Abstract
Fast-growing broiler chicks are susceptible to Se deficiency diseases including exudative diathesis (ED). Our objective was to determine if ED could be induced by feeding a current, practical diet and if the incidence was related to selenogenome expression in liver and muscle of chicks. Four groups of day-old broiler chicks (n = 60/group) were fed a corn-soy basal diet (BD; 14 μg Se/kg; produced in the Se-deficient area of Sichuan, China and not supplemented with Se or vitamin E), the BD and all-rac-α-tocopheryl acetate at 50 mg/kg and Se (as sodium selenite) at 0.3 mg/kg, or both of these nutrients for 6 wk. A high incidence of ED and mortality of chicks were induced by the BD. The incidences and mortality were completely prevented by supplemental dietary Se but were only partially decreased by supplemental α-tocopherol acetate. Dietary Se deficiency decreased (P < 0.05) mRNA levels of 7 common selenoprotein genes (Gpx1, Gpx4, Sepw1, Sepn1, Sepp1, Selo, and Selk) in muscle and liver. Whereas supplementing α-tocopherol acetate enhanced (P < 0.05) only the muscle Sepx1 mRNA level, it actually decreased (P < 0.05) hepatic Gpx1, Seli, Txnrd1, and Txnrd2 mRNA levels. In conclusion, dietary Se protected chicks from the Se deficiency disease ED, probably by upregulating selenoprotein genes coding for oxidation- and/or lesion-protective proteins. The protection by vitamin E might be mediated via selenoproteins not assayed in this study and/or Se-independent mechanisms. The inverse relationship between hepatic expression of 4 redox-related selenoprotein genes and vitamin E status revealed a novel interaction between Se and vitamin E in vivo.
Introduction
Broiler chicks are fast growing and susceptible to dietary Se deficiency. Three classical Se deficiency diseases in chickens include: ED8, pancreatic atrophy, and nutritional muscular dystrophy (1). The role of Se and its interaction with vitamin E in preventing these diseases were intensively studied in the 1950s to 1970s (2–8). Attempts were also made by earlier researchers to elucidate how Se deficiency induced the pathogenesis of these disorders, but the resulting mechanisms remained largely nutritional (9). Although recent progress (see for review, 10, 11) in Se biology offers new molecular tools for scientists to better address this “old” question, a practical challenge is whether the classical Se deficiency diseases can be replicated by using currently available practical diets.
A total of 25 selenoprotein genes (12) have been identified in mammals. The effects of dietary Se concentrations on the expression of these genes as arrays have been studied in mice (13), rats (14), and pigs (15). Only 14 poultry homologs of these 25 mammalian selenoprotein genes can be directly assembled from the currently public available database (16). There has been no information on the responses of selenogenome to dietary Se changes in broiler chicks. Because these animals are extremely susceptible to dietary Se deficiency, revealing the regulation of dietary Se on their selenogenome will not only help understand the pathogenesis of ED and other Se deficiency diseases but will also provide useful clues to unveil the metabolic functions of new selenoproteins. Although dietary vitamin E is closely related to Se in preventing these diseases (9), no report to our knowledge is available to illustrate its effects on selenoprotein gene expression in vivo. Therefore, we conducted this experiment to determine: 1) if the classical Se deficiency disease ED could be replicated in chicks by feeding a low-Se, practical corn-soy diet from the Se-deficient area in China; 2) if the occurrence of ED was associated with selenoprotein gene expression in tissues; and 3) if selenoprotein gene expression was regulated by dietary vitamin E. Answers to these questions were intended to demonstrate the systematic correlation between the development of a classical Se deficiency disease (ED) and expression profiles of multiple newly discovered selenoproteins and to reveal novel roles of dietary vitamin E in regulating selenoprotein gene expression in broiler chicks.
Materials and Methods
Chickens, diet, and experimental design.
Our animal protocol and laboratory procedure followed the research guidelines of Sichuan Agricultural University, China. A total of 255 day-old male broiler poults (Wenjiang Zhengda Poultry) were selected. A total of 15 chicks were killed on d 1 for baseline analyses and the rest of the 240 birds were allotted into 4 dietary treatment groups (n = 60). The BD (Table 1) was composed of corn and soybean meal produced in the Se-deficient area of Sichuan, China and was not supplemented with Se or vitamin E (-Se-VE). The other 3 experimental diets were supplemented with all-rac-α-tocopheryl acetate (Sichuan Internet Aaron Biotechnology) at 50 mg/kg (-Se+VE), Se (as sodium selenite, Sichuan Internet Aaron Biotechnology) at 0.3 mg/kg (+Se-VE), or both of these nutrients (+Se+VE). The analyzed Se concentrations were 14, 26, 421, and 570 μg/kg of feed (as fed) for these 4 diets, respectively. All 4 experimental diets were supplemented with the antibiotic colistin sulfate (Sichuan Internet Aaron Biotechnology) at 40 mg/kg of feed (mixed with the trace element premix). Chicks were housed in battery brooder cages with raised wire floors and the temperature was maintained at 30, 28, and 25°C for the first, second, and subsequent weeks, respectively. Animals were provided free access to the designated diets in plastic troughs and deionized water in stainless steel troughs. The experiment lasted for 6 wk. Individual body weights of chicks were measured weekly and cage feed disappearance was recorded daily to calculate actual daily feed intake of individual chicks. Daily observations were made to record general health, clinical symptoms of Se deficiency diseases, and mortality. Incidences of ED, based on the gross appearance (4), were recorded in detail throughout.
