 (31.6) | 7 (38.9) |
| Boys | 5 (14.7) | 13 (68.4) | 11 (61.1) |
|
| |||
| Ethnicity, n (%) | |||
| White | 30 (88.2) | 10 (52.6) | 9 (50) |
| Other | 4 (11.8) | 9 (47.4) | 9 (50) |
Data are mean ± SD or n (%).
X2 = 18.7, p <.001
Epoch-by-epoch Agreement
To address aim 1 Table 3 shows the sensitivity, specificity, and accuracy values (mean + SD) for the epoch-by-epoch agreement between PSG and actigraphy at the low, medium, and high threshold for each group.
Table 3.
Sensitivity, Specificity, Accuracy of Epoch-by-Epoch Comparison Polysomnography and Actigraphy
| JIA | Asthma | Controls | |
|---|---|---|---|
| n = 34 | n = 19 | n = 18 | |
| Sensitivity | |||
| Low Thresholda | .98 ± .01 | .97 ± .02 | .98 ± .01 |
| Medium Threshold b | .96 ± .02 | .94 ± .03 | .95 ± .02 |
| High Threshold c | .93 ± .03 | .88 ± .04 | .90 ± .04 |
| Specificity | |||
| Low Threshold d | .38 ± .20 | .60 ± .16 | .55 ± .15 |
| Medium Threshold e | .51 ± .21 | .73 ± .13 | .69 ± .15 |
| High Threshold f | .62 ± .18 | .80 ± .11 | .77 ± .15 |
| Accuracy | |||
| Low Threshold | .88 ± .07 | .90 ± .04 | .90 ± .04 |
| Medium Threshold | .89 ± .06 | .89 ± .04 | .90 ± .03 |
| High Threshold | .88 ± .05 | .87 ± .04 | .87 ± .02 |
Data are mean ± SD. All p = Pair wise comparison, Tukey HSD
p<.002, JIA vs. asthma
p<.001, JIA vs. asthma
Both p<.001, JIA vs. asthma; JIA vs. control
Both p<.002, JIA vs asthma; JIA vs control
Both p<.002, JIA vs asthma; JIA vs control
Both p<.008, JIA vs asthma; JIA vs control
Sensitivity
Significant main effects were found for threshold (F(2,68)= 226, p<.001) and group (F(2,68) =10.1, p<.001), and the group by threshold interaction was also significant (F(4,136) = 5.8, p<.001). Pairwise group comparisons showed significant differences at the low (Tukey HSD p<.002), medium (p<.001), and high threshold (p<.001) between JIA and asthma groups. Significant group differences also were found at the high threshold) between and control groups (p<.009).
Specificity
Significant main effects were found for threshold (F(2,68)=626, p<.001) and group (F(2,68) = 11.5, p<.001), and the group by threshold interaction also was significant (F(4,136) = 4.3, p<.002). Pairwise group comparisons showed significant differences for specificity at the low (p<.001), medium (p<.001) and high threshold (p<.001) between JIA and asthma groups and at the low (p<.002), medium (p<.002), and high (p<.008) threshold between JIA and control groups.
Accuracy
Table 3 shows accuracy data for the 3 groups, which did not differ by group at any threshold.
