Abstract
Rationale: Isoniazid preventive therapy is effective in reducing the risk of tuberculosis (TB) in persons living with HIV (PLWH); however, screening must exclude TB disease before initiating therapy. Symptom screening alone may be insufficient to exclude TB disease in PLWH because some PLWH with TB disease have no symptoms. The addition of chest radiography (CXR) may improve disease detection.
Objectives: The objective of the present analysis was to compare the costs and effects of the addition of CXR to the symptom screening process against the costs and effects of symptom screening alone.
Methods: Using data from Botswana, a decision analytic model was used to compare a “Symptom only” policy against a “Symptom+CXR” policy. The outcomes of interest were cost, death, and isoniazid- and multidrug-resistant TB in a hypothetical cohort of 10,000 PLWH.
Measurements and Main Results: The Symptom+CXR policy prevented 16 isoniazid- and 0.3 multidrug-resistant TB cases; however, because of attrition from the screening process, there were 98 excess cases of TB, 15 excess deaths, and an additional cost of U.S. $127,100. The Symptom+CXR policy reduced deaths only if attrition was close to zero; however, to eliminate attrition the cost would be U.S. $2.8 million per death averted. These findings did not change in best- and worst-case scenario analyses.
Conclusions: In Botswana, a policy with symptom screening only preceding isoniazid-preventive therapy initiation prevents more TB and TB-related deaths, and uses fewer resources, than a policy that uses both CXR and symptom screening.
Keywords: tuberculosis, isoniazid preventive therapy, cost-effectiveness, human immunodeficiency virus, chest X-ray
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Isoniazid prophylactic therapy (IPT) reduces the risk of tuberculosis (TB) disease in HIV-infected persons. Screening for IPT must exclude TB disease in order to avoid treatment of TB with a single drug. The addition of chest radiography to symptom screening improves detection of TB but also presents logistical difficulties and increases cost to developing countries with high TB burdens.
What This Study Adds to the Field
Our economic analysis, even in best- and worst-case scenarios, showed that addition of the chest radiograph to symptom screening would result in an unacceptable loss of potential beneficiaries from receiving IPT. In Botswana, symptom screening alone before IPT is not only less costly, but it also prevents more TB deaths and cases.
Tuberculosis (TB) is a major health risk. An estimated one-third of the world's population is infected with Mycobacterium tuberculosis (1). Most of these individuals never develop TB disease or experience symptoms, a condition termed latent TB infection. However, persons living with HIV (PLWH) are at greatly increased risk of progressing to TB disease (2). The annual risk of TB disease in PLWH coinfected with M. tuberculosis is 5–10%, compared with a lifetime risk of less than 10% in HIV-uninfected individuals (3).
Botswana suffers a high HIV prevalence as well as one of the highest rates of TB worldwide: one-quarter of 15- to 49-year-olds were estimated to be infected with HIV (4), and the case notification rate of TB disease tripled between 1989 and 2002 to 623 cases per 100,000 population (5), and 84% of patients with TB were coinfected with HIV (6).
The progression from latent TB infection to TB disease can be prevented in PLWH through isoniazid (INH) preventive therapy (IPT), as demonstrated in clinical trials conducted in TB-endemic countries (7, 8). IPT substantially reduces the risk of TB disease in PLWH (9, 10) and is cost-effective in Africa (11–14).
Before commencing IPT, screening must exclude TB disease to avoid treatment of TB disease with a single drug, which could select for isoniazid-resistant (INH-R) microorganisms. World Health Organization (WHO, Geneva, Switzerland) guidelines recommend the use of chest radiography (CXR) in addition to symptom screening to exclude TB disease (15).
Since 2001, Botswana has implemented a nationwide IPT program for PLWH using symptom screening without CXR. During the pilot phase of this program, which used CXRs during screening, only 1 of 563 (0.2%) asymptomatic PLWH with TB disease were identified (16). Because of the low prevalence of TB disease in these asymptomatic PLWH, and because 18% of potential beneficiaries did not return for CXR during IPT screening (16), the individuals not returning for CXR remained at increased risk for TB disease because they would not have received IPT. An editorial accompanying this report highlighted the unnecessary increased cost to resource-constrained countries of CXR screening and urges the WHO to reconsider its recommendation of requiring a CXR for TB screening in asymptomatic PLWH. The Ministry of Health in Botswana decided that CXR screening should not be required routinely for asymptomatic PLWH during the IPT screening process, because—in its judgment—the harm from the high attrition outweighed the benefit of identifying few asymptomatic PLWH with TB disease.
From 2004 to 2006 a clinical trial for IPT was conducted, in which candidates from Botswana government clinics were screened (17). This clinical trial used symptom screening and CXR. Using CXR, 1.6% of asymptomatic PLWH were identified with prevalent TB disease (17). Although this proportion is substantially higher than the 0.2% found in the pilot study, decision-makers in Botswana were uncertain whether the benefits of using CXR to identify these additional cases of asymptomatic TB disease were sufficiently high to justify the costs and the risk of losing potential beneficiaries of IPT during the CXR screening process. TB control programs around Africa face a similar dilemma: on the one hand, CXR can decrease the number of PLWH who develop INH-R TB disease by avoiding provision of IPT to PLWH with asymptomatic TB disease; on the other hand, requiring CXR has negative consequences due to the obstacle presented to the patient resulting in attrition, as well as increased costs. The objective of the present analysis was to compare the costs and effects of the addition of CXR to the symptom-screening process against the costs and effects of symptom screening alone.
We compared three screening policies preceding IPT on the basis of the incremental cost per averted case of INH-R TB disease, multidrug-resistant (MDR) TB disease, any TB disease, and death. The three policies examined were as follows: (1) “Symptom” policy—IPT preceded by symptom screening alone, which is the current policy of the National IPT Program; (2) “Symptom+CXR” policy—IPT preceded by symptom and CXR screening; and (3) “Symptom+CXR+Tracking” policy—IPT preceded by symptom and CXR screening combined with an intensive effort to prevent any attrition during the CXR-screening process.
METHODS
A decision analytic model was designed (18) to estimate the costs and effects of three screening policies in a cohort of 10,000 PLWH (Figure E1 in the online supplement). The analysis was conducted from the Botswana health-care perspective with an analytic horizon of 3 years (duration of IPT is 0.5 yr plus the potential benefit from IPT of 2.5 yr [19]), using a discount rate of 3%. We assumed that 1.6% of asymptomatic PLWH had TB disease (17). For those PLWH who failed to receive IPT because of attrition, we assumed a baseline annual incidence of TB disease of 4.1% (3, 20–22). For the Symptom+CXR+Tracking policy we assumed 0% attrition. Parameter values were derived from the Botswana IPT Trial (17) and the literature (Table 1).
