Abstract
Rationale: Studies of long-term functional outcomes of elderly survivors of mechanical ventilation (MV) are limited to local samples and biased retrospective, proxy-reported preadmission functional status.
Objectives: To assess the impact on disability of hospitalization with MV, compared with hospitalization without MV, accounting for prospectively assessed prior functional status.
Methods: Retrospective population-based longitudinal cohort study of Medicare beneficiaries age 65 and older enrolled in the Medicare Current Beneficiary Survey, 1996–2003.
Measurements and Main Results: Premeasures and postmeasures of disability included mobility difficulty and weighted activities of daily living disability scores ranging from 0 (not disabled) to 100 (completely disabled) based on self-reported health and functional status collected 1 year apart. Among 54,771 person-years (PY) of observation over 7 calendar years of data, 42,890 PY involved no hospitalization, 11,347 PY involved a hospitalization without MV, and 534 PY included a hospitalization with MV. Mortality at 1 year was 8.9%, 23.9%, and 72.5%, respectively. The level of disability at the postassessment was substantially higher for a prototypical patient who survived after hospitalization with MV (adjusted activities of daily living disability score [95% confidence interval] 14.9 [12.2–17.7]; adjusted mobility difficulty score [95% confidence interval] 25.4 [22.4–28.4]) compared with an otherwise identical patient who survived hospitalization without MV (11.5 [11.1–11.9] and 22.3 [21.8–22.9]) or who was not hospitalized (8.0 [7.9–8.1] and 13.4 [13.3–13.6]).
Conclusions: The greater marginal increase in disability among survivors of MV compared with survivors of hospitalization without MV is larger than would be predicted from prior functional status.
Keywords: Medicare, intensive care, mechanical ventilation, quality of life, functional status
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
A recent literature review found conflicting evidence regarding long-term outcomes of elderly survivors of intensive care; five studies demonstrated that functional status after discharge was unchanged from baseline, whereas three found a significant decline. A major weakness of all of these studies was retrospective ascertainment of preadmission functional status, typically by proxy. Proxy respondents typically overestimate dependence, biasing comparisons between preadmission and postadmission functional status toward the null.
What This Study Adds to the Field
Using a nationally representative population-based sample with preadmission functional status measures and a hospitalized control group, survivors of mechanical ventilation experience a much greater increase in disability than survivors of hospitalization without mechanical ventilation.
The use of intensive care and, in particular, mechanical ventilation (MV) among aged Medicare beneficiaries has increased markedly in the last decade (1). Data on long-term mortality associated with use of MV in the elderly are available from both local and national samples (2–6), but reports of physical function and quality of life are fewer and are limited to local samples (7–11). For example, in a study by Chelluri and coworkers (11) of 817 adults who were mechanically ventilated for more than 48 hours in a tertiary referral medical center, 57% of survivors needed caregiver assistance at 1 year. Rates of dependency were much higher for those over age 65. The mean number of activities of daily living (ADL) and instrumental ADL deficiencies were 1.35 and 3.38, respectively, and mean SF-36 physical function score was 45.5.
In addition to the limitation inherent in a local tertiary care sample, it is impossible to assess the impact of the acute care experience without a measure of prehospital physical function. A review of the literature on outcomes of elderly survivors of intensive care found that all studies used retrospective reports of prehospitalization physical function, often by proxy (12). However, serious doubts have been raised about the accuracy of both proxy and retrospective assessments of physical function (13). It is recognized that proxies generally overestimate dependence (14, 15). Yet if findings from these studies are to be used as the basis for clinical decision making, better informed consent, or health policy, a high level of confidence in the accuracy is required.
The purpose of the current study was, therefore, to estimate the level of disability among elderly survivors of MV, conditional on prehospitalization functional status, in a national probability sample of Medicare beneficiaries. Some of the results of this study have been previously reported in the form of an abstract (16).