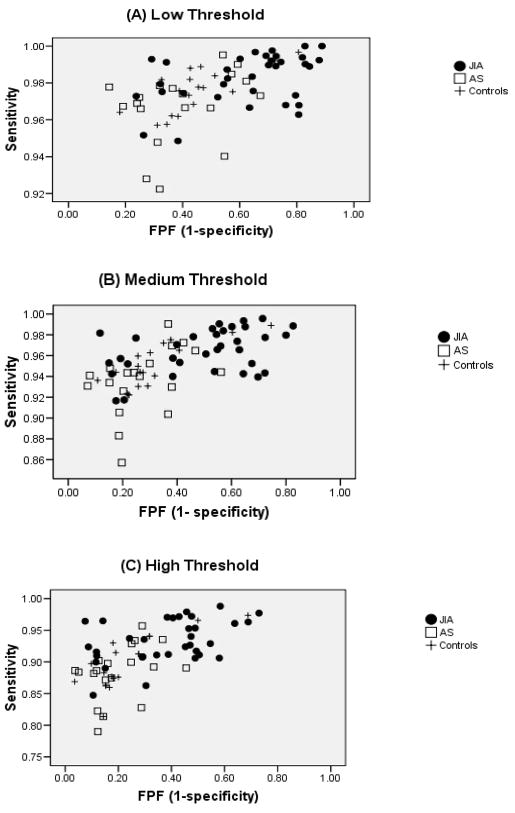
Figure 1A, B, and C shows 1-specificity (false positive fraction [FPF]) versus sensitivity by group, at the low, medium, and high thresholds, respectively. The FPF was higher in the JIA group at all thresholds compared to the asthma and control groups, which suggests that actigraphy identified sleep when PSG did not.
Figure 1. Actigraphy Sensitivity vs. 1-specificity.
Data represent low (A), medium (B), and high (C) actigraphy thresholds. JIA = ●, Asthma (AS) = □, + = controls. False positive fraction (FPF) is defined as the fraction or probability of actigraphy reporting sleep when PSG detects wake. The figures show the discrimination for each subject with respect to threshold. Each point represents the decision criterion (true positive vs. false positive) for each child at a given threshold.
Sleep Parameters Concordance
To address aim 2, Table 4 shows PSG derived and actigraphy derived TST, SE, WASO with the latter scored at low, medium and high thresholds for the 3 groups. PSG derived TST (F(2,68) = .43, p=.65), SE (F(2,68) = 1.1, p=.36),, WASO (F(2,68) = 2.1, p=.14) did not differ among the groups. However significant group differences were found for actigraphy TST at medium (F(2,68) = 4.0, p<.02) and high (F(2,68) = 5.3, p<.008) thresholds; actigraphy SE at the low (F(2,68) = 8.2, p<.001), medium (F(2,68) = 9.7, p<.001), and high (F(2,68) = 10.7, p<.001) thresholds; and actigraphy WASO at the low (F(2,68) = 13.9, p<.001), medium (F(2,68) = 14.5, p<.001), and high (F(2,68) = 14.6, p<.001) thresholds.
