Abstract
Diagnostic bronchoscopy has undergone two major paradigm shifts in the last 40 years. First, the advent of flexible bronchoscopy gave chest physicians improved access to the tracheobronchial tree with a rapid learning curve and greater patient comfort compared with rigid bronchoscopy. The second paradigm shift has evolved over the last 5 years with the proliferation of new technologies that have significantly enhanced the diagnostic capabilities of flexible bronchoscopy compared with traditional methods. At the forefront of these new technologies is endobronchial ultrasound. In its various forms, endobronchial ultrasound has improved diagnostic yield for pulmonary masses, nodules, intrathoracic adenopathy, and disease extent, thereby reducing the need for more invasive surgical interventions. Various navigational bronchoscopy systems have become available to increase flexible bronchoscope access to small peripheral pulmonary lesions. Furthermore, various modalities of airway assessment, including optical microscopic imaging technologies, may play significant roles in the diagnosis of a variety of pulmonary diseases in the future. Finally, the combination of new diagnostic bronchoscopy technologies and novel approaches in molecular analysis and biomarker assessment hold promise for enhanced diagnosis and personalized management of many pulmonary disorders. In this review, we provide a contemporary review of diagnostic bronchoscopy developments over the past decade.
Keywords: endobronchial ultrasound, lung cancer, autofluorescence, tomography, confocal
The first inspection and therapeutic intervention in the tracheobronchial tree was performed by Gustav Killian in 1897. Using a rigid bronchoscope, Killian extracted a pork bone from a patient's right mainstem bronchus in an era when foreign body airway obstruction was an often fatal event. Chevalier Jackson further advanced endoscopic airway evaluation with the development of improved lighting, unique foreign body removal instruments, and rigid bronchoscopy training programs. The rigid bronchoscope was the standard instrument for pulmonary diagnostic and therapeutic procedures for nearly 70 years, but had restricted general application due to its limited ability to evaluate beyond the trachea and mainstem bronchi, the requisite technical expertise, and a lack of optimal anesthetics for patient comfort.
The pulmonary diagnostic landscape underwent a paradigm shift with the introduction of flexible bronchoscopy (FB) by Shigeto Ikeda in 1967. With FB, inspection, biopsy, and airway interventions could be learned quickly with less technical rigor and better patient tolerance as compared with rigid bronchoscopy. Flexible bronchoscopy was adopted rapidly and widely by most chest physicians for pulmonary disease diagnosis and evaluation. The last 10 years have seen technological advances that have transformed FB by expanding its applications in pulmonary medicine. Herein, we will provide a contemporary overview of diagnostic bronchoscopy advances that have altered the landscape for pulmonary disorder evaluation.
AVAILABLE DIAGNOSTIC TOOLS
Four main diagnostic tools have been developed for use during bronchoscopy to obtain diagnostic material: bronchoalveolar lavage(BAL), brushings, forceps biopsies, and needle aspirations. Since bronchoscopy inception more than 100 years ago, these diagnostic modalities have been hampered by limited ability to ensure direct localization of pulmonary nodules, masses, infiltrates, or lymph nodes. However, recent technological developments have improved the ability to localize these lesions, to obtain diagnostic tissue, and to prevent unnecessary surgical intervention.
ENDOBRONCHIAL ULTRASOUND
Among the new diagnostic modalities available to chest physicians, endobronchial ultrasound (EBUS) has unquestionably had the most profound impact. Two major barriers to EBUS development existed: ultrasound size and sound wave transmission in air-filled structures. Ultrasound engineering advances allowed the former barrier to be overcome. The latter was surmounted by developing an integrated fluid-filled balloon surrounding the EBUS probe, thereby allowing for a sound wave–transducing medium interface to exist between the ultrasound probe and the airway wall (i.e., ultrasonographic coupling).
Ultrasound frequency is an important consideration for EBUS application. Lower frequencies give better penetration depth with less resolution; higher frequencies provide better spatial resolution, but less penetration depth. For EBUS applications, the frequencies range from 7.5 to 30 MHz. Currently, there are three EBUS probes available for different applications: (1) ultra-miniature radial probes (20 and 30 MHz; Olympus, Tokyo, Japan), (2) radial balloon probe (20 MHz; Olympus), and (3) convex probe or curvilinear EBUS (CP EBUS).
ULTRA-MINIATURE RADIAL PROBES
Small peripheral pulmonary nodule evaluation remains a common dilemma for chest physicians. A balance between pretest probability of a specific diagnosis and the complication risk associated with a biopsy method must be assessed. Surgical biopsy offers a superior yield at the cost of increased cost and morbidity. Computed tomography (CT)-guided transthoracic needle aspiration has a high yield, but has a 15 to 30% pneumothorax risk (1, 2). Although FB has the advantage of a low complication risk, it has been hampered by a significantly lower diagnostic yield than these other modalities. Flexible bronchoscopy sensitivity for peripheral pulmonary nodules has ranged from 36 to 86% and depends on several factors, including lesion size, distance from the proximal airways, and biopsy instruments chosen (3, 4).
Ultra-miniature radial EBUS (UM-EBUS) introduction has improved the diagnostic yield of peripheral pulmonary nodule biopsy (Figure 1A). With the UM-EBUS probe, the usual normal lung “snowstorm” appearance is replaced by a focal ultrasound alteration which can be marked by fluoroscopy, a guide sheath (GS), or both fluoroscopy and GS to guide biopsies (Figure 1B). In a randomized comparison of traditional fluoroscopically guided transbronchial biopsies (TBB) versus UM-EBUS–guided TBB, there was no statistical difference in establishing a diagnosis for lesions greater than 3 cm; however, for lesions smaller than 3 cm and for lesions smaller than 2 cm, the sensitivity of EBUS-guided TBB remained at 75 and 71%, whereas that of standard TBB fell dramatically to 31 and 23% (respectively) (5). Similar improvements in diagnostic sensitivity for lesions less than 3 cm in size and for lesions that are fluoroscopically invisible have been reported to be between 67 and 77% in other studies (6–8). Using GS-only assisted UM-EBUS localization and biopsy, Yoshikawa and colleagues demonstrated a 61.8% yield for peripheral nodules, suggesting that fluoroscopy exposure may not be necessary (9). Several factors have demonstrated a higher likelihood of obtaining diagnostic tissue using UM-EBUS: lesion size, UM-EBUS probe completely within a lesion (as opposed to adjacent to it), presence of a lesional air bronchus sign on CT scan, location in the middle lobe or lingula, or distance from the visceral pleura (6, 8–10). Furthermore, the diagnostic modality chosen may also improve the diagnostic yield. Transbronchial needle aspiration (TBNA) addition to conventional diagnostic procedures (TBB and BAL) in UM-EBUS localized lesions not only improved the malignant diagnostic yield from 56.5 to 79.2%, but also TBNA plus conventional diagnostic procedures maintained its improved diagnostic yield whether the UM-EBUS probe was within (63.6%) or adjacent to the lesion (59.1%) (11).
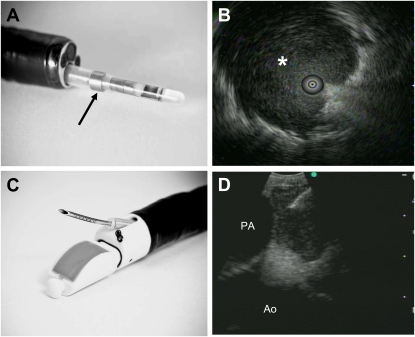
Figure 1.