METHODS
Overview
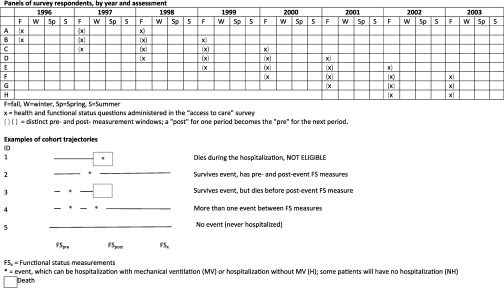
We conducted a retrospective longitudinal cohort study of Medicare beneficiaries 65 and older using 7 years (1996–2003) of data from the Medicare Current Beneficiary Survey (MCBS). MCBS conducts in-person interviews with each beneficiary four times per year for 4 years. We linked each beneficiary's annual report of health and functioning during the autumn “access to care” survey (“pre”) with their report 1 year later (“post”), conditional on whether they were hospitalized without MV or with MV during the 12 months between assessments (Figure 1). If a beneficiary had more than one hospitalization in the year, at least one of which involved MV, we categorized that beneficiary's exposure as “hospitalized with MV.” We focused on survivors of MV, rather than intensive care unit (ICU), because there is heterogeneity of ICU admission thresholds across hospitals and regions (17) and because the driving force underlying differences in post-ICU outcome is receipt of MV (18).
Figure 1.
Schematic of the Medicare Current Beneficiary Survey (MCBS) study cohort. The rows represent the MCBS cohort (each cohort participates for 4 yrs) and the columns represent the calendar years, broken down by season. The annual “access to care” survey is administered in the fall of each year. The completion date of this survey became the arbitrary start (and, 1 year later, end) of each beneficiary's person-year (PY) of data. Beneficiaries could contribute up to 3 “pre-post” PY of comparison data over their 4 years of participation in the MCBS. We categorized each PY of data according to the patient's Medicare claims for the year. If the person was hospitalized with mechanical ventilation (MV) one or more times in the year, we categorized them as “hospitalized with MV.” If they were hospitalized one or more times in the year without MV (and never had a hospitalization with MV) we categorized them as “hospitalized without MV.” If they were never hospitalized, we categorized them as “not hospitalized.” If they died during the hospitalization, they were not eligible for this study because they were not “survivors” of MV or hospitalization without MV. If they died before the next autumn, they were censored (missing because of death). If they were alive the next autumn, but did not respond to the survey, they were censored (missing because of nonresponse). As expected, this survivor and nonresponse and survivor bias were greater for the MV group. Assuming these decedents and nonresponders were systematically more disabled, our results underestimate the degree of disability among survivors of MV.
Data
The MCBS is a continuous survey of a nationally representative sample of aged, disabled, and institutionalized Medicare beneficiaries sponsored by Centers for Medicare and Medicaid Services (CMS), with oversampling of the oldest old and minorities. MCBS conducts in-person interviews with each sampled beneficiary four times per year for 4 years, after which they rotate off the panel; the sample is refreshed annually. Proxy informants (about 9.7% overall) are used for beneficiaries who are unable to participate in the interview because of illness or difficulty communicating. Questions related to health and functional status are asked once a year during the autumn “access to care” round. Conditional response rates for the second, third, and fourth year are 88.9%, 94.7%, and 96.8%, respectively, yielding progressively declining attrition rates of 11.1%, 5.3%, and 3.2%. Empiric analysis of MCBS finds that conditional response rates are not different for those in poor health versus those in better health (19). Moreover, existing nonresponse weighting procedures address the impact of attrition on estimates for most measures. We drew hospitalization records from linked Medicare inpatient claims files provided by CMS.
Sample
We restricted the analyses to beneficiaries aged 65 and older who were community-dwelling in the “pre” period and not enrolled in a group health plan (group health plans do not submit claims data to Medicare, limiting their usefulness for this study). Each beneficiary could contribute up to 3 person-years (PY) of observation over their 4 years of participation in the MCBS.
Functional Outcomes
We used data elements from the health status and functioning questionnaire to develop two indices of disability: a mobility difficulty score and a weighted ADL disability score (see the online supplement for detail).
We used the validated Rosow-Breslau Functional Health Scale (20) to calculate the mobility difficulty score from three items (difficulty walking two to three blocks, lifting 10 lb, and stooping or kneeling). We calculated the mobility difficulty score by assigning scores to each degree of difficulty (0 = none; 1 = a little; 2 = some; 3 = a lot; and 4 = cannot do) and summing across difficulty domains. These mobility difficulty items capture functional limitation likely to precede ADL disability.