TABLE 1.
Composition of basal diet (BD) (as fed)1
| Ingredients | Content, g/kg |
| Corn | 789.0 |
| Roasted soybean meal | 150.0 |
| CaCO3 | 10.0 |
| CaHPO4 | 21.0 |
| NaCl | 3.0 |
| Choline | 2.0 |
| Trace mineral premix2 | 5.0 |
| Vitamin premix3 | 0.5 |
| Amino acid premix4 | 19.5 |
| Total | 1000.0 |
| Nutrient composition (calculated) | |
| Metabolic energy, MJ/kg | 3.2 |
| Crude protein, % | 20.1 |
| Lysine, % | 0.8 |
| Methionine, % | 0.5 |
| Methionine + cysteine, % | 0.7 |
| Calcium, % | 1.0 |
| Available phosphorus, % | 0.5 |
The analyzed Se concentration in the BD was 14 g/kg.
Trace mineral premix provided per kg diet: FeSO4⋅7H2O, 379 mg; CuSO4⋅5H2O, 31.3 mg; ZnSO4⋅7H2O, 177 mg; MnSO4⋅5H2O, 154 mg; KI, 0.5 mg; and antibiotics colistin sulfate, 40 mg.
Vitamin premix provided per kg diet: retinyl acetate, 1500 mg; cholecalciferol, 200 mg; menadione, 5 mg; thiamin, 1.8 mg; riboflavin, 3.6 mg; calcium pantothenate, 10 mg; niacin, 35 mg; pyridoxol, 3.5 mg; -biotin, 0.15 mg; and folacin, 0.55 mg (without all-rac-α-tocopheryl acetate).
Amino acid premix provided per kg diet: l-lysine, 4630 mg; dl-methionine, 3160 mg; l-threonine, 2010 mg; ltryptophan; 356 mg; l-isoleucine, 2020 mg; l-valine, 1610 mg; l-phenylalanine, 2690 mg; l-arginine, 2570 mg; and l-glycine, 90 mg.
Sample collection and preparation.
At the end of the study, chicks (n = 8–15/group) were killed by decapitation to collect blood, liver, and pectoral muscle samples. After immediate dissection on an ice-cold surface, liver was perfused with and muscle was washed with ice-cold isotonic saline before being minced with surgical scissors. The minced samples were divided into aliquots, snap-frozen in liquid nitrogen, and stored at −80°C until use. Plasma samples were prepared by centrifugation of the whole blood (sodium EDTA as anticoagulant, 2200 × g for 15 min, 5804R Centrifuge, F45–30–11 rotor, Eppendorf) and stored at −80°C.
Biochemical assays.
Plasma and feed Se concentrations were measured using the hydride generation-atomic fluorescence spectrometer (AFS-3200,Yongtuo Instruments) (17) against the standard reference of Se [GBW (E) 080441, National Research Center for Certified Reference Materials]. Two independent samples were taken from each experimental diet for the total Se analysis. Plasma α-tocopherol concentrations were determined using HPLC (EX1600, Wufeng Instruments) against the standard reference of α-tocopherol (GB-T5009.82–2003, National Research Center for Certified Reference Materials). Activities of GPX were measured using a GPX assay kit (A005, Nanjing Jiancheng Bioengineering Institute) and an UV-visible spectrophotometer (DU800, Beckman). Concentrations of protein were determined using the Bradford method (18).
Real-time qPCR analysis of selenoprotein mRNA levels.
To determine the effects of dietary Se and vitamin E on the mRNA expression of 14 selenoprotein genes assembled from the currently publicly available database (19), we isolated total mRNA from liver and muscle (50–100 mg tissue) of the 5 most representative chicks from each group (based on ED incidences and growth performance). The RNA sample preparation, qPCR procedure, and relative mRNA abundance qualification were the same as previously described by our group (15). Primers (Supplemental Table 1) for the 14 selenoprotein genes and 2 reference genes: β-actin (Actb) and glyceraldehyde 3-phosphate dehydrogenase (Gapdh), were designed using Primer Express 3.0 (Applied Biosystems).
Statistical analysis.
The main effects of dietary Se and vitamin E were analyzed as 2 × 2 factorial arrangement of treatments by 2-way ANOVA (SPSS for Windows 13.0). Mean comparisons were conducted by using a Bonferroni t test when the main effect was significant. If an interaction between dietary Se and vitamin E was found, a mean comparison were done conditionally. Data were presented as mean ± SE and the significance level was set at P < 0.05.
