Abstract
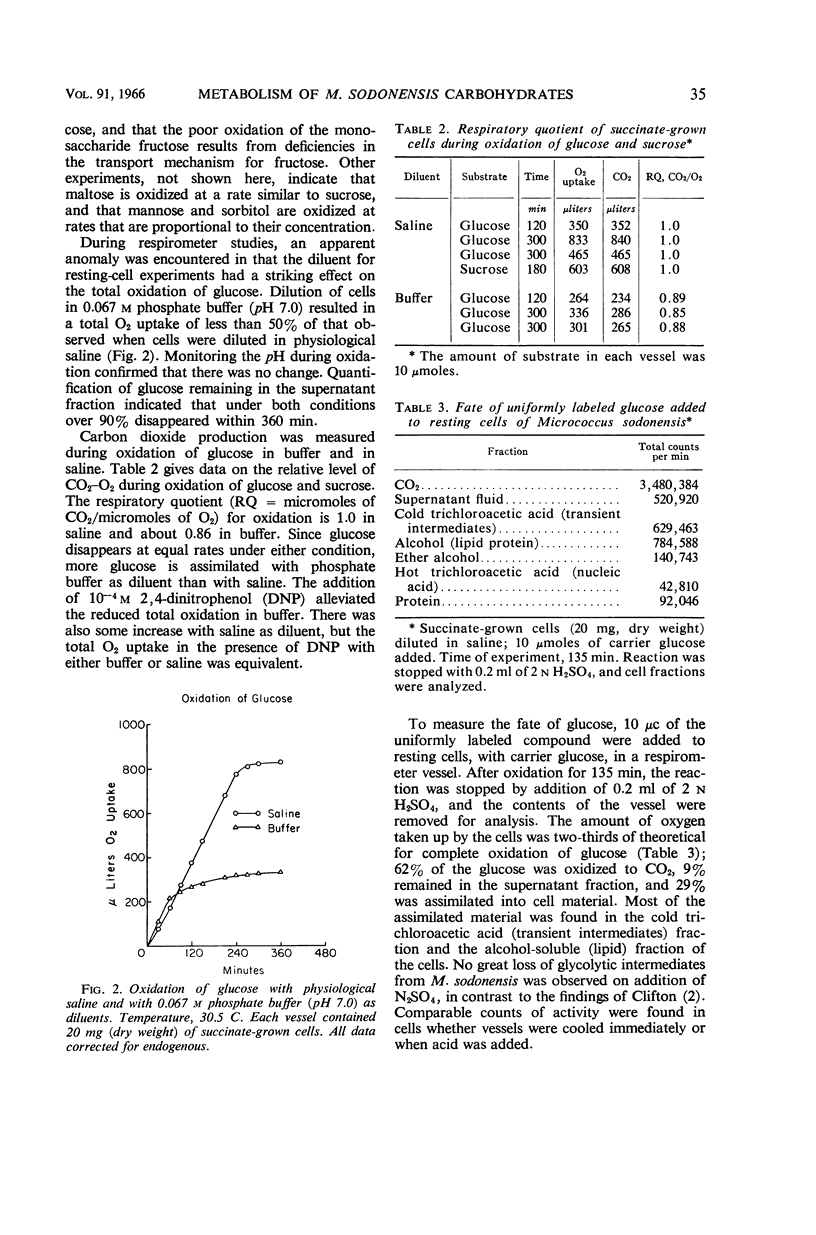
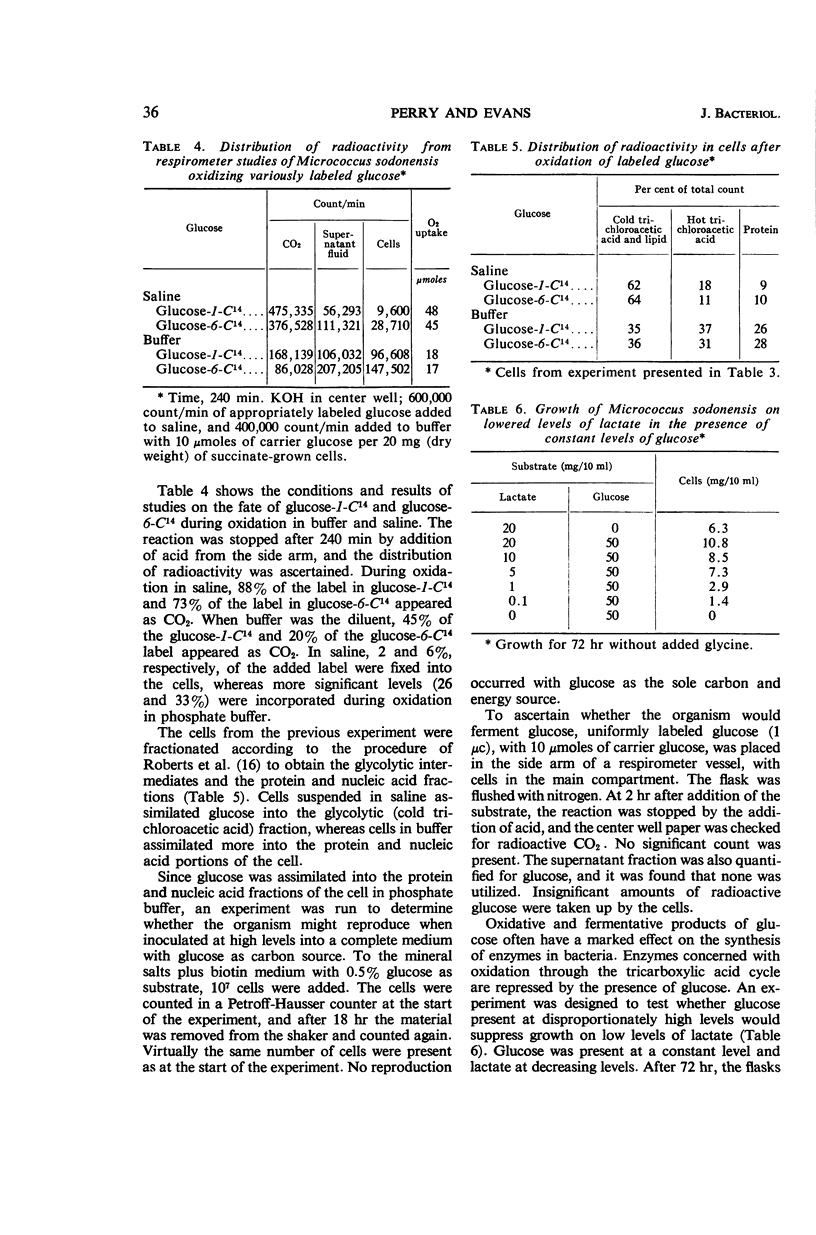
Perry, Jerome J. (North Carolina State University, Raleigh), and James B. Evans. Oxidation and assimilation of carbohydrates by Micrococcus sodonensis. J. Bacteriol. 91:33–38. 1966.—Micrococcus sodonensis is a biotin-requiring strict aerobe that cannot utilize carbohydrates as sole sources of carbon and energy. However, addition of mannose, glucose, sucrose, or maltose to a medium on which the organism can grow resulted in an increase in total growth. M. sodonensis oxidized these sugars without induction, thus indicating the presence of constitutive enzymes for their transport, activation, and metabolism. Under appropriate nonproliferating cell conditions, glucose was readily incorporated into essential constituents of the cell. When glucose-1-C14 and glucose-6-C14 were oxidized by nonproliferating cells, the label was found in both the protein and nucleic acid fractions of the cell. The respiratory quotients of cells oxidizing glucose in saline and in phosphate buffer indicated assimilation of sugar carbon in buffer and virtually no assimilation in saline. The ability of M. sodonensis to completely oxidize glucose and to grow on intermediates of glucose oxidation but not on glucose suggests that glucose may suppress or repress some reaction(s) necessary for growth, and that growth substrates either derepress or circumvent this block.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AARONSON S. Biotin assay with a coccus, Micrococcus sodonensis, nov. sp. J Bacteriol. 