Table 4.
Sleep Parameters between Polysomnography and Actigraphy
| JIA | Asthma | Controls | |
|---|---|---|---|
| n = 34 | n = 19 | n = 18 | |
| Total Sleep Time(mins) | |||
| Polysomnography | 485 ± 48.9 | 479 ± 66.9 | 468 ± 75.1 |
| Actigraphy | |||
| Low threshold | 512 ± 39.0 | 481 ± 66.3 | 482 ± 68.7 |
| Medium threshold a | 496 ± 37.9 | 457 ± 66.2 | 462 ± 68.9 |
| High threshold b | 474 ± 37.0 | 428 ± 65.0 | 435 ± 72.3 |
| Sleep Efficiency, % | |||
| Polysomnography | 84 ± 8.9 | 81 ± 10.1 | 82 ± 8.4 |
| Actigraphy | |||
| Low threshold c | 89 ± 5.2 | 81 ± 9.3 | 84 ± 7.5 |
| Medium threshold d | 86 ± 5.7 | 77 ± 9.9 | 80 ± 8.1 |
| High threshold e | 82 ± 6.3 | 72 ± 9.9 | 75 ± 8.9 |
| Wake After Sleep Onset, % | |||
| Polysomnography | 11.3 ± 8.6 | 16.2 ± 10.3 | 14.0 ± 6.0 |
| Actigraphy | |||
| Low threshold f | 5.0 ± 3.2 | 12.1 ± 8.1 | 9.8 ± 3.1 |
| Medium threshold g | 8.0 ± 4.3 | 16.5 ± 9.0 | 13.7 ± 4.3 |
| High threshold h | 12.1 ± 5.5 | 21.8 ± 9.2 | 18.8 ± 5.6 |
Data are mean ± SD. All p = Pair wise comparison, Tukey HSD
p= .05 JIA vs. asthma
Both p<.04 JIA vs. asthma, JIA vs. control
p<.001 JIA vs. asthma
p<.001 JIA vs. asthma; p<.04, JIA vs. control
p<.001 JIA vs. asthma; p<.02 JIA vs. control
p<.001 JIA vs. asthma; p = .007 JIA vs. control
p<.001 JIA vs. asthma; p = .005 JIA vs. control
p<.001 JIA vs. asthma; p =.003 JIA vs. control
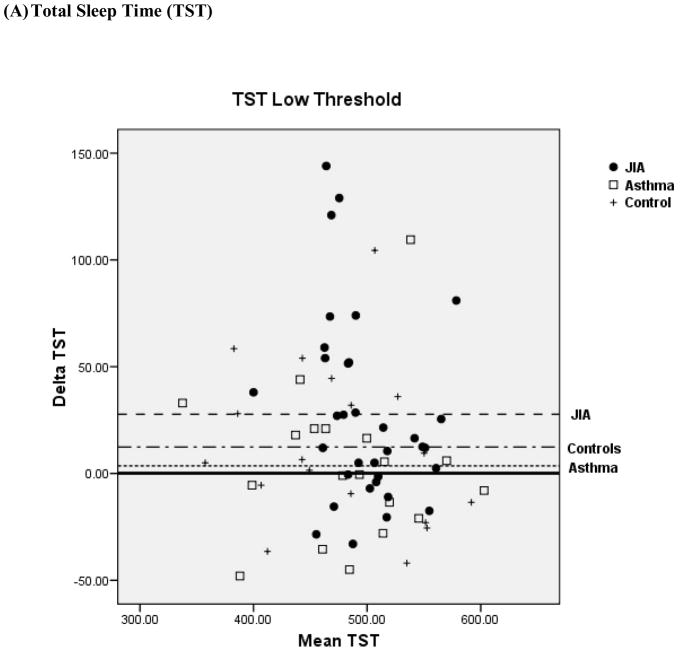
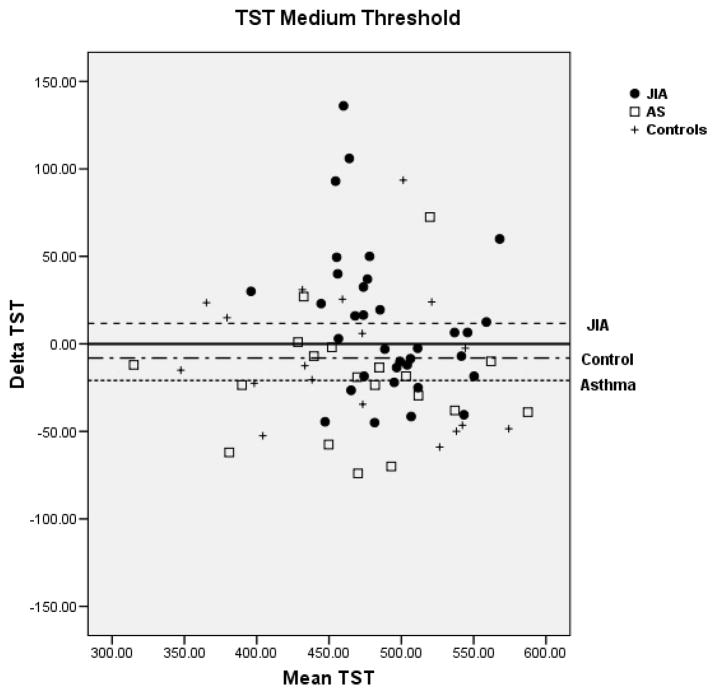
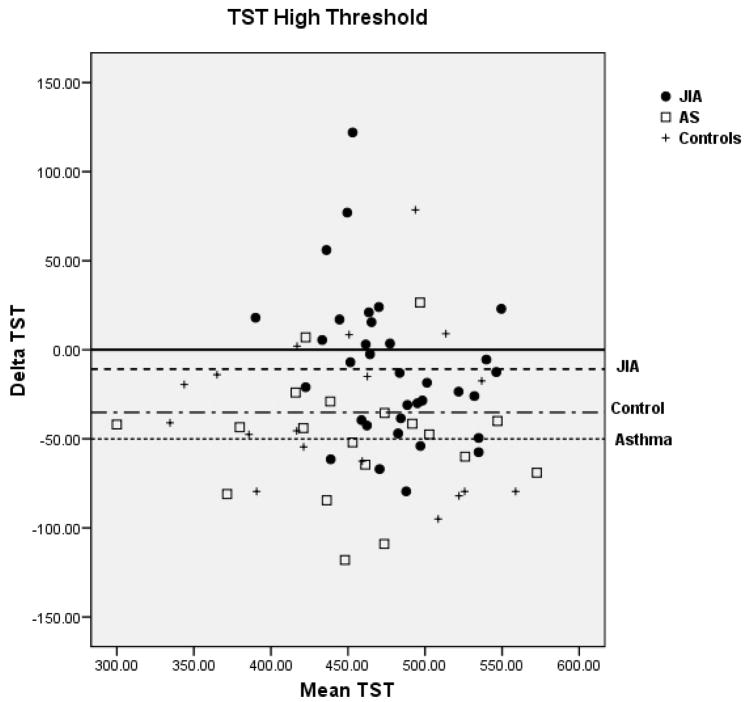
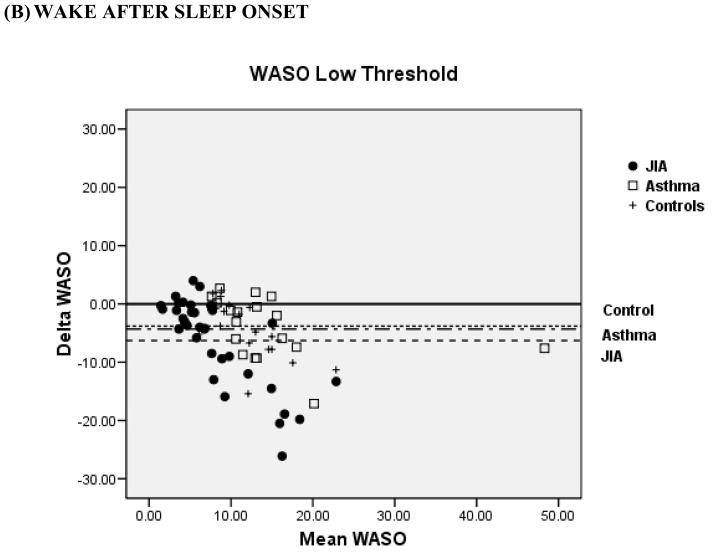
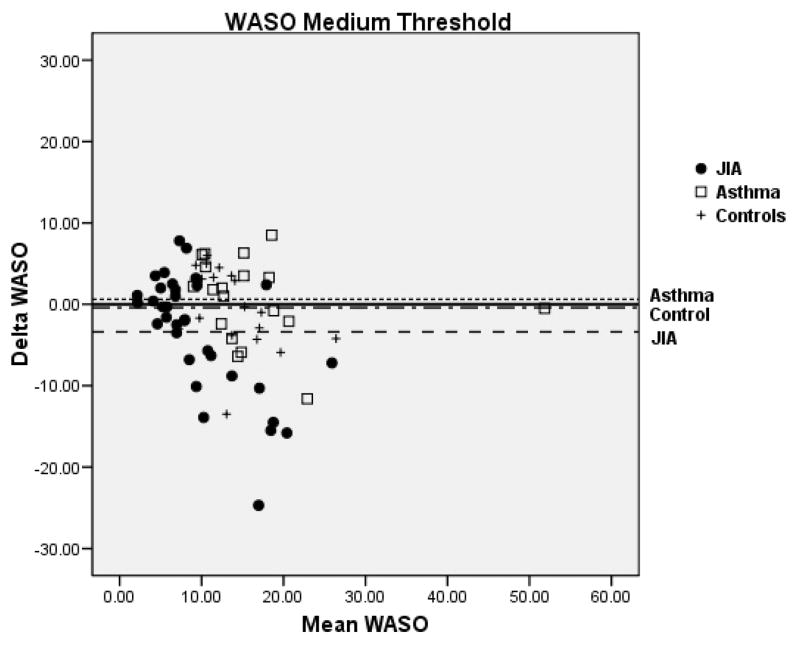