We used the validated modified Katz Activities of Daily Living Scale (21) to calculate ADL disability score (level of difficulty and receipt of assistance with bathing, dressing, getting in or out of bed or chairs, eating, walking, and toileting). We further weighted ADL disability score using the validated weighting scheme developed by Finch and coworkers (22) using magnitude estimation to convert functional status to a continuous scale. This approach assigns greater weight to loss of ADLs associated with greater disability (e.g., loss of toileting is weighted heavier than loss of ability to dress).
Finally, we normalized both the mobility difficulty score and the weighted ADL disability score to a 100-point scale for ease of interpretation: a score of 0 is not disabled and of 100 is completely disabled. Thus, every value between 0 and 100 can be interpreted as a percentage of complete mobility or ADL disability.
Independent Variables
The primary independent variable was MV, identified from inpatient claims files using the International Classification of Diseases 9th edition procedure codes for MV (96.X) in any of the six procedure fields or a diagnosis-related group code indicating MV (475 or 483). Additional model covariables included age; sex; race; marital status; income; baseline cognitive score (a four-point score [0–3] created by summing three dichotomous survey questions: memory loss, problems making decisions, and trouble concentrating); baseline normalized weighted ADL disability score or normalized mobility score (depending on outcome modeled); comorbidity (count of Charlson comorbidities classified using administrative data [(23]); cerebrovascular accident (CVA) as the reason for hospitalization; number of hospitalizations in the 1-year period; and days since the last hospitalization before the “post” survey date. We used mean value imputation for missing data on income.
Statistical Analysis
We report mean “pre” functional status among beneficiaries not hospitalized between a preobservation and postobservation period, among those hospitalized at least once but without MV, and among those hospitalized at least once with MV. To assess the marginal impact on disability of hospitalization with MV, compared with hospitalization without MV, we fitted separate generalized linear models for each outcome (weighted ADL disability score and mobility difficulty score) using the γ distribution to address the skewness of outcome data and accounted for repeated measures among beneficiaries using an autoregressive correlation. We estimated separate regressions for hospitalized (including a dummy for MV vs. no MV) and nonhospitalized beneficiaries. Variables for the number of comorbidities, hospitalization, and cognitive score were entered as continuous variables. We standardized age and days since the last hospitalization to adjust for nonlinearity and for ease of interpretation. Finally, for ease of presentation, we calculated adjusted scores for a “prototypical” person with average age and cognitive score, baseline disability or difficulty score equal to 10, no CVA, and Charlson count equal to 1. We performed data management and statistical analyses using SAS Version 9.2 (SAS, Cary, NC).
We conducted the study under a data use agreement with the CMS and received approval from the University of Pittsburgh Institutional Review Board.
RESULTS
Sample
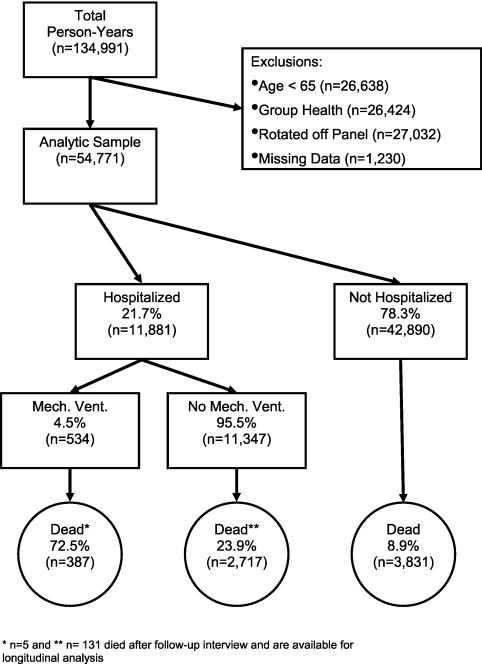
Among 134,991 PY of observation over 7 years, we excluded 26,638 for age less than 65, 26,424 for enrollment in group health, 27,032 for rotation off the panel, and 1,230 for missing “post” data. This left 54,771 PY of observation among 26,072 unique beneficiaries. A total of 42,890 PY involved no hospitalization, 11,347 PY involved a hospitalization without MV, and 534 PY included a hospitalization with MV (Figure 1). The respective rates of missing “post” data for these three groups was 1.9%, 2.8%, and 8.1% and the respective 1-year mortality rate was 8.9%, 23.9%, and 72.5%. The interval between discharge and “post” assessment for those hospitalized with or without MV averaged 127.9 (SD 95.9) and 161.6 (SD 101.5) days, respectively.