1955 Jan;69(1):67–69. doi: 10.1128/jb.69.1.67-69.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLIFTON C. E. OXIDATIVE ASSIMILATION BY BACILLUS MEGATERIUM. J Bacteriol. 1963 Jun;85:1365–1370. doi: 10.1128/jb.85.6.1365-1370.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M. G., Campbell J. J. OXIDATIVE ASSIMILATION OF GLUCOSE BY PSEUDOMONAS AERUGINOSA. J Bacteriol. 1962 Oct;84(4):784–792. doi: 10.1128/jb.84.4.784-792.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epps H. M., Gale E. F. The influence of the presence of glucose during growth on the enzymic activities of Escherichia coli: comparison of the effect with that produced by fermentation acids. Biochem J. 1942 Sep;36(7-9):619–623. doi: 10.1042/bj0360619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSHANOVITCH V. N., PALKINA N. A., BURD G. I. THE ROLE OF LACTATE IN REGULATION OF THE ENZYMATIC SYSTEM SYNTHESIS PARTICIPATING IN THE ACETATE OXIDATION IN STAPHYLOCOCCUS AUREUS. Biochem Biophys Res Commun. 1963 Sep 10;13:12–19. doi: 10.1016/0006-291x(63)90154-2. [DOI] [PubMed] [Google Scholar]
- KLEIN H. P., DOUDOROFF M. The mutation of Pseudomonas putrefaciens to glucose utilization and its enzymatic basis. J Bacteriol. 1950 Jun;59(6):739–750. doi: 10.1128/jb.59.6.739-750.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOPPER P. H. The assimilation of glucose by resting cells of Escherichia coli. J Bacteriol. 1954 May;67(5):507–510. doi: 10.1128/jb.67.5.507-510.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Studies on some methane-utilizing bacteria. Arch Mikrobiol. 1958;30(1):91–118. doi: 10.1007/BF00509229. [DOI] [PubMed] [Google Scholar]