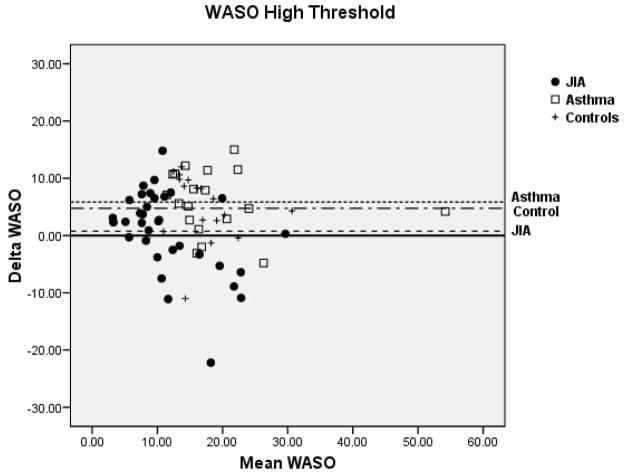
Bland-Altman plots for TST and WASO between PSG and actigraphy for the low, medium, and high threshold are shown in Figure 2 for the 3 groups. Compared to the low and high thresholds, actigraphy showed the least amount of overestimation or underestimation in the identification of TST and WASO at the medium threshold for all 3 groups. Mean difference for TST at the medium threshold was 11 min for JIA, −20 min for asthma, and −8 min for controls. Mean difference for WASO at the medium threshold was −3 min for JIA, 0.61 min for asthma, and −0.42 min for controls. In contrast, TST at the high threshold showed the least underestimation in the JIA group (−10.9 min) but the most underestimation in the asthma group (−50.1 min). WASO at the high threshold was only slightly overestimated (0.75 min) in the JIA group, but it was overestimated (5.8 min) in the asthma group.
Figure 2.
Bland-Altman plots between Polysomnography and Actigraphy for Total Sleep Time (TST) (A) and Wake After Sleep Onset (WASO) (B). Data represent the mean bias ± standard deviation which provides an estimate of the degree of variation of the difference between polysomnography and actigraphy measures of the TST and WASO. JIA = ●, Asthma (AS) = □, + = controls.
Discussion
The findings from this study are the first report of PSG and actigraphy concordance in 9-to-11 year-old children with JIA, asthma, and healthy controls. Overall, findings showed that actigraphy had the least overestimation or underestimation of sleep or wakefulness at the medium