Compared with those hospitalized without MV, those who received MV in the year were similar ages, but more likely to be males, be black, have worse baseline mobility and ADL disability scores, and be cognitively impaired (see table in Appendix 1 in the online supplement). Reflecting the survivor bias, baseline mobility, ADL disability, and cognitive scores were similar between those hospitalized with and without MV in the analytic sample (Table 1). Those surviving MV had a higher Charlson comorbidity count, more hospitalizations, and were more likely to be recently discharged and residing in a nursing home than those surviving hospitalization without MV (Table 1).
TABLE 1.
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF THE SAMPLE INCLUDED IN PRE-POST ANALYSES,* MEDICARE CURRENT BENEFICIARY SURVEY 1996–2003
| Not Hospitalized (n = 39,107 PY) |
Hospitalized, Without MV (n = 8,771 PY) |
Hospitalized, With MV (n = 152 PY) |
|||||
|---|---|---|---|---|---|---|---|
| Mean or % | SD | Mean or % | SD | Mean or % | SD | P Value† | |
| “Pre” (baseline) measures | |||||||
| Age, yr | 75.6 | 6.9 | 77.6 | 7.3 | 75.9 | 6.7 | 0.005 |
| Female, % | 58.3 | 59.1 | 58.6 | 0.895 | |||
| Black, % | 8.3 | 9 | 9.9 | 0.713 | |||
| Other non-white race, % | 3.2 | 2.2 | 2.6 | 0.697 | |||
| Married, % | 55.2 | 48.2 | 43.4 | 0.243 | |||
| Income >$25,000, % | 38.6 | 33.1 | 27.4 | 0.127 | |||
| Pre-ADL disability score | 5.3 | 13.8 | 10.9 | 19.5 | 9.8 | 19.3 | 0.470 |
| Premobility difficulty score | 21.0 | 22.6 | 32.4 | 26.5 | 33.9 | 25.7 | 0.482 |
| Cognitive score‡ | 0.23 | 0.67 | 0.39 | 0.86 | 0.32 | 0.76 | 0.343 |
| Clinical measures between “pre” and “post” assessments | |||||||
| Charlson comorbidity count | — | 1.0 | 1.0 | 1.8 | 1.1 | <0.001 | |
| CVA during the year, % | — | 3.9 | 5.9 | 0.213 | |||
| No. of hospitalizations | — | 1.6 | 1.1 | 2.8 | 2.5 | <0.001 | |
| Days since last hospitalization, d | — | 161.6 | 101.6 | 127.9 | 95.8 | <0.001 | |
| Nursing home resident at “post,” % | 0.8 | 7.2 | 12.5 | 0.013 | |||
Definition of abbreviations: ADL = activities of daily living; MV = mechanical ventilation; PY = person years of observation.
Beneficiaries who survived from fall interview to fall interview; the exception is 194 nursing home residents who died before the fall interview, but whose health and functional status were abstracted from the last available nursing home record before death.
Comparison between hospitalized without MV versus hospitalized with MV; t test or chi-square for discrete variables.
The cognitive score is a four-point score (0–3) created by summing three dichotomous survey questions (memory loss, problems making decisions, and trouble concentrating).