Two forms of endobronchial ultrasound (EBUS). (A) The ultra-miniature (UM)-EBUS contains a circulating ultrasound crystal that provides a 360° view of the surrounding structures when full airway ultrasonographic coupling occurs. This probe is inserted through a guide sheath (arrow; Olympus Corporation, Tokyo, Japan), which can be left behind in the airway on UM-EBUS probe removal to direct instruments for biopsy. (B) This UM-EBUS image demonstrates a focal lesion (asterisk) surrounding the probe. (C) The convex probe (CP)-EBUS TBNA (BF-UC160F-OL8; Olympus Corporation, Tokyo, Japan) videobronchoscope has an integrated ultrasound probe that scans 90° perpendicular from the longitudinal axis, a 35° forward oblique video view, and a 2.0-mm working channel through which a dedicated biopsy needle can be passed. Distal tip dimples on the needle provide an echogenic surface to reflect ultrasound waves to allow needle visualization. (D) This CP-EBUS image demonstrates a left paratracheal lymph node with the ascending aorta (Ao), the pulmonary artery (PA), and the needle present in the lymph node.
Although the majority of UM-EBUS investigations have focused on pulmonary nodules, in suspected tuberculosis cases with negative sputum, UM-EBUS–guided biopsy and washings improved the overall tuberculosis diagnostic yield from 58.3 to 80.8% (12). Collectively, these studies have demonstrated that UM-EBUS can enhance the diagnostic yield for parenchymal lesions when compared with traditional fluoroscopic localization and biopsy.
RADIAL BALLOON PROBE
To overcome the ultrasonographic coupling problem with the UM-EBUS probes in the central airways, a radial EBUS probe was developed that is inserted into an outer sheath with a distal tip balloon. The balloon is filled with saline to provide a fluid medium to allow for sound wave transmission from the probe to the airway wall. The radial balloon EBUS (RB-EBUS; Olympus Corporation, Tokyo, Japan) probe provides a less than 1-mm resolution with a 360° visualization of paratracheal and peribronchial structures. Five to seven layers of the tracheal and proximal bronchial wall have been described with RB-EBUS (13).
RB-EBUS CLINICAL APPLICATIONS
Intrathoracic Adenopathy
Several studies with RB-EBUS investigated its role in localization and biopsy of mediastinal and hilar adenopathy. Although the diagnostic yield for RB-EBUS TBNA was consistently superior to conventional anatomic TBNA (14–16), it has been supplanted by CP-EBUS for this purpose (see below) and will not be discussed further in this context.
Tumor Invasion and Extension
A common lung cancer staging dilemma is whether a paratracheal tumor invades the tracheal wall, which would preclude definitive surgical resection. The presence of tracheal wall invasion by CT scan is often ambiguous and may lead to surgical exploration with discovery of tracheal involvement and abandonment of planned definitive surgery. RB-EBUS has a very high sensitivity (93–95%) at determining airway wall involvement with malignancy (13, 17). In a study of patients with central thoracic malignancies potentially involving the proximal airways, patients underwent sequential CT scan, RB-EBUS, and surgical resection with histologic analysis. RB-EBUS performance at differentiating airway involvement far surpassed that of CT scan with an improvement in accuracy from 51 to 94% (18). Similar results have been demonstrated in patients with thyroid or esophageal cancer potentially involving the trachea (19). These studies demonstrate that RB-EBUS can discriminate tumor stage in a minimally invasive fashion better than other imaging modalities to reduce unnecessary exploratory thoracotomy.
Adjunct to Therapeutic Interventions
Treatment for carcinoma in situ (CIS) or very early-stage central lung cancer may include photodynamic therapy (PDT), but because of limited penetration depth, PDT is most effective in the absence of transmural airway wall invasion. In a study of 18 patients with early-stage local cancers, RB-EBUS was used successfully to select PDT monotherapy (intracartilaginous extension only) versus surgical resection (extracartilaginous extension) with excellent long-term outcomes (20). Moreover, when performing therapeutic bronchoscopy for malignant airway obstruction, the location of surrounding structures and the depth and length of airway involvement can be uncertain. In a large observational study of more than 1,100 bronchoscopies using RB-EBUS, management decisions, including terminating debulking maneuvers near vessels, adjusting stent size, and referral for surgery, were altered in 43% of cases (21). Similarly, stent failure at relieving airflow obstruction could be predicted if RB-EBUS demonstrated extension of cartilage destruction beyond the stent choke point (22). RB-EBUS diagnostic information can also assist in surgical planning, as demonstrated in a patient with a right middle lobe carcinoid who underwent a successful wedge bronchoplasty instead of a bilobectomy (23). Used in these various diagnostic scenarios, RB-EBUS can allow for optimal clinical decisions and therapeutic interventions.
RB-EBUS has also found diagnostic application in nonmalignant thoracic diseases. In relapsing polychondritis (RP), a hyaline cartilage autoimmune disease resulting in loss of cartilaginous airway support leading to tracheobronchomalacia, RB-EBUS can demonstrate airway edema and cartilage fragmentation and destruction. This RP RB-EBUS pattern differed substantially compared with post-tracheotomy or excessive dynamic airway collapse RB-EBUS patterns (24, 25), thereby lending support to the RP diagnosis and to guide appropriate therapy. In addition, Soja and colleagues have recently demonstrated that RB-EBUS performed on patients with asthma effectively differentiated individual airway wall layer thickening that negatively correlated with FEV1 (26). Applying RB-EBUS in this diagnostic fashion to evaluate airway wall thickness and remodeling may have major implications for assessing efficacy of pharmacologic and nonpharmacologic asthma or other obstructive lung disease treatments.
CP EBUS-TBNA
The CP EBUS bronchoscope has a built-in curvilinear ultrasound transducer with a larger distal diameter (6.9 mm) compared with a standard bronchoscope. White light video bronchoscopy occurs at a 35° oblique angle with EBUS at 90° from the longitudinal axis. Dedicated biopsy needles (21- or 22-gauge) are inserted through the 2-mm working channel to perform aspirations (Figure 1C). Real-time EBUS imaging displays needle penetration through the tracheobronchial wall into the target during the biopsy maneuver (Figure 1D). If there is difficulty in achieving adequate EBUS images because of poor ultrasonographic coupling, a saline-filled balloon surrounding the transducer can be used to improve image quality. In addition, Doppler capabilities allow vascular structure differentiation, which should minimize unintended vascular puncture.
LUNG CANCER STAGING
Accurate lung cancer staging is vital for making therapeutic decisions, avoiding unnecessary surgery, and assessing prognosis. Hilar and mediastinal lymph node evaluation is fundamental to staging, but the primary noninvasive staging modalities—CT and positron emission tomography scans—are limited by both false-positive and false-negative findings. Therefore, pathologic confirmation of lymph node involvement should be pursued to verify appropriate lung cancer stage and to guide subsequent therapeutic options.
Invasive mediastinal lung cancer staging can be accomplished by cervical mediastinoscopy (CM), thoracoscopy, or TBNA. Although the traditional gold standard is CM, it has limited access to many lymph node stations, requires general anesthesia, has a 2% morbidity and 0.08% mortality risk, and is more costly than TBNA (27). In addition, a review of nearly 41,000 lung cancer cases in the United States demonstrated that the CM gold standard was performed in only 27% of patients with nodal tissue acquired in 47% of those cases (28). Unfortunately, anatomic TBNA yield has been extremely variable and depends on many factors, including training, technique, experience, lymph node location and size, and on-site cytologic evaluation (29). Many of these limitations are due to lack of confident lymph node localization and intranodal needle insertion and have been surmounted with CP EBUS development.
Many well-designed studies have demonstrated that the diagnostic yield from CP EBUS-TBNA can approach that of the gold standard, CM, with sensitivity and negative predictive value (NPV) of 90 to 95%, such that diagnostic surgical procedures (e.g., CM, video-assisted thoracoscopic surgery) can be avoided in many cases (30–33, see Table 1). When compared with CT or positron emission tomography, CP EBUS-TBNA is superior at detecting mediastinal and hilar metastatic disease, thereby allowing for more accurate staging (34–38). In several of these studies, lymph node size was less than 10 mm, demonstrating CP EBUS can localize, biopsy, and identify regional nodal metastases in radiographically “normal” lymph nodes. Although nodal size may not impact on adequate sample acquisition, Lee and colleagues have demonstrated that the yield per lymph node aspiration plateaued at 95% after the third needle pass (70% first pass, 84% second pass) (39). Moreover, a recent prospective, crossover trial compared CP EBUS-TBNA yield to CM with surgical lymph node dissection at resection as the gold standard in patients with suspected lung cancer. Although the overall pathologic nodal stage determination did not differ between CP EBUS-TBNA (93%) and CM (82%), the overall per lymph node diagnostic yield was higher for CP EBUS-TBNA than CM (91 vs. 78%) with a significant difference seen in the subcarinal location (24%) (40). This study was the first to compare directly the CM gold standard to CP EBUS-TBNA in patients with suspected lung cancer with the demonstration that CP EBUS-TBNA may be superior to CM, particularly in certain lymph node stations, but will need confirmation in future studies.
