Abstract
Purpose
Advances in neurobiology are providing new opportunities to investigate the neurological systems underlying motor speech control. This study explores the perceptual characteristics of the speech of three genotypes of spino-cerebellar ataxia (SCA) as manifest in four different speech tasks.
Methods
Speech samples from 26 speakers with SCA were perceptually rated by experienced listeners. The genotypes were: SCA1, SCA5, or SCA6. The speech tasks were: diadochokinesis, word repetition, sentence reading, and picture description. The speech samples were rated using two sets of dimensions characterized as primary (e.g., articulation, rate, and rhythm) or secondary (e.g., imprecise consonants, excess and equal stress, and harsh voice).
Results
On primary dimensions, SCA6 was the most impaired generally. Articulation was the most severely affected dimension and the diadochokinesis task was most effective in revealing speech impairments. On secondary dimensions, picture description was the task most likely to produce abnormal speech. The SCA groups shared articulatory problems but differed with respect to abnormal voice features.
Conclusions
These results support previous characterizations of ataxic dysarthria, and provide further information about the speech characteristics of genetic subtypes. Task demands affect perceptual ratings. Voice characteristics may be key to differentiating ataxic subtypes. As the genetic disorders that affect speech become better understood, more detailed characterizations of motor control systems should emerge.
1.0. Introduction
Ataxia is a collection of neurological signs and symptoms produced by a variety of etiologies. The principle feature of ataxia is motor incoordination, which commonly extends to limb movements, gait, eye movements, swallowing, and speech. An ataxia can be acquired or hereditary. Acquired ataxias can be the result of a range of neuropathologic processes including stroke, tumor, toxin, or paraneoplastic degeneration, with associated neurologic impairments as a function of the extent and location of the lesion or lesions (Perlman, 2006). In contrast with the acquired ataxias, the hereditary ataxias are increasingly associated with specific genetic abnormalities and consequent neuropathophysiology at the molecular and cellular levels (Bird, 2009).
One class of hereditary ataxias is referred to as spinocerebellar ataxia (SCA). These are progressive neurodegenerative autosomal dominant diseases. Individuals with SCA commonly show gradually progressive gait unsteadiness, generalized incoordination of the extremities, and dysarthria due to degeneration of the cerebellum, and in some SCAs, the brainstem and spinal cord (Gomez & Subramony, 2003; Schöls, Bauer, Schmidt, Schulte, & Riess, 2004). Individuals with SCA are highly heterogenous and previous classification schemes based on clinical presentation, age of onset, rate of progression, hereditary pattern, and associated clinical features have been replaced by recent advances in the molecular genetics that allow the identification of genetic abnormalities associated with each SCA type.
The first genetically identified SCA (SCA1) is associated with an excessive number of trinucleotide repeats on chromosome 6 (Orr et al., 1993). SCA1 involves degeneration of the cerebellum and brainstem, typically has its onset during the third or fourth decade of life in affected individuals, and progresses over decades. SCA6 is also a progressive cerebellar degenerative disease associated with an excessive number of trinucleotide repeats, in this case on chromosome 19 (Zhuchenko et al., 1997; Gomez et al., 1997). SCA6 is often said to produce a “pure cerebellar syndrome” since gross neuropathological degenerative changes are restricted to the cerebellum. Age of onset is about the fifth decade of life. SCA6 has been reported to show a mild presentation and slow progression. Unlike SCA1 and SCA6, SCA5 is not a trinucleotide repeat disease. The associated genetic abnormality has been located on chromosome 11 (Ranum, Schut, Lundgren, & Livingston, 1994) and is believed to be a spectrin mutation (Ikeda et al., 2005). It commonly has its onset during the third or fourth decade of life. The disease usually progresses slowly compared to other SCAs and is described as clinically milder than other SCAs.
In spite of their neuropathological and genetic differences, SCA1, 5, and 6 all share dysarthria as a significant clinical feature. Further, a Positron Emission Tomography (PET) study of regional cerebral blood flow during speech repetition in a mixed sample of SCA1, 5 and 6 subjects who provided the speech samples used in this study showed that as a group, these SCAs had the same relationship between repetition rate and regional blood flow found in normal speakers (Sidtis, Strother, & Rottenberg, 2003). As the repetition rate, measured in syllables per second, increased, blood flow increased in Broca’s area and and decreased in the right caudate nucleus. Unlike the normal speakers, though, increased repetition rate in the SCAs was also associated with an increase in blood flow in the right cerebellum and a decrease in flow in the left superior temporal region (Sidtis, Gomez, Groshong, Strother, & Rottenberg, 2006). The cerebellar and temporal changes were viewed as a consequence of and adaptation to cerebellar degeneration, respectively.
Schalling, Hammarberg, & Hartelius (2007) studied the speech of a large group of progressive hereditary ataxic subjects, 12 with an SCA determined by genetic testing (SCA 2, 3, 7, and 8) and nine with a clinical diagnosis, using acoustic and perceptual measures. Their results suggested that the perceptual judgements reflected two major factors. The first factor reflected speech timing and articulation, driven by the following judgments: imprecise consonants, monotony, prolonged intervals, imprecise vowels, stereotypic intonational patterns, equalized stress, and short phrases. The second factor reflected voice quality, driven by the perception of harshness, strained-strangled voice, and glottal fry. However, the variability on each of these perceptual dimensions was high in the SCA group, limiting the clinical utility of individual dimensions in separating normal controls from the mixed group of SCA subjects.
Few studies have examined the speech characteristics of specific SCAs. Schalling & Hartelius (2004) provided a detailed speech characterization in three subjects with SCA3 or SCA 7 using acoustic analysis focusing primarily on durational measures. The tasks were sustained phonation, alternating motion rate/sequential motion rate tasks (syllable repetition), sentence and paragraph reading, story telling, and conversation. Speech rates, including pause time, were calculated for reading, story telling, conversation, and syllable repetition, while syllable and pause durations were the measures for syllable repetition. Fundamental frequency values were derived for continuous speech and sustained phonation. Since the same speech characteristics were not assessed across all task modes, but were selected for each task, the effect of task on ataxic features could not be readily determined from this study. Depending on the task examined, subjects demonstrated decreased speech rate, increased pause duration, increased duration variability of alternating motion rate, and vocal instability.
Schalling, Hammarberg & Hartelius (2008) subsequently conducted a three-year longitudinal study with six subjects with SCA (SCA2, SCA3, SCA 7) and three other subjects with a degenerative cerebellar disorder, utilizing a range of speech tasks. However, acoustic analysis was performed on syllable repetition, and perceptual ratings were performed on paragraph reading. Acoustic analyses indicated reductions in speech rate over time. Perceptual ratings indicated that articulatory and prosodic features were more severely affected than voice quality. Speech in the group of SCAs studied reflected typical characteristic of ataxic dysarthria, but SCA sub-typing was not attempted, and effects of speech parameters across speech task measures were not reported or compared.
A longitudinal study of our SCA subjects who were identified prior to significant symptom onset examined changes in regional cerebral blood flow and speech (Sidtis, Strother, Groshong, Rottenberg, & Gomez, 2010). Over a two year period, speech timing shifted towards equal intervals in a syllable repetition task (pa-ta-ka), and blood flow was reduced in the cerebellum but increased in Broca’s area. These studies suggest that in SCA, articulatory and phonatory abnormalities may progress at different rates, that cerebellar degeneration may be accompanied by neocortical compensation, and that significant signs of disease progression can be documented over a two-to-three year period, at least during the early stages of the disease.
1.1. Goals of the study
The present study was undertaken to examine the clinical characteristics of the speech of individuals from three genotypes of SCA who participated in studies of brain activity during speech (Sidtis et al., 2006; 2010), and to further determine if specific speech abnormalities could be associated with each SCA type. Given the limited knowledge of possible differences in disordered speech across the newly identified genotypes of SCA, this is a descriptive study. Inferential statistics were used to determine if genotypic differences existed in the perceptual ratings of speech dimensions commonly used in clinical evaluation. For the subset of speech samples from SCA subjects judged to be abnormal on any of these primary dimensions, additional perceptual ratings, inferential statistics, and statistical discovery techniques were employed to examine potential relationships among the three genotypes, speech task, and a larger number of perceptual dimensions widely used in clinical characterizations. Although perceptual studies do not have the objective quality of acoustic studies, they can have greater functional saliency for clinicians as well as providing a first step towards acoustic analyses, especially given the acoustic complexity of many of the perceptual dimensions of disordered speech.
