Abstract
The National Institutes of Health has developed a comprehensive research program that includes research centers of excellence, individual research projects, small business projects, contracts, and interagency agreements to conduct basic, translational, and clinical research aimed at the discovery and/or identification of better medical countermeasures against chemical threat agents. Chemical threats include chemical warfare agents, toxic industrial and agricultural chemicals, and toxins and other chemicals that could be used intentionally as an act of terror or by large-scale accidents or natural disasters. The overarching goal of this research program is to enhance our medical response capabilities during an emergency. The program is named Countermeasures Against Chemical Threats (CounterACT). It supports translational research, applying ideas, insights, and discoveries generated through basic scientific inquiry to the treatment or prevention of mortality and morbidity caused by chemical threat agents. The categories of research supported under this program include creation and development of screening assays and animal models for therapy development, identification of candidate therapeutics, obtaining preliminary proof-of-principle data on the efficacy of candidate therapeutics, advanced efficacy and preclinical safety studies with appropriate animal models using Good Laboratory Practices (GLP), and clinical studies, including clinical trials with new drugs. Special consideration is given to research relevant to people who are particularly vulnerable, including the young, the elderly, and individuals with pre-existing medical conditions.
Keywords: chemical terrorism, translational research, National Institutes of Health
Industrial chemicals are relatively inexpensive and easy to obtain, and they have the potential to cause mass casualties when released intentionally as an act of terror, or by large-scale accidents or natural disasters. The traditional chemical warfare agents (CWAs) were developed during the first and second World Wars. They include chemicals such as sarin, soman, VX, sulfur mustard, and others. Several stockpiles of the chemicals still remain around the world today and represent a possible terrorism risk. Historically, there have been several chemical attacks that have resulted in mass casualties. For example, sulfur mustard and nerve agents were used against Iraqi Kurdish villages in the late 1980s, and more recently, nerve agents were used by the Japanese cult organization Aum Shinrikyo in two separate attacks against civilians in Japan (1–3). The civilian chemical threat spectrum includes chemical warfare agents, toxic industrial and agricultural chemicals, toxins, and other highly toxic chemicals (Table 1).
TABLE 1.
EXAMPLES OF CHEMICAL THREAT AGENTS
| Class | Agent | Target(s) |
|---|---|---|
| Traditional Chemical Warfare Agents (CWAs) | Sarin (GB) | Nerve |
| Soman (GD) | Nerve | |
| VX | Nerve | |
| Sulfur mustard (HD) | Lung, skin | |
| Toxic Industrial Chemicals (TICs) | Cyanide | Blood, cellular |
| Chlorine | Lung | |
| Phosgene | Lung | |
| Toxic Agricultural Chemicals | Parathion | Nerve |
| Chlorpyrifos | Nerve | |
| Insecticides | Disulfoton | Nerve |
| TETS | Nerve | |
| Strychnine | Blood, cellular | |
| Rodenticides | Sodium fluoroacetate | Blood, cellular |
Toxic industrial chemicals (TICs) such as cyanide, ammonia, pesticides, acids, and others are manufactured and stored in large volume at industrial facilities and transported across the nation for various uses. Agricultural chemicals of concern include insecticides such as parathion and rodenticides such as tetramethylenedisulfotetramine (TETS). The threat from these chemicals includes potential unintentional releases as well, due to large-scale industrial accidents or natural disasters like hurricanes. The threat from CWAs is mitigated by restricted access, difficulty in synthesis of purified agent, and international treaties against their use. But the TICs are not regulated as strictly, and many chemicals are readily available or stored in large enough quantities to pose a serious threat to human health if released. According to a 2003 report published by the General Accounting Office (4), the U.S. Environmental Protection Agency (EPA) has identified 123 chemical plants in the United States where a terrorist attack or accident could potentially expose more than one million people to a cloud of toxic gas. One of the most ubiquitous chemical threat agents is chlorine, which is the focus of the symposium reported in this issue. Unfortunately, there are too many examples of accidents in which chlorine has caused human mortality and morbidity, including the 2005 train disaster in Graniteville, South Carolina which killed nine and required treatment for chlorine exposure in many others (5, 6).
THE COUNTERACT PROGRAM
Requirements for an effective response to a civilian chemical attack or large-scale accident or natural disaster include: (1) post-exposure treatments that are effective within an often short therapeutic time window; (2) drugs and devices that can be used by medical personnel at the scene of the event or in a pre-hospital setting to treat many individuals; (3) drugs and devices that are appropriate for a diverse population including the pediatric, elderly, and individuals with pre-existing medical conditions; and (4) rapid and effective diagnostic technologies to determine the chemical agent and pathophysiology. Using these guiding principles, the National Institutes of Health (NIH) developed the Countermeasures Against Chemical Threats (CounterACT) Research Program, which supports the development of improved medical countermeasures that could be used in the event of chemical terrorist attack or accident.
The CounterACT Research Program includes Research Centers of Excellence, individual research projects, small business projects, contracts, and interagency agreements with the Department of Defense (see www.ninds.nih.gov/counteract). The network conducts basic, translational, and clinical research aimed at the discovery and/or identification of better medical countermeasures against chemical threat agents, and their movement through the regulatory process. The overarching goal of this research program is to enhance our medical response capabilities during an emergency.