- MACQUILLAN A. M., HALVORSON H. O. Physiological changes occurring in yeast undergoing glucose repression. J Bacteriol. 1962 Jul;84:31–36. doi: 10.1128/jb.84.1.31-36.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B., NEIDHARDT F. C. The effect of glucose on the induced biosynthesis of bacterial enzymes in the presence and absence of inducing agents. Biochim Biophys Acta. 1956 Aug;21(2):324–334. doi: 10.1016/0006-3002(56)90016-6. [DOI] [PubMed] [Google Scholar]
- MARTINEZ R. J., RITTENBERG S. C. Glucose dissimilation by Clostridium tetani. J Bacteriol. 1959 Feb;77(2):156–163. doi: 10.1128/jb.77.2.156-163.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY J. J., EVANS J. B. Oxidative metabolism of lactate and acetate by Micrococcus sodonensis. J Bacteriol. 1960 Jan;79:113–118. doi: 10.1128/jb.79.1.113-118.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY J. J., EVANS J. B. Role of potassium in the oxidative metabolism of Micrococcus sodonensis. J Bacteriol. 1961 Oct;82:551–555. doi: 10.1128/jb.82.4.551-555.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRASTERS K. C., WINKLER K. C. CARBOHYDRATE METABOLISM OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Nov;33:213–229. doi: 10.1099/00221287-33-2-213. [DOI] [PubMed] [Google Scholar]
- WITTENBERGER C. L., HAAF A. S. LACTATE-DEGRADING SYSTEM IN BUTYRIBACTERIUM RETTGERI SUBJECT TO GLUCOSE REPRESSION. J Bacteriol. 1964 Oct;88:896–903. doi: 10.1128/jb.88.4.896-903.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]