Results
Growth performance, plasma Se and #x03B1-tocopherol concentrations, plasma and tissue GPX activities, and incidence of ED.
The final body weight and overall daily gain of chicks were additively decreased (P < 0.05) by dietary Se (34–38%) and vitamin E (7–10%) deficiencies (Table 2). Daily feed intake and gain/feed efficiency were decreased (~20%; P < 0.05) by dietary Se deficiency. Dietary vitamin E deficiency decreased (P < 0.05) plasma α-tocopherol concentrations by 84 and 77% in the –Se and +Se chicks, respectively, whereas dietary Se deficiency decreased (P < 0.05) plasma Se concentrations by 92 and 87% in the –VE and +VE chicks, respectively. Dietary vitamin E deficiency also decreased (P < 0.05) plasma Se concentrations by 50 and 16% in the –Se and +Se chicks, respectively. Plasma GPX activity as well as liver and muscle GPX activities were decreased (P < 0.05) by dietary Se deficiency (Table 3). At wk 2, the –Se chicks had ∼50% of the GPX activities as in the +Se chicks. Whereas the relative percentage of plasma GPX activity dropped to ∼10% at wk 4 and 6, liver GPX activity in the –Se chicks remained 29% of that in the +Se chicks at wk 6. Plasma GPX activity was decreased (P < 0.05) by dietary vitamin E deficiency in both the –Se (14%) and +Se (10%) chicks at wk 4, but the groups did not differ at wk 2 or 6.
TABLE 2.
Effects of dietary Se and vitamin E concentrations on growth performance and plasma concentrations of Se and α-tocopherol in chicks1
| Se,2mg/kg | 0 | 0 | 0.3 | 0.3 |
| Vitamin E,3mg/kg | 0 | 50 | 0 | 50 |
| Initial weight, g | 42 ± 1 (60) | 43 ± 1 (60) | 42 ± 1 (60) | 43 ± 1 (60) |
| Final weight, g | 1051 ± 37a (8) | 1202 ± 68b (28) | 1706 ± 41c (30) | 1844 ± 83d (30) |
| Body gain, g/d | 26 ± 1a (–4) | 28 ± 1b (–) | 40 ± 1c (–) | 43 ± 1d (–) |
| Feed intake, g/d | 68 ± 3a (–) | 76 ± 4a (–) | 88 ± 4b (–) | 91 ± 6b (–) |
| Gain/feed, g/kg | 379 ± 10a (–) | 364 ± 20a (–) | 451 ± 20b (–) | 472 ± 30b (–) |
| Plasma Se,5μmol/L | 0.2 ± 0.0a (5) | 0.4 ± 0.0b (5) | 2.6 ± 0.3c (5) | 3.1 ± 0.3d (5) |
| Plasma vitamin E,6nmol/L | 4.4 ± 0.8a (5) | 27.3 ± 4.0b (5) | 6.6 ± 1.9a (5) | 28.1 ± 6.3b (5) |
Values are means ± SE ( ). Means in a row with superscripts without a common letter differ, P < 0.05.
As sodium selenite (Sichuan Internet Aaron Biotechnology).
As all- -α-tocopheryl acetate (Sichuan Internet Aaron Biotechnology).
= 8–60 surviving chicks.
Initial plasma Se concentration of day-old chicks was 1.2 ± 0.0 mol/L (n = 5).
VE = α-tocopherol. The initial plasma concentration of day-old chicks was 230.6 ± 150.0 nmol/L ( = 5).
TABLE 3.
Effects of dietary Se and vitamin E concentrations on plasma and tissue glutathione peroxidase activity in chicks1
| unit/mg protein2 | ||||
| Se,3mg/kg | 0 | 0 | 0.3 | 0.3 |
| Vitamin E, mg/kg | 0 | 50 | 0 | 50 |
| Plasma | ||||
| wk 2 | 3.5 ± 0.5a | 4.0 ± 0.5a | 7.0 ± 0.7b | 7.7 ± 1.6b |
| wk 4 | 1.9 ± 0.2a | 2.2 ± 0.5b | 16.7 ± 1.2c | 18.5 ± 0.8d |
| wk 6 | 1.8 ± 0.3a | 2.2 ± 0.4a | 20.4 ± 0.9b | 20.6 ± 1.2b |
| Muscle | ||||
| wk 2 | 2.7 ± 0.5a | 2.8 ± 0.7a | 4.7 ± 0.9b | 5.8 ± 0.5b |
| wk 4 | 1.5 ± 0.6a | 1.8 ± 0.3a | 7.0 ± 0.6b | 7.7 ± 0.8b |
| wk 6 | 1.4 ± 0.5a | 1.4 ± 0.4a | 7.4 ± 0.8b | 8.6 ± 1.8b |
| Liver | ||||
| wk 2 | 4.7 ± 1.7a | 5.1 ± 1.8a | 10.2 ± 1.1b | 10.1 ± 1.2b |
| wk 4 | 2.4 ± 1.2a | 2.5 ± 1.2a | 7.2 ± 1.3b | 7.7 ± 2.0b |
| wk 6 | 2.4 ± 0.9a | 2.4 ± 0.9a | 8.3 ± 1.2b | 10.1 ± 0.9b |
Values are means ± SE, = 6. Means in a row without a common letter differ, P < 0.05.