threshold for TST and WASO for all three groups. However, compared to PSG, actigraphy was accurate in the identification of sleep and wakefulness in 9 to 11 year old healthy children at all three thresholds, and less accurate in children with JIA and asthma. Our results highlight the importance of evaluating actigraphy determined sleep parameters at each threshold when using a device and software that enables this level of discrimination since sensitivity, specificity and accuracy may differ with children with various chronic illnesses. As noted by others actigraphy is not as accurate a measure of sleep parameters compared to PSG for certain clinical populations (Spruyt et al., 2011; Paquet et al., 2007; Insana et al., 2010; Hyde et al., 2007; Owens et al., 2009; Sitnick et al., 2008). Our findings of low specificity in the identification of nocturnal wakefulness across all three groups is consistent with those of others (Sitnick et al., 2008; Hyde et al., 2007; Paquet et al., 2007). These observations could be attributed to the probability of more sleep epochs compared to wake epochs identified during the nighttime hours. However, in comparison to the other groups the false positive fraction was higher in the JIA group, which suggests that actigraphy may be less likely to identify wakefulness in JIA. This finding might be explained by sleep fragmentation and brief episodes of wake associated with mild sleep disordered breathing that we have identified in JIA (Ward et al., 2010) and as noted in children with obstructive sleep apnea (Hyde et al., 2007).