A secondary goal of this study was to specify differences in speech characteristic presentations that appear in different speech tasks. The effects of task demands on measurable parameters of voice, fluency and articulation have been reported. Both perceptual and acoustic studies reveal that dysarthric features vary significantly with speech task (Kempler & Van Lancker, 2002; Sidtis, Rogers, Godier, Tagliati & Sidtis, 2010). Differences arise from the kinds of demands placed by specific tasks (reading, spontaneous speech, repetition) on the cerebral systems involved in planning and execution of speech. These factors are compounded by current controversies around which tasks are the most useful or revelatory for motor speech evaluation (Weismer, 2006; Kent, Kent, Rosenbek, Vorperian, & Weismer, 1997; Folkins, Moon, Luschei, Robin, Tye-Murray & Moll, 1995; Ziegler, 2002). By examining the same perceptual characteristics on all tasks, the relative sensitivity of each task to revealing specific dysarthric characteristics could be assessed. So, the descriptive goal of this study involves a multi-dimensional approach: SCA genotypes by speech task by perceptual dimension. Based on clinical experience, some tasks are expected to reflect ataxic dysarthria across tasks. On the other hand, certain tasks may be more sensitive to genotype-specific speech problems.
Another consideration is disease severity, which takes on new meaning with genetic testing. Subjects who would not have reached clinical attention based on the severity of their signs and symptoms can now be studied having been correctly classified by family membership and genetic testing. We have included several such individuals. However, to increase our sensitivity to potential genotypic differences across a range of severity, we have also included annual follow-up data on a subset of subjects who were followed for up to three years. In a previous PET study, these subjects demonstrated progressive declines of cerebellar blood flow and changes in speech timing over the period of study (Sidtis, Strother, Groshong, Rottenberg, & Gomez, 2010). As a complex of neurologic signs and symptoms that varies with SCA type, the concept of severity in ataxia is not straightforward. Similarly, the degree of cerebellar atrophy is not likely to have the same functional impact in SCAs with and without brainstem involvement. In the present study, we relied on a composite score of ratings on four primary dimensions of speech to estimate severity.
2.1. Material and methods
The speech samples obtained from subjects representing three genotypes of SCA, consisting of four speech tasks commonly used in motor speech evaluation, were rated perceptually based on one or two sets of speech dimensions. Perceptual speech ratings have been widely used in clinical settings to evaluate and diagnose dysarthrias. Despite recent technological advances in the instrumental assessment of dysarthria, perceptual assessment is still considered a primary clinical tool because it is convenient, economical, and robust (Kent, 1996). Profiles of the perceptual characteristics of different SCAs, as they vary systematically with commonly utilized speech tasks, may yield some insights into the functional significance of affected neural systems and eventually provide clinical information useful in evaluation procedures. As previously stated, this is a descriptive study in which it is expected that the SCA genotypes will share some features in their dysarthria profiles and differ in others. Further, based on clinical experience, it is expected that some of these features will be consistent across tasks while other features may be unique to task and genotype combinations.
2.2. Speech samples
A total of 106 previously recorded speech samples from 32 evaluations of 26 speakers with SCA were used for the clinical ratings. These subjects originally participated in a positron tomography study of speech production in SCA (Sidtis, Gomez, Groshong, Strother, & Rottenberg, 2006). Five of the speakers had second annual follow-up evaluations (3 SCA1 and 2 SCA5) and one of these (1 SCA1) had a third annual follow-up evaluation. The SCA subjects with follow-up examinations entered the study at a very early stage of their disease but were found to have significant changes in their speech and brain function over the course of follow-up (Sidtis, Strother, Groshong, Rottenberg, & Gomez, 2010). As SCA is progressive disease, the follow-up evaluations were included in this study to capture a wider range of severity within SCA types. This was important as genetic identification enabled several SCA subjects to be entered into the parent study with minimal signs and symptoms. The mean age of the SCA speakers at the time of recording was 42.3 ± 18.5 yrs (range = 18 – 84 yrs). Fifteen were male and 11 were female. There were three SCA subgroups: SCA1, SCA5, and SCA6. The SCA1 group was comprised of 15 speakers (8 males and 7 females) with a mean age of 33.9 ± 14.8 yrs (range = 18 – 58 yrs). The SCA5 group consisted of 11 speakers (3 males and 8 females) with a mean age of 49.3 ± 15.5 yrs (range = 22 – 68). The SCA6 group was composed of 6 speakers (2 males and 4 females) with a mean age of 50.8 ± 25.1 (range = 24 – 84). The SCA groups are summarized in Table 1.
Table 1.
Summary of the demographic characteristics, mean diadochokinetic (DDK) rates for /pa-ta-ka/ repetition, and mean global impairment scores (GIS) of the three SCA groups.
| Descriptors | SCA 1 (n = 15) | SCA 5 (n = 11) | SCA 6 (n = 6) |
|---|---|---|---|
| Age (yrs) | 33.9 ± 14.8 | 49.3 ± 15.5 | 50.8 ± 25.1 |
| M/F | 8/7 | 3/8 | 2/4 |
| DDK (syllables/sec) | 3.8 ± 1.7 | 3.6 ± 1.0 | 3.4 ± 1.0 |
| GIM (grand mean) | 2.0 ± 1.1 | 1.8 ± 0.8 | 2.2 ± 1.1 |
The speech samples were drawn from a multi-purpose examination of individuals with SCA as part of an interdisciplinary research program. Individuals and families with specific SCAs were recruited from across the United States to participate in several days of study. The limited time available with the subjects required that examination items served multiple purposes. The samples used for this study represented four speech tasks: diadochokinesis, word repetition, sentence repetition, and picture description. The diadochokinesis involved repeating the syllables /pa-ta-ka/ as quickly as possible on a single breath. In the word repetition task, speakers were asked to repeat words from the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983). The sentence repetition task involved repeating statements (e.g., Sue could give pancakes to the boys, He will never visit the zoo, etc.) spoken by the examiner. The sentences were phonetically balanced but were primarily designed to assess prosody. In the picture description task, speakers were requested to describe the “Cookie theft” picture from the Boston Diagnostic Aphasia Examination (Goodglass & Kaplan, 1983). These tasks were chosen in consideration of previous findings that repetition and spontaneous speech affect motor speech parameters differently (Kempler & Van Lancker, 2002; Sidtis et al., 2010; Ziegler & Wessel, 1996). Further, repetition at different levels of linguistic structure might be similarly expected to exact disparate demands in the speech production system. Thus the repetition tasks utilized here represent three levels of linguistic structure (syllable, word, and sentence). Picture description was used to simulate spontaneous speech while constraining the range of verbal material.
The ataxic speakers performed one to four of the tasks. All of the 32 recorded sessions included the diadochokinetic task. There were 24 samples of the word repetition task, 27 samples of the sentence repetition task, and 23 samples of the picture description task, yielding 106 speech samples for the perceptual rating protocol.
Speech samples were recorded using a Marantz Cassette Recorder (Model PMD201). For the listening task, the tapes were digitized at a 44kHz sampling rate using Marantz professional CD recorder (Model CDR300). Then, from each speech sample, portions of approximately 6 to 10-seconds in length were extracted. The samples were recorded onto compact disks for the listening task.
2.3. Raters
Ten students enrolled in a speech-language pathology Master’s Degree program participated as raters in this study. The raters were native speakers of American English. The mean age of the participants was 23.1 ± 1.2 yrs (range = 22 – 25 yrs).
2.4. Speech dimensions
For the listening task, two sets of speech dimensions were used: Primary dimensions and secondary dimensions. Primary dimensions were developed to evaluate the degree of overall speech impairment, and secondary dimensions were used to examine a detailed profile of the speech characteristics identified as most deviant in individuals with cerebellar lesions (Duffy, 2005; Ackermann & Hertrich, 1994; Hartelius, Runmarker, Anderson, & Nord, 2000).