1 mol glutathione oxidized/min at 37°C.
See Table 2 for supplemental forms of VE and Se.
The incidence of ED and other diseases such as pancreatic atrophy (data not shown) occurred in –Se chicks as early as wk 2. Thereafter (from wk 3 to 6), 36 chicks showed symptoms of ED and 21 chicks died in the –Se-VE group. Meanwhile, 11 chicks showed symptoms of ED and 1 chick died in the –Se+VE group. Autopsy of these 2 groups of chicks (Supplemental Fig. 1) showed the typical greenish, gelatinous edema, with subcutaneous hemorrhage under the skin of dependent portions of the body (1). These signs appeared mostly under the breast, but also under the wings, the ventral surface of the face, and down the legs. No incidence of ED or other diseases such as pancreatic atrophy was observed throughout in the +Se chicks.
Muscle mRNA levels of selenoprotein genes.
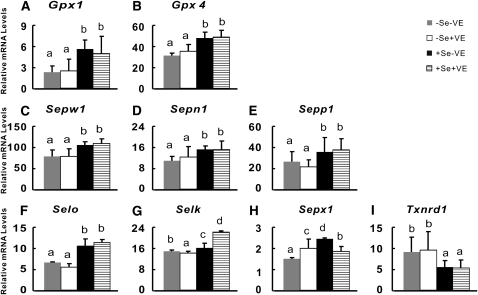
A total of 9 of the 14 assayed selenoprotein genes in muscle were affected (P < 0.01) by dietary Se, whereas only 2 selenoprotein genes (Selk and Sepx1) were affected (P < 0.01) by dietary vitamin E and/or its interaction with Se (Supplemental Table 2). The –Se chicks had 24–55% lower (P < 0.05) muscle mRNA levels of Gpx1, Gpx4, Sepw1, Sepn1, Sepp1, and Selo than those of +Se chicks ( . In contrast, muscle mRNA levels of Txnrd1 (Fig. 1I) were actually 73% greater (P < 0.05) in the –Se chicks than in those of +Se chicks. Muscle mRNA levels of Selk (Fig. 1G) were decreased (5%; P < 0.05) in the –Se chicks and increased (38%; P < 0.05) in the +Se chicks by dietary vitamin E supplementation, respectively. However, the converse was true for the effects of dietary vitamin E on muscle mRNA levels of Sepx1, with a 37% increase (P < 0.05) in the –Se chicks and 21% decrease (P < 0.05) in the +Se chicks. In contrast, muscle mRNA levels of Sels, Selt, Sep15, Seli, and Txnrd2 were not affected by dietary Se or vitamin E (Supplemental Table 3).
FIGURE 1.
Effects of dietary Se and vitamin E (VE) concentrations on relative mRNA abundance of 9 selenoprotein genes in muscle of chicks. Data are means ± SE, n = 5. Means for a variable without a common letter differ, P < 0.05.
Liver mRNA levels of selenoprotein genes.
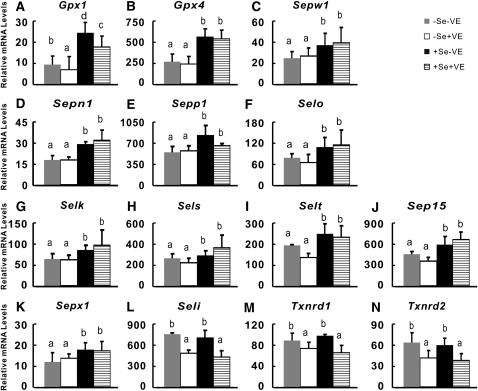
A total of 11 of the 14 assayed selenoprotein genes in liver were affected (P < 0.01) by dietary Se, whereas 4 selenoprotein genes were affected (P < 0.01) by dietary vitamin E (Supplemental Table 4). The –Se chicks had 23–62% lower (P < 0.05) liver mRNA levels of Gpx1, Gpx4, Sepw1, Sepn1, Sepp1, Selo, Selk, Sels, Selt, Sep15, and Sepx1 than those of the +Se chicks ( ). Notably, the +VE chicks had 25–35% lower (P < 0.05) liver mRNA levels of Gpx1, Seli, Txnrd1, and Txnrd2 ( ) than those of –VE chicks at both levels of dietary Se concentrations.
FIGURE 2.
Effects of dietary Se and vitamin E (VE) concentrations on relative mRNA abundance of 14 selenoprotein genes in liver of chicks. Data are means ± SE, n = 5. Means for a variable without a common letter differ, P < 0.05.