TABLE 1.
CLINICAL MODEL PARAMETERS AND DATA SOURCES
| Name | Description | Base-Case Estimate | Estimate in Reference | Reference | Range |
|---|---|---|---|---|---|
| Attrition | Attrition when adding CXR to IPT screening in programmatic setting | 18% | 18% | 16 | 0–24% |
| 24% | 24 | ||||
| 10% | 48 | ||||
| TB incidence | Annual incidence of TB (HIV-infected, regardless of CD4 count—no ART, TST ignored) | 4.1% | 9.7/100 py | 20 | 0.7–10% |
| 0.7–9.1/100 py | 3 | ||||
| 1.5–10/100 py | 22 | ||||
| 2.9/100 py | 21 | ||||
| Efficacy IPT | Efficacy of IPT in HIV+, TST ignored | 37.5% | 42% reduction | 9 | 33–42% |
| 33% reduction | 10 | ||||
| Duration IPT efficacy | Duration of the efficacy of IPT | 2.5 yr | 1.0 yr | 49 | 1–2.5 yr |
| 2.5 yr | 19 | ||||
| Risk INH-R TB | Risk of INH-R TB as a consequence of INH-monotherapy | 14% | 14% | 31 | 10–50% |
| 68% | 41 | ||||
| 48% | 39 | ||||
| 36% | 40 | ||||
| Abnormal CXR | Proportion of CXR in asymptomatic PLWH read as abnormal | 11% | 11% | IPT clinical trial | 5–20% |
| True abnormal CXR | Proportion of abnormal CXRs that are interpreted correctly as abnormal | 70% | 70% | IPT clinical trial (28) | 61–75% |
| 68% | 50 | ||||
| True abnormal CXR with TB disease | Proportion of abnormal CXRs that are interpreted correctly as abnormal, that also have TB disease | 14% | 14% | IPT clinical trial | 10–30% |
| False normal CXR | Proportion of truly abnormal CXRs that were misinterpreted as normal | 4% | 5.5% | IPT clinical trial (28, 29) | 1–9% |
| False normal CXR with TB | Proportion of truly abnormal CXRs that were misinterpreted as normal that have TB disease | 14% | 14% | IPT clinical trial | 10–30% |
| Normal CXR misread as abnormal | Proportion of normal CXRs that were misinterpreted as abnormal | 31% | 31% | IPT clinical trial | 10–30% |
| 25–39% | 28 | ||||
| 25–32% | 29 | ||||
| Treatment success, background TB | Treatment success rate for TB disease with any resistance | 70%* | 85% | 51 | 65–85% |
| 72% | 52 | ||||
| 72% | 33 | ||||
| Treatment success, INH-R TB | Treatment success rate for INH-R TB | 67%† | 82% | 51 | 62–82% |
| Treatment success, MDR TB | Treatment success rate for MDR TB | 40%* | 52% | 51 | 30–52% |
| Mortality, background TB | Mortality in PLWH during TB treatment without regard for drug susceptibility | 15% | 0–13% | 34 | 10–25% |
| 33%‡ | 53 | ||||
| 40%‡ | 8 | ||||
| 11% | 33 | ||||
| 7%§ | 51 | ||||
| 6%§ | 52 | ||||
| Mortality, INH-R TB | Mortality in PLWH during DOTS treatment for INH-R TB | 15%‖ | 2%§ | 51 | 10–25% |
| Mortality, MDR TB | Mortality DOTS MDR TB | 40% | 72–98% | 35 | 20–60% |
| 17% | 52 | ||||
| Background rifampicin resistance | Background resistance for rifampicin in Botswana | 2% | 2% | 30 | 0.5–10% |
Definition of abbreviations: ART = antiretroviral therapy; CXR = chest X-ray; DOTS = directly observed therapy short course; INH-R = isoniazid resistant; IPT = isoniazid preventive therapy; MDR = multidrug resistant; PLWH = persons living with HIV; py = person years; TB = tuberculosis; TST = tuberculin skin test.
Treatment success adjusted down because of poorer response in HIV-infected adults.
Treatment success for INH-R is not established for PLWH and so a conservative estimate is used. In the IPT trial treatment success for patients with INH-R TB, all of whom had cavitary disease, was 100% (four of four).
These statistics are quoted from the pre-ART era and likely reflect outcomes from that period. Treatment outcomes are better in Botswana, where ART is widely available.
Presumably these statistics were derived from mostly HIV-uninfected patients with TB.
A more conservative base-case statistic was used in our model than the reference cited in order to reflect statistics more typical for Botswana and HIV-infected patients with TB.
The incremental cost–effectiveness ratio (ICER) was calculated as the difference in costs divided by the difference in health effects between policies. Health effects were expressed in terms of deaths and new cases of TB disease, subdivided as background resistant, INH-R, and MDR TB disease. Primary and secondary outcomes were the incremental cost per death averted and per case of INH-R TB disease averted, respectively.
Screening to Identify PLWH Who Should Receive IPT
The Symptom policy screening procedures were identical to those of the National IPT Program: individuals with fever or cough of any duration, weight loss, AIDS-defining illness (23), a physical examination suspicious for TB disease, or no proof of HIV infection were excluded. A positive tuberculin skin test (TST) was not required, in accordance with WHO recommendations (15), and also because the addition of TST could result in 18–24% attrition (24, 25). In the Symptom+CXR and Symptom+CXR+Tracking policies, any asymptomatic individual not excluded by the Symptom policy was referred for CXR. PLWH with abnormal CXRs were assumed to receive an evaluation, to determine TB disease with three sputum smears and a clinical assessment by a physician; PLWH with a negative evaluation subsequently received IPT.
Attrition was defined as the refusal of PLWH to obtain a CXR or not receiving a CXR either due to the nonavailability of CXRs, or nonreturn for the CXR. We incorporated CXR interpretation errors by clinicians into the model. Proportions of truly abnormal CXRs interpreted as normal and truly normal CXRs interpreted as abnormal were derived from the Botswana IPT Trial (26) and are similar to results of other studies (27–29).
IPT
Success of IPT was defined as no TB disease for 2.5 years and IPT was assumed to impose a risk reduction of 37.5% in PLWH regardless of TST status, which translates into an annual TB incidence of 2.6% (instead of 4.1%) after receipt of IPT (9, 10). As these data are derived from intent-to-treat analyses from clinical trials, we assumed this efficacy incorporated typical adherence levels. We assumed a 14% risk of selecting INH-R TB in prevalent TB disease among asymptomatic PLWH with abnormal CXRs initiating IPT (see Discussion). We also assumed that beyond the background rate of infection with INH-R TB, IPT does not select for additional INH-R organisms.