The speech samples represented four tasks: syllable repetition, word repetition, sentence repetition, and story description. Each sample was rated with respect to the presence of abnormality on the primary dimensions of articulation, rate, rhythm, and prosody. For the picture description task, an intelligibility rating was also obtained. Abnormal articulation is most often cited as a dysarthric feature of ataxia. We selected both rhythm and prosody as dimensions even though, in some contexts, rhythm is considered a subcomponent of prosody (Sidtis & Van Lancker Sidtis, 2003). Rhythm is a complex concept. In English, rhythm depends on accent placement, the sequencing of reduced and unreduced vowels, and syllable structures (Ladefoged, 2005). For evaluation of ataxic speakers, “excess and equal stress” represents a disorder of rhythm, and “monopitch” is a disorder of prosody (Duffy, 2005); similarly, an “irregular rhythm of repetitive movement” and a breakdown in prosody (Murdoch, 2004; Duffy, 2005) are separately described for the disorder. We therefore requested raters to evaluate “rhythm” as referring to regular and normal syllable accents in the sample (called pitch accents in Ladefoged, 2005), and “prosody” as the melodic contour of the intonational entity (syllabic unit, word, or sentence).
A scale of one to five was used, with scores of 2 or greater considered abnormal. If a speech sample was rated as abnormal on any of the primary dimensions, the sample was also rated on secondary dimensions. Samples not rated as abnormal were not subjected to further analysis. This strategy enabled a more efficient evaluation of the speech samples allowing more time for raters to focus on abnormal tokens for additional ratings on secondary dimensions. Secondary dimensions, which were derived from the Mayo Clinic protocol (Duffy, 2005), were used to examine detailed characteristics of speech samples that were judged as impaired. Secondary dimensions consisted of irregular articulatory breakdown, imprecise consonants, distorted vowels, prolonged phonemes, excess and equal stress, excess loudness variation, hypernasality, voice tremor, harsh voice, breathy voice, and strained-strangled voice. Primary and secondary dimensions are summarized in Table 2.
Table 2.
Primary and secondary dimensions used for rating the ataxic speech samples in this study. Primary dimensions were used to identify the presence of a speech abnormality. The secondary dimensions were used to further characterize abnormal speech. For the picture description task, intelligibility was used as an additional primary dimension as this task produced spontaneous rather than repeated speech.
| Type of measures | Speech dimensions | Purpose |
|---|---|---|
| Primary dimensions | Articulation Rate Rhythm Speech prosody |
To determine whether the speech samples show any impairment and to examine the area of speech difficulty. |
| Secondary dimensions | Irregular articulatory breakdown Imprecise consonants Distorted vowels Prolonged phonemes Excess and equal stress Excess loudness variation Hypernasality Voice tremor Harsh voice Breathy voice Strained-strangled voice |
To determine detailed characteristics of speech samples that were judged as impaired. |
2.5. Listening task
A total of 106 speech samples were presented to each rater individually through Sony headphones using a Marantz professional CD player (Model CDR 3000). The raters were asked to perceptually judge each speech sample for the speech dimensions provided. Perceptual rating of each speech dimension was done using a five-point equal-appearing interval scale. Listeners were instructed to rate each dimension according to the Mayo descriptions, which were provided by the experimenter. Listeners were encouraged to ask questions if the description of the dimension was unclear. For all the dimensions, a rating of “one” represented normal and a rating of “five” represented the most severe deviation from normal. Speech samples were played twice, but could be played again if the raters requested. The raters were instructed to take as much time as necessary. The raters were blinded with respect to the SCA status of the speakers who provided the speech samples.
Before the actual listening task, a training session was given to all raters to familiarize them with the procedure. The training session involved reviewing definitions of the speech dimensions that were to be used in the listening task. Then, raters were asked to rate a total of eight speech samples from three speakers with SCA for training. Samples for the training trials were taken from speakers with SCA who were not included in the rating study. During the training trials the listeners were encouraged to discuss the speech dimensions and rating procedure with the experimenter to insure clarity.
2.6. Rating reliability
Inter-listener reliability for each speech dimension and each task were calculated using Cronbach’s alpha. Reliability ranged from 0.4 to 0.96 across dimensions and tasks with a mean reliability of 0.81. For the speech dimensions across tasks, hypernasality had the lowest reliability (Cronbach’s α = 0.53) while intelligibility had the highest reliability (Cronbach’s α = 0.97). For the speech tasks across dimensions, picture description had the highest reliability (Cronbach’s α = 0.86) while diadochokinesis had a somewhat lower reliability (Cronbach’s α = 0.73).
2.7. Statistical analysis
To examine the effects of SCA, speech dimension, and task, a series of mixed model and repeated measure analyses of variance (ANOVA) was conducted for primary and secondary dimensions. Speech dimension and task were within-subject variables, SCA was a between-subject variable. Each SCA speech sample represented an observation. Inter-rater reliability was high across tasks and dimensions and there was no main effect of rater, so each rater was treated as a replication.
For between-group pair-wise comparisons by t-test, equal variances were not assumed. For within-group pair-wise comparisons, equal variances were assumed. Because of the number of comparisons, and the restriction to samples judged in the first rating phase as abnormal, alpha levels with probabilities less than 0.03 were considered significant.
The ratings of the secondary dimensions were also subjected to two exploratory analyses. Since SCA type is a mutually exclusive categorical variable, a discriminant function analysis was used to determine if there was a pattern in the secondary ratings that could provide a classification of the three genotypes. Within each genotype, stepwise multiple linear regression analyses were performed to determine if there were linear combinations of secondary dimension rating scores that could successfully predict the overall severity of the speech disorder, a continuous variable. These exploratory analyses were employed to complement the results of the inferential statistics that were employed to establish group differences.
3.0. Results
As noted earlier, the characterization of disordered speech as a function of genotype and task requires a multidimensional description. The results reflect the effects of three factors: perceptual dimension, genotype, and task. To facilitate this characterization and allow the reader to review the results with a focus on any one of the three factors, the primary dimension results are presented from the perspective of each factor. Although this results in some redundancy, it allows greater accessibility to the effects of each dimension as it interacts with the other two. For simplicity, a summary of the results for the primary dimensions follows the presentation by factor.
The secondary perceptual dimensions are presented individually with reference to task effects, genotype effects, and their interaction, when present. This section is also followed by a summary of the main results for the secondary dimensions. The results of the two exploratory analyses, discriminant function analysis and multiple linear regression are presented last. These analyses address the profiles of the perceptual characteristics of each SCA, and the predictors of severity of the global speech impairment measure, respectively. Each of these analyses contains summary sections as well.
3.1. Global impairment measure (GIM)
A global measure of impairment was obtained by averaging the ratings across all of the primary measures and tasks. This global measure of impairment was used to provide a general picture of the severity of each SCA type across primary dimensions and tasks (GIM, Table 1). With this global measure, the SCA6 group was rated as more impaired than the SCA5 group [t(98.8) = −2.24; p = 0.027]. No other group differences were observed with the global measure. However, significant SCA group differences were observed as a function of task and primary measure.
3.2. Genotype
3.2.1. SCA1
All of the diadochokinesis samples produced by the SCA1 speakers had abnormal ratings. Ninety-seven percent of the sentence repetition and picture description samples and 47% of the word repetition samples had abnormal ratings. Across tasks, SCA1 articulation was worse than rate [t(149) = 2.43; p = 0.016], rhythm [t(149) = 2.68; p = 0.008], and prosody [t(149) = 2.71; p = 0.0008]. No other pair-wise comparisons were significant in the primary measures averaged across tasks.
3.2.2. SCA5
All of the diadochokinesis samples produced by the SCA5 speakers had abnormal ratings. Ninety-seven percent of the sentence repetition, 62% of the picture description samples and 44% of the word repetition samples had abnormal ratings. Averaged across tasks, articulation was worse than rate [t(109) = 3.39; p = 0.001], rhythm [t(109) = 3.14; p = 0.002], and prosody [t(109) = 4.07; p < 0.0001]. No other pair-wise comparisons were significant in the primary measures averaged across tasks.
3.2.3. SCA6
All of the diadochokinesis samples produced by the SCA6 speakers had abnormal ratings. Ninety-five percent of the sentence repetition, 82% of the picture description samples and 65% of the word repetition samples had abnormal ratings. None of the pair-wise contrasts among the primary measures averaged across tasks were significant.