Discussion
The classical Se deficiency disease ED was successfully replicated in broiler chicks fed a practical corn-soy diet produced in the Se-deficient area of China. Chicks fed the Se-deficient diets manifested typical clinical signs of ED and showed poor growth performance, decreased plasma concentrations of Se and α-tocopherol, and low plasma GPX activity and liver and muscle GPX activities (7). Although the analyzed dietary Se concentrations were discrepant from the respective designated levels, these apparent discrepancies may just underscore the common technical challenges of sampling and quantification for trace element analysis of complete diets. Because we did not add any inorganic Se to the 2 –Se diets, all Se contents in those diets were from intrinsic source of corn, soybean, and other ingredients and should be the same or similar. Despite this, plasma and tissue GPX activities indicated that the dietary treatments produced appropriate body Se status as intended. Because supplementing the BD with Se at 0.3 mg/kg completely prevented the incidence of ED and other Se deficiency diseases (data not shown), we attempted to identify common responses of selenoprotein gene expression profiles to dietary Se in 2 major tissues, muscle and liver of chicks, that may be related to the overall protective mechanisms of Se. Indeed, 7 selenoprotein genes, including Gpx1, Gpx4, Sepw1, Sepn1, Sepp1, Selo, and Selk, were downregulated to a similar extents between the 2 tissues by dietary Se deficiency. Coincidently, 6 of these 7 genes code for glutathione peroxidases (Gpx1 and Gpx4) (20, 21), redox protein (Selk) (22, 23), and antioxidant proteins with high expression or amount in muscle (Sepw1 and Sepn1) (24–32) and in plasma (Sepp1) (33). Although the function for the protein encoded by Selo (13, 34) is not postulated, a collective loss of the functions associated with the other 6 genes in liver and muscle might be sufficient to induce liver necrosis and muscular dystrophy, respectively, in the Se-deficient chicks (1). Presumably, similar changes in capillaries (35) may serve as the molecular pathogenesis of ED. However, the severe tissue lesion and the lack of guidance in the literature precluded us from collecting usable capillary samples for a direct analysis in the present study. The lack of appropriate antibodies or sensitive proteomic procedures disallowed us to conduct further functional assessments at the protein level.
In liver, 4 additional selenoprotein genes (Sep15, Sels, Selt, and Sepx1) were downregulated by dietary Se deficiency. These genes code for proteins associated with redox control [Sep15 (36) and Sels (37, 38)], regulation of Ca2+ homeostasis and neuroendocrine secretion (Selt) (39), and schizophrenia (Sepx1) (40). It is unclear if the mRNA decreases of these 4 selenoprotein genes in liver actually led to protein changes and directly linked to the Se deficiency diseases. Because Txnrd1 codes for thioredoxin reductase-1 that protects protein from oxidation (41, 42), the intriguing elevation of its mRNA in muscle of the Se-deficient chicks might be induced by the elevated oxidative stress. Because supplementing α-tocopherol acetate at 50 mg/kg into the BD greatly decreased the mortality and incidence of ED in chicks, a new question arises as to whether this previously recognized protection (5) was mediated by regulating selenoprotein gene expression. Among the 14 genes assayed, only Sepx1 mRNA level was increased 33% by this supplementation in the muscle of –Se chicks. That supplementation actually caused a 5% decrease (P < 0.05) in Selk mRNA in the –Se chicks, although that change might be functionally negligible. Seemingly, the protection by supplemental vitamin E was mediated via other selenoprotein genes not assayed in the present study or previously postulated mechanisms, including scavenging free radicals directly (43) or enhancing enteric absorption (44, 45) and postabsorptive utilization (9, 46) of Se. The last mechanism was somewhat implicated in the present study, because plasma Se concentrations were elevated by dietary vitamin E supplementation at both levels of dietary Se.
The downregulation of hepatic Gpx1, Seli, Txnrd1, and Txnrd2 mRNA by the vitamin E supplementation represents a novel finding of the present study. In fact, all these genes except Gpx1 were affected only by dietary vitamin E. Seemingly, transcriptions of these genes and/or their mRNA stabilities were responsive to liver vitamin E status, although the GPX1 mRNA decreases led to no enzyme activity change. Because these 4 selenoprotein genes code for key redox enzymes (41, 42, 45, 47), an inverse relationship between their mRNA levels and vitamin E status implies a possible novel functional coordination between Se and vitamin E in vivo. Furthermore, this inverse relationship was concurrent with an elevated plasma Se concentration in the +VE groups. These combined changes might allow limited body Se in Se deficiency to be mobilized and redistributed for the most critical functions, resulting in a delay or attenuation of Se depletion and pathogenesis in susceptible tissues. Sensitive assays using radioactive or stable isotope tracers of Se should be developed to test this hypothesis in future studies. Because this Se-sparing action of vitamin E did not upregulate expression of any assayed selenoprotein genes in the present study except Sepx1, viable candidates for the presumed critical functions in chicks may be among the other 11 unstudied mammalian homologs. Implications of the discrepant roles of vitamin E and its strong interaction with dietary Se on muscle expressions of Selk and Sepx1 remain unclear. Furthermore, an alternative hypothesis should also be tested in the future if the apparent effects of vitamin E on selenoprotein mRNA levels were somewhat confounded by possible differences between the groups in dietary Se intake or growth.