TABLE 1.
SELECTED STUDIES OF MEDIASTINAL AND HILAR LYMPH NODE STAGING WITH ENDOBRONCHIAL ULTRASOUND–TRANSBRONCHIAL NEEDLE ASPIRATION
| Study | Selection Criteria | N | Mean LN Size (mm) | Sensitivity (%) | NPV (%) | Prevalence* (%) |
|---|---|---|---|---|---|---|
| Yasufuku, et al. (30) | LN ≥ 10 mm or clinical suspicion | 108 | 19 (L), 13 (S) | 94.6 | 89.5 | 68.5 |
| Herth, et al. (31) | LN ≥ 10 mm | 502 | 16 | 94.4 | 11.0 | 98.2 |
| Herth, et al. (36) | LN ≤ 10 mm, PET negative | 100 | 7.9 | 88.9 | 98.9 | 9.0 |
| Bauwens, et al. (32) | Any size LN, PET positive | 106 | 14.4 | 95.0 | 90.6 | 57.5 |
| Lee, et al. (39) | LN 5-20 mm | 105 | 15.2 (L), 8.6 (S) | 94.3 | 96.9 | 33.3 |
| Wallace, et al. (94) | Any size LN | 150 | NR | 69.0 | 88.1 | 30.4 |
| Ernst, et al. (40) | LN ≥ 10 mm | 66 | 15.6 | 86.6 | 77.6 | 61.7 |
| Hwangbo, et al. (37) | LN 5-20 mm | 117 | 14.4 (L), 7.9 (S) | 90.0 | 96.7 | 25.6 |
Definition of abbreviations: L = long axis; LN = lymph node; NPV = negative predictive value; NR = not recorded; PET = positron emission tomography; S = short axis.
Studies were selected based on minimum study size of 50 subjects and presentation of sufficient data to calculate sensitivity, NPV, and prevalence. All data calculated based on patient as unit of analysis except for study by Ernst et al. (40), which is presented with lymph node as unit of analysis.
Prevalence of lymph nodes with tumor involvement.
Mediastinal restaging following neoadjuvant therapy in patients with nonbulky N2 disease remains controversial, and CP EBUS-TBNA may provide a safe alternative to repeat CM. Herth and colleagues retrospectively reviewed 124 cases of patients with proven stage IIIA, N2-positive lung cancer who underwent neoadjuvant chemotherapy followed by restaging CP EBUS-TBNA and subsequent surgical resection and lymphadenectomy (41). Although the CP EBUS-TBNA sensitivity in this setting is lower than newly diagnosed patients (76%), the yield paralleled that reported with CM in this setting (70–75%) (42–44). Similar to lung cancer staging, establishing lymph node involvement in malignant pleural mesothelioma has importance to determine therapeutic options. In a recent retrospective review, Rice and colleagues demonstrated that although all staging modalities had suboptimal performance, CP EBUS-TBNA outperformed CM (sensitivity, 59 vs. 28%, respectively) to determine lymph node involvement in malignant pleural mesothelioma (45).
In addition to an important role in establishing staging information, CP EBUS-TBNA application to evaluate recurrent lymphadenopathy in the setting of prior malignancy was extremely effective in confirming malignant recurrence (97.4% sensitivity) (46). Moreover, in a patient population with extrapulmonary malignancies and possible pulmonary metastases being considered for metastasectomy, CP EBUS-TBNA demonstrated mediastinal and/or hilar lymph node metastases in 56% of patients, thereby guiding the decision regarding metastasectomy (47). CP EBUS-TBNA has also proved successful in the diagnosis of other metastatic tumors to mediastinal or hilar lymph nodes, including renal cell carcinoma (48), chondrosarcoma (49), and thyroid cancer (50).
OTHER DIAGNOSTIC APPLICATIONS OF CP EBUS-TBNA
Intrathoracic adenopathy in the appropriate clinical setting raises the concern for sarcoidosis or lymphoma, and most patients with this finding undergo diagnostic evaluation with biopsy. Using CP EBUS-TBNA, several groups have demonstrated that the sensitivity for recovering noncaseating granulomas characteristic of sarcoidosis is 85 to 94% (51–53), which compared favorably to the 48 to 72% yield from conventional TBNA (54, 55). A recent randomized controlled trial in patients with suspected sarcoidosis compared CP EBUS-TBNA with anatomic TBNA using a 19-gauge core needle. CP EBUS-TBNA improved the diagnostic yield by 29.5% compared with anatomic TBNA (83.3 vs. 53.8%, respectively) (56).
Because CP EBUS-TBNA has the capacity for central vascular structure evaluation, several case reports have demonstrated intravascular thrombus or tumor invasion into central vascular structures (57, 58) (Figures 2A and 2B). A novel proof-of-concept pilot study used CP EBUS-TBNA in patients with CT angiography-confirmed pulmonary emboli (PE) to determine if the emboli could be visualized. Of the 101 CT angiography–documented PE, 97 (96%) PE could be visualized, with at least one clot seen in every patient (59). Although this approach to PE diagnosis may not find broad application, CP EBUS intrathoracic vasculature evaluation may play a critical role in those patients in whom contrast cannot be administered or who are too medically unstable to undergo CT angiography.
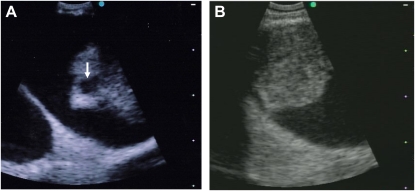
Figure 2.
Vascular lesions detected by convex probe endobronchial ultrasound (CP EBUS). (A) A 56-year-old man with progressive dyspnea and a CT scan demonstrating mediastinal adenopathy and proximal pulmonary artery filling defects interpreted as pulmonary embolism. CP EBUS–TBNA was performed of the mediastinal lymph nodes and scanning of the vasculature demonstrated the intraluminal filling defects with cystic structures (arrow). TBNA demonstrated only blood and the patient underwent catheter-directed endovascular biopsy, which confirmed high-grade angiosarcoma. (B) A 65-year-old smoking woman (75 pack-years) presented with cough and dyspnea and was found to have a right hilar mass with mediastinal adenopathy. Mediastinal node CP EBUS-TBNA confirmed adenocarcinoma, but scanning lower into the right hilar area demonstrated tumor vascular invasion into the right pulmonary artery inferior branch.
EBUS LIMITATIONS
Every technology has limitations, and EBUS is no exception. The recent development of EBUS translates into most chest physicians' lacking adequate training and/or experience with this technology. The American College of Chest Physicians (ACCP) recommendations for attaining EBUS competence is 50 supervised procedures, followed by 20 per year to maintain competence (60). Courses offered through the ACCP and other institutions can provide initial EBUS training, but this is often simulator- and animal model–based, and subsequent supervision and performance feedback on patient procedures are lacking.
Technical limitations also exist. Because of the inability to direct the UM-EBUS probe in certain anatomic locations, it can be difficult to localize small peripheral pulmonary lesions, particularly in the upper lobes, because of the scope angulation required. Some of these limitations may be overcome by using guide sheaths or combining EBUS and navigation technologies as discussed below. Tracheal RB-EBUS is limited by the inability to achieve ultrasonographic coupling and a full 360° view for an extended period due to airway obstruction. Although CP EBUS improved on RB-EBUS for real-time lymph node sampling, the right and left paratracheal positions can be difficult to maintain ultrasonographic coupling while attempting airway needle puncture, particularly at the tracheobronchial angle. Difficult needle puncture also can occur in elderly patients with narrower intercartilaginous spaces and cartilage calcification, as well as in scirrhous nodal processes, such as nodular sclerosing Hodgkin disease. Moreover, in patients undergoing conscious sedation bronchoscopy, significant cough while attempting ultrasonographic coupling can limit procedural success. From an economic perspective, a fully-equipped EBUS bronchoscopy suite would cost approximately $125,000. For this investment, there is minimal additional professional fee reimbursement and no additional facility fee reimbursement above standard bronchoscopy.