3.3. Primary dimensions
3.3.1. Main effects of primary dimensions
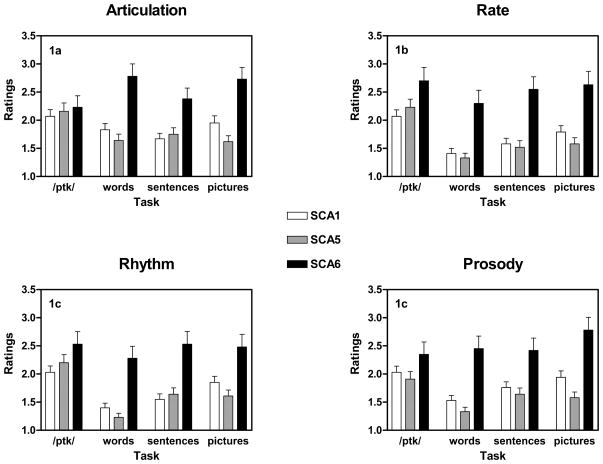
Across SCA types and tasks, articulation was rated as significantly more impaired than rate [t(319) = 3.26; p = 0.001], rhythm [t(319) = 3.7; p < 0.001], and prosody [t(319) = 4.35; p < 0.001]. There were no significant pair-wise differences in severity ratings among rate, rhythm, and prosody. The mean scores are presented as a function of dimension, task, and SCA in Figure 1.
Figure 1.
The four primary dimensions used in this study. Means ± standard errors are presented for each task and SCA group.
3.3.2. Articulation
There was a significant effect of task in articulation [F(3, 678) = 3.74; p = 0.011]. Diadochokinesis articulation scores reflected significantly more impairment than that observed for sentence [t(269) = 5.53; p < 0.001] and word repetition [t(238) = 2.84 p = 0.005]. Articulation during picture description was also more impaired than articulation during sentence repetition [t(229) = −3.43 p = 0.001].
There was a significant effect of SCA type on articulation [F(2, 226) = 7.75; p = 0.001]. SCA6 had the highest articulatory impairment rating and SCA5 had the lowest impairment rating on this dimension, but the individual pair-wise SCA group comparisons were not significant.
SCA type interacted with task as well on this dimension [F(6, 678) = 7.96; p < 0.001]. Articulation during picture description was worse in SCA6 compared to SCA5 [t(58.1) = −4.78; p < 0.001] and SCA1 [t(70.5) = −3.17; p = 0.002]. Articulation during word repetition was also worse in SCA6 compared to SCA5 [t(56.9) = −4.55; p < 0.001] and SCA1 [t(59.6) = −3.83; p < 0.001]. Articulation scores for each SCA type and task are presented in Figure 1A.
3.3.3. Rate
There was a significant effect of task on rate judgments [F(3, 678) = 31.79; p < 0.001]. Across SCA types, diadochokinesis rate was judged as significantly more impaired than sentence repetition rate [t(269) = 5.53; p < 0.001], picture description rate [t(229) = 5.53; p < 0.001], and word repetition rate [t(238) = 2.84 p < 0.001]. Rate during sentence repetition [t(238) = −4.32; p < 0.001] and picture description [t(228) = −5.55; p < 0.001] was also judged to be more impaired than word repetition rate. Finally, rate during picture description was judged to be more impaired than sentence repetition rate [t(229) = −2.74; p = 0.007].
There was also a significant effect of SCA type [F(2, 226) = 11.74; p < 0.001]. SCA6 rates were judged as more impaired than SCA5 rates [t(95.8) = −2.69; p = 0.008].
There was a significant interaction between SCA type and task for rate [F(6, 678) = 2.53; p = 0.02]. SCA6 was more impaired in rate than SCA5 for picture description [t(55.4) = −4.01; p < 0.001], sentence repetition [t(102.3) = −2.78; p = 0.006], and word repetition [t(47.5) = −3.97; p < 0.001]. SCA6 was also more impaired in rate than SCA1 for picture description [t(57.5) = −3.15; p = 0.003], sentence repetition [t(100.5) = −3.0; p = 0.003], and word repetition [t(51.1) = −3.58; p = 0.001]. There were no pair-wise differences between SCA1 and SCA5. Rate scores for each SCA type and task are presented in Figure 1B.
3.3.4. Rhythm
There was a significant effect of task on rhythm [F(3, 678) = 29.08; p < 0.001]. Across SCA types, diadochokinesis rhythm was significantly more impaired than rhythm during sentence repetition [t(269) = 5.53; p < 0.001], rhythm during picture description [t(229) = 5.53; p < 0.001], and rhythm during word repetition [t(238) = 2.84 p < 0.001]. Rhythm during sentence repetition [t(238) = −4.32; p < 0.001] and picture description [t(228) = −5.55; p < 0.001] was also more impaired than rhythm during word repetition. Finally, rhythm during picture description was more impaired than rhythm during sentence repetition [t(229) = −2.74; p = 0.007].
There was a significant effect of SCA type on rhythm [F(2, 226) = 10.13; p < 0.001]. As with rate, there was a trend of SCA6 rhythms being more impaired than SCA5 rhythms although the pairwise comparison was not significant.
There was also a significant interaction between SCA type and task [F(6, 678) = 4.36; p < 0.001]. As with the rate measure, rhythm in SCA6 was more impaired than SCA 5 for picture description [t(56.0) = −3.51; p = 0.001], and word repetition [t(46.6) = −4.67; p < 0.001]. SCA6 was also more impaired in rhythm than SCA1 for picture description [t(58.3) = −2.49; p = 0.016], sentence repetition [t(96.1) = −2.94; p = 0.004], and word repetition [t(51.6) = −3.85; p < 0.001]. There were no pair-wise differences between SCA 1 and SCA 5. Rhythm scores for each SCA type and task are presented in Figure 1C.
3.3.5. Prosody
There was a significant effect of task on prosody [F(3, 678) = 10.68; p < 0.001]. Across SCA types, diadochokinesis prosody was significantly more impaired than prosody during sentence repetition [t(269) = 3.27; p = 0.001] and word repetition [t(238) = 6.69 p < 0.001]. Prosody during sentence repetition [t(238) = −4.21; p < 0.001] and picture description [t(228) = −5.88; p < 0.001] was also more impaired than prosody during word repetition. Finally, prosody during picture description was more impaired than prosody during sentence repetition [t(229) = −2.76; p = 0.006]. There was a significant effect of SCA type [F(2, 226) = 10.9; p < 0.001], but pairwise contrasts only indicated that SCA6 prosody had tendency of being more impaired than SCA5 prosody [t(94.8) = −2.16; p = 0.034].
There was also a significant interaction between SCA type and task [F(6, 678) = 3.96; p = 0.001]. SCA6 was more impaired in prosody than SCA5 for picture description [t(54.0) = −4.85; p < 0.001], and word repetition [t(47.2) = −4.72; p < 0.001]. SCA6 was also more impaired in prosody than SCA1 for picture description [t(60.1) = −3.27; p = 0.002], and word repetition [t(51.5) = −3.82; p < 0.001]. SCA1 was more impaired than SCA5 in the prosody of their picture descriptions [t(185.3) = 2.44; p = 0.016]. Prosody scores for each SCA type and task are presented in Figure 1D.
3.4 Tasks
3.4.1. Diadochokinesis
As noted above, all of the diadochokinesis samples produced by each SCA type were rated as abnormal. Consequently, there were no significant SCA differences.
3.4.2. Word repetition
SCA6 performed more poorly on word repetition than SCA1 [t(54.7) = −4.28; p < 0.001] and SCA5 [t(49.2) = −5.12; p < 0.001]. SCA1 and SCA5 did not differ on this task. This is consistent with the percentage of samples rated as abnormal by each SCA type.
3.4.3. Sentence repetition
SCA6 performed more poorly than SCA1 [t(105.3) = −2.5; p = 0.014]. However, SCA5 did not differ from either SCA1 or SCA6 on sentence repetition. While the SCA types had comparable rates of impairment in their sentence repetition samples, the magnitudes of the ratings reflected the greater severity of speech abnormalities during sentence repetition in the SCA6 group compared to the SCA1 group.
3.4.4. Picture description
SCA6 performed more poorly than SCA1 [t(63.8) = −3.43; p = 0.001] and SCA5 [t(56.5) = −4.9; p < 0.001]. SCA1 and SCA5 did not differ on picture description scores averaged across primary measures. However, on the intelligibility ratings, which were only obtained for the picture description task, SCA6 was more impaired that than both SCA1 [t(71) = − 2.45; p = 0.017] and SCA5 [t(47.9) = − 5.27; p < 0.001], and SCA1 was also more impaired than SCA5 [t(175.7) = 3.7; p < 0.001].