SCOPE OF RESEARCH
The CounterACT Program is focused on the development of therapeutics and diagnostic tools for chemical threat agents. Research areas supported within this program include the development and validation of in vitro and animal models for efficacy screening of compounds, efficacy screening of compounds using these models, advanced efficacy and preclinical safety studies with appropriate animal models (including nonhuman primates) using current Good Laboratory Practices (GLP), and clinical studies, including clinical trials with new drugs. The program only supports translational research, defined as the process of applying ideas, insights, and discoveries generated through basic scientific inquiry to the treatment or prevention of human disease. Some examples of preclinical studies the program supports include:
Creation and development of validated screening assays for therapy development
Creation and validation of animal models of chemical effects on humans
Identification of candidate therapeutics using primary or secondary screening efforts
Development of preliminary proof-of-principle data on the efficacy of candidate therapeutics
Alternate routes of administration for new or approved therapies that would be safe, effective, and easy to administer during a mass casualty scenario
Alternate formulations of existing therapeutics that possess physical and chemical characteristics that improve their pharmacokinetics and produce longer shelf lives
Preclinical safety studies, formulation, and current Good Manufacturing Practice (cGMP) synthesis are also supported by the CounterACT program. This is done in part by a contract facility dedicated to this effort. Some more advanced studies the program supports include definitive efficacy studies for U.S. Food and Drug Administration (FDA) approval under the Animal Rule, and Phase I/II clinical trials in humans when appropriate.
Because of the urgency in need and lengthy time and expense in bringing a new compound to regulatory approval, investigators are encouraged to consider drugs that are already approved by the FDA for other indications. Some of these drugs have been shown to be effective in treating victims of chemical exposures, and in some cases, the length of time to regulatory approval for a new indication may be shorter than for a novel compound. CounterACT only supports translational research that is clearly relevant to the development of new or improved therapeutic drugs that will enhance our medical response capabilities during an emergency. New medical countermeasures that have no practical use during a mass casualty situation are not considered. Because many chemical threats have rapid modes of action, the drug should act rapidly to counter these effects. Drugs that are only effective if given prior to chemical insult (pretreatment), or those that must be given within a very short period (1–15 minutes) after the insult are of lower priority. Drugs that are only effective when administered intravenously are also of low priority, since their use would be impractical in a mass casualty situation. Special emphasis is placed on research relevant to people who are particularly vulnerable, including the young, the elderly, and individuals with pre-existing medical conditions.
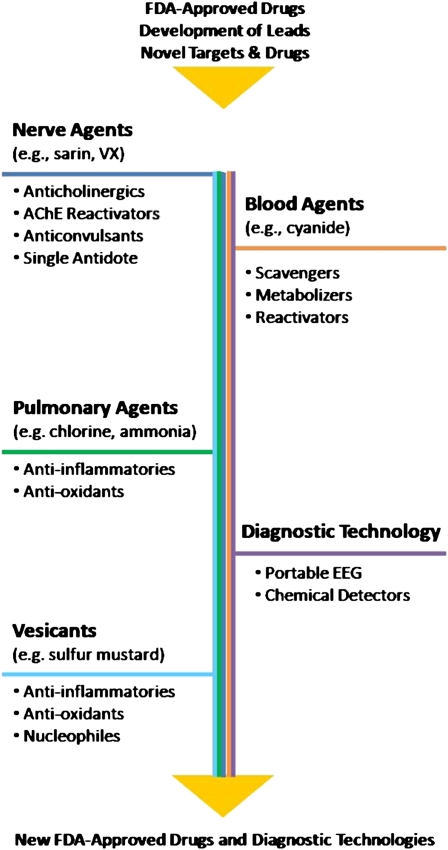
There are currently several different classes of therapeutics under study by CounterACT-supported investigators for the various chemical threat agents. These classes include anti-inflammatory compounds, antioxidants, anticholinergic drugs, enzyme reactivators, and others (Figure 1). Although the toxicities of the various threat agents are exerted by different mechanisms, the ideal medical countermeasure is envisioned to have protective effects against more than one class of chemical threat agent. For example, an anti-inflammatory or antioxidant drug that may be effective in preventing the formation of blisters and lesions due to a cutaneous exposure to a vesicating chemical could, in theory, be efficacious against some of the pulmonary toxicities of an inhalation exposure to a TIC, such as chlorine. Similarly, CounterACT investigators are working on antioxidants and other compounds that show promise as treatments for both chemical and radiation injuries. Broad spectrum activity makes a countermeasure much more attractive for procurement by local and federal health agencies, and possible inclusion in the Strategic National Stockpile.
Figure 1.
CounterACT research programs.