NAVIGATIONAL BRONCHOSCOPY
Another recent advance in the evaluation of peripheral pulmonary lesions and mediastinal and hilar adenopathy has been the development of navigational bronchoscopy systems. The first Food and Drug Administration–approved system, based on electromagnetic navigation (EMN; InReach System' superDimension, Ltd., Herzliya, Israel), uses an electromagnetic board to generate a magnetic field around the patient, a magnetic sensor probe, an extended working channel, and three-dimensional integration of CT scan reconstruction and FB position. In essence, this EMN system works on the same triangulation principle as a global positioning system and allows the bronchoscopist to direct the FB through the airways to the target. Several studies have demonstrated EMN diagnostic sensitivity to range between 67% and 74%, independent of lesion size (61–63). Although these studies demonstrated an improved yield compared with historical fluoroscopic biopsy methods, the only comparative randomized controlled study of EMN alone, UM-EBUS alone, or EMN followed by confirmatory UM-EBUS found a significantly higher yield with the combined approach compared with UM-EBUS or EMN alone (combination 88%, UM-EBUS 69%, EMN 59%) (64). This study suggested that EMN may be helpful, but the best diagnostic yield for small peripheral lesions may come from combined diagnostic modalities.
Beyond diagnostic use, novel EMN applications in targeted cancer therapeutic delivery, including EMN-guided stereotactic radiosurgery fiducial placement or implantation of radiotherapy monitoring devices, can be achieved (65, 66). Two new navigational bronchoscopy systems recently have received Food and Drug Administration 510K clearance for diagnostic bronchoscopy application (LungPoint; Broncus Technologies, Inc, Mountain View, CA, and SPiN Drive System; Veran Medical Technologies, St. Louis, MO), but neither has been evaluated in a clinical trial to date to confirm their efficacy.
Similar to EBUS, navigational bronchoscopy systems may be limited in general application by their high capital cost and training necessary for optimal system use. At the current time, the greatest experience and yield with these technologies has occurred in centers of excellence with results unlikely to be reproducible in less experienced centers. More importantly, the lack of randomized comparative studies with this technology raises concerns about its role compared with traditional or ultrasound-guided approaches.
AUTOFLUORESCENCE BRONCHOSCOPY AND NARROW BAND IMAGING
Autofluorescence bronchoscopy (AFB) was developed as a novel screening approach to identify central intraepithelial moderate or severe dysplasia, CIS, or microinvasive neoplasia in patients at risk for lung cancer. Although AFB may be able to localize these early lesions with greater sensitivity than white light bronchoscopy (WLB) (67–72), prospective longitudinal lesion assessment demonstrated that only 0 to 9% of moderate dysplastic foci and 0 to 32% of severe dysplastic foci progress to CIS or invasive cancer (73–75), and 60 to 65% of moderate/severe dysplastic lesions regressed or resolved spontaneously (73). The uncertainty of central airway dysplastic lesion natural history combined with the increasing incidence of peripheral adenocarcinomas not accessible to bronchoscopic visualization makes it unlikely that AFB will find a role as a routine screening tool for lung cancer in large populations. The ACCP has recommended AFB use, if available, in the following settings: (1) normal chest radiography with severe dysplasia, CIS, or carcinoma on sputum cytology; (2) patients considered for curative endobronchial therapy to treat CIS; and (3) patients with known central airway severe dysplasia or CIS undergoing periodic bronchoscopy (72).
Narrow band imaging (NBI) uses a unique filter to select light wavelengths that preferentially are absorbed by hemoglobin, thereby permitting superior microvasculature detection. Because angiogenesis occurs preferentially in dysplastic and neoplastic lesions, NBI may identify early dysplastic lesions better than WLB or AFB. Early studies with NBI in high-risk patients demonstrated its ability to detect lesions that could not be visualized by WLB with a similar sensitivity to AFB (76, 77). A recent study compared WLB, AFB, and NBI in the same patients who presented for airway surveillance and revealed similar sensitivity between AFB and NBI, but improved NBI specificity for detecting abnormal lesions (78). Although current clinical applications for AFB and NBI in the general pulmonary population do not exist, they may play an important role in future risk stratification, prognostication, and/or chemoprevention trials in high-risk patients
OPTICAL COHERENCE TOMOGRAPHY
Optical coherence tomography (OCT) is the optical ultrasound analog whereby near-infrared light transit time and reflection is used rather than sound waves and provides a macroscopic optical cross-sectional view of hollow organs. By using light instead of sound waves, OCT overcomes two major ultrasound limitations in the lung: (1) the inability to image through air, and (2) poor spacial resolution. Early ex vivo studies demonstrated that OCT could identify the microstructural anatomic alterations in the various airway wall layers (79, 80). Interestingly, when compared with histologic biopsy specimens from abnormal areas, OCT image patterns were unique and different depending on whether dysplastic, invasive cancer, or inflammatory changes were seen on the biopsy (81). In a combined modality approach, Lam and colleagues performed AFB on patients at high risk for lung cancer followed by OCT and abnormal AFB lesion biopsy. They demonstrated that OCT could identify quantitative epithelial thickness differences between invasive cancer and CIS and between dysplasia and metaplasia/hyperplasia. In addition, cell nuclei characteristics became more discernible by OCT with moderate dysplasia or higher lesional grade (82). Because OCT can measure airway wall thickness, Coxson and colleagues evaluated the correlation between airway wall thickness as measured by OCT or CT scan and FEV1% predicted. In 44 former or current smokers, they showed a strong correlation between OCT and CT wall area measurements and these measurements correlated significantly with FEV1% predicted (83). Although these studies are preliminary pilot investigations, they suggest that OCT may be able to provide an “optical biopsy” with information about microinvasive airway lesion evolution, airway wall remodeling in obstructive lung diseases, or interstitium alterations in idiopathic interstitial pneumonia (IIP). These data may provide diagnostic information, but more importantly, longitudinal evaluation in an individual patient may allow therapeutic tailoring in the future.
FIBERED CONFOCAL FLUORESCENCE MICROSCOPY
Fibered confocal fluorescence microscopy (FCFM) is based on confocal microscopy that allows thin-section biological specimen imaging by replacing the microscope's objective with a flexible fiberoptic miniprobe that can be introduced through an FB (Figure 3A). This technology does not rely on light reflectance as in OCT, but rather on cellular and tissue autofluorescence on laser excitation. An initial FCFM study identified that the predominant source of airway wall autofluorescence occurred from the subepithelial layer elastin fibers, and alterations in this elastin network can be detected in microinvasive and invasive proximal airway lesions (84). These same investigators demonstrated that FCFM can image alveolar and acinar elastin fiber alterations and alveolar macrophage accumulation in smoking patients compared with nonsmoking patients (85) (Figures 3B–3D). Therefore, although limited data are available, this technology may have evolving diagnostic and therapeutic monitoring application across many parenchymal lung diseases, including obstructive lung diseases, sarcoidosis, IIP, and microinvasive malignant lesions.
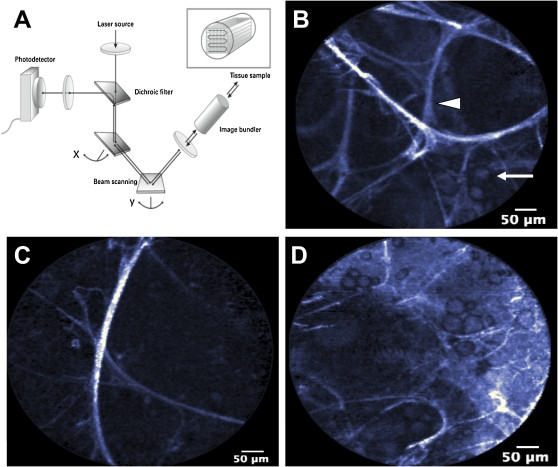
Figure 3.