3.4.5 Summary of primary dimensions
The results of the analyses of the primary dimensions can be summarized as follows. The SCA6 group was rated as most severely impaired among three SCA groups, and the SCA5 group was judged as least impaired. Task differences were observed across genotypes. The diadochokinesis task was most effective in revealing speech abnormalities in all SCA groups in that the highest percentage of abnormal samples was observed in all SCAs in this task. Word repetition produced the lowest number of abnormal samples in all SCA groups. Rating of speech dimensions differed across tasks. Articulation was the most severely affected primary dimension in all tasks, particularly in the diadochokinesis task. Rate, rhythm, and prosody were also impaired in the diadochokinesis task but to a lesser degree than articulation. Prosodic abnormalities were greater during picture description than during sentence repetition, and both were greater than prosodic abnormalities during word repetition.
3.5. Secondary dimensions, genotype, and task
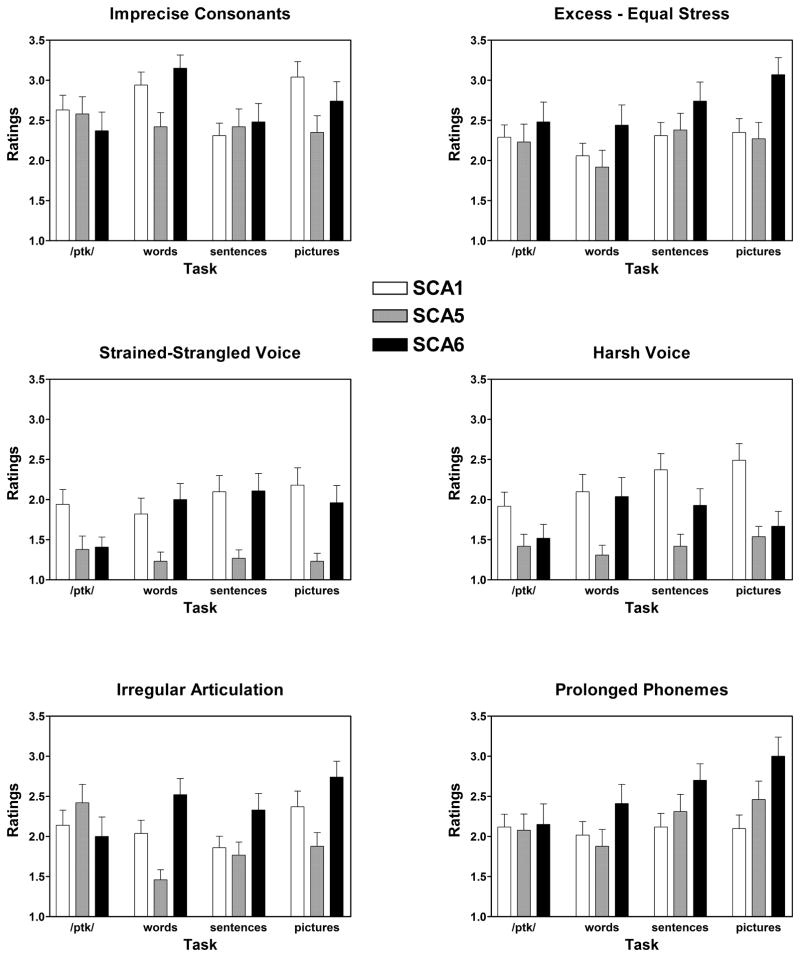
As previously indicated, the secondary dimensions represent a more detailed set of speech characteristics derived from the Mayo Clinic protocol. Listeners rated the secondary dimensions on the samples from any speaker whose speech was rated as abnormal on a primary dimension. The means, standard deviations, and rank for the 11 secondary speech dimensions are presented in Table 3. Selected results of the secondary dimensions that presented significant genotype effects, tasks effects or interaction are provided in Figure 2.
Table 3.
Means, standard deviations, and ranks of the ratings of the 11 secondary speech dimensions.
| Rank | Speech dimensions | Mean | Standard deviation |
|---|---|---|---|
| 1 | Imprecise consonants | 10.52 | 3.79 |
| 2 | Excess and equal stress | 9.34 | 3.75 |
| 3 | Prolonged phonemes | 8.83 | 3.85 |
| 4 | Irregular articulatory breakdown | 8.56 | 3.74 |
| 5 | Distorted vowels | 7.99 | 3.6 |
| 6 | Harsh voice | 7.56 | 4.26 |
| 7 | Strained-strangled voice | 7.0 | 4.01 |
| 8 | Breathy voice | 6.19 | 3.38 |
| 9 | Hypernasality | 6.1 | 3.12 |
| 10 | Voice tremor | 5.6 | 3.18 |
| 11 | Excess loudness variation | 5.54 | 2.38 |
Figure 2.
Selected secondary dimension measures applied to speech samples judged as abnormal on a primary dimension. As in Figure 1, means ± standard errors are presented for each test and SCA group.
3.5.1. Irregular articulatory breakdown
As expected, there was a significant effect of task [F(3, 297) = 4.81; p = 0.003] with more irregular articulatory breakdown during diadochokinesis than during word repetition [t(116) = 2.209; p = 0.029] and sentence repetition [t(143) = 2.596; p = 0.01], and more articulatory breakdown during picture description than during sentence repetition [t(116) = −3.226; p = 0.002] and word repetition [t(116) = −3.23; p = 0.002]. There was a task by SCA type interaction [F(6, 297) = 5.85; p < 0.001] as well. SCA6 had greater irregular articulatory breakdown than SCA5 on picture description [t(60.2) = −3.67; p = 0.001] and word repetition [t(44.74) = −4.75; p < 0.001]. SCA1 had greater irregular articulatory breakdown than SCA5 on word repetition [t(82.9) = 3.16; p = 0.002].
3.5.2. Imprecise consonants
Again, there was a significant effect of task [F(3, 297) = 5.99; p = 0.001]. Picture description [t(115) = −2.15; p = 0.033] and word repetition [t(116) = −3.77; p < 0.001] had more imprecise consonants than sentence repetition. There was a task by SCA type interaction [F(6, 297) = 3.40; p = 0.003]. Both SCA6 [t(63.6) = −3.94; p < 0.001] and SCA1 [t(89.1) = 3.62; p < 0.001] had more imprecise consonants than SCA5 on word repetition.
3.5.3. Distorted vowels
There was a significant effect of task [F(3, 297) = 9.35; p < 0.001]. Vowel distortion during picture description was worse than during sentence repetition [t(116) = −3.32; p = 0.001] and diadochokinesis [t(128) = −4.0; p < 0.001]. Vowel distortion during word repetition was also worse than during diadochokinesis [t(115) = −3.55; p = 0.001]. There was also a task by SCA type interaction [F(6, 297) = 2.48; p < 0.024], but no pair-wise comparisons were significant.
3.5.4. Prolonged phonemes
There was a significant effect of task [F(3, 297) = 7.38; p < 0.001]. The prolonged phonemes dimension was rated as worse during picture description than during word repetition [t(108) = −3.39; p = 0.001] and diadochokinesis [t(128) = −3.51; p = 0.001]. Prolonged phonemes during sentence repetition was also more marked than during word repetition [t(116) = −2.81; p = 0.006]. There was a task by SCA type interaction [F(6, 297) = 2.52; p = 0.022] but none of the pairwise SCA comparisons were significant.
3.5.5. Excess and equal stress
There was a significant effect of task [F(3, 297) = 5.83; p = 0.001]. Picture description [t(108) = −4.47; p < 0.001] and sentence repetition [t(116) = −4.04; p < 0.001] were worse than word repetition. There were no significant SCA effects.
3.5.6. Excess loudness variation
There was a significant effect of task [F(3, 297) = 2.92; p = 0.035]. Diadochokinesis was worse than word repetition [t(115) = 2.73; p = 0.007]. There was a task by SCA type interaction [F(6, 297) = 2.66; p < 0.016], but none of the pairwise comparisons were significant.
3.5.7. Hypernasality
Task was significant [F(3, 297) = 5.70; p = 0.001] with sentence repetition [t(142) = −2.68; p = 0.008] and word repetition [t(115) = −3.17; p = 0.002] having more pronounced hypernasality than diadochokinesis.
3.5.8. Voice tremor
There were no significant differences as a function of task, SCA type, or their interaction.