MDR TB is combined INH-R and rifampicin-resistant TB. From the new cases of INH-R TB disease, we estimated the proportion of new MDR TB cases using the background rifampicin monoresistance rate of 2% (30), assuming that selection for INH-R microorganisms occurs at the same rate in pan-susceptible as in rifampicin-monoresistant cases.
Adverse events due to IPT are uncommon (0.75%) (31), and therefore their consequences were negligible for this analysis.
TB Treatment
TB disease in Botswana is treated in accordance with the WHO directly observed therapy short course (DOTS) recommendations (32). Successful TB treatment was defined as no TB disease for 3 years. We assumed that all PLWH with TB disease, whether detected during screening or not, received TB treatment once within the model's time frame and that patients were not previously treated. The outcomes of TB treatment were categorized as “Death,” “Success” (cured, completed treatment), or “Failure” (transfer, default, failure). TB treatment outcomes differed according to microorganism susceptibility: background resistance (i.e., includes pan-susceptible and TB with any resistance), INH-R, and MDR. The mortality of INH-R TB was assumed to be equal to that of TB disease with “background resistance.” In Botswana, empiric treatment for MDR TB disease consists of daily observed treatment with amikacin (an injected drug), ethionamide, pyrazinamide, and ciprofloxacin for 6 months, followed by 18 months of ethionamide and ciprofloxacin. TB treatment success rates for PLWH are similar to those for HIV-uninfected individuals, but they suffer higher rates of TB mortality (33, 34). TB treatment success and mortality from MDR TB are much poorer for PLWH than for HIV-uninfected persons but are likely ameliorated with antiretroviral therapy (ART) (33, 35).
Cost Data
Costs, reported in 2008 U.S. dollars, were calculated by multiplying resource use by unit costs (Table 2). The costs of labor and drugs were derived from Botswana's 2008 government salary scale and Central Medical Stores Price List, respectively (36). Using resource-based costing, we calculated the marginal costs of IPT, that is, the costs of adding one HIV-infected person to the IPT program. We assumed that PLWH lost after being sent for CXR (attrition) did not have a CXR performed and therefore incurred no CXR costs.
TABLE 2.
COST PARAMETERS IN 2008 U.S. DOLLARS AND DATA SOURCES
| Item | Resource Use | Unit | Cost |
|---|---|---|---|
| Total cost of IPT per additional patient | $16.97 | ||
| Nurse screening time (15 min during six follow-up visits) | 90 | min | $13.81 |
| Isoniazid (INH) 6 mo | 182 | DDD | $2.31 |
| Pyridoxine (B6) months | 182 | DDD | $0.85 |
| Total cost per CXR | $13.50 | ||
| Equipment costs per CXR | $2.86 | ||
| Film cost per CXR | $2.94 | ||
| Radiographer: administer CXR | 20 | min | $2.92 |
| Medical officer: reading CXR | 10 | min | $4.78 |
| Total cost per patient for DOTS (pan-susceptible) | $288.28 | ||
| Pharmacist: observation (10 min daily for 182 d) | 1,820 | min | $278.92 |
| Standard 182 d of DOTS treatment (HRZE2HR4) | 182 | DDD | $9.36 |
| Total cost per patient for DOTS (INH-R) | $294.40 | ||
| Pharmacist: observation (10 min daily for 182 d) | 1,820 | min | $278.92 |
| Standard 182 d of DOTS treatment (HRZE6) | 182 | DDD | $15.48 |
| Total cost per patient for second-line DOTS (MDR TB) | $3,865.61 | ||
| Nurse: observation and injection (10 min daily for 6 mo) | $279.23 | ||
| Pharmacist: observation (10 min daily for 18 mo) | $838.28 | ||
| Medical officer: medical evaluation (20 min monthly for 24 mo) | $229.24 | ||
| 6 mo of Amik+Ethio+PZA+Cipro and 18 mo of Ethio+Cipro | $2,518.86 | ||
| Total cost of evaluation for TB disease | $23.40 | ||
| Nurse: intake | 15 | min | $2.30 |
| Medical officer: clinical evaluation | 25 | min | $11.94 |
| Lab technician: performance of three sputum smears | 15 | min | $2.30 |
| Materials for sputum smear microscopy | 3 | $6.86 | |
| Total cost per patient for patient tracking | $30.39 | ||
| Assistant health educator: time spent tracking patient | 6 | h | $17.53 |
| Telephone airtime | $2.14 | ||
| Vehicle use cost | $10.71 |
Definition of abbreviations: Amik = amikacin; Cipro = ciprofloxacin; CXR = chest X-ray; DDD = daily defined dose; DOTS = directly observed anti-TB therapy short course; Ethio = ethionamide; HR4 = isoniazid plus rifampicin for 4 months; HRZE2, HRZE6 = isoniazid plus rifampicin plus pyrazinamide plus ethambutol for 2 and 6 months, respectively; INH-R = isoniazid resistant; IPT = isoniazid preventive therapy; MDR TB = multidrug-resistant tuberculosis; PZA = pyrazinamide.
Salaries are based on the following hourly wages in Botswana: nurse (C4–C1 pay grade), $9.21; radiographer (C4–C2 pay grade), $8.77; medical officer (D4–D2 pay grade), $28.65; laboratory technician (C4–C2 pay grade), $9.20; and assistant health educator (B1–B5 pay grade), $2.92 [reported in Adjustment of Salary Scales and Review of Allowances 2008 DP 2/5 XI (115) 02/22/2008. Public Service Management Directive No. 1 of 2008].
The Symptom+CXR+Tracking policy employed a full-time tracker who would spend 6 hours tracking each lost person and succeed in having him obtain his CXR and initiate IPT.
Sensitivity and Scenario Analysis
We performed step-wise one-way sensitivity on all model parameters, to evaluate the relative impact on the primary and secondary outcomes of the Symptom+CXR policy relative to the Symptom policy. Clinical parameters for the base-case scenario were varied over the ranges shown in Table 1 and cost parameters were varied from 75 to 125% of the base-case value (Table 2).
In addition, we investigated worst- and best-case scenarios for all three policies to assess the simultaneous impact of changes in several parameters. Assumptions that differed from their base-case value are shown in Table 3 and are derived from references in Table 1.
TABLE 3.
VALUES ASSUMED FOR MODEL PARAMETERS IN BASE-CASE, WORST-CASE, AND BEST-CASE SCENARIOS
| Base-Case Scenario (%) | Worst-Case Scenario (%) | Best-Case Scenario (%) | |
|---|---|---|---|
| Risk of selecting INH-R TB in asymptomatic HIV-infected adults with prevalent TB disease | 14 | 50 | 10 |
| Abnormal CXR rate in asymptomatic HIV-infected adults | 11 | 20 | 5 |
| Prevalent TB disease in asymptomatic HIV-infected adults with abnormal CXRs | 14 | 30 | 10 |
| Efficacy of IPT in HIV-infected adults (regardless of TST status) | 37.5 | 33 | 42 |
Definition of abbreviations: CXR = chest X-ray; INH-R = isoniazid resistant; IPT = isoniazid preventive therapy; TB = tuberculosis; TST = tuberculin skin test.