Epoch-by-epoch Agreement
Concordance between actigraphy and PSG for sensitivity to measure sleep was high for the three groups. This finding is not surprising since the probability of actigraphy identifying sleep epochs compared to wake epochs are high during the night and improve with longer sleep duration.
Unlike sensitivity, specificity was lower in JIA children. As mentioned above this finding may be related to mild sleep disordered breathing that we recently reported in JIA (Ward et al., 2010). When children with arthritis are awake they may move less during sleep, particularly if they have painful joints leading actigraphy identification of sleep when the child is actually awake. Alternatively, since there were more girls in the JIA group compared to asthma and control groups, group differences in actigraphy recognition of wakefulness might be related to girls but not boys being more likely to lie awake without moving as has been noted in adult women with insomnia (Lichstein et al., 2006). Unfortunately, we did not have sufficient sample to evaluate gender differences within the groups. JIA and asthma are chronic conditions that probably impact sleep quality differently and more research is necessary to replicate our findings and explore reasons behind group differences. Previous studies in infants and children have also shown that actigraphy is less accurate in identifying wake episodes in certain clinical populations (Spruyt et al., 2011; Insana et al., 2010; Owens et al., 2009; Sitnick et al., 2008).
Sleep Parameters Concordance
The Bland-Altman plots highlight the close concordance between PSG and actigraphy for both TST and WASO at the medium threshold in healthy children. This finding is inconsistent with a recent study that found poor concordance between PSG and actigraphy for both TST and WASO at the medium threshold in 4-to-9 year-old healthy children who wore the same Actiwatch device (Actiwatch 64 ™, Mini-Mitter Philips Respironics, Bend, OR) (Spruyt et al., 2011). The difference in concordance may be attributed to the inclusion of younger children and/or the use of a 1 minute epoch sampling in the Spruyt et al. study. The manufacturer recommends use of the medium threshold. However further studies are needed on PSG and actigraphy concordance at low, medium, and high threshold to determine which threshold for scoring actigraphy would yield the most sensitive, specific and accurate measure of sleep and of nocturnal wakefulness in healthy children of different ages and to assess the impact of different recorded epoch lengths on these measures.
In JIA and asthma, the Bland-Altman analysis revealed striking differences in the underestimation of sleep scored at the high threshold. The asthma group showed nearly 5 times the amount of bias compared to the JIA group. This finding may be explained by more nighttime movement, differing disease mechanisms, and/or disease related symptoms (frequent night cough) in asthma children. The difference in TST and WASO for the JIA children may also reflect particular distinct mechanisms, low disease activity, or a combination of disease-related symptoms (pain, fatigue) and medication use. Unlike the JIA children, the asthma children had an underestimation of TST at the medium threshold and an underestimation of WASO at the low and medium thresholds. Unlike the JIA and healthy children, the low threshold for TST had the least amount of bias (3.6 minutes) in the asthma group. Future studies examining PSG and actigraphy concordance in children with chronic conditions may want to include videosomnography to understand the nature of the movements recorded by the actigraph. Our findings highlight that changes in sleep are likely not uniform across pediatric chronic conditions, and that recommendations regarding thresholds for scoring actigraphy from healthy children are not adequate for assuming the same quality of validity of actigraphy data in children with chronic conditions.
Limitations
There are several limitations worth noting in this study. First, the small number of asthma (n=19) and control (n=18) children compared to the JIA (n=34) children restricts the generalizability of the findings and the types of data analysis that could be conducted. Second, only one type of actigraph device was studied. Future studies could compare various actiwatch devices. Additional comparison studies are needed between PSG and actigraphy concordance in different pediatric chronic conditions compared to healthy control children.
In conclusion, there is a paucity of data on PSG and actigraphy concordance in children with chronic conditions. The extent of sleep disturbances as measured by actigraphy in pediatric chronic conditions has not been well studied. Given the unobtrusiveness and cost-effectiveness of actigraphy in pediatric chronic illness’ it is important for clinicians and researcher to understand the differences in scoring threshold algorithms and their strengths and limitations. Although actigraphy may not be as sensitive for certain populations it remains an ecologically useful device to measure aspects of sleep and wake. Additional research on PSG and actigraphy concordance will provide more nuanced use in children with chronic health conditions.