3.5.9. Harsh voice
There was a significant effect of task [F(3, 297) = 4.65; p = 0.003]. Voice was rated as more harsh for both picture description [t(128) = −2.85; p = 0.005] and sentence repetition [t(142) = −3.06; p = 0.003] compared to diadochokinesis. There was also an effect of SCA type [F(2, 99) = 5.16; p = 0.007]. Task interacted with SCA type [F(6, 297) = 3.45; p = 0.003]. For picture description, harsh voice was the one secondary dimension in which SCA1 was worse than both SCA6 [t(89.7) = 2.69; p = 0.008] and SCA5 [t(96.3) = 4.12; p < 0.001]. Voice was also rated as harsher for SCA1 compared to SCA5 for sentence [t(85.8) = 3.74; p < 0.001] and word [t(81.3) = 2.79; p = 0.007] repetition.
3.5.9.1. Breathy voice
There was a significant effect of task [F(3, 297) = 3.23; p = 0.023]. Word repetition was rated more breathy for SCA6 compared to SCA5 [t(44.7) = −4.75; p < 0.001].
3.5.9.2. Strained-strangled voice
SCA1 had a greater strained-strangled voice rating than SCA5 [t(179.7) = 2.84; p = 0.005]. There was also an interaction between task and SCA type [F(6, 297) = 3.04; p = 0.007]. SCA6 was worse than SCA5 on picture description [t(41.9) = −3.15; p = 0.003] and word repetition [t(44.9) = −3.40; p = 0.001]. SCA1 was also worse than SCA5 on picture description [t(85.7) = 4.15; p < 0.001] and sentence repetition [t(75.7) = 3.32; p = 0.001].
3.5.9.3. Summary of secondary dimensions
On the secondary dimensions, the picture description task produced the highest percentage of abnormal speech samples among four different speech tasks. As with the primary dimensions, articulatory measures (imprecise consonants, prolonged phonemes, irregular articulatory breakdown) identified SCA6 as most impaired, whereas a voice dimension, harsh voice, separated SCA1 from both SCA6 and SCA5.
3.6. Secondary measures: Classifying SCA types
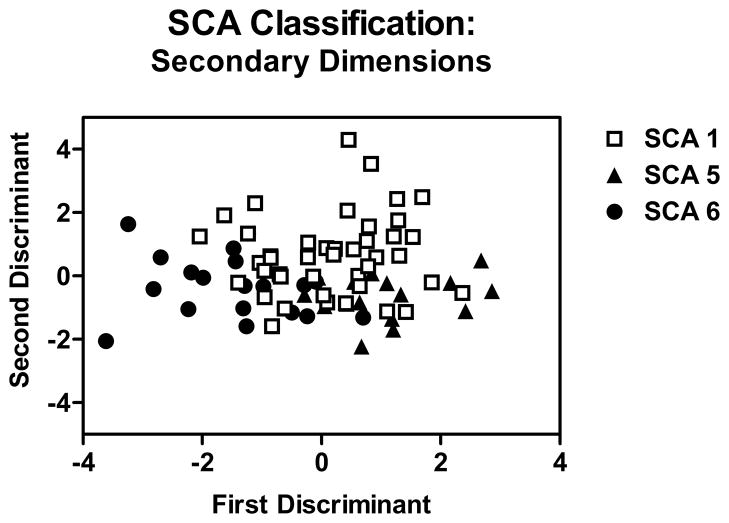
A discriminant function analysis was performed to determine if some combination of secondary dimensions can be used to classify the SCA types. Discriminant function analyses are commonly used to identify variables that contribute to the discrimination between groups. In the present study, secondary dimension scores on every task were entered as potential discriminants in classifying the three genotypes. The analysis revealed that the secondary dimensions that classified the SCA types were irregular articulatory breakdown during diadochokinesis and word repetition, harsh voice during picture description and word repetition, hypernasality during diadochokinesis and sentence repetition, and breathy voice during diadochokinesis. These speech dimensions contributed to two discriminant functions: The first function accounted for 69% of the variance and the second function accounted for 31% of the variance.
In order to find an optimal combination of variables that predict the classification of SCA types, correlation analyses between the two discriminant functions and speech dimensions was performed. The first function was negatively correlated (all p-values < 0.01) with irregular articulatory breakdown for word repetition (r = −0.529) and picture description (r = −0.338), and imprecise consonants during word repetition (r = −0.395). A negative score on the first discriminant function represents greater abnormality. The second function, which accounted for 31% of the variance, was positively correlated with three secondary dimensions, harsh voice, breathiness, and strained-strangled voice, on each of the four tasks. The correlations between the second discriminant and harsh voice were r = 0.725 for picture description, r = 0.524 for sentence repetition, r = 0.471 for diadochokinesis, and r = 0.425 for word repetition. Positive scores on this discriminant function represent greater abnormality. The results are presented in Figure 3.
Figure 3.
Results of a discriminant function analysis in which the secondary dimension scores were used to predict SCA type. Negative scores on the first discriminant represent greater impairment. This dimension reflects irregular articulation and imprecise consonants. Positive scores on the second discriminant represent greater impairment. This dimension reflected harsh voice, breathiness, and strained-strangled voice.
In summary, the secondary dimensions provided a basis for classifying the SCA types, although the groups did overlap. The first discriminant function reflected articulatory factors while the second discriminant function reflected voice quality. SCA6, a “pure cerebellar ataxia,” tended to have more severe articulatory problems, SCA1, a “mixed cerebellar ataxia,” more severe voice problems, and SCA5, also a “pure cerebellar ataxia” but clinically milder, was less impaired in both domains.
3.7.1. Secondary measures: Predicting severity by SCA type
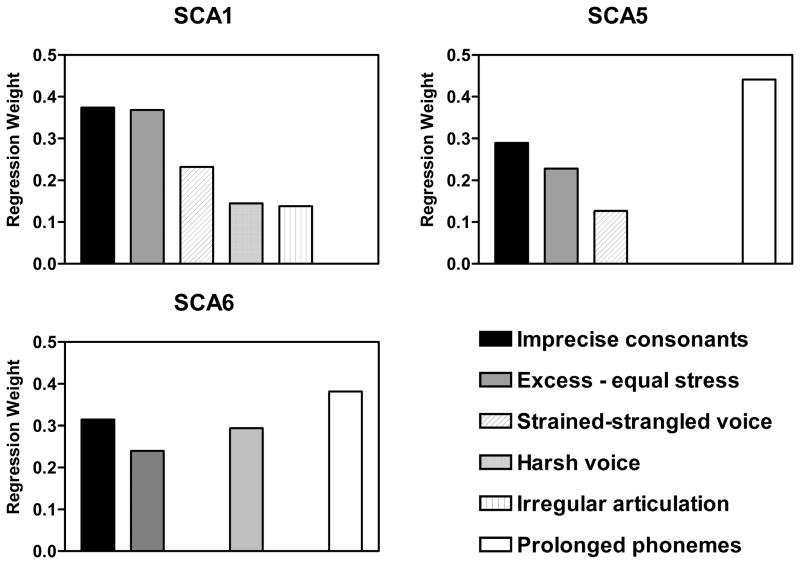
Another approach to characterizing the abnormal speech of each SCA employed multiple linear regression. A stepwise approach was used to predict the average severity score derived from the primary dimensions from the ratings on the secondary dimensions averaged across tasks. Separate predictive models were generated for each SCA type.
For SCA1, the linear regression yielded a significant model [F(5,107) = 21.01; p < 0.001] that accounted for 77% of the variance. In this model, the predictors were imprecise consonants, excess and equal stress, strained-strangled voice, harsh voice, and irregular articulatory breakdown. For SCA5, the linear regression yielded a significant model [F(4,85) = 54.81; p < 0.001] that accounted for 72% of the variance.
In this model, the predictors were prolonged phonemes, imprecise consonants, excess and equal stress, and strained-strangled voice. For SCA6, the linear regression yielded a significant model [F(4,43) = 32.94; p < 0.001] that accounted for 75% of the variance. In this model, the predictors were prolonged phonemes, harsh voice, imprecise consonants, and excess and equal stress. The regression weights for each SCA predictive model are presented in Figure 4.
Figure 4.
The results of multiple linear regression analyses in which secondary dimension scores, averaged across tasks, were used to predict overall severity in each SCA group. Overall severity was estimated by the average of the primary dimension scores.