All other parameters remained identical to the base-case values shown in Table 1.
RESULTS
Base-Case Analysis
Table 4 shows cases and costs for a cohort of 10,000 PLWH over a time frame of 3 years. The baseline Symptom policy resulted in 618 new cases of TB disease and 21.6 new INH-R cases in asymptomatic PLWH with prevalent TB disease who were started on IPT. The Symptom+CXR policy reduced these cases of INH-R disease by 16 (−74%). On average, the Symptom+CXR policy cost an incremental $12.71 per patient ($127,100 for the cohort), resulting in an ICER of $7,900 per averted INH-R case (Table 5). Although reducing resistance, the Symptom+CXR policy indirectly increased the number of new cases of TB disease by +15.8%, due to attrition from the IPT program. These additional TB cases increase the total cost of this option and they also increase deaths by 13%. The Symptom+CXR policy is dominated by the Symptom policy, that is, switching from the Symptom to the Symptom+CXR policy increases deaths as well as costs.
TABLE 4.
EFFECT OF THREE PRE-ISONIAZID PROPHYLACTIC THERAPY SCREENING POLICIES ON DEATHS, NEW ISONIAZID-RESISTANT TUBERCULOSIS DISEASE, NEW MULTIDRUG-RESISTANT TUBERCULOSIS DISEASE, AND NEW TUBERCULOSIS DISEASE CASES FOR A COHORT OF 10,000 HIV-INFECTED ADULTS IN BOTSWANA OVER THREE YEARS
| Policy | New INH-R TB Disease | New MDR TB Disease | New TB Disease | Deaths | Total Costs |
|---|---|---|---|---|---|
| Symptom (baseline) | 21.63 | 0.44* | 618.50 | 116.53 | $395,100 |
| Symptom+CXR | 5.61 | 0.11* | 716.20 | 131.11 | $522,200 |
| Symptom+CXR+Tracking | 6.84 | 0.14* | 618.50 | 116.45 | $607,600 |
| Policy | Difference in New INH-R TB Disease | Difference in New MDR TB Disease | Difference in New TB Disease | Difference in Deaths | Difference in Costs |
| Symptom (baseline) | 0 | 0 | 0 | 0 | $0 |
| Symptom+CXR | −16.02 (−74%) | −0.33 (−74%) | 97.7 (16%) | 14.58 (13%) | $127,100 |
| Symptom+CXR+Tracking | −14.79 (−68.4%) | −0.301 (−68.3%) | 0 (0%) | −0.075 (−0.1%) | $212,500 |
Definition of abbreviations: CXR = chest X-ray; INH-R TB= isoniazid-monoresistant TB; MDR TB = multidrug-resistant TB; TB = tuberculosis.
The three policies are as follows: “Symptom,” in which only symptoms are used to exclude persons with TB disease; “Symptom+CXR,” in which chest radiography is added to assist in the screening process; “Symptom+CXR+Tracking,” in which both chest radiography and active patient tracking to achieve 0% attrition are added. Shown are the differences in cases and costs in comparison with the baseline “Symptom” policy.
These numbers indicate less than 1 case in the cohort of 10,000 HIV-infected adults.
TABLE 5.
INCREMENTAL COST–EFFECTIVENESS RATIOS OF TWO POLICIES EXPRESSED IN 2008 U.S. DOLLARS PER CASE OF ISONIAZID-RESISTANT TUBERCULOSIS AVERTED AND PER DEATH AVERTED RELATIVE TO “SYMPTOM” POLICY FOR A COHORT OF 10,000 HIV-INFECTED ADULTS
| Policy | Incremental Cost–Effectiveness Ratio in Dollars per Isoniazid-resistant TB Case Averted | Incremental Cost–Effectiveness Ratio in Dollars per Death Averted |
|---|---|---|
| Symptom+CXR | +$7,933 | Dominated |
| Symptom+CXR+Tracking | +$14,368 | +$2,816,061 |
Definition of abbreviations: CXR = chest X-ray; TB = tuberculosis.
The two policies shown are as follows: “Symptom+CXR,” in which chest radiography is added to assist in the screening process; and “Symptom+CXR+Tracking,” in which both chest radiography and active patient tracking to achieve 0% attrition are added. Both are compared with the baseline policy “Symptom,” in which only symptoms are used to exclude persons with TB disease. “Dominated” means that the policy is less effective (i.e., there are more deaths than with the “Symptom” policy; Table 4) and more costly than the baseline policy. A positive value means that the policy is more effective but also more costly than the baseline “Symptom” policy. The value of the cost–effectiveness ratio is the result of the incremental costs divided by the incremental effects. These ratios can be derived from the values in Table 4, but results may differ slightly due to rounding.
The Symptom+CXR+Tracking policy has an additional cost of $30 per person (Table 2) and offers all of the benefits of preventing INH-R TB as the Symptom+CXR policy, without the increase in the number of cases of TB disease due to attrition (and consequently deaths). However, the costs of the Symptom+CXR+Tracking policy are highest among the evaluated policies, due to additional tracking costs and the costs of more PLWH receiving CXRs and IPT. This policy results in an ICER of $14,400 per INH-R TB case averted, or $2,816,000 per death averted (Table 5). The increase in new INH-R and MDR TB cases with this policy compared with the Symptom+CXR policy is caused by the increased number of persons who receive IPT and become at risk of INH-R TB.
There was less than one case of MDR TB per 10,000 PLWH as a consequence of any policy: 0.44, 0.11, and 0.14 in the Symptom, Symptom+CXR, and Symptom+CXR+Tracking options, respectively.