Functional Outcomes
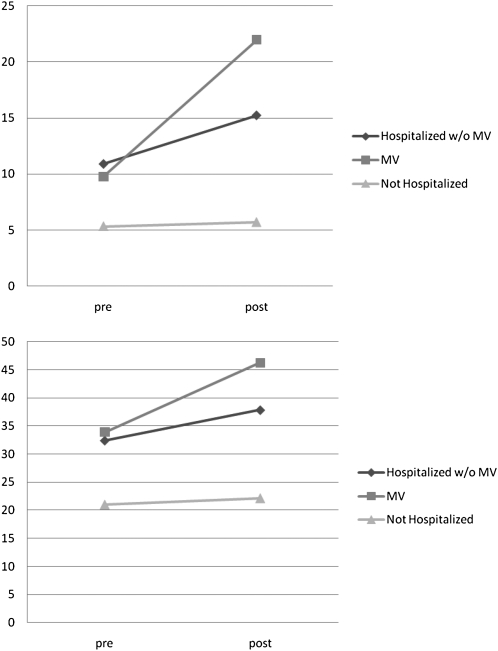
The ADL disability and mobility difficulty scores range from 0 (not disabled) to 100 (completely disabled) and can be interpreted as a percentage of complete disability on the measure. Among survivors, “post” period ADL disability and mobility difficulty were substantially greater among those who received MV (crude ADL disability score [95% confidence interval] 22.5 [18.8–26.2]; crude mobility difficulty score [95% confidence interval] 46.2 [42.7–49.6]) compared with those who were hospitalized without MV (15.3 [14.2–16.4] and 37.9 [36.8–39]) and those who were not hospitalized (5.7 [5.4–6] and 22 [21.7–22.4]). Not only is the absolute “post” score worse, but the increase (worsening) of the score from the “pre” period to the “post” period was also much greater among survivors of MV compared with survivors of hospitalization without MV and with nonhospitalized controls (Figure 2). This is particularly easy to appreciate for the survivors of hospitalization, because those with and without MV had the same mean “pre” period ADL disability and mobility difficulty scores (Figure 3). To adjust for potential confounders in Table 1, we present the adjusted ADL disability and mobility difficulty scores for a prototypical patient (with average age and cognitive score, baseline disability or difficulty score of 10, no CVA, and a Charlson comorbidity count of 1) who was not hospitalized, hospitalized without MV, or hospitalized with MV during the year in Table 2. Compared with surviving a hospitalization without MV, the prototypical patient surviving a hospitalization with MV experiences 30% greater ADL disability (11.5 vs. 14.9) and 14% greater mobility difficulty (22.3 vs. 25.4) in the “post” period.
Figure 2.
Person-years of observation. We used 8 years of Medicare Current Beneficiary Survey data, with each participating beneficiary contributing to up to four annual measures of health and physical functioning and inpatient claims before rotating off the panel.
Figure 3.
Unadjusted 1-year change in activities of daily living (ADL) disability (top panel) and mobility difficulty (bottom panel) scores. Nonhospitalized beneficiaries show small increases in disability over the year. Among those who are hospitalized, survivors of mechanical ventilation (MV) experience steeper increases in disability than those who did not receive MV (differences in the unadjusted “post” scores between hospital survivors without MV and with MV for ADL disability [P = 0.0008] and mobility [P = 0.0002]).
TABLE 2.
ADJUSTED 1-YEAR DISABILITY SCORES FOR A PROTOTYPICAL ELDERLY MV SURVIVOR COMPARED WITH A SURVIVOR HOSPITALIZED WITHOUT MV AND A SURVIVOR NOT HOSPITALIZED, MEDICARE CURRENT BENEFICIARY SURVEY 1996–2003
| Disability Score | Not Hospitalized Adjusted Mean (95% CI) | Hospitalized, no MV Adjusted Mean (95% CI) | Hospitalized, With MV Adjusted Mean (95% CI) |
|---|---|---|---|
| ADL disability | 8.0 (7.9–8.1) | 11.5 (11.1–11.9) | 14.9 (12.2–17.7) |
| Mobility difficulty | 13.4 (13.3–13.6) | 22.3 (21.8–22.9) | 25.4 (22.4–28.4) |
Definition of abbreviations: ADL = activities of daily living; CI = confidence interval; MV = mechanical ventilation.
Adjusted for prior functional status and cognitive score, demographics, Charlson comorbidity count, stroke in the year, number of hospitalizations, and days since last hospitalization. Scores are the average level of disability, ranging from 0 = not disabled to 100 = completely disabled. Scores under each scenario are calculated for the same prototypical person with average age and cognitive score, baseline ADL disability or mobility difficulty score = 10, no cerebrovascular accident, and Charlson count = 1.
DISCUSSION
Our findings offer the first nationally representative estimates of functional status outcomes for aged MV survivors using a prospective population-based sample. We document a larger marginal increase in disability among survivors of MV compared with survivors of hospitalization without MV. Although numerically small, this increase is greater than would be predicted from prior functional status and has clinical implications.