Compared with rodents (13, 14) or pigs (15), broiler chicks in the present study had a higher percentage of genes assayed responsive to dietary Se but to a less extent. Whereas 9 genes in muscle and 11 genes in liver of the 14 selenoprotein genes assayed in chicks were significantly affected by dietary Se deficiency, only 3 genes (Gpx1, Selh, and Sepw1) in mice (13), 5 genes (Gpx1, Gpx3, Sepw1, Selh, and Selk) in rats (14), and 3 genes (Gpx1, Sepw1, and Txnrd1) in pigs (15) were highly or moderated regulated by dietary Se. Moreover, a number of selenoprotein genes, including Gpx4, Sep15, Sepn1, Selo, Sels, Selt, or Sepx1, were regulated by dietary Se in chicks in the present study but not in rodents of previous studies (13, 14). Although Gpx1 was still the most affected selenoprotein gene by dietary Se in chicks, as in the case of rodents (13, 14, 20), dietary Se deficiency in the former caused relatively modest decreases in liver Gpx1 mRNA or GPX activity (55–80% vs. >90%) compared with the latter. Another unique feature of selenoprotein transcripts in broilers is that the mRNA abundance of Gpx1relative to that of Gpx4 was ~3–12% lower in both liver and muscle. Similar to that shown in turkeys (48), liver Gpx4 mRNA levels in broilers were regulated by body Se status. As mentioned above, liver Gpx4 mRNA or GPX4 activity was little affected by dietary Se in rodents (48, 49). Among the 14 selenoprotein genes assayed in the present study, Sepw1 ranked first in mRNA abundance, followed by Sels, Sep15, and Gpx4 in muscle. Meanwhile, Seli had the highest mRNA abundance, followed by Sepp1, Sep15, Gpx4, and Sels in liver. Apart from possible experimental differences, some of these features may imply a unique avian selenogenome derived by an evolution divergence from the mammalian species.
Acknowledgments
X.G.L., X.J.X, and K.N.W. designed the research; J.Q.H., D.L.L., H.Z., L.H.S., and X.L. conducted the experiments and analyzed the data; J.Q.H., L.H.S., and X.G.L. wrote the paper; and X.G.L. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Supported in part by the Chang Jiang Scholars Program of the Chinese Ministry of Education (X.G.L.), the Chinese Natural Science Foundation (30628019, 30700585, and 30871844), and the NIH DK 53018 (X.G.L.).
Supplemental Figure 1 and Supplemental Tables 1–4 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Abbreviations used: BD, basal diet; ED, exudative diathesis; GPX, glutathione peroxidase; -Se, Se-deficient; +Se, Se-adequate; VE, vitamin E; -VE, vitamin E deficient; +VE, vitamin E adequate.
Literature Cited
- 1.NRC Selenium in nutrition. Revised ed Washington, DC: National Academy Press; 1983 [Google Scholar]
- 2.Creech BG, Rahman M, Reid B, Couch J. Exudative diathesis in chicks. J Nutr. 1958;64:55–65 [DOI] [PubMed] [Google Scholar]
- 3.Creech BG, Feldman GL, Ferguson TM, Reid BL, Couch JR. Exudative diathesis and vitamin E deficiency in turkey poults. J Nutr. 1957;62:83–95 [DOI] [PubMed] [Google Scholar]
- 4.Mathias M, Hogue D. Effect of selenium, synthetic antioxidants, and vitamin E on the incidence of exudative diathesis in the chick. J Nutr. 1971;101:1399–402 [DOI] [PubMed] [Google Scholar]
- 5.Noguchi T, Cantor A, Scott M. Mode of action of selenium and vitamin E in prevention of exudative diathesis in chicks. J Nutr. 1973;103:1502–11 [DOI] [PubMed] [Google Scholar]
- 6.Schwarz K. Development and status of experimental work on factor 3-selenium. Fed Proc. 1961;20:666–73 [PubMed] [Google Scholar]
- 7.Thompson J, Scott M. Role of selenium in the nutrition of the chick. J Nutr. 1969;97:335–42 [DOI] [PubMed] [Google Scholar]
- 8.Thompson J, Scott M. Impaired lipid and vitamin E absorption related to atrophy of the pancreas in selenium-deficient chicks. J Nutr. 1970;100:797–809 [DOI] [PubMed] [Google Scholar]
- 9.Combs G, Jr, Scott M. Antioxidant effects on selenium and vitamin E function in the chick. J Nutr. 1974;104:1297–303 [DOI] [PubMed] [Google Scholar]
- 10.Beckett GJ, Arthur JR. Selenium and endocrine systems. J Endocrinol. 2005;184:455–65 [DOI] [PubMed] [Google Scholar]