Sensitivity Analysis
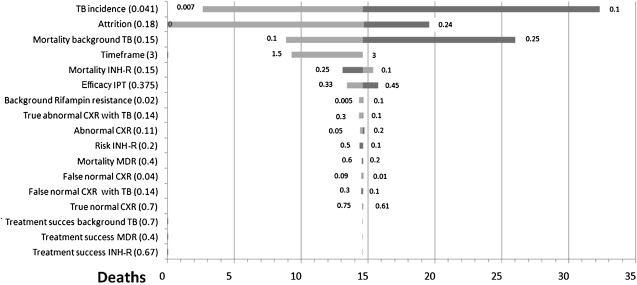
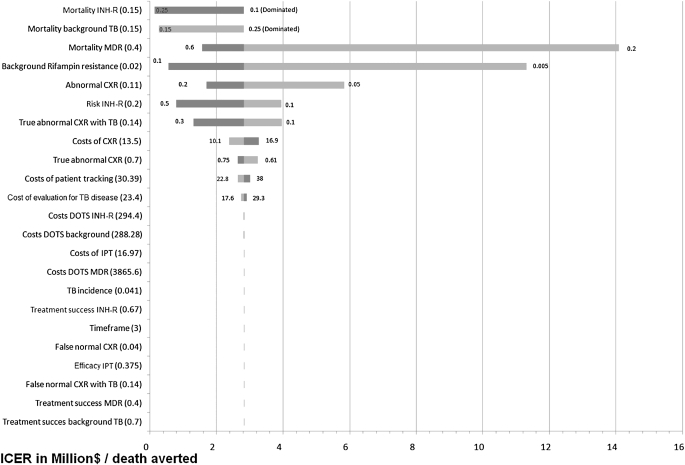
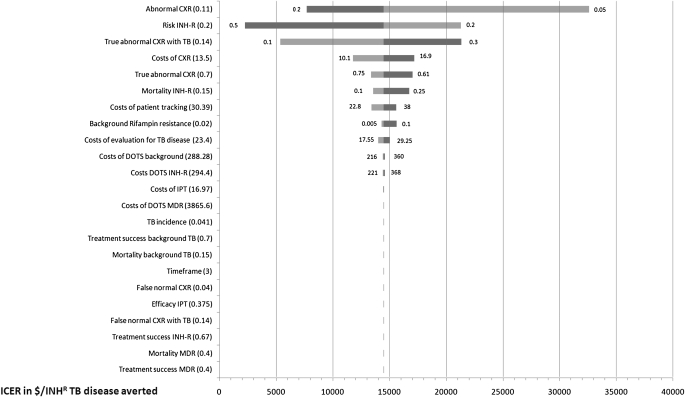
The tornado diagram in Figure 1 shows that even when using extreme values for clinical model parameters, the Symptom+CXR policy indirectly causes more deaths than the Symptom policy. Symptom+CXR reduces deaths only when the attrition is less than 0.2%. It is noteworthy that with a higher incidence of TB disease, this screening policy becomes even less beneficial. The tornado diagram in Figure 2 shows the impact of varied model parameters on the cost per death averted of the Symptom+CXR+Tracking policy relative to the Symptom policy. The ICER drops below $0.5 million per death averted only when using extreme values for the “background rifampin resistance,” “risk of INH-R,” “mortality INH-R,” and “Mortality background TB.” Figure 3 shows the impact of varying model parameters on the cost per case of INH-R TB disease averted, of the Symptom+CXR+Tracking policy relative to the Symptom policy.
Figure 1.
Tornado diagram showing the change in the number of incremental deaths in a cohort of 10,000 persons living with HIV (PLWH), using the “Symptom+CXR” policy versus the “Symptom” policy, through variation of the model parameters. Parameters are ordered from top to bottom by their impact on model outcome. Dark bars represent the upper limit and lighter bars the lower limit of the parameter value. The base-case value is shown in parentheses and 14.5 deaths represents the base-case comparison of the two policies, that is, there are 14.5 more deaths with the “Symptom+CXR” policy than the “Symptom” policy. The “Symptom+CXR” policy reduces deaths only when the attrition is near 0%. CXR = chest radiograph; INH-R = isoniazid-resistant TB disease; IPT = isoniazid preventive therapy; MDR = multidrug-resistant tuberculosis disease; TB = tuberculosis; Timeframe = post-IPT TB-free survival.
Figure 2.
Tornado diagram showing the change in the incremental cost–effectiveness ratio (ICER) of the “Symptom+CXR+Tracking” policy (0% attrition) compared with the “Symptom” policy, expressed in millions of U.S. dollars per death averted in a cohort of 10,0000 HIV-infected adults. Model parameters were varied as shown in Table 1 and cost parameters were varied from 75 to 125% of the base-case values shown in Table 2. Parameters are ordered from top to bottom by their impact on model outcome. Dark bars represent the upper limits and lighter bars the lower limits of the parameter value. The base-case values are shown in parentheses on the left and $2.8 million per death averted is the base-case ICER of the “Symptom+CXR+Tracking” policy compared with the “Symptom” only policy for a cohort of 10,000 HIV-infected adults. Not shown is the incremental cost–effectiveness ratio of using the lower limit for the mortality of isoniazid-resistant TB (10%) or the upper limit for the mortality of background resistant TB (25%), because in these cases the “Symptom+CXR” policy is dominated by the “Symptom” policy (the latter has fewer deaths and is less costly). CXR = chest radiograph; DOTS = directly observed therapy for TB disease; INH-R = isoniazid-resistant TB disease; IPT = isoniazid preventive therapy; MDR = multidrug-resistant tuberculosis disease; TB = tuberculosis.
Figure 3.
Tornado diagram showing the change in the incremental cost–effectiveness ratio of the “Symptom+CXR+Tracking” policy (0% attrition) compared with the “Symptom” policy, expressed in millions of U.S. dollars per new case of isoniazid-resistant TB averted in a cohort of 10,000 HIV-infected adults. Model parameters were varied as shown in Table 1 and cost parameters were varied from 75 to 125% of the base-case values shown in Table 2. Parameters are ordered from top to bottom by their impact on model outcome. Dark bars represent the upper limits and lighter bars the lower limits of the parameter value. The base-case values are shown in parentheses on the left and $14,400 represents the base-case ICER of adding CXR and Tracking per isoniazid-resistant case of TB averted in a cohort of 10,000 HIV-infected adults screened. CXR = chest radiograph; DOTS = directly observed therapy for TB disease; INH-R = isoniazid-resistant TB disease; IPT = isoniazid preventive therapy; MDR = multidrug-resistant tuberculosis disease; TB = tuberculosis.
Under the assumptions of the worst-case as well as the best-case scenarios, Symptom+CXR is also dominated by the Symptom policy, that is, switching from the Symptom to the Symptom+CXR policy increases deaths as well as costs. In the worst-case scenario, the ICER of the Symptom+CXR+Tracking policy compared with the Symptom policy is much lower than for our base-case scenario, at $906 per INH-R TB case averted, or $151,000 per death averted. In the best-case scenario the ICER of the Symptom+CXR+Tracking policy compared with the Symptom policy is much higher than for our base-case scenario, at $53,600 per INH-R TB case averted, or $17.0 million per death averted.