In a recent review of long-term outcomes of elderly survivors of intensive care, Hennessy and coworkers (12) identified 16 studies with functional status or health-related quality of life; 14 were single site and 2 were multicenter; 9 were prospective; and most used consecutive sampling. Inclusion criteria and instruments for outcome ascertainment varied widely, with most including any ICU survivor, not just those with MV. Among those focusing on ADLs and physical functioning, five demonstrated no change between functional status pre-ICU admission and post-ICU discharge (7, 24–27), whereas three found a significant decline (10, 28, 29). Health-related quality of life was generally unchanged. A major weakness of all of these studies was retrospective ascertainment of preadmission functional status, typically by proxy. Proxy respondents typically overestimate dependence, biasing pre–post comparisons toward the null (14, 15). Our study overcomes this weakness by using prospectively collected interview data on functional status.
We believe that our findings swing the balance in this controversy toward the conclusion that survivors of MV experience substantial declines in functional status. Those hardy enough to survive into the postdischarge period actually had better baseline functional status than survivors of hospitalization without MV. Hence, the relatively larger decrement in ADL disability and mobility difficulty scores is likely attributable to more than just the restricted mobility of acute illness (30) and probably includes a cognitive component (31). The reasons for the late morbidity after MV are many, and include the stress and nature of critical illness and the therapies and environment of intensive care. In recent years, there have been efforts to optimize care in the ICU to reduce some of these sequelae. However, many problems that develop in the ICU are difficult to eradicate and some ICU interventions and management strategies necessary to prevent immediate death are associated with unavoidable complications. In the care of other diseases and conditions, such as stroke and traumatic brain injury, the need for dedicated, prolonged care beyond the initial critical episode has long been recognized. This has led to the organization of dedicated services, such as multidisciplinary rehabilitation services, and wide recognition among primary care providers and other physicians of the benefits of early referral to such services. Frail geriatric populations requiring hospitalization have also benefited from a programmatic approach to inpatient care (32) and rehabilitation postdischarge. In contrast, there is no systematic approach to care following MV even though some hypothesize that a course of MV may induce many of the anatomic and physiologic characteristics of frailty. Given that patients with frailty (33), hospital-acquired ADL deficiencies (34), declines in mobility (35), and recent MV (18) have poor longer-term survival, interventions focused on improving function and mobility in this population could impact mortality and functional status.
Our study is subject to several limitations. With the exception of CVA, which directly impacts physical functioning, we did not seek to differentiate between the episodes of critical illness that precipitated the need for MV from the MV itself, such as respiratory arrest and resuscitation. We did not seek to differentiate between shorter and longer episodes of MV or to disentagle relationships between post-MV cognitive function from physical function due to the relatively small numbers of beneficiaries with any MV event. We did not have clinical data about presence and duration of delirium. The study period reflects the epidemiology of posthospitalization survival and disability 7–14 years ago. Although there has been secular decrease in hospital mortality from MV in recent years, 90-day survival has not improved, with deaths shifting into the postacute setting (18, 36, 37). Therefore, our estimates may underestimate the magnitude of disability in 2010. Finally, our analyses suffer from survivor bias and nonresponse bias, which also underestimates the magnitude of disability. Specifically, those who survived to hospital discharge, but who died before the next autumn survey and those who were alive but did not respond were likely more disabled.
Significant disability among survivors of MV has implications for patients' treatment goals; many elders might not elect to receive a high burden intervention if they knew it would result in substantial disability (38). Outcomes for prolonged MV patients are significantly worse than expected by patients' surrogates and physicians (39). Clinicians should consider accurate predictions of disability risk and mortality risk when making decisions about the use of MV with elderly patients. Future research should explore the predictors of better long-term outcomes of MV.