Flexible confocal fibered microscopy. (A) Schematic representation of fibered confocal fluorescence microscopy, whereby laser light excites biologic tissue generating autofluorescence, which is detected to generate an image (Cellvizio; Mauna Kea Technologies, Paris, France). (B) Normal alveolar architecture demonstrating alveolar septa (arrowhead) with a few scattered alveolar macrophages (arrow). (C) Emphysematous lung with loss of many alveolar septa and large alveolar spaces. (D) Fairly well-preserved alveolar architecture, but with increased alveolar macrophage number.
DIAGNOSTIC BRONCHOSCOPY MOLECULAR AND BIOMARKER APPLICATIONS
In the last 5 years, the advent of targeted agents has improved survival rates in both recurrent and metastatic non-small cell lung cancer (NSCLC). It is increasingly apparent that both histological analysis and molecular typing will be necessary to determine appropriate therapy for patients with NSCLC. For example, treatment with epidermal growth factor receptor (EGFR) inhibitors is most beneficial in patients with tumors that harbor activating EGFR mutations (86). Because the majority of patients with NSCLC present with unresectable disease, mutation testing is often limited by insufficient material obtained during diagnostic FB. New methods have been developed to allow EGFR molecular typing of small-volume samples obtained by EBUS-TBNA or transbronchial biopsy (87–89). Similarly, aberrant DNA methylation of several genes involved in tumor progression and expression of several cell cycle proteins were assessed from EBUS-TBNA samples and appeared to correlate with chemotherapy response (90, 91). In addition to molecular typing, advanced histological analysis increasingly is important as the choice of specific cytotoxic agents now relies on distinguishing among the various NSCLC histologies. For example, pemetrexed appears to have preferential activity in adenocarcinoma, compared with squamous cell or large cell carcinoma (92). With differential NSCLC subtype responses to specific chemotherapy now becoming more evident and with more molecular targeted NSCLC therapies in development, the ability to obtain adequate tissue from diagnostic FB has gained tremendous importance. The improved diagnostic yield with current bronchoscopic technologies should allow for more material to be available for immunohistochemical and molecular studies that will be required for personalized treatment decisions.
Evolving research in many pulmonary diseases (e.g., chronic obstructive pulmonary disease, asthma, IIPs, lung cancer, sarcoidosis, etc.) is investigating how to use diagnostic FB specimens to measure various biomarkers to assess risk, to establish diagnoses, and to monitor and adjust therapy. Most of these investigations use standard diagnostic FB approaches, but use specimens in a novel fashion. For example, an 80-gene biomarker set from proximal airway epithelial cells in patients undergoing FB for presumed NSCLC has shown in a preliminary study to distinguish with 90% sensitivity between smokers with and without lung cancer (93). Should any of these investigations demonstrate improved risk assessment, diagnostic ability, or therapeutic decision customization, the diagnostic FB role could expand to diseases in which FB traditionally has had a minimal role (asthma, chronic obstructive pulmonary disease, IIPs).
SUMMARY
The addition of new technologies to diagnostic FB has expanded the role for and the diagnostic yield of FB. EBUS has been at the forefront of these developments predominantly for application in parenchymal pulmonary lesions and mediastinal and hilar adenopathy. In fact, in experienced centers, CP EBUS rivals CM for lung cancer staging in a less-invasive, potentially more cost-effective fashion. Navigational bronchoscopy may have its greatest application in combination with UM-EBUS to maximize diagnostic yield in parenchymal lesions. Novel in situ optical biopsy technologies (OCT and FCFM) may find an expanding diagnostic role in a broad array of pulmonary disorders. Of potentially greatest significance is the promise that diagnostic specimens obtained with assistance from these new technologies may allow for molecular and biomarker assessment not only for diagnostic purposes but more importantly for tailoring therapeutic interventions.
Supplementary Material
Acknowledgments
The authors thank Edmund Moon, M.D. for his expertise and assistance in photograph acquisition and preparation.
Originally Published in Press as DOI: 10.1164/rccm.201002-0186CI on April 8, 2010
Author Disclosure: A.R.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.V. received expert witness fees from McGuire Woods, LLP for $10,001–$50,000, industry-sponspored grants from Allegro Diagnostics for $50,001–$100,000, Matsushita Electronics for $50,001–$100,000, and Merck & Co. for $10,001–$50,000. D.H.S. received consultancy fees from Broncus Technologies for $1,001 $5,000 and Spiration, Inc. for $5,001–$10,000. D.H.S. also serves on the advisory board for Mesothelioma Applied Research Foundation for less than $1,000 and CSA Medical for less than $1,000 and owns stock for less than $1,000. D.H.S. received a sponsored grant from NCI for $50,001–$100,000.
References
- 1.Yeow KM, Su IH, Pan KT, Tsay PK, Lui KW, Cheung YC, Chou AS. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 2004;126:748–754. (See comment). [DOI] [PubMed] [Google Scholar]
- 2.Ohno Y, Hatabu H, Takenaka D, Higashino T, Watanabe H, Ohbayashi C, Sugimura K. CT-guided transthoracic needle aspiration biopsy of small (≤20 mm) solitary pulmonary nodules. AJR Am J Roentgenol 2003;180:1665–1669. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest 2003;123:115S–128S. [DOI] [PubMed] [Google Scholar]
- 4.Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000;117:1049–1054. [DOI] [PubMed] [Google Scholar]
- 5.Paone G, Nicastri E, Lucantoni G, Dello Iacono R, Battistoni P, D'Angeli AL, Galluccio G. Endobronchial ultrasound-driven biopsy in the diagnosis of peripheral lung lesions. Chest 2005;128:3551–3557. [DOI] [PubMed] [Google Scholar]
- 6.Kurimoto N, Miyazawa T, Okimasa S, Maeda A, Oiwa H, Miyazu Y, Murayama M. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959–965. [DOI] [PubMed] [Google Scholar]
- 7.Herth FJ, Eberhardt R, Becker HD, Ernst A. Endobronchial ultrasound-guided transbronchial lung biopsy in fluoroscopically invisible solitary pulmonary nodules: a prospective trial. Chest 2006;129:147–150. [DOI] [PubMed] [Google Scholar]
- 8.Yamada N, Yamazaki K, Kurimoto N, Asahina H, Kikuchi E, Shinagawa N, Oizumi S, Nishimura M. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest 2007;132:603–608. [DOI] [PubMed] [Google Scholar]
- 9.Yoshikawa M, Sukoh N, Yamazaki K, Kanazawa K, Fukumoto S, Harada M, Kikuchi E, Munakata M, Nishimura M, Isobe H. Diagnostic value of endobronchial ultrasonography with a guide sheath for peripheral pulmonary lesions without X-ray fluoroscopy. Chest 2007;131:1788–1793. [DOI] [PubMed] [Google Scholar]