DISCUSSION
Adding CXR to a “Symptom” policy will reduce new INH-R and MDR TB cases; however, because of attrition by requiring CXR, this indirectly increases TB cases and TB deaths because fewer PLWH will benefit from IPT. Attrition must be close to 0% before deaths indirectly caused by a “Symptom+CXR” policy equal those in a “Symptom Only” policy. Although adding CXR also increases costs, a policy that includes intensive tracking efforts to eliminate attrition is not cost-effective by WHO standards at an incremental cost–effectiveness ratio of about $2.8 million per death averted. According to the WHO, ICERs greater than three times the GDP (3 × $6,982 = $20,946 for Botswana in 2008) per disability-adjusted life-year can be considered not cost-effective (37).
A rapid point-of-care diagnostic test with high sensitivity and specificity would be highly desirable to improve pre-IPT screening. By producing immediate results, such a hypothetical test would avoid attrition and patient-tracking costs. Ideally the net costs (the costs of screening minus the savings from averted disease and its treatment) of this strategy should be the same or lower than symptom screening alone, which would be the case if the hypothetical test cost is less than or equal to $0.3 per test per patient (data not shown). Until such a test becomes available, our analysis shows that provision of IPT to PLWH in endemic settings should not be hampered by additional screening modalities that would lead to more deaths in PLWH and unacceptably high costs to society. Investigators at Yale University (Ithaca, NY) used a mathematical model to determine the impact on the prevalence of INH-R TB of varying sensitivities of TB detection during screening for IPT (38). They included transmission of TB within the population and made an extreme assumption that 100% of PLWH with TB disease receiving IPT developed INH-R TB. Their model showed that improving TB screening sensitivity from 60 to 90% did not have a significant impact on INH-R TB transmission rates whereas IPT reduced TB by at least 12 cases per 100,000 PLWH each year. We investigated the consequences of adding CXR to the screening process in terms of cost, deaths, as well as the development of INH-R TB. Both the Yale analysis and ours agree that increasing the sensitivity of screening for TB disease before IPT is not of great societal value.
Our analysis has several important limitations. For a number of model parameters uncertainty existed around their values because of the paucity of published data; nevertheless, our conclusions remained robust in sensitivity and scenario analysis. For example, the outcome of our model is strongly driven by the risk of selecting for INH-R organisms when providing IPT to PLWH with asymptomatic TB disease and abnormal CXR at screening. This risk is not well established. Studies conducted in the precombination chemotherapy era in primarily symptomatic patients with cavitary (i.e., high bacillary load) TB disease showed that 36–68% developed INH-R disease because they were maintained on single-drug therapy for 6–12 months (39–41). The screening context of the present analysis is one in which PLWH with TB are asymptomatic, few have cavitary disease (14%, or 3 of 22 in the Botswana Trial; T.S., personal communication), and all are screened monthly for symptoms of TB. Furthermore unpublished data from the Botswana Trial showed that one of seven participants with radiographically evident disease who were taking INH and who developed symptomatic TB disease had INH-R TB (T.S., personal communication). When we varied this parameter to its upper limit (50% which was seen in the pre-DOTS era in symptomatic patients with cavitary TB receiving INH monotherapy) simultaneously with several others in our worst-case scenario, “Symptom+CXR” remained an unacceptable policy because of the increase in deaths due to attrition. Another example is factors that could affect the efficacy of IPT, such as adherence to therapy and the rate of TST positivity, which were not specifically modeled. However, the efficacy of IPT in our model was derived from intent-to-treat analyses, which would incorporate such inefficiencies, and, additionally, we reduced the efficacy of IPT in scenario analysis, which did not affect our major conclusion.
Our model did not incorporate the effects of the transmission of INH-R TB bacilli, which is estimated to be 70% as transmissible as pan-susceptible TB (42). This concern is unlikely to be of significance because TB transmission would occur not only among about 1.6% of participants with asymptomatic TB disease but also among a similar proportion lost through attrition due to the added requirement of a screening CXR. Furthermore, those enrolled in IPT are monitored monthly for TB symptoms, but those lost to the health care system are more likely to have a prolonged period of transmission before returning to the clinic. Finally, the majority of pulmonary TB in Botswana is smear negative (33), which presents a lower risk of transmission than smear-positive TB (43).
We examined models that included the costs of hospitalization during DOTS (∼$400 on average per patient) and the cost–effectiveness rankings were essentially unchanged; hence in this article we present the simpler models without hospitalization costs.
We did not include the effect of ART in reducing TB incidence, even though its beneficial effect is established (20, 44) and ART is widely available in Botswana (45). The reasons for not modeling the ART effect include the following: (1) approximately 70% of Botswana's PLWH do not receive ART because of the eligibility requirement of a CD4+ lymphocyte count below 250 cells/mm (45), whereas all HIV-infected adults are eligible for IPT; (2) although there are preliminary data showing the superior efficacy of the combination of ART and IPT (46, 47), this is not yet well established; and (3) the results of this analysis will be more generalizable to PLWH in TB-endemic countries that are resource constrained, as ART is not widely available in the majority of such countries whereas a 6-month course of IPT could be more readily available.
Our analysis implies that IPT would exert its greatest benefit when more PLWH receive prophylaxis. Therefore there is a need to increase the awareness for IPT among potential beneficiaries. The Ministry of Health in Botswana could promote awareness of the benefit of IPT among PLWH, as it has so successfully for ART. This source of motivation may also reduce attrition during the screening process and the 6-month follow-up period.
CONCLUSIONS
In Botswana, the negative effects of adding CXR screening to symptom screening for IPT outweigh the beneficial effect of preventing INH-R and MDR TB, because of attrition during the screening process. A CXR screening policy is beneficial only when attrition is close to 0%, but such a policy is unrealistic and unaffordable for resource-constrained countries such as Botswana.
Supplementary Material
Supported by a grant from the Centers for Disease Control and Prevention (Atlanta, GA).
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention.
This article has an online supplement, which is available from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201004-0620OC on December 10, 2010
Author Disclosure: T.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.B. has received a consulting fee from BaseCase and Becton Dickson Co. M.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. O.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.P. has served on the advisory board for SHIRE, MAPI, GlaxoSmithKline, Sanofi, and other pharmaceutical companies and has received grants from Sanofi, GlaxoSmithKline, and Wyeth; and is part owner of HECTA Consultancy. G.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC; WHO Global Surveillance and Monitoring Project. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country [consensus statement]. JAMA 1999;282:677–686. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Prevention and treatment of tuberculosis among patients infected with human immunodeficiency virus: principles of therapy and revised recommendations. MMWR Recomm Rep 1998;47(RR-20):1–58. [PubMed] [Google Scholar]
- 3.Girardi E, Raviglione MC, Antonucci G, Godfrey-Faussett P, Ippolito G. Impact of the HIV epidemic on the spread of other diseases: the case of tuberculosis. AIDS 2000;14:S47–S56. [PubMed] [Google Scholar]
- 4.Gaborone Central Statistics Office. Botswana AIDS impact survey, BAIS 2005. Gaborone, Botswana: Gaborone Central Statistics Office; 2005.