Supplementary Material
Acknowledgments
The authors thank Aiju Men for her programming assistance, Drs. Stephanie Studenski and Lakshmipathi Chelluri for their design and analysis suggestions, Professor Julie M. Donhue for sharing her data, the Centers for Medicare and Medicaid Services for providing the data, and Tami Merriman at the Research Data Assistance Center for her technical assistance. This study was supported by a grant from the NIA-funded University of Pittsburgh Claude D. Pepper Older Americans Independence Center (P30 AG024827). Dr. Barnato was supported by a career development award from the NIA (K08 AG21921). The NIA had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Howard Degenholtz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supported by a grant from the NIA-funded University of Pittsburgh Claude D. Pepper Older Americans Independence Center (P30AG024827). A.E.B. was supported by a career development award from the NIA (K08AG21921).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201002-0301OC on November 5, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Barnato AE, McClellan MB, Kagay CR, Garber AM. Trends in inpatient treatment intensity among Medicare beneficiaries at the end of life. Health Serv Res 2004;39:363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen IL, Lambrinos J. Investigating the impact of age on outcome of mechanical ventilation using a population of 41,848 patients from a statewide database. Chest 1995;107:1673–1680. [DOI] [PubMed] [Google Scholar]
- 3.Ely EW, Evans GW, Haponik EF. Mechanical ventilation in a cohort of elderly patients admitted to an intensive care unit. Ann Intern Med 1999;131:96–104. [DOI] [PubMed] [Google Scholar]
- 4.Kurek CJ, Dewar D, Lambrinos J, Booth FV, Cohen IL. Clinical and economic outcome of mechanically ventilated patients in New York State during 1993: analysis of 10,473 cases under DRG 475. Chest 1998;114:214–222. [DOI] [PubMed] [Google Scholar]
- 5.Pesau B, Falger S, Berger E, Weimann J, Schuster E, Leithner C, Frass M. Influence of age on outcome of mechanically ventilated patients in an intensive care unit. Crit Care Med 1992;20:489–492. [DOI] [PubMed] [Google Scholar]
- 6.Zilberberg MD, Epstein SK. Acute lung injury in the medical ICU: comorbid conditions, age, etiology, and hospital outcome. Am J Respir Crit Care Med 1998;157:1159–1164. [DOI] [PubMed] [Google Scholar]
- 7.Chelluri L, Pinsky MR, Donahoe MP, Grenvik A. Long-term outcome of critically ill elderly patients requiring intensive care. JAMA 1993;269:3119–3123. [PubMed] [Google Scholar]
- 8.Weinert CR, Gross CR, Kangas JR, Bury CL, Marinelli WA. Health-related quality of life after acute lung injury. Am J Respir Crit Care Med 1997;156:1120–1128. [DOI] [PubMed] [Google Scholar]
- 9.Niskanen M, Ruokonen E, Takala J, Rissanen P, Kari A. Quality of life after prolonged intensive care. Crit Care Med 1999;27:1132–1139. [DOI] [PubMed] [Google Scholar]
- 10.Montuclard L, Garrouste-Orgeas M, Timsit JF, Misset B, De Jonghe B, Carlet J. Outcome, functional autonomy, and quality of life of elderly patients with a long-term intensive care unit stay. Crit Care Med 2000;28:3389–3395. [DOI] [PubMed] [Google Scholar]
- 11.Chelluri L, Im KA, Belle SH, Schulz R, Rotondi AJ, Donahoe MP, Sirio CA, Mendelsohn AB, Pinsky MR. Long-term mortality and quality of life after prolonged mechanical ventilation. Crit Care Med 2004;32:61–69. [DOI] [PubMed] [Google Scholar]
- 12.Hennessy D, Juzwishin K, Yergens D, Noseworthy T, Doig C. Outcomes of elderly survivors of intensive care: a review of the literature. Chest 2005;127:1764–1774. [DOI] [PubMed] [Google Scholar]
- 13.Lum TY, Lin WC, Kane RL. Use of proxy respondents and accuracy of minimum data set assessments of activities of daily living. J Gerontol A Biol Sci Med Sci 2005;60:654–659. [DOI] [PubMed] [Google Scholar]
- 14.Santos-Eggimann B, Zobel F, Berod AC. Functional status of elderly home care users: do subjects, informal and professional caregivers agree? J Clin Epidemiol 1999;52:181–186. [DOI] [PubMed] [Google Scholar]
- 15.Long K, Sudha S, Mutran EJ. Elder-proxy agreement concerning the functional status and medical history of the older person: the impact of caregiver burden and depressive symptomatology. J Am Geriatr Soc 1998;46:1103–1111. [DOI] [PubMed] [Google Scholar]
- 16.Degenholtz HB, Barnato A, Men A. National study of functional status after prolonged mechanical ventilation in the elderly. Presented at the Gerontological Society of America's 2007 Annual Scientific Meeting. San Francisco, CA.