In summary, imprecise consonants and excess and equal stress had strong predicting power to overall severity in all SCA groups. Irregular articulatory breakdown was a predictor for SCA1 and prolonged phonemes was a predictor for SCA5 and SCA6. Strained-strangled voice was a predictor for SCA1 and SCA5. Harsh voice was a predictor for SCA1 and SCA6.
4.0. Discussion
The present investigation was conducted to determine the clinical characteristics of the speech of individuals from three genotypes of SCA through perceptual ratings. Further, the effects of different task demands on the ratings produced by the different genetic groups were examined. Speech samples from three groups of SCA speakers were perceptually rated based on two sets of speech dimensions. The aim was to provide a multi-dimensional characterization of the abnormal speech produced on different speech tasks by subjects suffering from different SCAs.
Articulation was the most impaired primary dimension across all SCAs and tasks. This is consistent with the traditional characterization of ataxic dysarthria produced by a variety of etiologies (Murdoch, 2004). Articulatory imprecision has been reported as one of the most salient perceptual features of ataxic dysarthria (Duffy, 2005). The presence of the perceptually recognized articulatory impairment in ataxic dysarthria has also been confirmed by studies using instrumental measurements (Kent, Netsell, & Abbs, 1975; Kent, Kent, Duffy, Thomas, Weismer, & Stuntebeck, 2000).
The articulation impairment of the SCA speakers was pronounced across all four tasks utilized in this study. A similar result was found by perceptual rating study conducted by Zeplin and Kent (1996). That study reported that while severely affected speech dimensions in subjects with dysarthria tended to differ across speech tasks, there were some speech dimensions that were affected regardless of the tasks. They called these speech dimensions “core dimensions.” One of the core dimensions in ataxic dysarthria was articulatory impairment (imprecise consonants), which corresponds to the present findings.
There has been considerable discussion about which speech tasks to use in assessing motor speech function and what their unique contributions can be (Weismer, 2006; Folkins, Moon, Luschei, Robin, Tye-Murray & Moll, 1995; Ziegler & Wessler, 1996; Ziegler, 2002). In this study, diadochokinesis was the most effective task in revealing abnormal speech across all SCAs on the primary dimensions. Word repetition, as expected, revealed the least impairment. This is also in accordance with previous work that demonstrated that syllable repetition reflected ataxic dysarthria speech abnormalities fairly consistently, whereas word production was found to be of little value, demonstrating little impairment (Kent, Kent, Rosenbek, Vorperian, & Weismer, 1997). Ziegler (2002) demonstrated that syllable repetition was more useful than natural sentence production in revealing timing difficulties in ataxic subjects.
In this study, picture description was the task most likely to produce abnormal speech on the secondary dimensions. This finding is in agreement with previous studies that have reported significantly poorer intelligibility in spontaneously generated utterances in comparison with the same utterance-types in repetition and reading modes (Kempler & Van Lancker, 2002) as well as significant differences in acoustic measures of fluency and voice quality, when the same phrase-types produced in the two speech tasks were compared (Sidtis et al., 2010). This difference suggests, as in previous studies, that while rapid repetition, maximum performance tasks elicit the cardinal features of ataxic dysarthria, the demands of natural speech exhibit a broader and more variable range of speech abnormality that may reveal phenotypic differences in SCA subgroups.
One of the aims of the present study was to examine whether the three different SCA genotypes produced different patterns of impairment. In general, while all of the SCAs were impaired on articulatory dimensions, SCA6 was more impaired than both SCA1 and SCA5. In contrast, voice dimensions were more impaired in SCA1 than both SCA6 and SCA5. This is an important point. Had the differences between SCA types been uniform across dimensions, the results would not be distinguishable from those resulting from a general effect of disease severity. While disease severity, however problematic, remains an significant issue, relative genotypic differences across speech dimensions suggests that with greater experience, some of these SCA distinctions may prove to be reliable.
The presence of subgroups in ataxic dysarthria based on articulation and voice features has been reported by several studies (Grémy, Chevrie-Muller & Garde, 1967; Joanette & Dudley, 1980; Schalling, Hammarberg, & Hartelius, 2007). Grémy, Chevrie-Muller and Garde identified two different subgroups of ataxic dysarthria: one group had difficulties of both laryngeal irregularities and articulation, and the other group had impairments only in articulation. Similarly, in their study with speakers with Friedreich’s ataxia, Joanette and Dudley (1980) conducted a factor analysis, which resulted in two main factors referred to as a “general dysarthric factor,” where imprecise consonants and prolonged phonemes weighted most heavily, and “phonatory stenosis factor,” which included voice dimensions such as harshness, strained-strangled voice quality. Based on these two main factors, subjects with Friedreich’s ataxia could be categorized into three groups: The first group demonstrated a general dysarthria without phonatory stenosis, the second group was characterized by phonatory stenosis with mild general dysarthria, and the third group showed mild impairments in both speech factors. The authors pointed out that different neurological involvement may have produced such groupings. They also suggested that articulation problem may reflect cerebellum involvement, and voice impairment may be associated with lower brainstem damage.
As noted previously, Schalling, Hammarberg, & Hartelius (2007) also found that articulatory and voice abnormalities contributed to ataxic dysarthria in a mixed group of SCAs. The present study extends the previous work in ataxic dysarthria by reaffirming that articulatory problems are common across the SCA types studied (i.e., core dimensions) but further suggesting that voice changes are more likely to reflect phenotypic abnormalities. The pathophysiology of the articulatory and voice changes in the ataxias is likely more complicated than the cerebellum and brain stem contributions suggested by Joanette and Dudley (1980) in Friedreich’s ataxia. In the present study, both the most affected (SCA6) and the least affected (SCA5) groups are classified as “pure” cerebellar syndrome because of primary cerebellar pathology while SCA1 is a “mixed” cerebellar syndrome because of brainstem involvement. While the dimensions of imprecise consonants and excess and equal stress predicted severity in each of our SCA groups, harsh voice was a predictor of severity in SCA1 and SCA6 while strained-strangled voice was a predictor of severity in SCA1 and SCA5. Although the research experience with speech and the understanding of the specific SCA pathophysiology is currently limited, the present results suggest that the SCAs may provide significant insights into the neurological control of voice in the future as the different neuropathologies of the SCAs are better understood at a systems level.
Although the heterogeneity of the phenotypic presentation of each genotype reduces the diagnostic role of the pattern of speech abnormalities in SCA identification, this is not necessary with the availability of genetic testing. The observed differences suggest that just as neurology refers to the “ataxias” rather than simply “ataxia,” ataxic dysarthria may be better characterized as a plural, the “ataxic dysarthrias,” representing a constellation of abnormalities with a central feature of articulatory incoordination. Further, the interactions between genotypes and tasks in the present study reinforce this plurality and demonstrate the importance of the selection of tasks in the motor speech examination. The identification of the core feature of ataxic speech is quite straightforward. A simple maximum performance test like syllable repetition appears to be highly sensitive. The characterization of voice changes associated with the range of cerebellar, brainstem, and other sites of neuropathology across the range of the SCAs appears to be best captured by conversational or monologue speech rather than repetition. While not currently diagnostic, documenting the voice changes in the different SCAs may provide a valuable resource in the future.
5.0. Conclusions
While complicating the clinical process, the range of neuropathologies associated with the SCAs represents an opportunity to better understand motor speech control from the perspective of a new class of “lesion” studies. As the neurological and neuropathological knowledge base regarding the SCAs expands, there will be new opportunities to study the effects of progressive pathology in the cerebellum alone or in combination with different brainstem structures by selecting the appropriate SCA, which was not possible in traditional lesion studies. Further, it may be possible to study the effects of specific pathophysiologies (abnormal proteins, calcium channel disruptions, changes in cerebellar-striatal-cortical networks) on specific aspects (e.g., articulation, timing, voice) of the motor speech system. The importance of dysarthria as a clinical feature of the SCAs indicates that the proper evaluation of speech in these individuals can contribute significantly to their clinical care and to the scientific understanding of the neurology of motor speech control.
Research Highligts.
Three genotypes of spinocerebellar ataxia (SCA) shared articulatory problems.
Individual genotypes differed on voice abnormalities.
Voice differences did not correspond to the presence or absence of brainstem pathology.
Articulatory problems were most efficiently detected with rapid syllable repetition.
Voice problems were most efficiently detected with monologue speech.