- 10.Fielding DI, Robinson PJ, Kurimoto N. Biopsy site selection for endobronchial ultrasound guide-sheath transbronchial biopsy of peripheral lung lesions. Intern Med J 2008;38:77–84. [DOI] [PubMed] [Google Scholar]
- 11.Chao TY, Chien MT, Lie CH, Chung YH, Wang JL, Lin MC. Endobronchial ultrasonography-guided transbronchial needle aspiration increases the diagnostic yield of peripheral pulmonary lesions: a randomized trial. Chest 2009;136:229–236. [DOI] [PubMed] [Google Scholar]
- 12.Lin SM, Chung FT, Huang CD, Liu WT, Kuo CH, Wang CH, Lee KY, Liu CY, Lin HC, Kuo HP. Diagnostic value of endobronchial ultrasonography for pulmonary tuberculosis. J Thorac Cardiovasc Surg 2009;138:179–184. [DOI] [PubMed] [Google Scholar]
- 13.Kurimoto N, Murayama M, Yoshioka S, Nishisaka T, Inai K, Dohi K. Assessment of usefulness of endobronchial ultrasonography in determination of depth of tracheobronchial tumor invasion. Chest 1999;115:1500–1506. [DOI] [PubMed] [Google Scholar]
- 14.Herth FJ, Becker HD, Ernst A. Ultrasound-guided transbronchial needle aspiration: an experience in 242 patients. Chest 2003;123:604–607. [DOI] [PubMed] [Google Scholar]
- 15.Herth F, Becker HD, Ernst A. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest 2004;125:322–325. (See comment). [DOI] [PubMed] [Google Scholar]
- 16.Plat G, Pierard P, Haller A, Hutsebaut J, Faber J, Dusart M, Eisendrath P, Sculier JP, Ninane V. Endobronchial ultrasound and positron emission tomography positive mediastinal lymph nodes. Eur Respir J 2006;27:276–281. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka F, Muro K, Yamasaki S, Watanabe G, Shimada Y, Imamura M, Hitomi S, Wada H. Evaluation of tracheo-bronchial wall invasion using transbronchial ultrasonography (TBUS). Eur J Cardiothorac Surg 2000;17:570–574. [DOI] [PubMed] [Google Scholar]
- 18.Herth F, Ernst A, Schulz M, Becker H. Endobronchial ultrasound reliably differentiates between airway infiltration and compression by tumor. Chest 2003;123:458–462. [DOI] [PubMed] [Google Scholar]
- 19.Wakamatsu T, Tsushima K, Yasuo M, Yamazaki Y, Yoshikawa S, Koide N, Fujimori M, Koizumi T. Usefulness of preoperative endobronchial ultrasound for airway invasion around the trachea: esophageal cancer and thyroid cancer. Respiration 2006;73:651–657. [DOI] [PubMed] [Google Scholar]
- 20.Miyazu Y, Miyazawa T, Kurimoto N, Iwamoto Y, Kanoh K, Kohno N. Endobronchial ultrasonography in the assessment of centrally located early-stage lung cancer before photodynamic therapy. Am J Respir Crit Care Med 2002;165:832–837. [DOI] [PubMed] [Google Scholar]
- 21.Herth F, Becker HD, LoCicero J III, Ernst A. Endobronchial ultrasound in therapeutic bronchoscopy. Eur Respir J 2002;20:118–121. [DOI] [PubMed] [Google Scholar]
- 22.Miyazawa T, Miyazu Y, Iwamoto Y, Ishida A, Kanoh K, Sumiyoshi H, Doi M, Kurimoto N. Stenting at the flow-limiting segment in tracheobronchial stenosis due to lung cancer. Am J Respir Crit Care Med 2004;169:1096–1102. [DOI] [PubMed] [Google Scholar]
- 23.Kim MP, Ernst A, DeCamp MM, Gangadharan SP. Endobronchial ultrasound-facilitated video-assisted lobectomy with wedge bronchoplasty for typical carcinoid tumor of the right middle lobe. Chest 2008;133:1474–1476. [DOI] [PubMed] [Google Scholar]
- 24.Miyazu Y, Miyazawa T, Kurimoto N, Iwamoto Y, Ishida A, Kanoh K, Kohno N. Endobronchial ultrasonography in the diagnosis and treatment of relapsing polychondritis with tracheobronchial malacia. Chest 2003;124:2393–2395. [DOI] [PubMed] [Google Scholar]
- 25.Murgu S, Kurimoto N, Colt H. Endobronchial ultrasound morphology of expiratory central airway collapse. Respirology 2008;13:315–319. [DOI] [PubMed] [Google Scholar]
- 26.Soja J, Grzanka P, Sladek K, Okon K, Cmiel A, Mikos M, Mikrut S, Pulka G, Gross-Sondej I, Nizankowska-Mogilnicka E, et al. The use of endobronchial ultrasonography in assessment of bronchial wall remodeling in patients with asthma. Chest 2009;136:797–804. [DOI] [PubMed] [Google Scholar]
- 27.Lemaire A, Nikolic I, Petersen T, Haney JC, Toloza EM, Harpole DH Jr, D'Amico TA, Burfeind WR. Nine-year single center experience with cervical mediastinoscopy: complications and false negative rate. Ann Thorac Surg 2006;82:1185–1189. [DOI] [PubMed] [Google Scholar]
- 28.Little AG, Rusch VW, Bonner JA, Gaspar LE, Green MR, Webb WR, Stewart AK. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051–2056. [DOI] [PubMed] [Google Scholar]
- 29.Holty JE, Kuschner WG, Gould MK. Accuracy of transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: a meta-analysis. Thorax 2005;60:949–955. (See comment). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasufuku K, Chiyo M, Koh E, Moriya Y, Iyoda A, Sekine Y, Shibuya K, Iizasa T, Fujisawa T. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer 2005;50:347–354. [DOI] [PubMed] [Google Scholar]
- 31.Herth FJ, Eberhardt R, Vilmann P, Krasnik M, Ernst A. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax 2006;61:795–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bauwens O, Dusart M, Pierard P, Faber J, Prigogine T, Duysinx B, Nguyen B, Paesmans M, Sculier JP, Ninane V. Endobronchial ultrasound and value of PET for prediction of pathological results of mediastinal hot spots in lung cancer patients. Lung Cancer 2008;61:356–361. [DOI] [PubMed] [Google Scholar]
- 33.Szlubowski A, Kuzdzal J, Kolodziej M, Soja J, Pankowski J, Obrochta A, Kopinski P, Zielinski M. Endobronchial ultrasound-guided needle aspiration in the non-small cell lung cancer staging. Eur J Cardiothorac Surg 2009;35:332–335. [DOI] [PubMed] [Google Scholar]
- 34.Herth FJ, Ernst A, Eberhardt R, Vilmann P, Dienemann H, Krasnik M. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically normal mediastinum. Eur Respir J 2006;28:910–914. [DOI] [PubMed] [Google Scholar]
- 35.Yasufuku K, Nakajima T, Motoori K, Sekine Y, Shibuya K, Hiroshima K, Fujisawa T. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest 2006;130:710–718. [DOI] [PubMed] [Google Scholar]
- 36.Herth FJ, Eberhardt R, Krasnik M, Ernst A. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest 2008;133:887–891. [DOI] [PubMed] [Google Scholar]
- 37.Hwangbo B, Kim SK, Lee HS, Lee HS, Kim MS, Lee JM, Kim HY, Lee GK, Nam BH, Zo JI. Application of endobronchial ultrasound-guided transbronchial needle aspiration following integrated PET/CT in mediastinal staging of potentially operable non-small cell lung cancer. Chest 2009;135:1280–1287. [DOI] [PubMed] [Google Scholar]
- 38.Ernst A, Eberhardt R, Krasnik M, Herth FJ. Efficacy of endobronchial ultrasound-guided transbronchial needle aspiration of hilar lymph nodes for diagnosing and staging cancer. J Thorac Oncol 2009;4:947–950. [DOI] [PubMed] [Google Scholar]
- 39.Lee HS, Lee GK, Lee HS, Kim MS, Lee JM, Kim HY, Nam BH, Zo JI, Hwangbo B. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest 2008;134:368–374. [DOI] [PubMed] [Google Scholar]