Table 1.
Measures for Actigraphic Analysis
| Actigraphy | Polysomnography | Total | |
|---|---|---|---|
| Sleep | Wake | ||
| Sleep | True Sleep (TS) | False Wake (FW) | TS + FW |
| Wake | False Sleep (FS) | True Wake (TW) | FS + TW |
| Total | TS + FS | FW + TW | TS + FS + TW + FW |
Sensitivity = TS/(TS+FS); Specificity = TW/(FW + TW); Accuracy = (TS + TW)/(TS + FS+ TW+ FW) Kushida, et al., 2001
Acknowledgments
The authors thank the children and families who helped with this research. We thank Robert Burr, MSEE, PhD, Research Professor, for his assistance with the data analysis and Shao-Yu Tsai, PhD, RN, Assistant Professor, National Taiwan University for her assistance with the data entry. We thank Linda Peterson, Research Coordinator, Dr. Laurie Beitz, MD, and the staff in the Rheumatology Clinic for recruiting the participants. We thank Ernie Tolentino, Laboratory Manager, and the sleep laboratory staff, James Rothermel, Taryn Jenkins, David Krizan, and Paul Wilkinson for recording, processing and scoring of the sleep data. We also thank Hieke Nuhsbaum for data entry and Salimah Man, Yuen Song, Tuyet Nguyen. Sarah Shapro, and Whitney Jewell for helping with data collection and processing. This research was supported by grants from the National Institute of Nursing Research, T32 NR00710, NR08136, NR08238, the Center for Women’s Health and Gender Research, NR04011, the National Center for Research Resources (NCRR), M01-RR-00037, the Center for Research on Management of Sleep Disturbances, NR011400, and Pulmonary Center Training grant, T72 MC 0007.
Footnotes
Conflict of interests: None for all the authors.
References
- Berger AM, Wielgus KK, Young-McCaughan S, Fischer P, Farr L, Lee KA. Methodological challenges when using actigraphy in research. J Pain Symptom Manage. 2008;36:191–199. doi: 10.1016/j.jpainsymman.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- EPR-3. National Asthma Education and Prevention Program. Bethesda, MD: US Department of Health and Human Services; National Institutes of Health; National Heart, Lung, and Blood Institute; 2007. Expert panel report 3: Guidelines for the diagnosis and management of asthma. 07–4051 NIH Publication. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf. [Google Scholar]
- Hyde M, O’Driscoll DM, Binette S, et al. Validation of actigraphy for determining sleep and wake in children with sleep disordered breathing. J Sleep Res. 2007;16:213–216. doi: 10.1111/j.1365-2869.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- Insana SP, Gozal D, Montgomery-Downs HE. Invalidity of one actigraphy brand for identifying sleep and wake among infants. Sleep Med. 2010;11:191–196. doi: 10.1016/j.sleep.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Louis G, Kripke DF, Cole RJ, Assmus JD, Langer RD. Sleep detection with an accelerometer actigraph: comparisons with polysomnography. Physiol Behav. 2001;72:21–28. doi: 10.1016/s0031-9384(00)00355-3. [DOI] [PubMed] [Google Scholar]
- Jean-Louis G, Zizi F, von Gizycki H, Hauri P. Actigraphic assessment of sleep in insomnia: application of the Actigraph Data Analysis Software (ADAS) Physiol Behav. 1999;65:659–663. doi: 10.1016/s0031-9384(98)00213-3. [DOI] [PubMed] [Google Scholar]
- Kieckhefer GM, Lentz MJ, Tsai SY, Ward TM. Parent-child agreement in report of nighttime respiratory symptoms and sleep disruptions and quality. J Pediatr Health Care. 2009;23:315–326. doi: 10.1016/j.pedhc.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieckhefer GM, Ward TM, Tsai SY, Lentz MJ. Nighttime sleep and daytime nap patterns in school age children with and without asthma. J Dev Behav Pediatr. 2008;29:338–344. doi: 10.1097/DBP.0b013e318182a99e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Hahn EK, Grizas AP, et al. Wrist actigraphic scoring for sleep laboratory patients: algorithm development. J Sleep Res. 2010;19:612–619. doi: 10.1111/j.1365-2869.2010.00835.x. [DOI] [PubMed] [Google Scholar]
- Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29:232–239. [PubMed] [Google Scholar]
- Littner M, Kushida CA, Anderson WM, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–341. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- Owens J, Sangal B, Sutton VK, Bakken R, Allen AJ, Kelsey D. Subjective and objective measures of sleep in children with attention-deficit/hyperactivity disorder. Sleep Medicine. 2009;10:446–456. doi: 10.1016/j.sleep.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Paquet J, Kawinska A, Carrier J. Wake detection capacity of actigraphy during sleep. Sleep. 2007;30:1362–1369. doi: 10.1093/sleep/30.10.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe Sullivan M. The Statistical Evaluation of Medical Tests for Classification and Prediction. New York: Oxford University Press; 2003. [Google Scholar]
- Pollak CP, Stokes PE, Wagner DR. Direct comparison of two widely used activity recorders. Sleep. 1998;21:207–212. doi: 10.1093/sleep/21.2.207. [DOI] [PubMed] [Google Scholar]
- Ramagopal M, Scharf SM, Roberts DW, Blaisdell CJ. Obstructive sleep apnea and history of asthma in snoring children. Sleep Breath. 2008;12:381–92. doi: 10.1007/s11325-008-0174-x. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring systems for sleep stages in human subjects. Los Angeles CA: Brain Information Service, Brain Research Institute, UCLA; 1968. [Google Scholar]
- Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;6:113–124. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Hauri PJ, Kripke DF, Lavie P. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18:288–302. doi: 10.1093/sleep/18.4.288. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Horowitz I, Wolach-Benodis L, Wolach B. Sleep and pulmonary function in children with well-controlled, stable asthma. Sleep. 1998;21:379–84. doi: 10.1093/sleep/21.4.379. [DOI] [PubMed] [Google Scholar]
- Sitnick SL, Goodlin-Jones BL, Anders TF. The use of actigraphy to study sleep disorders in preschoolers: some concerns about detection of nighttime awakenings. Sleep. 2008;31:395–401. doi: 10.1093/sleep/31.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruyt K, Gozal D, Dayyat E, Roman A, Molfese DL. Sleep assessments in healthy school- aged children using actigraphy: concordance with polysomnography. J Sleep Res. 2011;20:223–32. doi: 10.1111/j.1365-2869.2010.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulit LG, Storfer-Isser A, Rosen CL, Kirchner L, Redline S. Associations of obesity, sleep-disordered breathing, and wheezing in children. Amer J Resp Crit Care Med. 2005;171:659–664. doi: 10.1164/rccm.200403-398OC. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Labyak SE, Richardson LP, et al. Actigraphic sleep and daytime naps in adolescent girls with chronic musculoskeletal pain. J Pediatr Psychol. 2008;33:307–311. doi: 10.1093/jpepsy/jsm117. [DOI] [PubMed] [Google Scholar]
- Van de Water AT, Holmes A, Hurley DA. Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography—a systemic review. J Sleep Res. 2011;20:183–200. doi: 10.1111/j.1365-2869.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- Ward TM, Brandt P, Archbold K, et al. Polysomnography and self-reported sleep, pain, fatigue, and anxiety in children with active and inactive juvenile rheumatoid arthritis. J Pediatr Psychol. 2008;33:232–241. doi: 10.1093/jpepsy/jsm121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward TM, Archbold K, Lentz M, Ringold S, Wallace CA, Landis CA. Sleep disturbance, daytime sleepiness, and neurocognitive performance in children with juvenile idiopathic arthritis. Sleep. 33:252–259. doi: 10.1093/sleep/33.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]