- 5.Botswana National Tuberculosis Control Program. Botswana National Tuberculosis Control Program annual report. Gaborone, Botswana: Epidemiology Section, Ministry of Health, 2002.
- 6.Gammino VM, Mboya JJ, Samandari T, Sheth A, Almquist J, Nkubito G, Jimbo W, Obita G, Roels TH, Wells CD, et al. Baseline evaluation of routine HIV testing among tuberculosis patients in Botswana. Int J Tuberc Lung Dis 2008;12:92–94. [PubMed] [Google Scholar]
- 7.Mwinga A, Hosp M, Godfrey-Faussett P, Quigley M, Mwaba P, Mugala BN, Nyirenda O, Luo N, Pobee J, Elliott AM, et al. Twice weekly tuberculosis preventive therapy in HIV infection in Zambia. AIDS 1998;12:2447–2457. [DOI] [PubMed] [Google Scholar]
- 8.Whalen C, Horsburgh CR Jr, Hom D, Lahart C, Simberkoff M, Ellner J. Site of disease and opportunistic infection predict survival in HIV-associated tuberculosis. AIDS 1997;11:455–460. [DOI] [PubMed] [Google Scholar]
- 9.Bucher HC, Griffith LE, Guyatt GH, Sudre P, Naef M, Sendi P, Battegay M. Isoniazid prophylaxis for tuberculosis in HIV infection: a meta-analysis of randomized controlled trials. AIDS 1999;13:501–507. [DOI] [PubMed] [Google Scholar]
- 10.Woldehanna S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev 2004;1:CD000171. [DOI] [PubMed] [Google Scholar]
- 11.Bell JC, Rose DN, Sacks HS. Tuberculosis preventive therapy for HIV-infected people in sub-Saharan Africa is cost-effective. AIDS 1999;13:1549–1556. [DOI] [PubMed] [Google Scholar]
- 12.Hausler HP, Sinanovic E, Kumaranayake L, Naidoo P, Schoeman H, Karpakis B, Godfrey-Faussett P. Costs of measures to control tuberculosis/HIV in public primary care facilities in Cape Town, South Africa. Bull World Health Organ 2006;84:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrestha RK, Mugisha B, Bunnell R, Mermin J, Hitimana-Lukanika C, Odeke R, Madra P, Adatu F, Blandford JM. Cost-effectiveness of including tuberculin skin testing in an IPT program for HIV-infected persons in Uganda. Int J Tuberc Lung Dis 2006;10:656–662. [PubMed] [Google Scholar]
- 14.Shrestha RK, Mugisha B, Bunnell R, Mermin J, Odeke R, Madra P, Hitimana-Lukanika C, Adatu-Engwau F, Blandford JM. Cost-utility of tuberculosis prevention among HIV-infected adults in Kampala, Uganda. Int J Tuberc Lung Dis 2007;11:747–754. [PubMed] [Google Scholar]
- 15.World Health Organization. Policy statement on preventive therapy against tuberculosis in people living with HIV—report of a meeting held in Geneva, 18–20 February 1998. WHO/TB/98.255. Geneva, Switzerland: World Health Organization; 1998.
- 16.Mosimaneotsile B, Talbot EA, Moeti TL, Hone NM, Moalosi G, Moffat HJ, Lee EJ, Kenyon TA. Value of chest radiography in a tuberculosis prevention programme for HIV-infected people, Botswana. Lancet 2003;362:1551–1552. [DOI] [PubMed] [Google Scholar]
- 17.Agizew TB, Arwady MA, Yoon JC, Nyirenda S, Mosimaneotsile B, Tedla Z, Motsamai O, Kilmarx PH, Wells CD, Samandari T. Tuberculosis in asymptomatic HIV-infected adults with abnormal chest radiographs screened for tuberculosis prevention. Int J Tuberc Lung Dis 2010;14:45–51. [PubMed] [Google Scholar]
- 18.TreeAge Software. TreeAge Pro 2009 software. Williamstown, MA: TreeAge Software; 2009.
- 19.Quigley MA, Mwinga A, Hosp M, Lisse I, Fuchs D, Porter JDH, Godfrey-Faussett P. Long-term effect of preventive therapy for tuberculosis in a cohort of HIV-infected Zambian adults. AIDS 2001;15:215–222. [DOI] [PubMed] [Google Scholar]
- 20.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet 2002;359:2059–2064. [DOI] [PubMed] [Google Scholar]
- 21.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis 2005;191:150–158. [DOI] [PubMed] [Google Scholar]
- 22.Glynn JR, Murray J, Bester A, Nelson G, Shearer S, Sonnenberg P. Effects of duration of HIV infection and secondary tuberculosis transmission on tuberculosis incidence in the South African gold mines. AIDS 2008;22:1859–1867. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance, African region. Geneva, Switzerland: World Health Organization; 2005.
- 24.Mugisha B, Bock N, Mermin J, Odeke RM, Miller B, tu-Engwau F, Granich R, Bunnell R. Tuberculosis case finding and preventive therapy in an HIV voluntary counseling and testing center in Uganda. Int J Tuberc Lung Dis 2006;10:761–767. [PubMed] [Google Scholar]
- 25.World Health Organization. Report of a “lessons learnt” workshop on the six ProTEST pilot projects in Malawi, South Africa and Zambia. 3–6 February 2004. WHO/HTM/TB/2004.336. Geneva, Switzerland: World Health Organization; 2004.
- 26.Agizew T, Bachhuber MA, Nyirenda S, Makwaruzi VZ, Tedla Z, Tallaksen RJ, Parker JE, Mboya JJ, Samandari T. Association of chest radiographic abnormalities with tuberculosis disease in asymptomatic HIV-infected adults. Int J Tuberc Lung Dis 2010;14:324–331. [PubMed] [Google Scholar]
- 27.Graham S, Das GK, Hidvegi RJ, Hanson R, Kosiuk J, Al ZK, Menzies D. Chest radiograph abnormalities associated with tuberculosis: reproducibility and yield of active cases. Int J Tuberc Lung Dis 2002;6:137–142. [PubMed] [Google Scholar]
- 28.World Health Organisation. Toman's tuberculosis case detection, treatment, and monitoring—questions and answers. Geneva, Switzerland: World Health Organization; 2004.