- 17.Angus DC, Marrie TJ, Obrosky DS, Clermont G, Dremsizov TT, Coley C, Fine MJ, Singer DE, Kapoor WN. Severe community-acquired pneumonia: use of intensive care services and evaluation of American and British thoracic society diagnostic criteria. Am J Respir Crit Care Med 2002;166:717–723. [DOI] [PubMed] [Google Scholar]
- 18.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA 2010;303:849–856. [DOI] [PubMed] [Google Scholar]
- 19.Kautter J, Khatutsky G, Pope GC, Chromy JR, Adler GS. Impact of nonresponse on Medicare current beneficiary survey estimates. Health Care Financ Rev 2006;27:71–93. [PMC free article] [PubMed] [Google Scholar]
- 20.Rosow I, Breslau N. A Guttman Health Scale for the aged. J Gerontol 1966;21:556–559. [DOI] [PubMed] [Google Scholar]
- 21.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv 1976;6:493–508. [DOI] [PubMed] [Google Scholar]
- 22.Finch M, Kane RL, Philp I. Developing a new metric for ADLS. J Am Geriatr Soc 1995;43:877–884. [DOI] [PubMed] [Google Scholar]
- 23.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-cm administrative databases. J Clin Epidemiol 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 24.Kass JE, Castriotta RJ, Malakoff F. Intensive care unit outcome in the very elderly. Crit Care Med 1992;20:1666–1671. [DOI] [PubMed] [Google Scholar]
- 25.Rockwood K, Noseworthy TW, Gibney RT, Konopad E, Shustack A, Stollery D, Johnston R, Grace M. One-year outcome of elderly and young patients admitted to intensive care units. Crit Care Med 1993;21:687–691. [DOI] [PubMed] [Google Scholar]
- 26.Broslawski GE, Elkins M, Algus M. Functional abilities of elderly survivors of intensive care. J Am Osteopath Assoc 1995;95:712–717. [PubMed] [Google Scholar]
- 27.McHugh GJ, Havill JH, Armistead SH, Ullal RR, Fayers TM. Follow up of elderly patients after cardiac surgery and intensive care unit admission, 1991 to 1995. N Z Med J 1997;110:432–435. [PubMed] [Google Scholar]
- 28.Udekwu P, Gurkin B, Oller D, Lapio L, Bourbina J. Quality of life and functional level in elderly patients surviving surgical intensive care. J Am Coll Surg 2001;193:245–249. [DOI] [PubMed] [Google Scholar]
- 29.Vazquez Mata G, Rivera Fernandez R, Gonzalez Carmona A, Delgado-Rodriguez M, Torres Ruiz JM, Raya Pugnaire A, Aguayo de Hoyos E. Factors related to quality of life 12 months after discharge from an intensive care unit. Crit Care Med 1992;20:1257–1262. [DOI] [PubMed] [Google Scholar]
- 30.Gill TM, Allore HG, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA 2004;292:2115–2124. [DOI] [PubMed] [Google Scholar]
- 31.Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJ, Carson SS, Curtis JR, Larson EB. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA 2010;303:763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Counsell SR, Holder CM, Liebenauer LL, Palmer RM, Fortinsky RH, Kresevic DM, Quinn LM, Allen KR, Covinsky KE, Landefeld CS. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of acute care for elders (ACE) in a community hospital. J Am Geriatr Soc 2000;48:1572–1581. [DOI] [PubMed] [Google Scholar]
- 33.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 34.Boyd CM, Landefeld CS, Counsell SR, Palmer RM, Fortinsky RH, Kresevic D, Burant C, Covinsky KE. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc 2008;56:2171–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc 2007;55:1727–1734. [DOI] [PubMed] [Google Scholar]
- 36.Barnato AE, Linde-Zwirble WT, Rickert T, Angus DC. Trends in hospital disposition among Medicare beneficiaries admitted to the ICU in the last 90 days of life: 39. Crit Care Med 2004;32:A10. [Google Scholar]
- 37.Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long-term acute care hospital utilization after critical illness. JAMA 2010;303:2253–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med 2002;346:1061–1066. [DOI] [PubMed] [Google Scholar]
- 39.Cox CE, Martinu T, Sathy SJ, Clay AS, Chia J, Gray AL, Olsen MK, Govert JA, Carson SS, Tulsky JA. Expectations and outcomes of prolonged mechanical ventilation. Crit Care Med 2009;37:2888–2894, quiz 2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.