THE COUNTERACT PULMONARY AGENT RESEARCH PROGRAM
Many TICs produced and transported in high volume in the United States can severely disrupt normal pulmonary function and lead to respiratory failure if individuals are exposed to high enough levels. The volatility of many TICs and CWAs is of particular concern because of the ease with which many people can be exposed by inhalation. Of the several hundred TICs, sulfuric acid, ammonia, chlorine, nitric acid, and ammonium nitrate are among the most abundant. Ammonia and alkali agents like sodium hydroxide, as well as acids such as hydrochloric and sulfuric acid, are highly corrosive to the upper airways. Sulfur mustard, a highly corrosive CWA, targets the upper airways and can cause acute inflammation, painful ulcerations, increased secretions, and difficulties in breathing and swallowing. Damage to the upper airways can lead to respiratory failure and death. Exposure can also lead to long-term health problems. For example, chronic respiratory problems such as scarring and narrowing of the trachea have been observed in Iranians exposed to sulfur mustard during the Iran–Iraq War of the 1980s (7). Many TICs may reach the lower respiratory tract and cause acute life-threatening injuries such pulmonary edema. These include ammonia, chlorine, phosgene, and perfluoroisobutene. Individuals at greatest risk are those with pre-existing cardiopulmonary disease. Chlorine is a greenish-yellow volatile gas with pungent odor and is 2.5 times heavier than air as a gas. It produces severe pain and irritation almost immediately within the conjunctiva and mucous membranes in the nasal passages and upper airway. Chlorine is the focus of the present symposium and is discussed in other articles in this issue.
The need for pre-hospital treatments for exposure to pulmonary agents is evident because most of the current treatments can only be administered in a controlled hospital setting, and many hospitals are ill-suited for a situation involving mass casualties among civilians. Inexpensive positive-pressure devices that can be used easily in a mass casualty situation, and drugs to prevent inflammation and pulmonary edema, are needed. Several drugs that have been approved by the FDA for other indications hold promise for treating chemically induced pulmonary edema. These include β2-agonists, dopamine, insulin, allopurinol, nonsteroidal anti-inflammatory drugs (NSAIDs), and others. Some of these potential drugs target the inflammatory response or the specific site(s) of injury. Others target surfactant or modulate the activity of ion channels that control fluid transport across lung membranes.
It is clear for chemical agents that affect the pulmonary tract that basic mechanistic research is needed to discover new targets for therapeutic development. Research is also needed to test the effectiveness of the many drugs already approved by the FDA for other diseases and disorders with similar pathologies. The anti-inflammatory and antioxidant drugs probably hold the greatest potential at present as a first step for treating mass chemical exposures. Research will require the identification and validation of appropriate in vitro systems and animal models for preclinical testing of drugs to treat chemically induced injury to the upper and lower respiratory tract. Since is clear that some of the chemicals may cause long-term chronic health effects, studies are also needed to fully characterize these effects for the purpose of developing effective medical interventions. Finally, some chemicals generate metabolic byproducts that could be used for diagnosis, but detection of these byproducts may not be possible until many hours after initial exposure. Diagnostic tools and biological markers associated with acute lung injury are needed to help guide medical interventions in both the pre- and in-hospital settings.
SUMMARY AND CONCLUSIONS
Part of the overall strategy to enhance emergency preparedness involves improving our medical response capabilities to reduce casualties and the strain on the health care system after an emergency event. The NIH has developed a research and development program to enhance the Strategic National Stockpile and better prepare health care professionals for an emergency event involving the release of toxic chemicals. New research has identified several opportunities to develop even better medical intervention strategies. These include better therapies that treat the most severe symptoms, antidotes based on basic knowledge of the specific chemical agents such as those that target their metabolic pathways, and broad-spectrum drugs that target common physiological mechanisms of injury, such as anti-inflammatory drugs that could be used to treat victims exposed to many different kinds of chemicals and radiation injury. For more information about the NIH CounterACT Research Program or available funding opportunities, see www.ninds.nih.gov/counteract.
Conflict of Interest Statement: Neither author has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Kortepeter MG, Cieslak TJ, Eitzen EM. Bioterrorism. J Environ Health 2001;63:21–24. [PubMed] [Google Scholar]
- 2.Morita H, Yanagisawa N, Nakajima T, Shimizu M, Hirabayashi H, Okudera H, Nohara M, Midorikawa Y, Mimura S. Sarin poisoning in Matsumoto, Japan. Lancet 1995;346:290–293. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki T, Morita H, Ono K, Maekawa K, Nagai R, Yazaki Y. Sarin poisoning in Tokyo subway. Lancet 1995;345:980. [PubMed] [Google Scholar]
- 4.United States Government Accounting Office. Homeland security. Voluntary initiatives are under way at chemical facilities, but the extent of security preparedness is unknown. Washington, DC: GAO-03-24R; 2003.
- 5.Van Sickle D, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, et al. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med 2009;27:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenck MA, Van Sickle D, Drociuk D, Belflower A, Youngblood C, Whisnant MD, Taylor R, Rudnick V, Gibson JJ. Rapid assessment of exposure to chlorine released from a train derailment and resulting health impact. Public Health Rep 2007;122:784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghanei M, Harandi AA. Long term consequences from exposure to sulfur mustard: a review. Inhal Toxicol 2007;19:451–456. [DOI] [PubMed] [Google Scholar]