- 29.Zellweger JP, Heinzer R, Touray M, Vidondo B, Altpeter E. Intra-observer and overall agreement in the radiological assessment of tuberculosis. Int J Tuberc Lung Dis 2006;10:1123–1126. [PubMed] [Google Scholar]
- 30.Nelson LJ, Talbot EA, Mwasekaga MJ, Ngirubiu PK, Mwansa RA, Notha M, Wells CD. Antituberculosis drug resistance and anonymous HIV surveillance in tuberculosis patients in Botswana, 2002. Lancet 2005;366:488–490. [DOI] [PubMed] [Google Scholar]
- 31.Mosimaneotsile B, Mathoma A, Chengeta B, Nyirenda S, Agizew T, Tedla Z, Motsamai O, Kilmarx PH, Wells CD, Samandari T. Isoniazid tuberculosis preventive therapy in HIV-infected adults accessing anti-retroviral therapy: a Botswana experience, 2004–2006. J Acquir Immune Defic Syndr 2010;54:71–77. [DOI] [PubMed] [Google Scholar]
- 32.Botswana Ministry of Health. Botswana National Tuberculosis Program manual for health care workers. Gaborone, Botswana: Ministry of Health; 2007.
- 33.Botswana National Tuberculosis Control Program. Botswana National Tuberculosis Control Program annual report. Gaborone, Botswana: Epidemiology Section, Ministry of Health, 2005.
- 34.El-Sadr WM, Perlman DC, Denning E, Matts JP, Cohn DL. A review of efficacy studies of 6-month short-course therapy for tuberculosis among patients infected with human immunodeficiency virus: differences in study outcomes. Clin Infect Dis 2001;32:623–632. [DOI] [PubMed] [Google Scholar]
- 35.Wells CD, Cegielski JP, Nelson LJ, Laserson KF, Holtz TH, Finlay A, Castro KG, Weyer K. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis 2007;196:S86–S107. [DOI] [PubMed] [Google Scholar]
- 36.Public Service Management Directive No. 1 of 2008. Adjustment of salary scales and review of allowances. DP 2/5 XI (115) 02/22/2008.
- 37.World Health Organization. CHOosing Interventions that are Cost Effective (WHO-CHOICE). Available from www.who.int/choice/en/. [DOI] [PMC free article] [PubMed]
- 38.Basu S, Maru D, Poolman E, Galvani A. Primary and secondary tuberculosis preventive treatment in HIV clinics: simulating alternative strategies. Int J Tuberc Lung Dis 2009;13:652–658. [PubMed] [Google Scholar]
- 39.Anonymous. Isoniazid in pulmonary tuberculosis; report on a trial conducted under the auspices of the Medical Research Council of Ireland. Ir J Med Sci 1953;6:421–432. [PubMed] [Google Scholar]
- 40.Fox W, Sutherland I. The clinical significance of positive cultures and of isoniazid-resistant tubercle bacilli during the treatment of pulmonary tuberculosis; report to the Tuberculosis Chemotherapy Trials Committee of the Medical Research Council. Thorax 1955;10:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selkon JB, Devadatta S, Kulkarni KG, Mitchison DA, Narayana AS, Nair CN, Ramachandran K. The emergence of isoniazid-resistant cultures in patients with pulmonary tuberculosis during treatment with isoniazid alone or isoniazid plus PAS. Bull World Health Organ 1964;31:273–294. [PMC free article] [PubMed] [Google Scholar]
- 42.van Soolingen D, Borgdorff MW, de Haas PE, Sebek MM, Veen J, Dessens M, Kremer K, van Embden JD. Molecular epidemiology of tuberculosis in The Netherlands: a nationwide study from 1993 through 1997. J Infect Dis 1999;180:726–736. [DOI] [PubMed] [Google Scholar]
- 43.Kenyon TA, Creek T, Laserson K, Makhoa M, Chimidza N, Mwasekaga M, Tappero J, Lockman S, Moeti T, Binkin N. Risk factors for transmission of Mycobacterium tuberculosis from HIV-infected tuberculosis patients, Botswana. Int J Tuberc Lung Dis 2002;6:843–850. [PubMed] [Google Scholar]
- 44.Santoro-Lopes G, de Pinho AM, Harrison LH, Schechter M. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin Infect Dis 2002;34:543–546. [DOI] [PubMed] [Google Scholar]
- 45.Ojikutu B, Makadzange AT, Gaolathe T. Scaling up ART treatment capacity: lessons learned from South Africa, Zimbabwe, and Botswana. Curr Infect Dis Rep 2008;10:69–73. [DOI] [PubMed] [Google Scholar]
- 46.Golub JE, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, King BS, Efron A, Moore RD, Chaisson RE, Durovni B. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS 2007;21:1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Golub JE, Pronyk P, Mohapy L, Thsabangu N, Struthers H, Gray GE, McIntyre JA, Chaisson RE, Martinson NA. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS 2009;23:631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Day JH, Charalambous S, Fielding KL, Hayes RJ, Churchyard GJ, Grant AD. Screening for tuberculosis before isoniazid preventive therapy among HIV-infected gold miners in South Africa. Int J Tuberc Lung Dis 2006;10:523–529. [PubMed] [Google Scholar]
- 49.Johnson JL, Okwera A, Hom DL, Mayanja H, Mutuluuza KC, Nsubuga P, Nakibali JG, Loughlin AM, Yun H, Mugyenyi PN, et al. Duration of efficacy of treatment of latent tuberculosis infection in HIV-infected adults. AIDS 2001;15:2137–2147. [DOI] [PubMed] [Google Scholar]
- 50.van Cleeff MR, Kivihya-Ndugga LE, Meme H, Odhiambo JA, Klatser PR. The role and performance of chest X-ray for the diagnosis of tuberculosis: a cost–effectiveness analysis in Nairobi, Kenya. BMC Infect Dis 2005;5:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Espinal MA, Kim SJ, Suarez PG, Kam KM, Khomenko AG, Migliori GB, Baez J, Kochi A, Dye C, Raviglione MC. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA 2000;283:2537–2545. [DOI] [PubMed] [Google Scholar]
- 52.Santha T, Garg R, Frieden TR, Chandrasekaran V, Subramani R, Gopi PG, Selvakumar N, Ganapathy S, Charles N, Rajamma J, et al. Risk factors associated with default, failure and death among tuberculosis patients treated in a DOTS programme in Tiruvallur District, South India, 2000. Int J Tuberc Lung Dis 2002;6:780–788. [PubMed] [Google Scholar]
- 53.Harries AD, Nyangulu DS, Kang'ombe C, Ndalama D, Glynn JR, Banda H, Wirima JJ, Salaniponi FM, Liomba G, Maher D, et al. Treatment outcome of an unselected cohort of tuberculosis patients in relation to human immunodeficiency virus serostatus in Zomba Hospital, Malawi. Trans R Soc Trop Med Hyg 1998;92:343–347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.