- 40.Ernst A, Anantham D, Eberhardt R, Krasnik M, Herth FJ. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008;3:577–582. [DOI] [PubMed] [Google Scholar]
- 41.Herth FJ, Annema JT, Eberhardt R, Yasufuku K, Ernst A, Krasnik M, Rintoul RC. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol 2008;26:3346–3350. [DOI] [PubMed] [Google Scholar]
- 42.Mateu-Navarro M, Rami-Porta R, Bastus-Piulats R, Cirera-Nogueras L, Gonzalez-Pont G. Remediastinoscopy after induction chemotherapy in non-small cell lung cancer. Ann Thorac Surg 2000;70:391–395. [DOI] [PubMed] [Google Scholar]
- 43.Van Schil P, van der Schoot J, Poniewierski J, Pauwels M, Carp L, Germonpre P, De Backer W. Remediastinoscopy after neoadjuvant therapy for non-small cell lung cancer. Lung Cancer 2002;37:281–285. [DOI] [PubMed] [Google Scholar]
- 44.Stamatis G, Fechner S, Hillejan L, Hinterthaner M, Krbek T. Repeat mediastinoscopy as a restaging procedure. Pneumologie 2005;59:862–866. [DOI] [PubMed] [Google Scholar]
- 45.Rice DC, Steliga MA, Stewart J, Eapen G, Jimenez CA, Lee JH, Hofstetter WL, Marom EM, Mehran RJ, Vaporciyan AA, et al. Endoscopic ultrasound-guided fine needle aspiration for staging of malignant pleural mesothelioma. Ann Thorac Surg 2009;88:862–868. [DOI] [PubMed] [Google Scholar]
- 46.Nosotti M, Tosi D, Palleschi A, Ferrero S, Rosso L. Transbronchial needle aspiration under direct endobronchial ultrasound guidance of PET-positive isolated mediastinal adenopathy in patients with previous malignancy. Surg Endosc 2009;23:1356–1359. [DOI] [PubMed] [Google Scholar]
- 47.Nakajima T, Yasufuku K, Iyoda A, Yoshida S, Suzuki M, Sekine Y, Shibuya K, Hiroshima K, Nakatani Y, Fujisawa T. The evaluation of lymph node metastasis by endobronchial ultrasound-guided transbronchial needle aspiration: crucial for selection of surgical candidates with metastatic lung tumors. J Thorac Cardiovasc Surg 2007;134:1485–1490. [DOI] [PubMed] [Google Scholar]
- 48.Nakajima T, Yasufuku K, Wong M, Iyoda A, Suzuki M, Sekine Y, Shibuya K, Hiroshima K, Iizasa T, Fujisawa T. Histological diagnosis of mediastinal lymph node metastases from renal cell carcinoma by endobronchial ultrasound-guided transbronchial needle aspiration. Respirology 2007;12:302–303. [DOI] [PubMed] [Google Scholar]
- 49.Nakajima T, Yasufuku K, Suzuki M, Sekine Y, Shibuya K, Hiroshima K, Fujisawa T. Histological diagnosis of spinal chondrosarcoma by endobronchial ultrasound-guided transbronchial needle aspiration. Respirology 2007;12:308–310. [DOI] [PubMed] [Google Scholar]
- 50.Chow A, Oki M, Saka H, Moritani S, Usami N. Metastatic mediastinal lymph node from an unidentified primary papillary thyroid carcinoma diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Intern Med 2009;48:1293–1296. [DOI] [PubMed] [Google Scholar]
- 51.Wong M, Yasufuku K, Nakajima T, Herth FJ, Sekine Y, Shibuya K, Iizasa T, Hiroshima K, Lam WK, Fujisawa T. Endobronchial ultrasound: new insight for the diagnosis of sarcoidosis. Eur Respir J 2007;29:1182–1186. [DOI] [PubMed] [Google Scholar]
- 52.Garwood S, Judson MA, Silvestri G, Hoda R, Fraig M, Doelken P. Endobronchial ultrasound for the diagnosis of pulmonary sarcoidosis. Chest 2007;132:1298–1304. [DOI] [PubMed] [Google Scholar]
- 53.Oki M, Saka H, Kitagawa C, Tanaka S, Shimokata T, Kawata Y, Mori K, Kajikawa S, Ichihara S, Moritani S. Real-time endobronchial ultrasound-guided transbronchial needle aspiration is useful for diagnosing sarcoidosis. Respirology 2007;12:863–868. [DOI] [PubMed] [Google Scholar]
- 54.Morales CF, Patefield AJ, Strollo PJ Jr, Schenk DA. Flexible transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest 1994;106:709–711. [DOI] [PubMed] [Google Scholar]
- 55.Trisolini R, Agli LL, Cancellieri A, Poletti V, Tinelli C, Baruzzi G, Patelli M. The value of flexible transbronchial needle aspiration in the diagnosis of stage I sarcoidosis. Chest 2003;124:2126–2130. [DOI] [PubMed] [Google Scholar]
- 56.Tremblay A, Stather DR, Maceachern P, Khalil M, Field SK. A randomized controlled trial of standard vs endobronchial ultrasonography-guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest 2009;136:340–346. [DOI] [PubMed] [Google Scholar]
- 57.Kennedy MP, Morice RC, Jimenez CA, Eapen GA. Endobronchial ultrasound appearance of pulmonary artery thrombus in a patient with thymic carcinoma. J Thorac Oncol 2007;2:862. [DOI] [PubMed] [Google Scholar]
- 58.MacEachern P, Dang B, Stather D, Tremblay A. Tumor invasion into pulmonary vessels viewed by endobronchial ultrasound. Journal of Bronchology 2008;15:206–207. [Google Scholar]
- 59.Aumiller J, Herth FJ, Krasnik M, Eberhardt R. Endobronchial ultrasound for detecting central pulmonary emboli: a pilot study. Respiration 2009;77:298–302. [DOI] [PubMed] [Google Scholar]
- 60.Ernst A, Silvestri GA, Johnstone D. American College of Chest Physicians. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest 2003;123:1693–1717. [DOI] [PubMed] [Google Scholar]
- 61.Gildea TR, Mazzone PJ, Karnak D, Meziane M, Mehta AC. Electromagnetic navigation diagnostic bronchoscopy: a prospective study. Am J Respir Crit Care Med 2006;174:982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eberhardt R, Anantham D, Herth F, Feller-Kopman D, Ernst A. Electromagnetic navigation diagnostic bronchoscopy in peripheral lung lesions. Chest 2007;131:1800–1805. [DOI] [PubMed] [Google Scholar]
- 63.Makris D, Scherpereel A, Leroy S, Bouchindhomme B, Faivre JB, Remy J, Ramon P, Marquette CH. Electromagnetic navigation diagnostic bronchoscopy for small peripheral lung lesions. Eur Respir J 2007;29:1187–1192. [DOI] [PubMed] [Google Scholar]
- 64.Eberhardt R, Anantham D, Ernst A, Feller-Kopman D, Herth F. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med 2007;176:36–41. [DOI] [PubMed] [Google Scholar]
- 65.Anantham D, Feller-Kopman D, Shanmugham LN, Berman SM, DeCamp MM, Gangadharan SP, Eberhardt R, Herth F, Ernst A. Electromagnetic navigation bronchoscopy-guided fiducial placement for robotic stereotactic radiosurgery of lung tumors: a feasibility study. Chest 2007;132:930–935. [DOI] [PubMed] [Google Scholar]
- 66.McGuire FR, Liming J, Ochran T, Kerley JM, McLemore TL. Real-time endobronchial ultrasound guided implantation of radiotherapy monitoring devices. Journal of Bronchology 2007;14:42–45. [Google Scholar]
- 67.Chhajed PN, Shibuya K, Hoshino H, Chiyo M, Yasufuku K, Hiroshima K, Fujisawa T. A comparison of video and autofluorescence bronchoscopy in patients at high risk of lung cancer. Eur Respir J 2005;25:951–955. [DOI] [PubMed] [Google Scholar]
- 68.Haussinger K, Becker H, Stanzel F, Kreuzer A, Schmidt B, Strausz J, Cavaliere S, Herth F, Kohlhaufl M, Muller KM, et al. Autofluorescence bronchoscopy with white light bronchoscopy compared with white light bronchoscopy alone for the detection of precancerous lesions: a European randomised controlled multicentre trial. Thorax 2005;60:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lam B, Wong MP, Fung SL, Lam DC, Wong PC, Mok TY, Lam FM, Ip MS, Ooi CG, Lam WK. The clinical value of autofluorescence bronchoscopy for the diagnosis of lung cancer. Eur Respir J 2006;28:915–919. [DOI] [PubMed] [Google Scholar]