- 11.Kohrle J, Jakob F, Contempre B, Dumont J. Selenium, the thyroid, and the endocrine system. Endocr Rev. 2005;26:944–84 [DOI] [PubMed] [Google Scholar]
- 12.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–43 [DOI] [PubMed] [Google Scholar]
- 13.Sunde RA, Raines AM, Barnes KM, Evenson JK. Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci Rep. 2009;29:329–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes KM, Evenson JK, Raines AM, Sunde RA. Transcript analysis of the selenoproteome indicates that dietary selenium requirements of rats based on selenium- regulated selenoprotein mRNA levels are uniformly less than those based on glutathione peroxidase activity. J Nutr. 2009;139:199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou J-C, Zhao H, Li J-G, Xia X-J, Wang K-N, Zhang Y-J, Liu Y, Zhao Y, Lei XG. Selenoprotein gene expression in thyroid and pituitary of young pigs is not affected by dietary selenium deficiency or excess. J Nutr. 2009;139:1061–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lobanov AV, Hatfield DL, Gladyshev VN. Reduced reliance on the trace element selenium during evolution of mammals. Genome Biol. 2008;9:R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno ME, Pérez-Conde C, Cámara C. Speciation of inorganic selenium in environmental matrices by flow injection analysis-hydride generation-atomic fluorescence spectrometry. Comparison of off-line, pseudo on-line and on-line extraction and reduction methods. J Anal At Spectrom. 2000;15:681–6 [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54 [DOI] [PubMed] [Google Scholar]
- 19.Jongeneel CV. Searching the expressed sequence tag (EST) databases: panning for genes. Brief Bioinform. 2000;1:76–92 [DOI] [PubMed] [Google Scholar]
- 20.Lei XG, Cheng WH. New roles for an old selenoenzyme: evidence from glutathione peroxidase-1 null and overexpressing mice. J Nutr. 2005;135:2295–8 [DOI] [PubMed] [Google Scholar]
- 21.Scimeca MS, Lisk DJ, Prolla T, Lei XG. Effects of gpx4 haploid insufficiency on GPx4 activity, selenium concentration, and paraquat-induced protein oxidation in murine tissues. Exp Biol Med (Maywood). 2005;230:709–14 [DOI] [PubMed] [Google Scholar]
- 22.Du S, Zhou J, Jia Y, Huang K. SelK is a novel ER stress-regulated protein and protects HepG2 cells from ER stress agent-induced apoptosis. Arch Biochem Biophys. 2010;502:137–43 [DOI] [PubMed] [Google Scholar]
- 23.Lu C, Qiu F, Zhou H, Peng Y, Hao W, Xu J, Yuan J, Wang S, Qiang B, Xu C. Identification and characterization of selenoprotein K: an antioxidant in cardiomyocytes. FEBS Lett. 2006;580:5189–97 [DOI] [PubMed] [Google Scholar]
- 24.Arbogast S, Beuvin M, Fraysse B, Zhou H, Muntoni F, Ferreiro A. Oxidative stress in SEPN1-related myopathy: From pathophysiology to treatment. Ann Neurol. 2009;65:677–86 [DOI] [PubMed] [Google Scholar]
- 25.Ferreiro A, Quijano-Roy S, Pichereau C, Moghadaszadeh B, Goemans N. Bönnemann C, Jungbluth H, Straub V, Villanova M, Leroy JP. Mutations of the selenoprotein N gene, which is implicated in rigid spine muscular dystrophy, cause the classical phenotype of multiminicore disease: reassessing the nosology of early-onset myopathies. Am J Hum Genet. 2002;71:739–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeong D, Kim T, Chung Y, Lee B, Kim I. Selenoprotein W is a glutathione-dependent antioxidant in vivo. FEBS Lett. 2002;517:225–8 [DOI] [PubMed] [Google Scholar]
- 27.Li J, Zhou J, Zhao H, Lei X, Xia X, Gao G, Wang K. Enhanced water-holding capacity of meat was associated with increased Sepw1 gene expression in pigs fed selenium-enriched yeast. Meat Sci. 2011;87:95–100 [DOI] [PubMed] [Google Scholar]
- 28.Loflin J, Lopez N, Whanger P, Kioussi C. Selenoprotein W during development and oxidative stress. J Inorg Biochem. 2006;100:1679–84 [DOI] [PubMed] [Google Scholar]
- 29.Moghadaszadeh B, Petit N, Jaillard C, Brockington M, Roy SQ, Merlini L, Romero N, Estournet B, Desguerre I, Chaigne D. Mutations in SEPN1 cause congenital muscular dystrophy with spinal rigidity and restrictive respiratory syndrome. Nat Genet. 2001;29:17–8 [DOI] [PubMed] [Google Scholar]