Acknowledgments
This work was supported by NIH/NIDCD R01 DC7658 and by a Founders Fellowship grant from the Steinhardt School of Culture, Education and Human Development at New York University. Dr. Joseph R. Duffy’s suggestion to examine this question is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann H, Hertrich I. Speech rate and rhythm in cerebellar dysarthria: an acoustic analysis of syllabic timing. Folia Phoniatrica et Logopaedica. 1994;46:70–78. doi: 10.1159/000266295. [DOI] [PubMed] [Google Scholar]
- Bird TD. Hereditary ataxia overview. In: Pagon RA, Bird TC, Dolan CR, Stephens K, editors. GeneReviews. Seattle, WA: University of Washington; 2009. (Internet NCBI Bookshelf ID: NBK1138;) [PubMed] [Google Scholar]
- Duffy JR. Motor speech disorders. St. Louis: Elsevier Mosby; 2005. [Google Scholar]
- Folkins JW, Moon JB, Luschei ES, Robin DA, Tye-Murray N, Moll KL. What can nonspeech tasks tell us about speech motor disabilities? Journal of Phonetics. 23(1–2):139–147. [Google Scholar]
- Gomez CM, Subramony SH. Dominantly inherited ataxias. Seminars in Pediatric Neurology. 2003;10:210–222. doi: 10.1016/s1071-9091(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Gomez CM, Thompson RM, Gammack JT, Perlman SL, Dobyns WB, Truwit CL, Anderson JH. Spinocerebellar ataxia type 6: Gaze-evoked and vertical nystagmus, Purkinje cell degeration, and variable age of onset. Annals of Neurology. 1997;42:933–950. doi: 10.1002/ana.410420616. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination (BDAE) Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Grémy F, Chevrie-Muller C, Garde E. Étude phoniatrique clinique et instrumentale des dysarthries I. – Technique – Résultats chez les malades présentant un syndrome cérébelleux. Revue Neurologique (Paris) 1967;116(5):401–426. [PubMed] [Google Scholar]
- Hartelius L, Runmarker B, Andersen O, Nord L. Temporal speech characteristics of individuals with multiple sclerosis and ataxic dysarthria: Scanning speech revisited. Folia Phoniatrica et Logopaedica. 2000;52:228–238. doi: 10.1159/000021538. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Dick KA, Weatherspoon MR, Gincel D, Armbrust KR, Dalton JC, Ranum LPW. Nature Genetics. 2006;38(2):184–190. doi: 10.1038/ng1728. [DOI] [PubMed] [Google Scholar]
- Joanette Y, Dudley JG. Dysarthric symptomatology of Friedreich’s Ataxia. Brain and Language. 1980;10:39–50. doi: 10.1016/0093-934x(80)90036-x. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Kempler D, Van Lancker D. Effect of speech task on intelligibility in dysarthria: A case study of Parkinson’s Disease. Brain and Language. 2002;80:449–464. doi: 10.1006/brln.2001.2602. [DOI] [PubMed] [Google Scholar]
- Kent RD, Netsell R, Abbs JH. Acoustic characteristics of dysarthria associated with cerebellar disease. Journal of Speech and Hearing Research. 1975;22:627–648. doi: 10.1044/jshr.2203.627. [DOI] [PubMed] [Google Scholar]
- Kent RD. Hearing and believing: some limits to the auditory-perceptual assessment of speech and voice disorders. American Journal of Speech-Language Pathology. 1996;5:7–23. [Google Scholar]
- Kent RD, Kent JE, Rosenbek JC, Vorperian HK, Weismer G. A speaking task analysis of the dysarthria in cerebellar disease. Folia Phoniatrica et Logopaedica. 1997;49:63–82. doi: 10.1159/000266440. [DOI] [PubMed] [Google Scholar]
- Kent RD, Kent JF, Duffy JR, Thomas JE, Weismer G, Stuntebeck S. Ataxic dysarthria. Journal of Speech, Language, and Hearing Research. 2000;43:1275–1289. doi: 10.1044/jslhr.4305.1275. [DOI] [PubMed] [Google Scholar]
- Ladefoged P. A course in phonetics. 5. Belmont, California: Thomson/Wadsworth Publishers; 2005. [Google Scholar]
- Murdoch BE. Subcortical brain mechanisms in speech motor control. In: Massen B, Kent RD, Peters HFM, van Lieshout PHHM, Hulstijn W, editors. Speech motor control in normal and disordered speech. Oxford, England: Oxford University Press; 2004. pp. 139–174. [Google Scholar]
- Orr HT, Chung M, Banfi S, Kwiatkowski TJ, Servadio A, Beaudet AL, Zoghbi HY. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nature Genetics. 1993;4:211–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- Perlman SL. Ataxias. Clinics in Geriatric Medicine. 2006;22:859–877. doi: 10.1016/j.cger.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Ranum LPW, Schut LJ, Lundgren JK, Orr HT, Livingston DM. Spinocerebellar ataxia type 5 in a family descended from the grandparents of President Lincoln maps to chromosome 11. Nature Genetics. 1994;8:80–284. doi: 10.1038/ng1194-280. [DOI] [PubMed] [Google Scholar]
- Schalling E, Hartelius L. Acoustic analysis of speech tasks performed by three individuals with spinocerebellar ataxia. Folia Phoniatrica et Logopaedica. 2004;56:367–380. doi: 10.1159/000081084. [DOI] [PubMed] [Google Scholar]
- Schalling E, Hammarberg B, Hartelius L. Perceptual and acoustic analysis of speech in individuals with spinocerebellar ataxia (SCA) Logopedics, Phoniatrics, Vocology. 2007;32:31–46. doi: 10.1080/14015430600789203. [DOI] [PubMed] [Google Scholar]
- Schalling E, Hammarberg B, Hartelius L. A longitudinal study of dysarthria in spinocerebellar ataxia (SCA): aspects of articulation, prosody, and voice. Journal of Medical Speech-Language Pathology. 2008;16:103–118. [Google Scholar]
- Schöls L, Bauer P, Schmidt T, Schulte T, Riess O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. The Lancet Neurology. 2004;3:291–304. doi: 10.1016/S1474-4422(04)00737-9. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Gomez C, Groshong A, Strother SC, Rottenberg DA. Mapping cerebral blood flow during speech production in hereditary ataxia. NeuroImage. 2006;31:246–254. doi: 10.1016/j.neuroimage.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Strother SC, Groshong A, Rottenberg DA, Gomez C. Longitudinal cerebral blood flow changes during speech in hereditary ataxia. Brain and Language. 2010;114:43–51. doi: 10.1016/j.bandl.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidtis D, Rogers T, Godier V, Tagliati M, Sidtis JJ. Voice and fluency changes as a function of speech task and deep brain stimulation. Journal of Speech Language and Hearing Research. 2010;53:1167–1177. doi: 10.1044/1092-4388(2010/09-0154). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidtis JJ, Strother SC, Rottenberg DA. Predicting performance from functional imaging data: Methods matter. NeuroImage. 2003;20:615–624. doi: 10.1016/S1053-8119(03)00349-5. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Van Lancker Sidtis D. A neurobehavioral approach to dysprosody. Seminars in Speech and Language. 2003;24:93–105. doi: 10.1055/s-2003-38901. [DOI] [PubMed] [Google Scholar]
- Weismer G. Philosophy of research in motor speech disorders. Clinical Linguistics & Phonetics. 2006;20 (5):315–349. doi: 10.1080/02699200400024806. [DOI] [PubMed] [Google Scholar]
- Zeplin J, Kent RD. Reliability of auditory-perceptual scaling of dysarthria. In: Robin D, Yorkston K, Beukelman DR, editors. Disorders of motor speech: Recent advances in assessment, treatment, and clinical characterization. Baltimore, MD: Brookes; 1996. pp. 145–154. [Google Scholar]
- Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, Lee CC. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nature Genetics. 1997;15:62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]
- Ziegler W. Task-related factors in oral motor control: Speech and oral diadochokinesis in dysarthria and apraxia of speech. Brain and Language. 2002;80:556–575. doi: 10.1006/brln.2001.2614. [DOI] [PubMed] [Google Scholar]
- Ziegler W, Wessel K. Speech timing in ataxic disorders. Neurology. 1996;47:208–214. doi: 10.1212/wnl.47.1.208. [DOI] [PubMed] [Google Scholar]