- 70.Ueno K, Kusunoki Y, Imamura F, Yoshimura M, Yamamoto S, Uchida J, Tsukamoto Y. Clinical experience with autofluorescence imaging system in patients with lung cancers and precancerous lesions. Respiration 2007;74:304–308. [DOI] [PubMed] [Google Scholar]
- 71.Edell E, Lam S, Pass H, Miller YE, Sutedja T, Kennedy T, Loewen G, Keith RL, Gazdar A. Detection and localization of intraepithelial neoplasia and invasive carcinoma using fluorescence-reflectance bronchoscopy: an international, multicenter clinical trial. J Thorac Oncol 2009;4:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kennedy TC, McWilliams A, Edell E, Sutedja T, Downie G, Yung R, Gazdar A, Mathur PN. American College of Chest Physicians. Bronchial intraepithelial neoplasia/early central airways lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:221S–233S. [DOI] [PubMed] [Google Scholar]
- 73.Bota S, Auliac JB, Paris C, Metayer J, Sesboue R, Nouvet G, Thiberville L. Follow-up of bronchial precancerous lesions and carcinoma in situ using fluorescence endoscopy. Am J Respir Crit Care Med 2001;164:1688–1693. [DOI] [PubMed] [Google Scholar]
- 74.Hoshino H, Shibuya K, Chiyo M, Iyoda A, Yoshida S, Sekine Y, Iizasa T, Saitoh Y, Baba M, Hiroshima K, et al. Biological features of bronchial squamous dysplasia followed up by autofluorescence bronchoscopy. Lung Cancer 2004;46:187–196. [DOI] [PubMed] [Google Scholar]
- 75.Breuer RH, Pasic A, Smit EF, van Vliet E, Vonk Noordegraaf A, Risse EJ, Postmus PE, Sutedja TG. The natural course of preneoplastic lesions in bronchial epithelium. Clin Cancer Res 2005;11:537–543. [PubMed] [Google Scholar]
- 76.Shibuya K, Fujisawa T, Hoshino H, Baba M, Saitoh Y, Iizasa T, Suzuki M, Otsuji M, Hiroshima K, Ohwada H. Fluorescence bronchoscopy in the detection of preinvasive bronchial lesions in patients with sputum cytology suspicious or positive for malignancy. Lung Cancer 2001;32:19–25. [DOI] [PubMed] [Google Scholar]
- 77.Vincent BD, Fraig M, Silvestri GA. A pilot study of narrow-band imaging compared to white light bronchoscopy for evaluation of normal airways and premalignant and malignant airways disease. Chest 2007;131:1794–1799. [DOI] [PubMed] [Google Scholar]
- 78.Herth FJ, Eberhardt R, Anantham D, Gompelmann D, Zakaria MW, Ernst A. Narrow-band imaging bronchoscopy increases the specificity of bronchoscopic early lung cancer detection. J Thorac Oncol 2009;4:1060–1065. [DOI] [PubMed] [Google Scholar]
- 79.Han S, El-Abbadi NH, Hanna N, Mahmood U, Mina-Araghi R, Jung WG, Chen Z, Colt H, Brenner M. Evaluation of tracheal imaging by optical coherence tomography. Respiration 2005;72:537–541. [DOI] [PubMed] [Google Scholar]
- 80.Tsuboi M, Hayashi A, Ikeda N, Honda H, Kato Y, Ichinose S, Kato H. Optical coherence tomography in the diagnosis of bronchial lesions. Lung Cancer 2005;49:387–394. [DOI] [PubMed] [Google Scholar]
- 81.Whiteman SC, Yang Y, Gey van Pittius D, Stephens M, Parmer J, Spiteri MA. Optical coherence tomography: real-time imaging of bronchial airways microstructure and detection of inflammatory/neoplastic morphologic changes. Clin Cancer Res 2006;12:813–818. [DOI] [PubMed] [Google Scholar]
- 82.Lam S, Standish B, Baldwin C, McWilliams A, leRiche J, Gazdar A, Vitkin AI, Yang V, Ikeda N, MacAulay C. In vivo optical coherence tomography imaging of preinvasive bronchial lesions. Clin Cancer Res 2008;14:2006–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coxson HO, Quiney B, Sin DD, Xing L, McWilliams AM, Mayo JR, Lam S. Airway wall thickness assessed using computed tomography and optical coherence tomography. Am J Respir Crit Care Med 2008;177:1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thiberville L, Moreno-Swirc S, Vercauteren T, Peltier E, Cave C, Bourg Heckly G. In vivo imaging of the bronchial wall microstructure using fibered confocal fluorescence microscopy. Am J Respir Crit Care Med 2007;175:22–31. [DOI] [PubMed] [Google Scholar]
- 85.Thiberville L, Salaun M, Lachkar S, Dominique S, Moreno-Swirc S, Vever-Bizet C, Bourg-Heckly G. Human in vivo fluorescence microimaging of the alveolar ducts and sacs during bronchoscopy. Eur Respir J 2009;33:974–985. [DOI] [PubMed] [Google Scholar]
- 86.Zhu CQ, da Cunha Santos G, Ding K, Sakurada A, Cutz JC, Liu N, Zhang T, Marrano P, Whitehead M, Squire JA, et al. National Cancer Institute of Canada Clinical Trials Group Study, B.R.21. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 2008;26:4268–4275. [DOI] [PubMed] [Google Scholar]
- 87.Inoue T, Miyazawa T, Kurimoto N, Fujita Y, Nakamura M, Oshige M, Ishida A, Saji J, Miyazu YM, Shirakawa T, et al. Gefitinib therapy for pulmonary adenocarcinoma with EGFR mutation diagnosed by transbronchial lung biopsy using endobronchial ultrasonography with guide sheath. Journal of Bronchology 2006;13:201–203. [Google Scholar]
- 88.Horiike A, Kimura H, Nishio K, Ohyanagi F, Satoh Y, Okumura S, Ishikawa Y, Nakagawa K, Horai T, Nishio M. Detection of epidermal growth factor receptor mutation in transbronchial needle aspirates of non-small cell lung cancer. Chest 2007;131:1628–1634. [DOI] [PubMed] [Google Scholar]
- 89.Nakajima T, Yasufuku K, Suzuki M, Hiroshima K, Kubo R, Mohammed S, Miyagi Y, Matsukuma S, Sekine Y, Fujisawa T. Assessment of epidermal growth factor receptor mutation by endobronchial ultrasound-guided transbronchial needle aspiration. Chest 2007;132:597–602. [DOI] [PubMed] [Google Scholar]
- 90.Nakajima T, Yasufuku K, Suzuki M, Fujiwara T, Shibuya K, Takiguchi Y, Hiroshima K, Kimura H, Yoshino I. Assessment of chemosensitivity-related aberrant methylation of non-small cell lung cancer by EBUS-TBNA. Journal of Bronchology and Interventional Pulmonology 2009;16:10–14. [DOI] [PubMed] [Google Scholar]
- 91.Mohamed S, Yasufuku K, Nakajima T, Hiroshima K, Kubo R, Iyoda A, Yoshida S, Suzuki M, Sekine Y, Shibuya K, et al. Analysis of cell cycle-related proteins in mediastinal lymph nodes of patients with N2-NSCLC obtained by EBUS-TBNA: relevance to chemotherapy response. Thorax 2008;63:642–647. [DOI] [PubMed] [Google Scholar]
- 92.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543–3551. [DOI] [PubMed] [Google Scholar]
- 93.Spira A, Beane JE, Shah V, Steiling K, Liu G, Schembri F, Gilman S, Dumas YM, Calner P, Sebastiani P, et al. Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nat Med 2007;13:361–366. [DOI] [PubMed] [Google Scholar]
- 94.Wallace MB, Pascual JM, Raimondo M, Woodward TA, McComb BL, Crook JE, Johnson MM, Al-Haddad MA, Gross SA, Pungpapong S, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA 2008;299:540–546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.