- 30.Petit N, Lescure A, Rederstorff M, Krol A, Moghadaszadeh B, Wewer UM, Guicheney P. Selenoprotein N: an endoplasmic reticulum glycoprotein with an early developmental expression pattern. Hum Mol Genet. 2003;12:1045–53 [DOI] [PubMed] [Google Scholar]
- 31.won Jeong D, Kim E, Kim T, Chung Y, Kim H, Kim I. Different distributions of selenoprotein W and thioredoxin during postnatal brain development and embryogenesis. Mol Cells. 2004;17:156–9 [PubMed] [Google Scholar]
- 32.Yeh J, Beilstein M, Andrews J, Whanger P. Tissue distribution and influence of selenium status on levels of selenoprotein W. FASEB J. 1995;9:392–6 [DOI] [PubMed] [Google Scholar]
- 33.Burk RF, Hill KE. Selenoprotein P: an extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu Rev Nutr. 2005;25:215–35 [DOI] [PubMed] [Google Scholar]
- 34.Pappas A, Zoidis E, Surai P, Zervas G. Selenoproteins and maternal nutrition. Comp Biochem Physiol B Biochem Mol Biol. 2008;151:361–72 [DOI] [PubMed] [Google Scholar]
- 35.Gries CL, Scott M. Pathology of selenium deficiency in the chick. J Nutr. 1972;102:1287–96 [DOI] [PubMed] [Google Scholar]
- 36.Korotkov KV, Kumaraswamy E, Zhou Y, Hatfield DL, Gladyshev VN. Association between the 15-kDa selenoprotein and UDP-glucose: glycoprotein glucosyltransferase in the endoplasmic reticulum of mammalian cells. J Biol Chem. 2001;276:15330–6 [DOI] [PubMed] [Google Scholar]
- 37.Curran JE, Jowett JBM, Elliott KS, Gao Y, Gluschenko K, Wang J, Azim DMA, Cai G, Mahaney MC, Comuzzie AG. Genetic variation in selenoprotein S influences inflammatory response. Nat Genet. 2005;37:1234–41 [DOI] [PubMed] [Google Scholar]
- 38.Gao Y, Feng HC, Walder K, Bolton K, Sunderland T, Bishara N, Quick M, Kantham L, Collier GR. Regulation of the selenoprotein SelS by glucose deprivation and endoplasmic reticulum stress-SelS is a novel glucose-regulated protein. FEBS Lett. 2004;563:185–90 [DOI] [PubMed] [Google Scholar]
- 39.Grumolato L, Ghzili H, Montero-Hadjadje M, Gasman S, Lesage J, Tanguy Y, Galas L, Ait-Ali D, Leprince J, Guerineau NC. Selenoprotein T is a PACAP-regulated gene involved in intracellular Ca2+ mobilization and neuroendocrine secretion. FASEB J. 2008;22:1756–68 [DOI] [PubMed] [Google Scholar]
- 40.Kakiuchi C, Ishiwata M, Nanko S, Ozaki N, Iwata N, Umekage T, Tochigi M, Kohda K, Sasaki T, Imamura A. Up-regulation of ADM and SEPX1 in the lymphoblastoid cells of patients in monozygotic twins discordant for schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:557–64 [DOI] [PubMed] [Google Scholar]
- 41.Nakamura H. Thioredoxin as a key molecule in redox signaling. Antioxid Redox Signal. 2004;6:15–7 [DOI] [PubMed] [Google Scholar]
- 42.Shan W, Zhong W, Zhao R, Oberley TD. Thioredoxin 1 as a subcellular biomarker of redox imbalance in human prostate cancer progression. Free Radic Biol Med. 2010;49:2078–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liebler DC. The role of metabolism in the antioxidant function of vitamin E. Crit Rev Toxicol. 1993;23:147–69 [DOI] [PubMed] [Google Scholar]
- 44.Combs G, Jr, Pesti G. Influence of ascorbic acid on selenium nutrition in the chick. J Nutr. 1976;106:958–66 [DOI] [PubMed] [Google Scholar]
- 45.de Haan JB, Bladier C, Griffiths P, Kelner M, O'Shea RD, Cheung NS, Bronson R, Silvestro MJ, Wild S, Zheng SS. Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide. J Biol Chem. 1998;273:22528–36 [DOI] [PubMed] [Google Scholar]
- 46.Combs G., Jr Differential effects of high dietary levels of vitamin A on the vitamin E- selenium nutrition of young and adult chickens. J Nutr. 1976;106:967–75 [DOI] [PubMed] [Google Scholar]
- 47.Horibata Y, Hirabayashi Y. Identification and characterization of human ethanolamine- phosphotransferase1. J Lipid Res. 2007;48:503–8 [DOI] [PubMed] [Google Scholar]
- 48.Sunde R, Hadley K. Phospholipid hydroperoxide glutathione peroxidase (Gpx4) is highly regulated in male turkey poults and can be used to determine dietary selenium requirements. Exp Biol Med (Maywood). 2010;235:23–31 [DOI] [PubMed] [Google Scholar]
- 49.Lei XG, Evenson JK, Thompson KM, Sunde RA. Glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase are differentially regulated in rats by dietary selenium. J Nutr. 1995;125:1438–46 [DOI] [PubMed] [Google Scholar]